Arbutin Stabilized Silver Nanoparticles: Synthesis, Characterization, and Its Catalytic Activity against Different Organic Dyes
Abstract
1. Introduction
2. Results
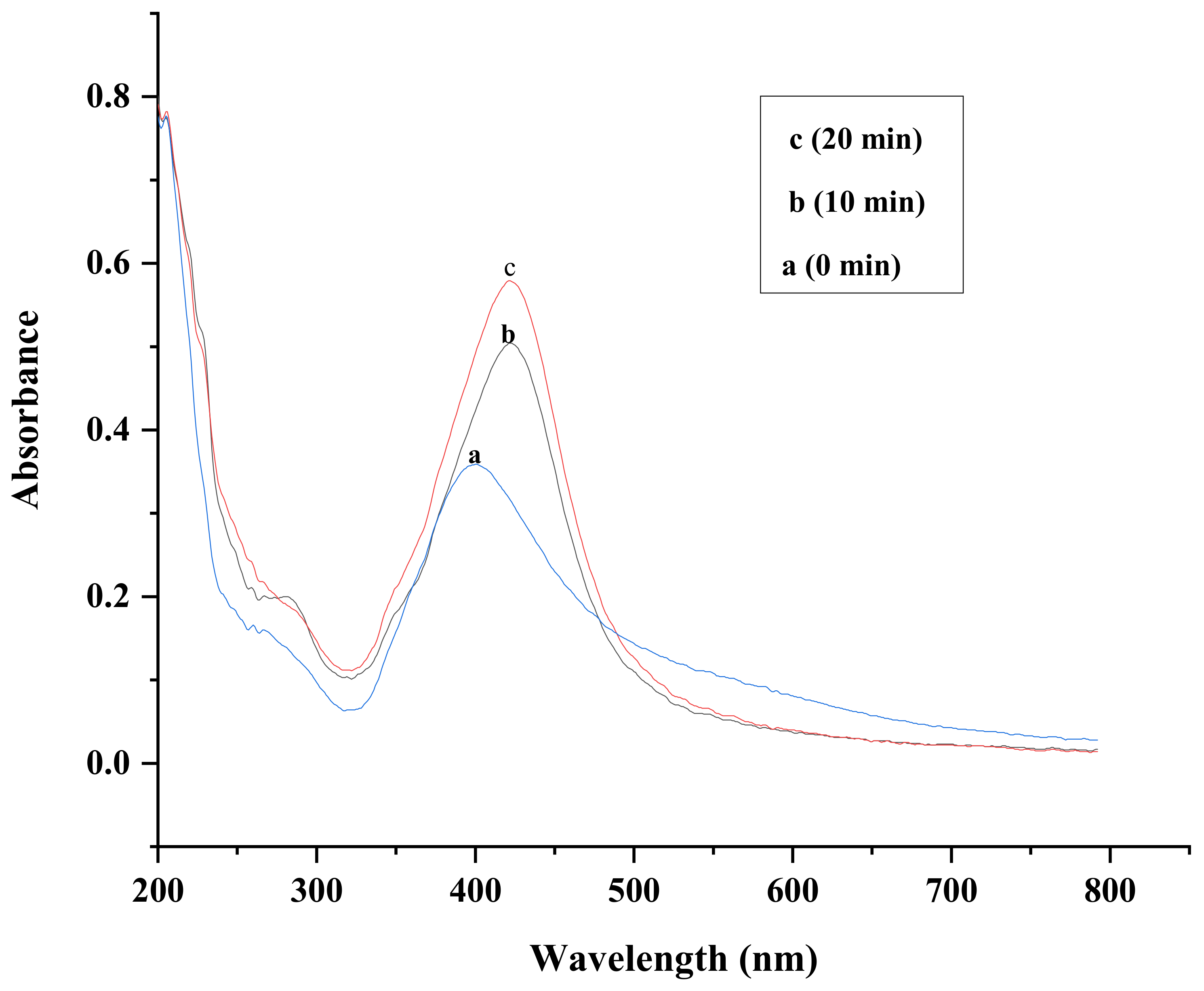
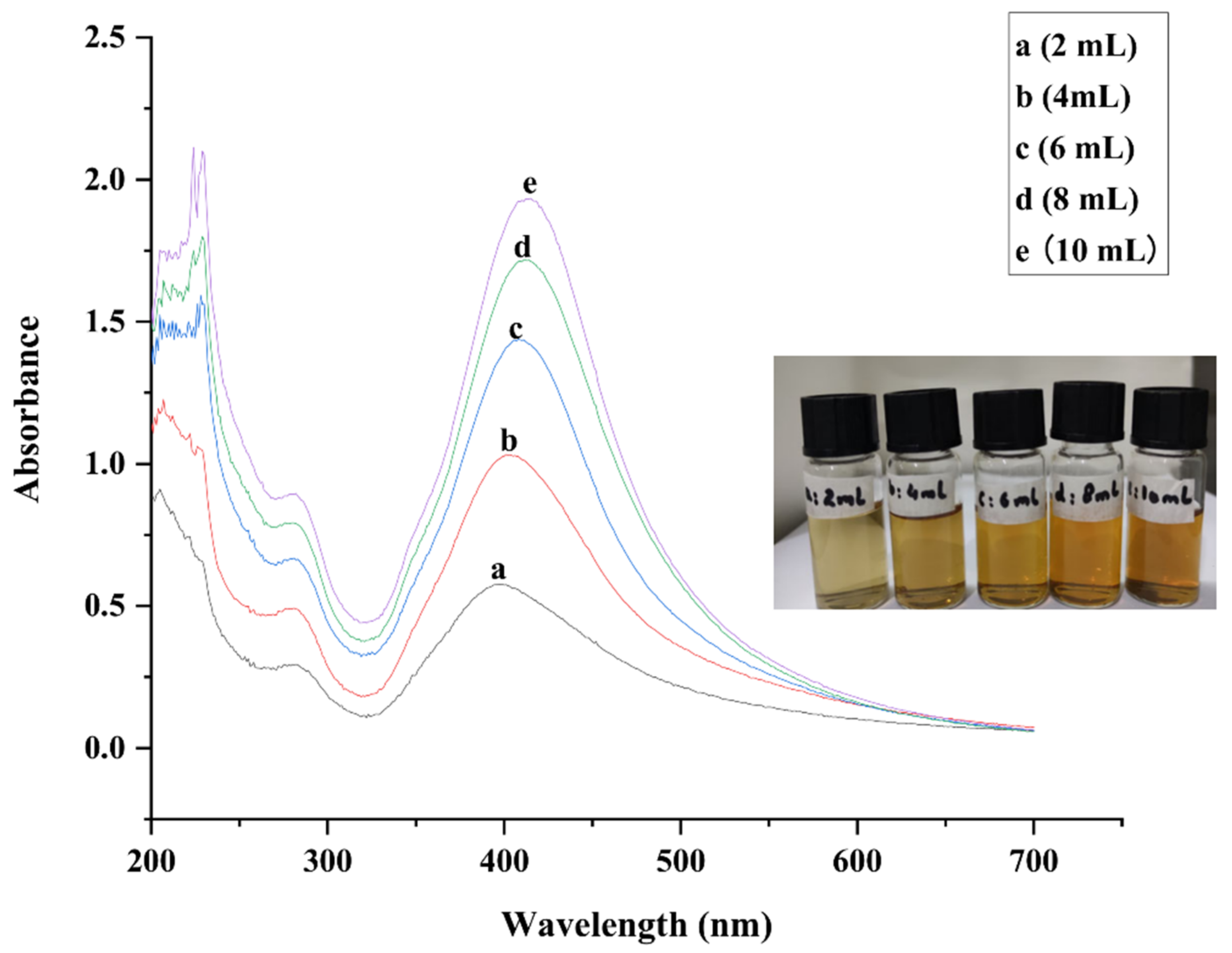
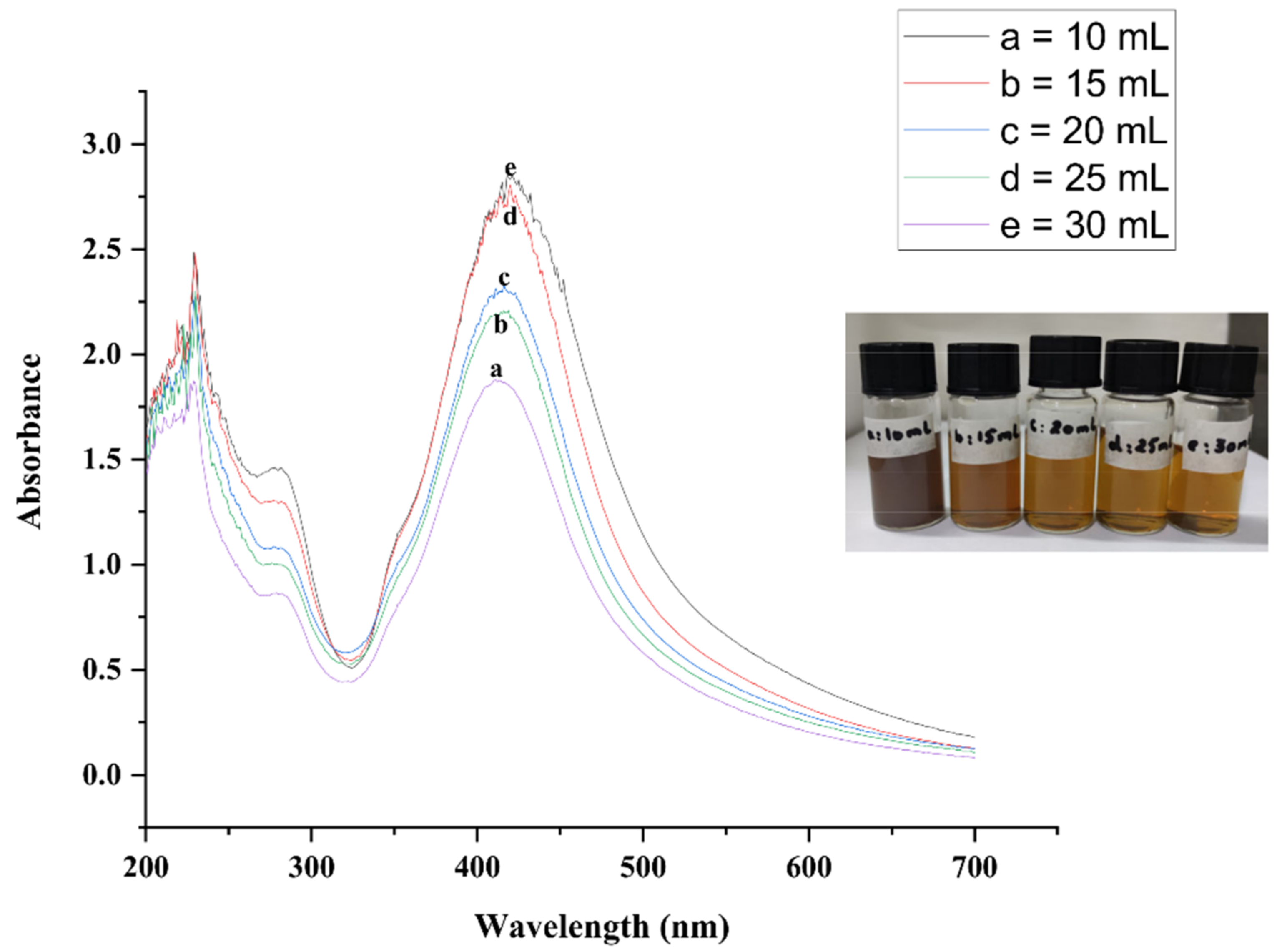
2.1. UV-Vis Spectroscopy
2.2. Dynamic Light Scattering and Zeta Potential
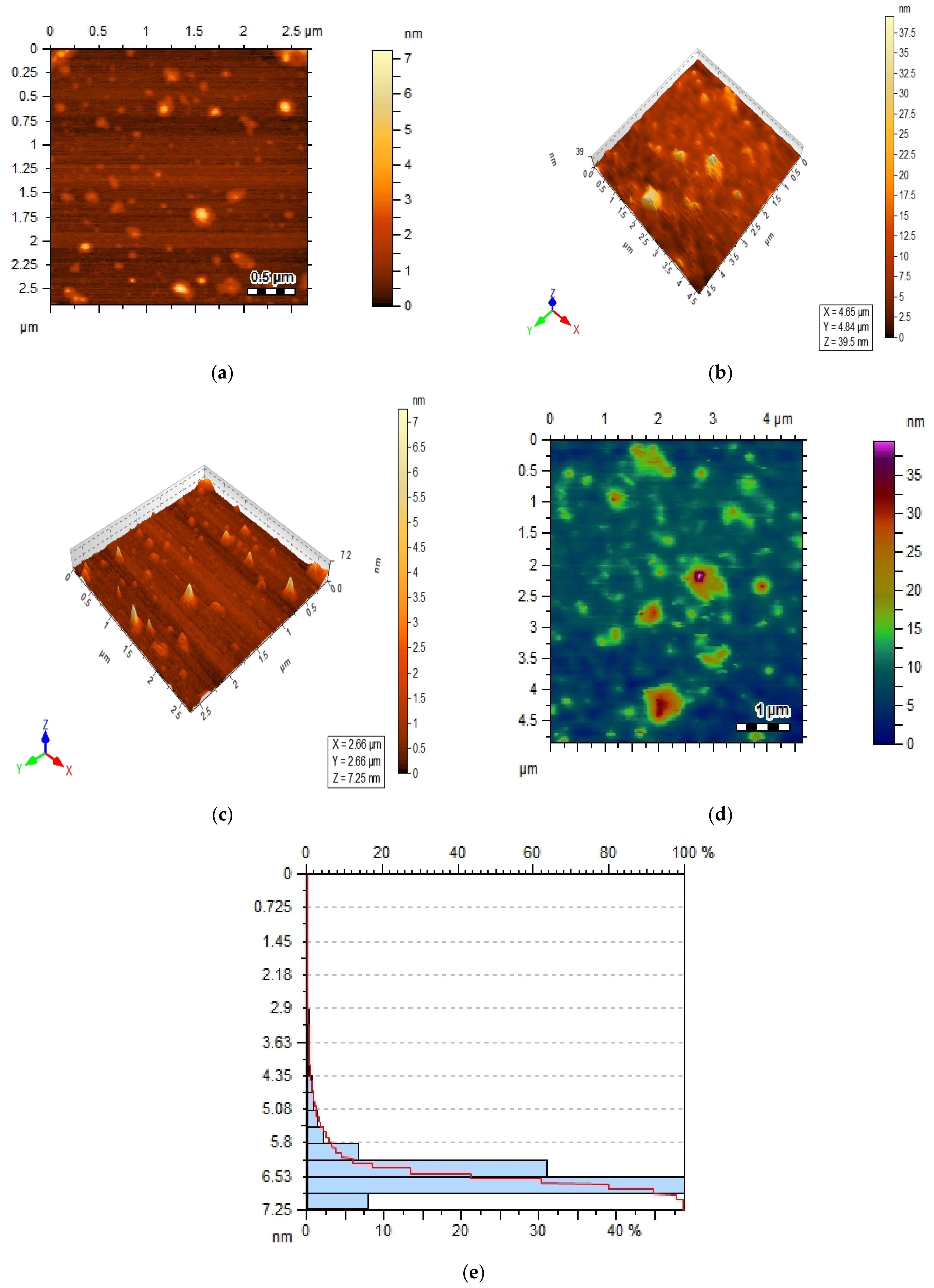
2.3. Atomic Force Microscopy
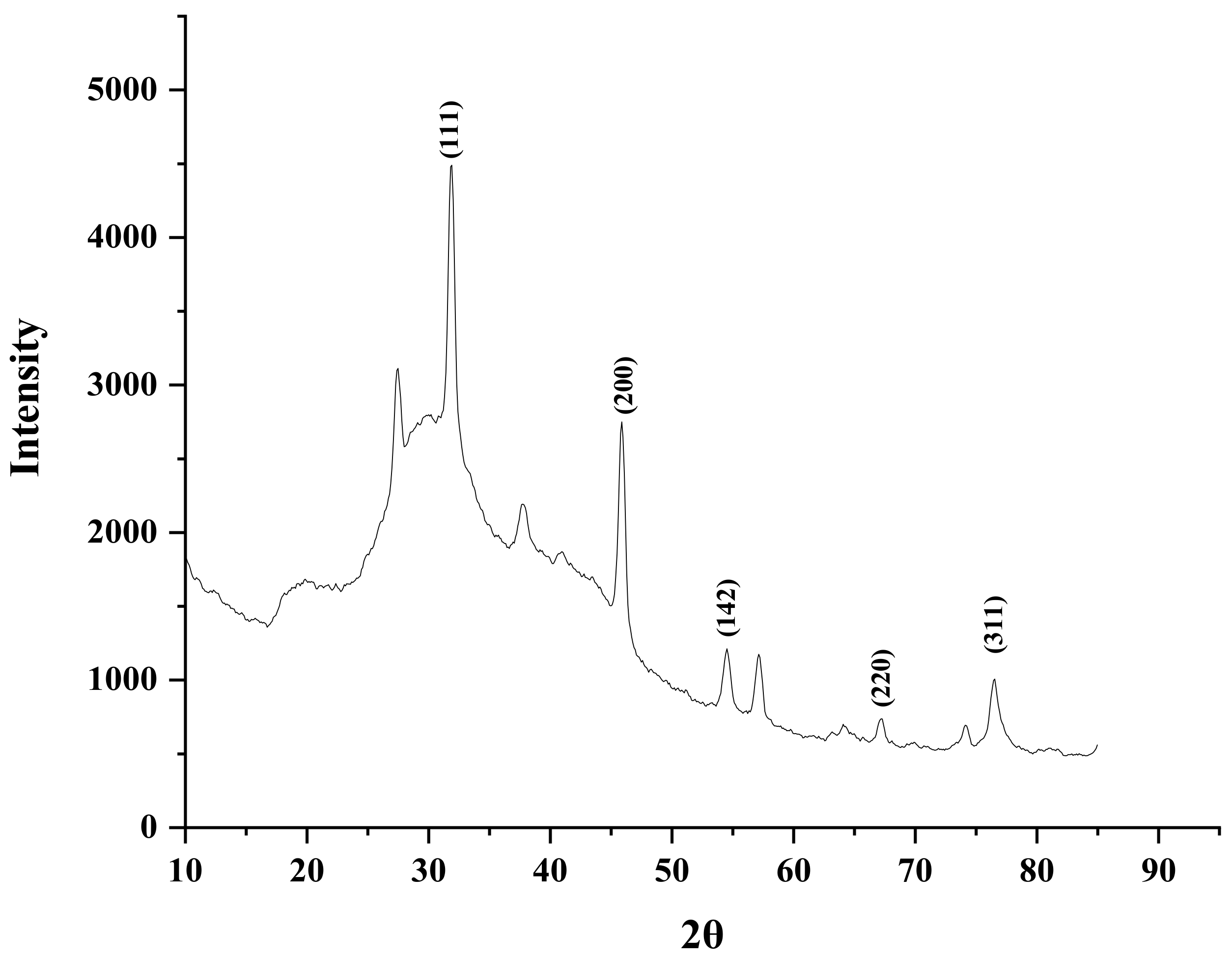
2.4. X-ray Diffraction
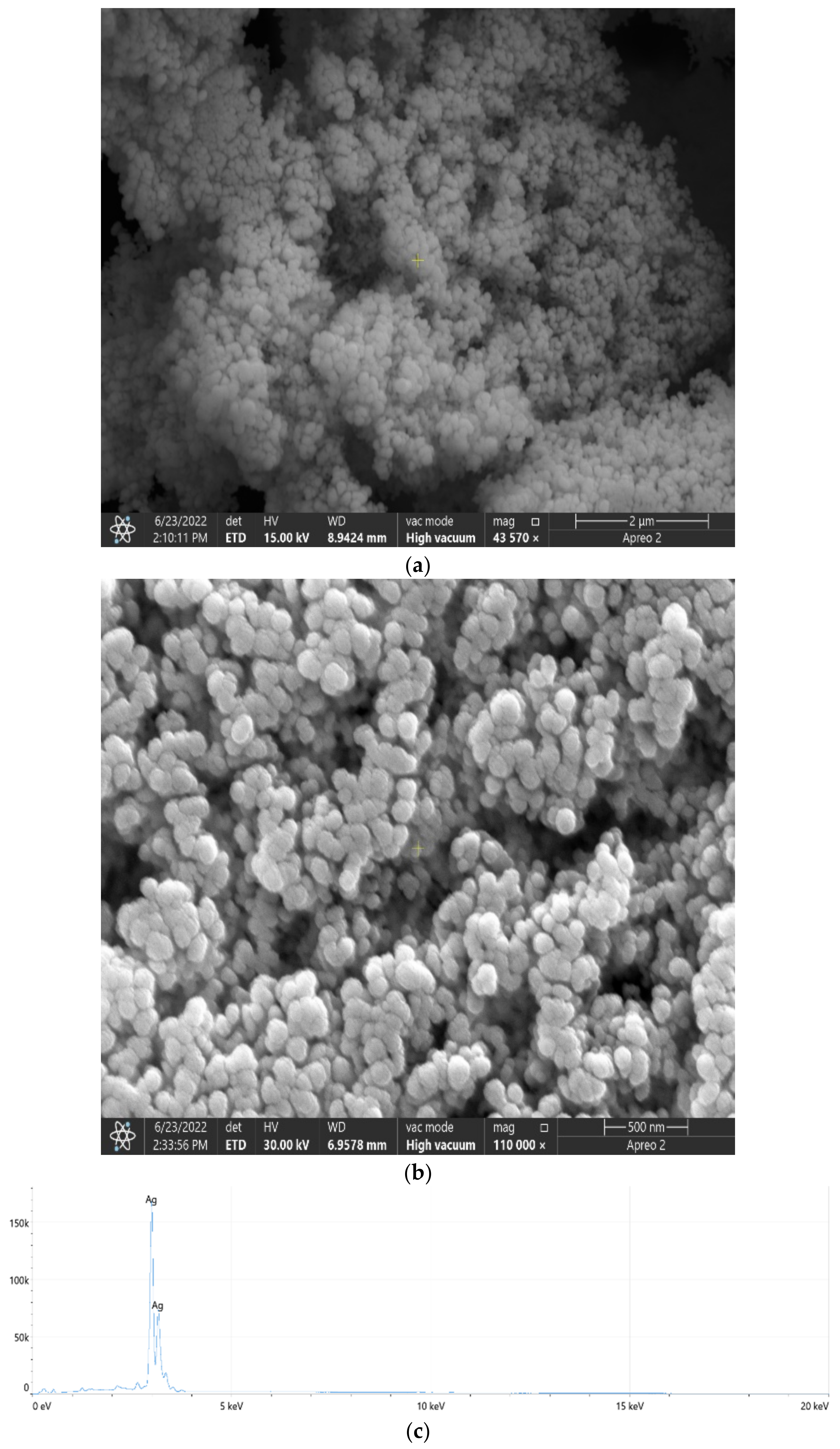
2.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
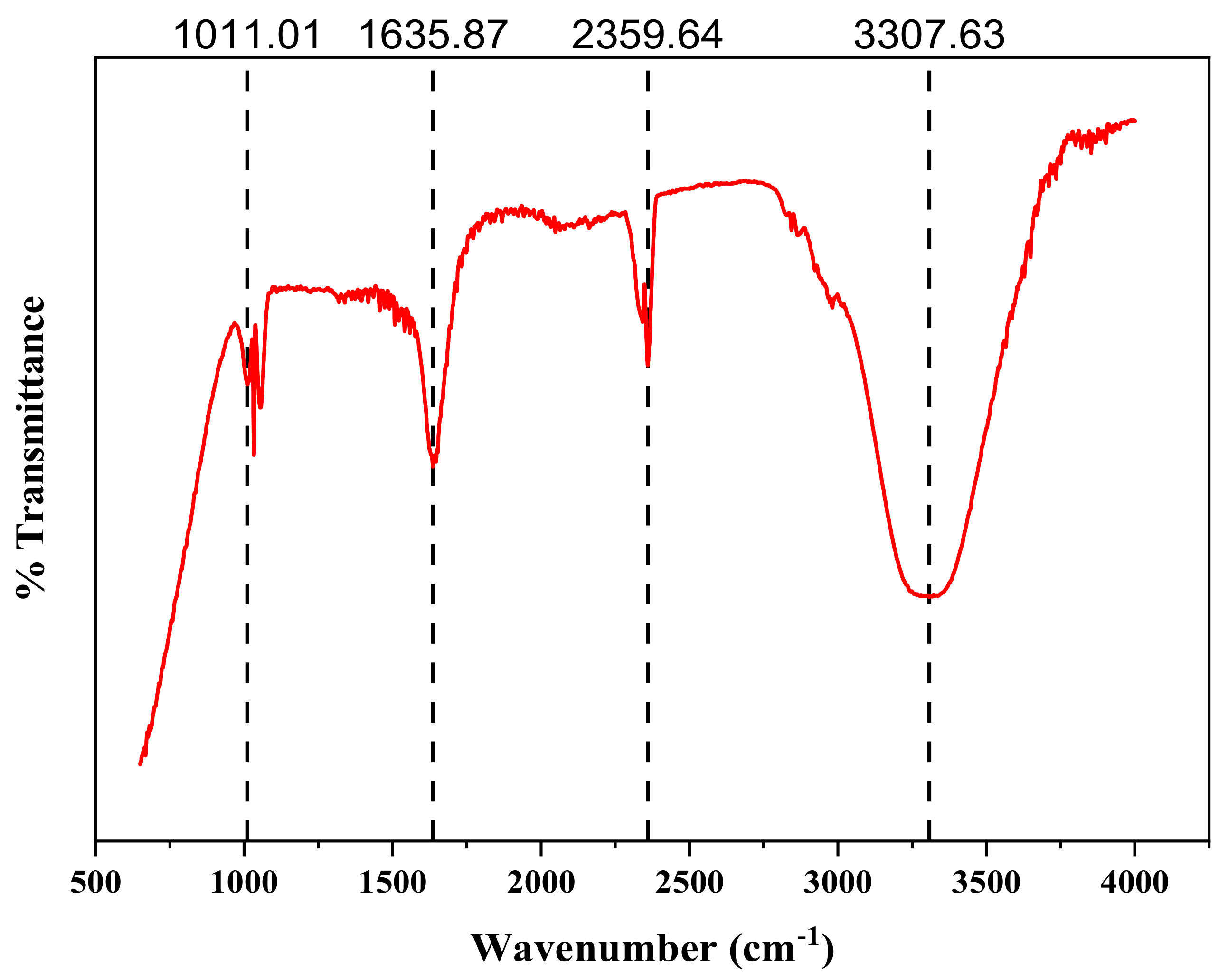
2.6. FTIR Analysis
2.7. Catalytic Activity
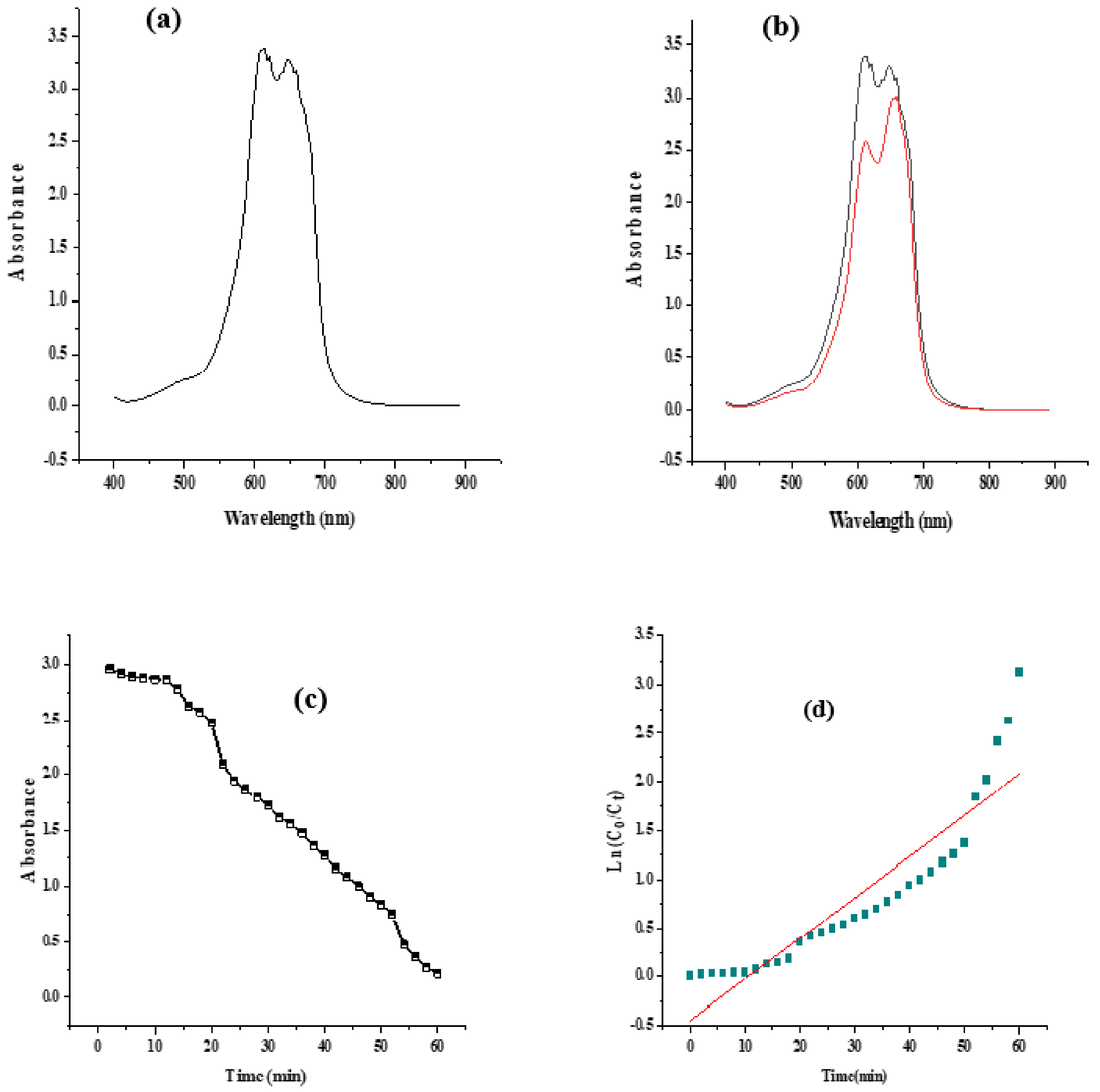
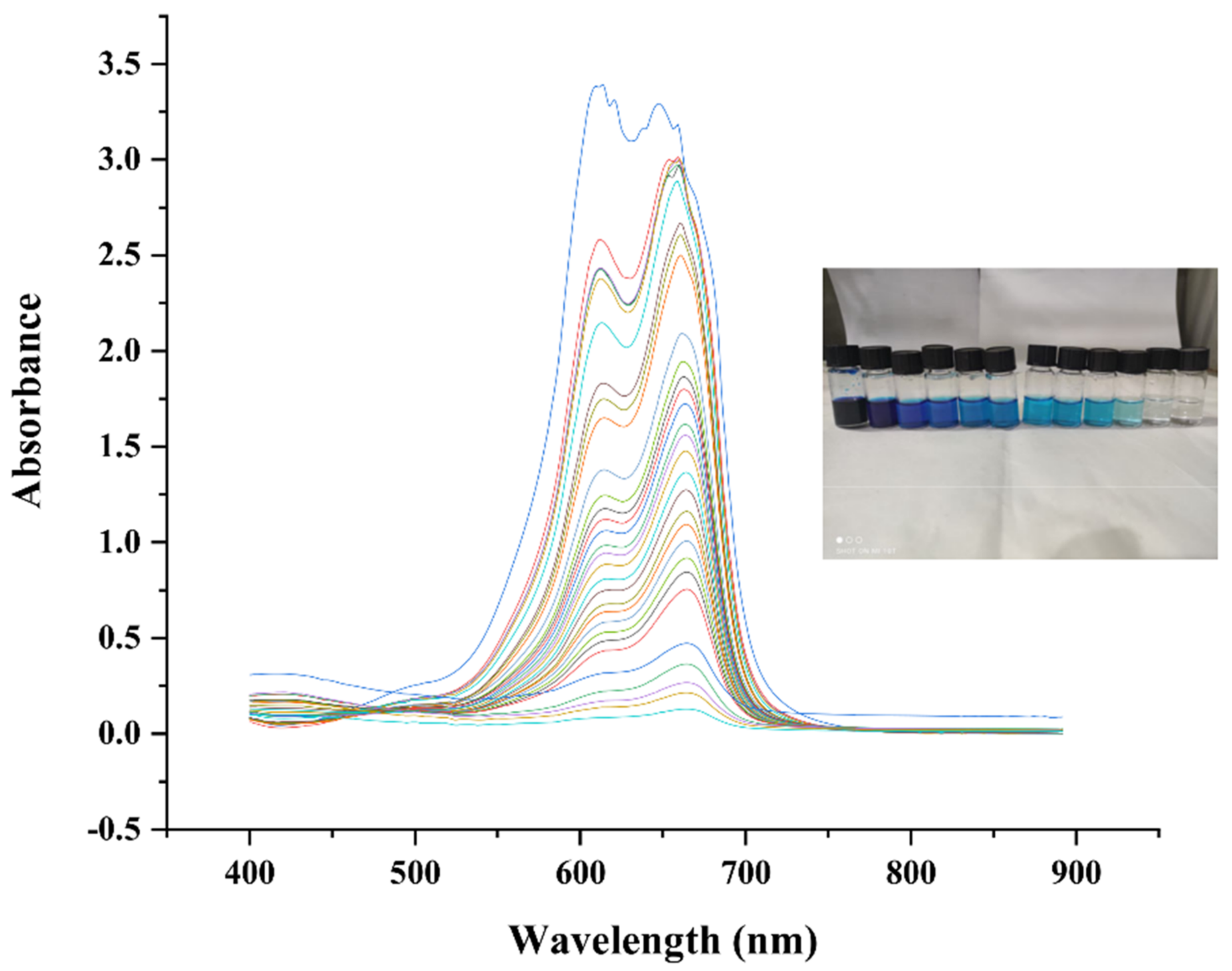
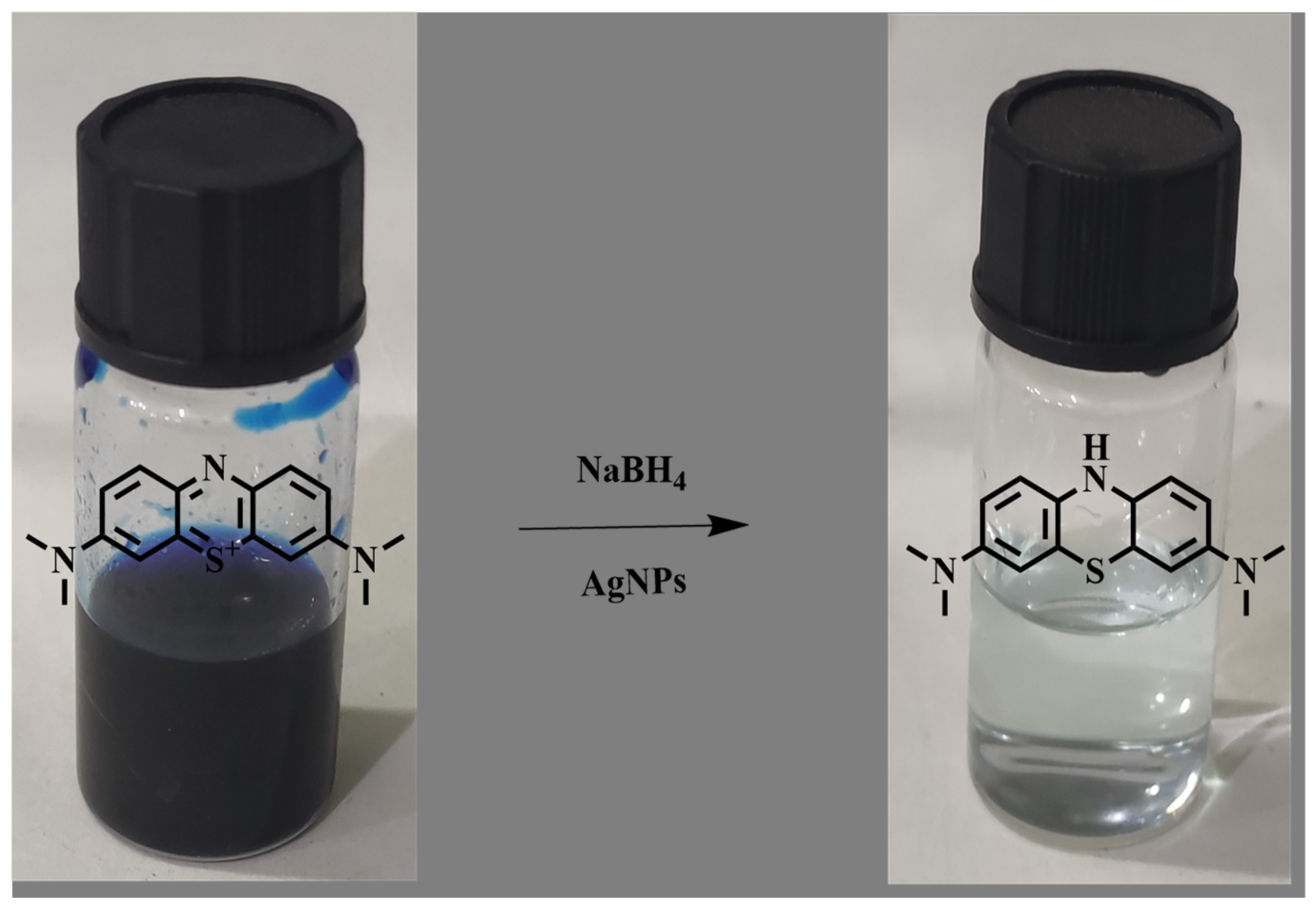
2.7.1. Catalytic Activity of Methylene Blue
2.7.2. Possible Mechanism of Degradation of Methylene Blue
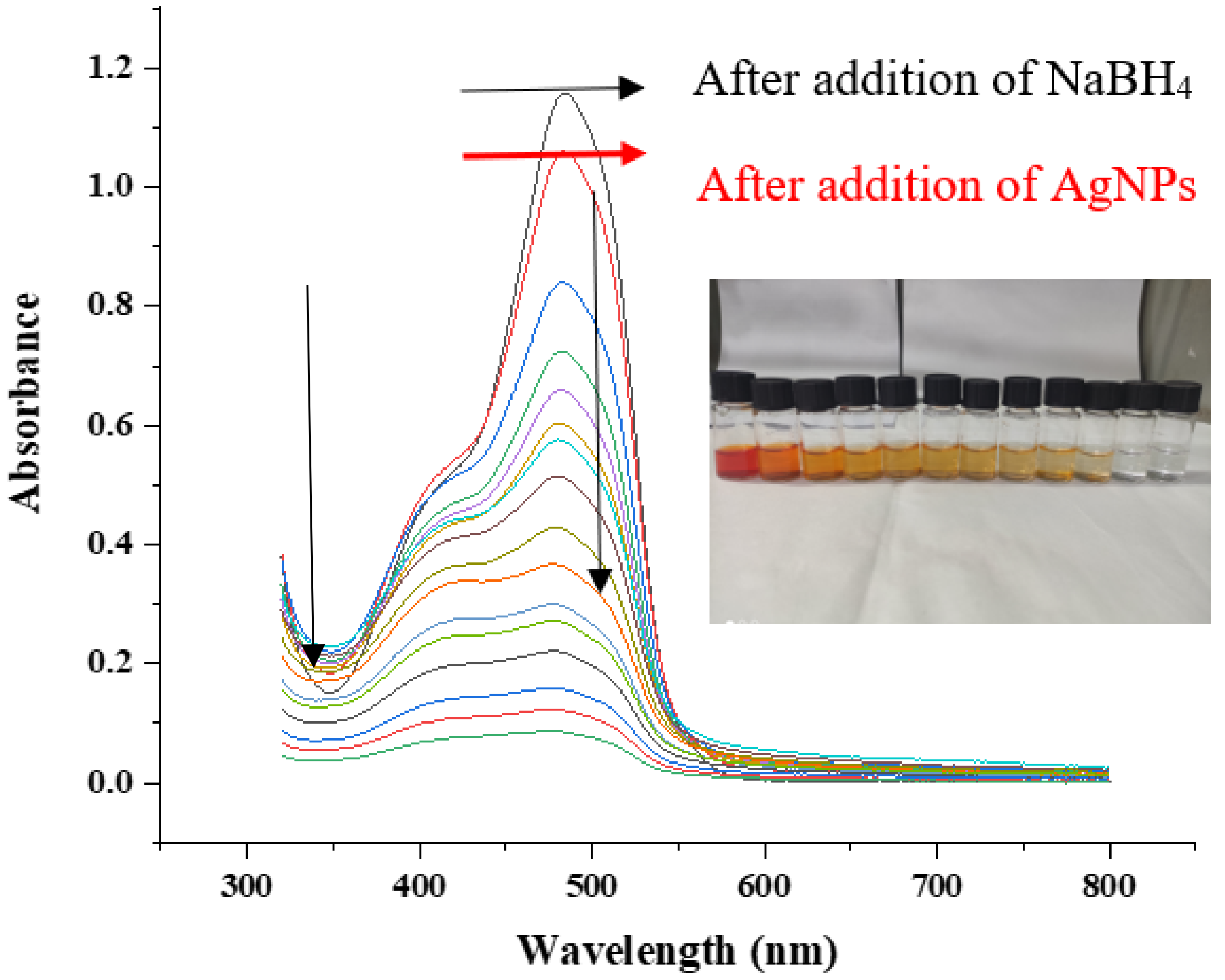
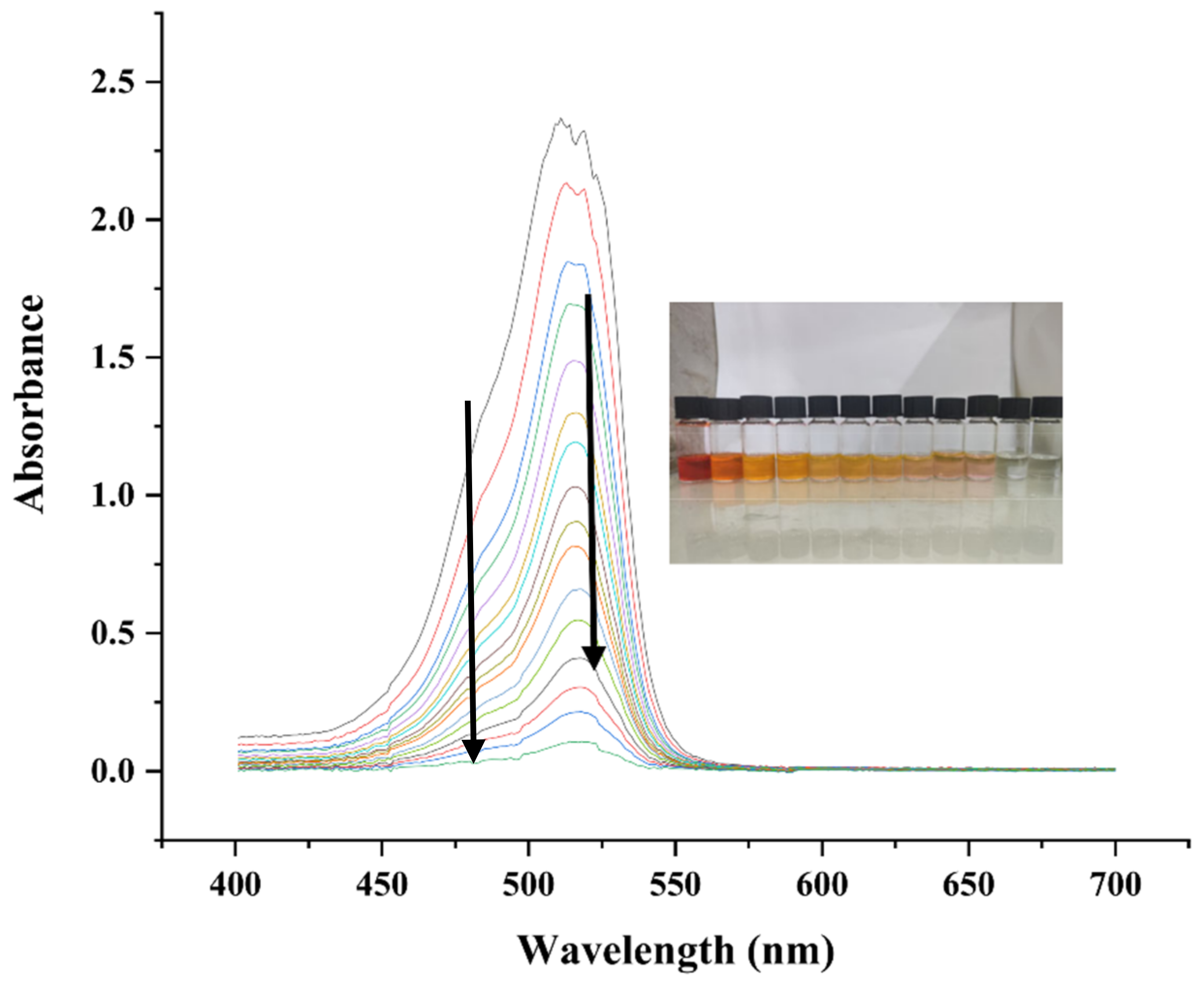
2.7.3. Catalytic Activity against Methyl Orange
2.7.4. Possible Mechanism of Catalytic Activity of Methyl Orange
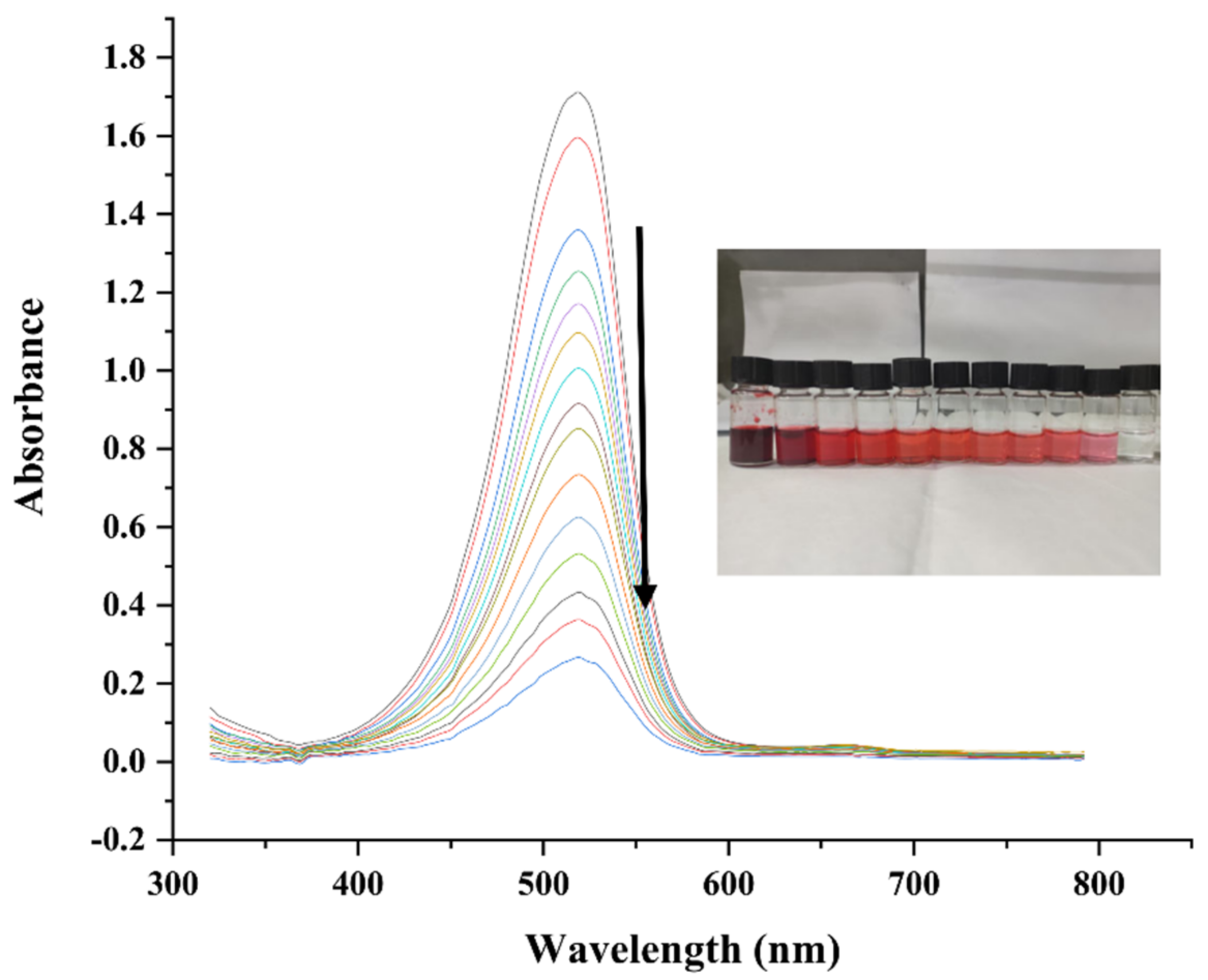
2.7.5. Catalytic Activity against Safranin O
2.7.6. Possible Mechanism of Degradation of Safranin O
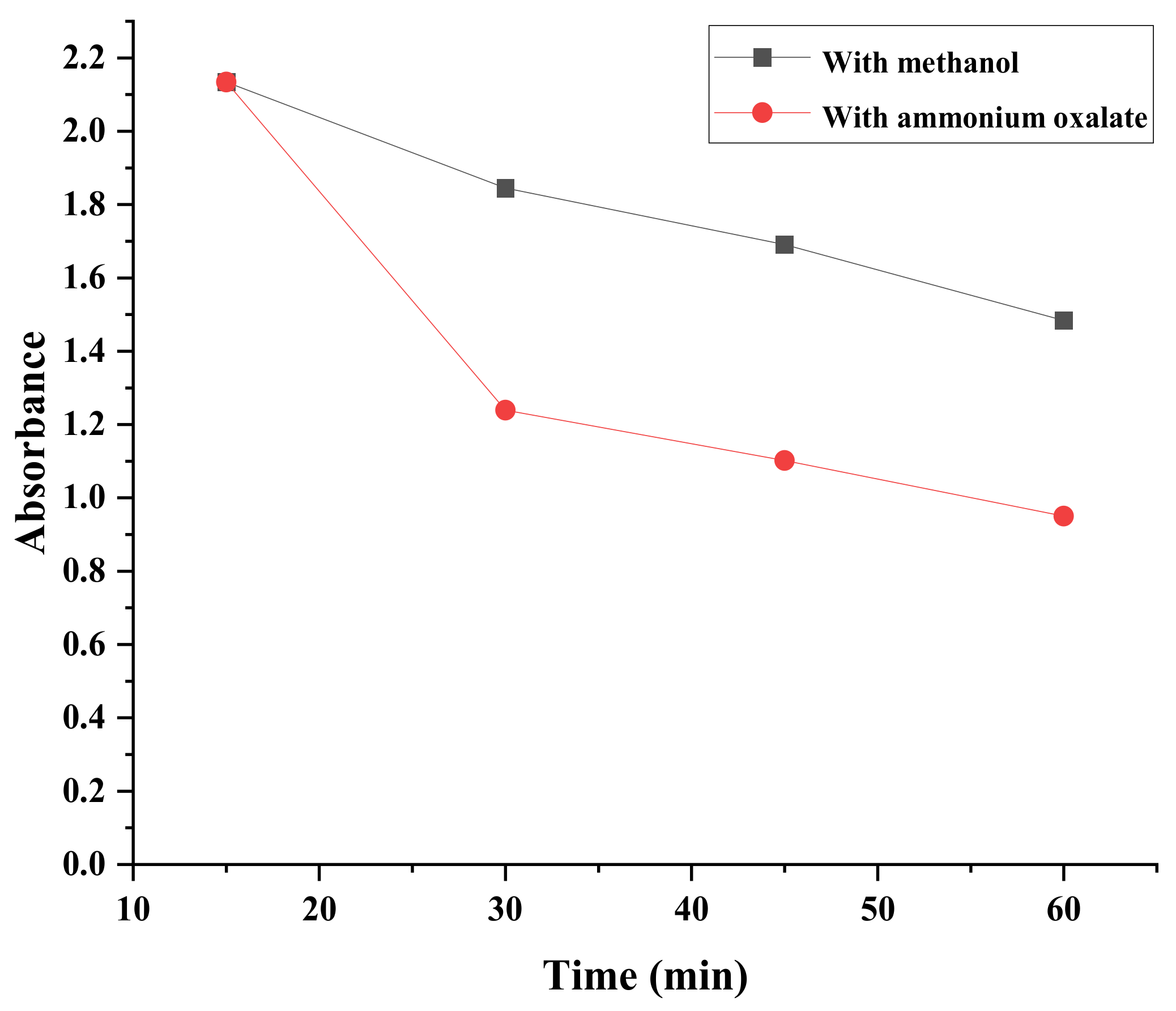
2.7.7. Catalytic Activity against Eosin Y
2.7.8. Possible Mechanism of Catalytic Degradation of Eosin Y
2.7.9. Quenching Study against Eosin Y
3. Experimental
3.1. Materials and Instrumentation
3.2. Synthesis of Silver Nanoparticles
3.3. Preparation of Dye’s Solution for Catalytic Activity
3.4. Characterization of Silver Nanoparticles Using UV-Vis, DLS, Zeta Potential, AFM, XRD, and SEM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 3, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnol. Adv. 2002, 20, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Fabrizi, E.; Palio, E.; De Maria, R. Colon cancer stem cells. J. Mol. Med. 2009, 11, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 10, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 15, 5580–5585. [Google Scholar] [CrossRef]
- Bin Hussein, M.Z.; Zainal, Z.; Yahaya, A.H.; Foo, D.W.V. Controlled release of a plant growth regulator, α-naphthalene acetate from the lamella of Zn–Al-layered double hydroxide nanocomposite. J. Control. Release 2002, 82, 417–427. [Google Scholar] [CrossRef]
- Mehrazar, E.; Rahaie, M.; Rahaie, S. Application of nanoparticles for pesticides, herbicides, fertilizers and animals feed management. Int. J. Nanopart. 2015, 1, 1–19. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 4, 655–663. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 29, 4411–4413. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 1, 76–83. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Alginate nanoparticles as antituberculosis drug carriers: Formulation development, pharmacokinetics and therapeutic potential. Indian J. Chest Dis. Allied Sci. 2006, 3, 171. [Google Scholar]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003, 9, 1261–1263. [Google Scholar] [CrossRef]
- Schabes-Retchkiman, P.S.; Canizal, G.; Herrera-Becerra, R.; Zorrilla, C.; Liu, H.B.; Ascencio, J.A. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt. Mater. 2006, 1, 95–99. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Yang, X. Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology 2007, 28, 285604. [Google Scholar] [CrossRef]
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Robson, M.C. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Inter. Wound J. 2007, 2, 114–122. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 2, 117–130. [Google Scholar] [CrossRef]
- Lansdown, A.B. Silver I: Its antibacterial properties and mechanism of action. J. Wound Care 2002, 4, 125–130. [Google Scholar] [CrossRef]
- Hugo, W.B. Types of antimicrobial agents. Disinfect. Preserv. Steriliz. 1992, 2, 7–88. [Google Scholar]
- Demling, R.H.; DeSanti, L. Effects of silver on wound management. Wounds 2001, 1, 4–15. [Google Scholar]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 4, 587–590. [Google Scholar] [CrossRef]
- Mujaddidi, N.; Nisa, S.; Al Ayoubi, S.; Bibi, Y.; Khan, S.; Sabir, M.; Qayyum, A. Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi J. Biol. Sci. 2021, 11, 6432–6440. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, L.; Zhang, H.; Min, Q.; Hou, W.; Zhu, J.J. Three-dimensional dendritic Pt nanostructures: Sonoelectrochemical synthesis and electrochemical applications. J. Phys. Chem. C 2008, 42, 16385–16392. [Google Scholar] [CrossRef]
- Mann, A.K.; Skrabalak, S.E. Synthesis of single-crystalline nanoplates by spray pyrolysis: A metathesis route to Bi2WO6. Chem. Mater. 2011, 4, 1017–1022. [Google Scholar] [CrossRef]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Mai, L.; Tian, X.; Xu, X.; Chang, L.; Xu, L. Nanowire electrodes for electrochemical energy storage devices. Chem. Rev. 2014, 23, 11828–11862. [Google Scholar] [CrossRef]
- Muhmood, T.; Cai, Z.; Lin, S.; Xiao, J.; Hu, X. Dimensions controllable synthesis of silver Nano-morphologies via moderate one step methodology. Adv. Powder Technol. 2021, 9, 3388–3394. [Google Scholar] [CrossRef]
- Kewes, G.; Herrmann, K.; Rodríguez-Oliveros, R.; Kuhlicke, A.; Benson, O.; Busch, K. Limitations of particle-based spasers. Phys. Rev. Lett. 2017, 23, 237402. [Google Scholar] [CrossRef]
- Chen, S.; Guan, Y.; Li, Y.; Yan, X.; Ni, H.; Li, L. A water-based silver nanowire ink for large-scale flexible transparent conductive films and touch screens. J. Mater. Chem. C 2017, 9, 2404–2414. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, T.; Jiang, W.; Yu, X.; Hu, Y.; Zhu, P.; Wong, C.P. Highly transparent triboelectric nanogenerator utilizing in-situ chemically welded silver nanowire network as electrode for mechanical energy harvesting and body motion monitoring. Nano Energy 2019, 59, 508–516. [Google Scholar] [CrossRef]
- Park, C.; Lee, B.; Kim, J.; Lee, H.; Kang, J.; Yoon, J.; Cho, S.J. Flexible Sensory Systems: Structural Approaches. Polymers 2022, 6, 1232. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Kim, K.K.; Won, P.; Hong, S.; Ko, S.H. Biomimetic chameleon soft robot with artificial crypsis and disruptive coloration skin. Nat. Commun. 2021, 1, 4658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, H.Q.; Sun, Y.; Zhou, W.; Wang, J. Facile synthesis of aqueous silver nanoparticles and silver/molybdenum disulfide nanocomposites and investigation of their nonlinear optical properties. Tungsten 2021, 4, 482–491. [Google Scholar] [CrossRef]
- Muhmood, T.; Ahmad, F.; Hu, X.; Yang, X. Silver Nanowires: A Focused Review about Their Synthesis, Properties, and Major Factors Limiting Their Commercialization. Nano Futures 2022, 6, 3. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dyecontaining effluents: A review. Bioresour. Technol. 1996, 3, 217–227. [Google Scholar] [CrossRef]
- Kruis, F.E.; Fissan, H.; Rellinghaus, B. Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater. Sci. Eng. B 2000, 69, 329–334. [Google Scholar] [CrossRef]
- Magnusson, M.H.; Deppert, K.; Malm, J.O.; Bovin, J.O.; Samuelson, L. Gold nanoparticles: Production, reshaping, and thermal charging. J. Nanopart. Res. 1999, 2, 243–251. [Google Scholar] [CrossRef]
- Jung, J.H.; Oh, H.C.; Noh, H.S.; Ji, J.H.; Kim, S.S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. 2006, 12, 1662–1670. [Google Scholar] [CrossRef]
- Mishra, G.; Tripathy, M. A critical review of the treatments for decolourization of textile effluent. Colourage 1993, 40, 35. [Google Scholar]
- Raghavacharya, C. Colour removal from industrial effluents: A comparative review of available technologies. Chem. Eng. World 1997, 7, 53–54. [Google Scholar]
- Slokar, Y.M.; Le Marechal, A.M. Methods of decoloration of textile wastewaters. Dyes Pigm. 1998, 4, 335–356. [Google Scholar] [CrossRef]
- Obaid, A.Y.; Al-Thabaiti, S.A.; El-Mossalamy, E.H.; Al-Harbi, L.M.; Khan, Z. Extracellular bio-synthesis of silver nanoparticles. Arab. J. Chem. 2017, 2, 226–231. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 4, 397–401. [Google Scholar] [CrossRef]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Takahara, P.M.; Rosenzweig, A.C.; Frederick, C.A.; Lippard, S.J. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature 1995, 6550, 649–652. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J. Phys. Chem. C 2010, 19, 8814–8820. [Google Scholar] [CrossRef]
- Leonard, K.; Ahmmad, B.; Okamura, H.; Kurawaki, J. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surf. B Biointerfaces 2011, 2, 391–396. [Google Scholar] [CrossRef]
- Naraginti, S.; Sivakumar, A. Eco-friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4-nitrophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 357–362. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 373–378. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.K.; Philip, D. Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, N.; Luo, J.; Liu, X. Green synthesis of silver nanoparticles by tannic acid with improved catalytic performance towards the reduction of methylene blue. Nano 2018, 1, 1850003. [Google Scholar] [CrossRef]
- Edison, T.J.I.; Sethuraman, M.G. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012, 9, 1351–1357. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Microwave-assisted green synthesis of silver nanoparticles and the study on catalytic activity in the degradation of dyes. J. Mol. Liq. 2015, 204, 184–191. [Google Scholar] [CrossRef]
- Barman, K.; Chowdhury, D.; Baruah, P.K. Bio-synthesized silver nanoparticles using Zingiber officinale rhizome extract as efficient catalyst for the degradation of environmental pollutants. Inorg. Nano-Met. Chem. 2020, 2, 57–65. [Google Scholar] [CrossRef]
- Muhmood, T.; Uddin, A. Fabrication of spherical-graphitic carbon nitride via hydrothermal method for enhanced photo-degradation ability towards antibiotic. Chem. Phys. Lett. 2020, 753, 137604. [Google Scholar] [CrossRef]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Green synthesis of silver nanoparticles using Nervalia zeylanica leaf extract and evaluation of their antioxidant, catalytic, and antimicrobial potentials. Parti. Sci. Technol. 2018, 37, 809–819. [Google Scholar] [CrossRef]
- Muthu, K.; Priya, S. Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 66–72. [Google Scholar] [CrossRef]
- Moon, S.A.; Salunke, B.K.; Saha, P.; Deshmukh, A.R.; Kim, B.S. Comparison of dye degradation potential of biosynthesized copper oxide, manganese dioxide, and silver nanoparticles using Kalopanax pictus plant extract. Korean J. Chem. Eng. 2018, 3, 702–708. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M.; Kumar, S.T.; Rao, M.V. Catalytic degradation of organic dyes using synthesized silver nanoparticles: A green approach. J. Bioremediat. Biodegrad. 2015, 5, 1. [Google Scholar]
- Rajamanikandan, R.; Shanmugaraj, K.; Ilanchelian, M. Concentration dependent catalytic activity of glutathione coated silver nanoparticles for the reduction of 4-nitrophenol and organic dyes. J. Clust. Sci. 2017, 3, 1009–1023. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Eco-friendly synthesis of silver and gold nanoparticles with enhanced antimicrobial, antioxidant, and catalytic activities. IET nanobiotechnol. 2018, 6, 850–856. [Google Scholar] [CrossRef]
- Lijuan, S.U.N.; Jiang, H.E.; Songsong, A.N.; ZHANG, J.; ZHENG, J.; Dong, R.E.N. Recyclable Fe3O4@ SiO2-Ag magnetic nanospheres for the rapid decolorizing of dye pollutants. Chinese J. Catal. 2013, 7, 1378–1385. [Google Scholar]
- Roy, A.; Bharadvaja, N. Silver nanoparticle synthesis from Plumbago zeylanica and its dye degradation activity. Bioinspired, Biomim. Nanobiomater. 2019, 2, 130–140. [Google Scholar] [CrossRef]
- Awais, M.; Khursheed, S.; Tehreem, R.; Mok, S.Y.; Siddiqui, G.U. pH-regulated rapid photocatalytic degradation of methylene blue dye via niobium-nitrogen co-doped titanium dioxide nanostructures under sunlight. Appl. Catal. A-Gen. 2022, 643, 118764. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Nosier, S.A.; El-Shazly, A.H.; Mubarak, A.A. Photocatalytic decolorization of methylene blue using TiO2/UV system enhanced by air sparging. Alex. Eng. J. 2018, 4, 3727–3735. [Google Scholar] [CrossRef]

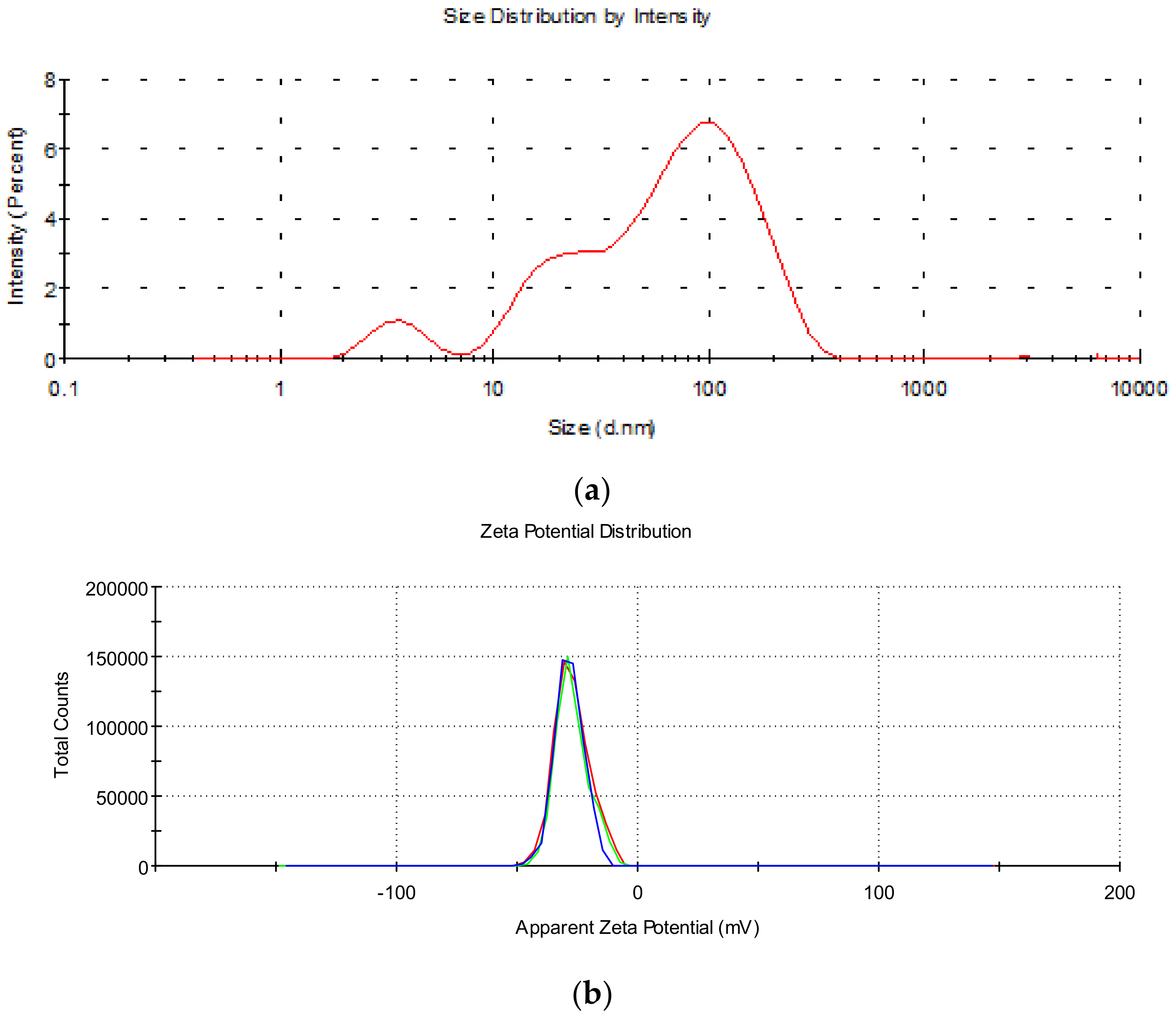






| Size (d.nm) | % Intensity | St. Dev. (d.nm) | |
|---|---|---|---|
| Peak 1 | 101.5 | 71.6 | 59.04 |
| Peak 2 | 19.20 | 18.8 | 5.815 |
| Peak 3 | 3.677 | 5.1 | 0.9729 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, U.; Akter, S.; Kaleem Qureshi, A.; Alhuthali, H.M.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A.; Farzana, M.; Alhazmi, A.Y.M.; et al. Arbutin Stabilized Silver Nanoparticles: Synthesis, Characterization, and Its Catalytic Activity against Different Organic Dyes. Catalysts 2022, 12, 1602. https://doi.org/10.3390/catal12121602
Farooq U, Akter S, Kaleem Qureshi A, Alhuthali HM, Almehmadi M, Allahyani M, Alsaiari AA, Aljuaid A, Farzana M, Alhazmi AYM, et al. Arbutin Stabilized Silver Nanoparticles: Synthesis, Characterization, and Its Catalytic Activity against Different Organic Dyes. Catalysts. 2022; 12(12):1602. https://doi.org/10.3390/catal12121602
Chicago/Turabian StyleFarooq, Umar, Shahina Akter, Ahmad Kaleem Qureshi, Hayaa M. Alhuthali, Mazen Almehmadi, Mamdouh Allahyani, Ahad Amer Alsaiari, Abdulelah Aljuaid, Maisha Farzana, Abdulfattah Yahya M. Alhazmi, and et al. 2022. "Arbutin Stabilized Silver Nanoparticles: Synthesis, Characterization, and Its Catalytic Activity against Different Organic Dyes" Catalysts 12, no. 12: 1602. https://doi.org/10.3390/catal12121602
APA StyleFarooq, U., Akter, S., Kaleem Qureshi, A., Alhuthali, H. M., Almehmadi, M., Allahyani, M., Alsaiari, A. A., Aljuaid, A., Farzana, M., Alhazmi, A. Y. M., & Shahab, M. (2022). Arbutin Stabilized Silver Nanoparticles: Synthesis, Characterization, and Its Catalytic Activity against Different Organic Dyes. Catalysts, 12(12), 1602. https://doi.org/10.3390/catal12121602







