Development of a Solid Catalyst Based on Pt Supported on Heterostructure (NaNbO3/NaNb3O8/NiO) Applied to the Photodegradation of Phenol in Seawater
Abstract
1. Introduction
2. Results and Discussions
2.1. Catalyst Characterization
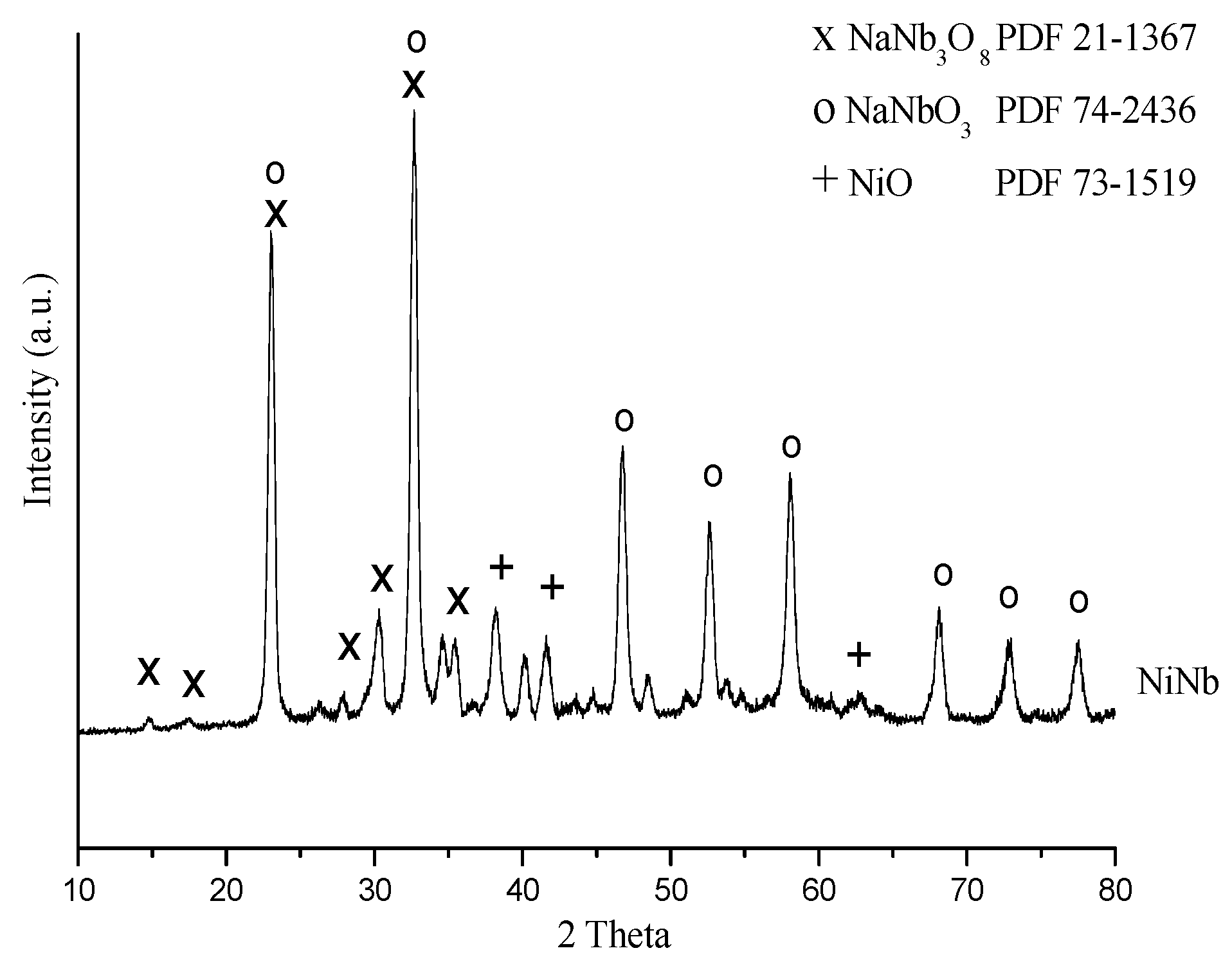
2.1.1. X-ray Diffraction (XRD) Analysis
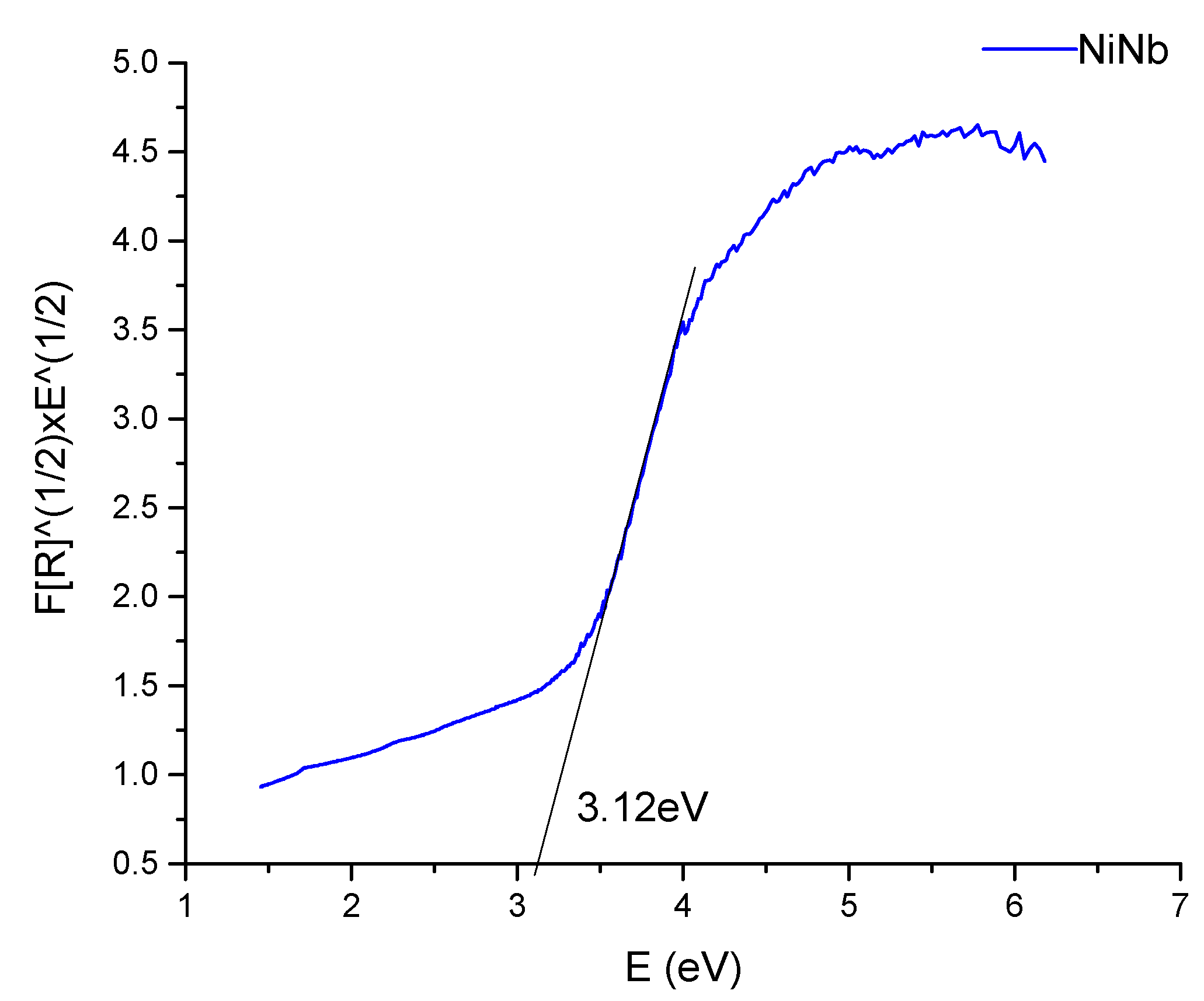
2.1.2. Diffuse Reflectance Spectroscopy (DRS) Analysis
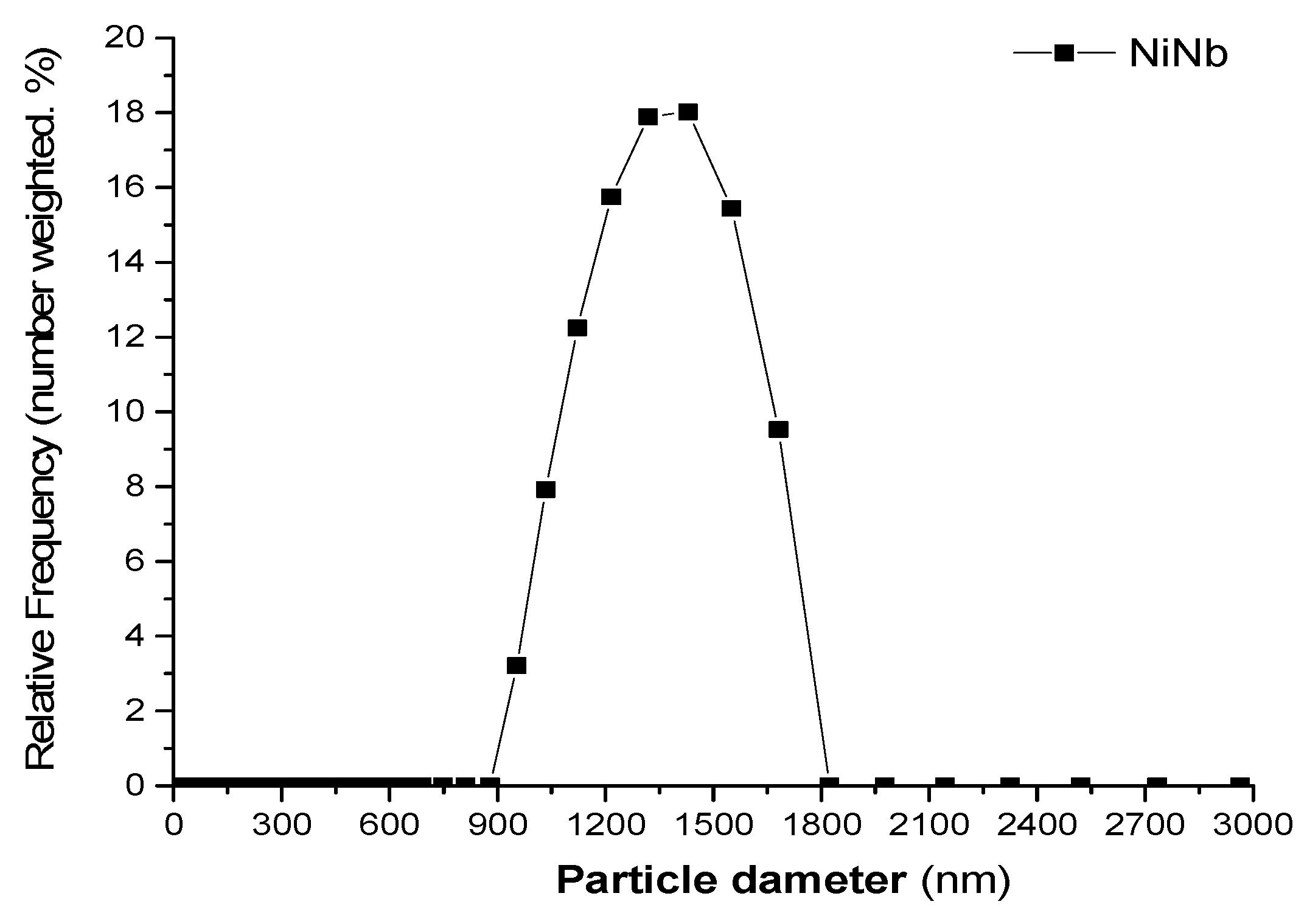
2.1.3. Surface Area (SBET), Particle Size Distribution, and Zeta Potential
2.1.4. Scanning Electron Microscope (SEM) Analysis
2.1.5. Total Reflection X-ray Fluorescence (TXRF) Analysis
2.1.6. Energy Dispersive X-ray Analysis (EDX)
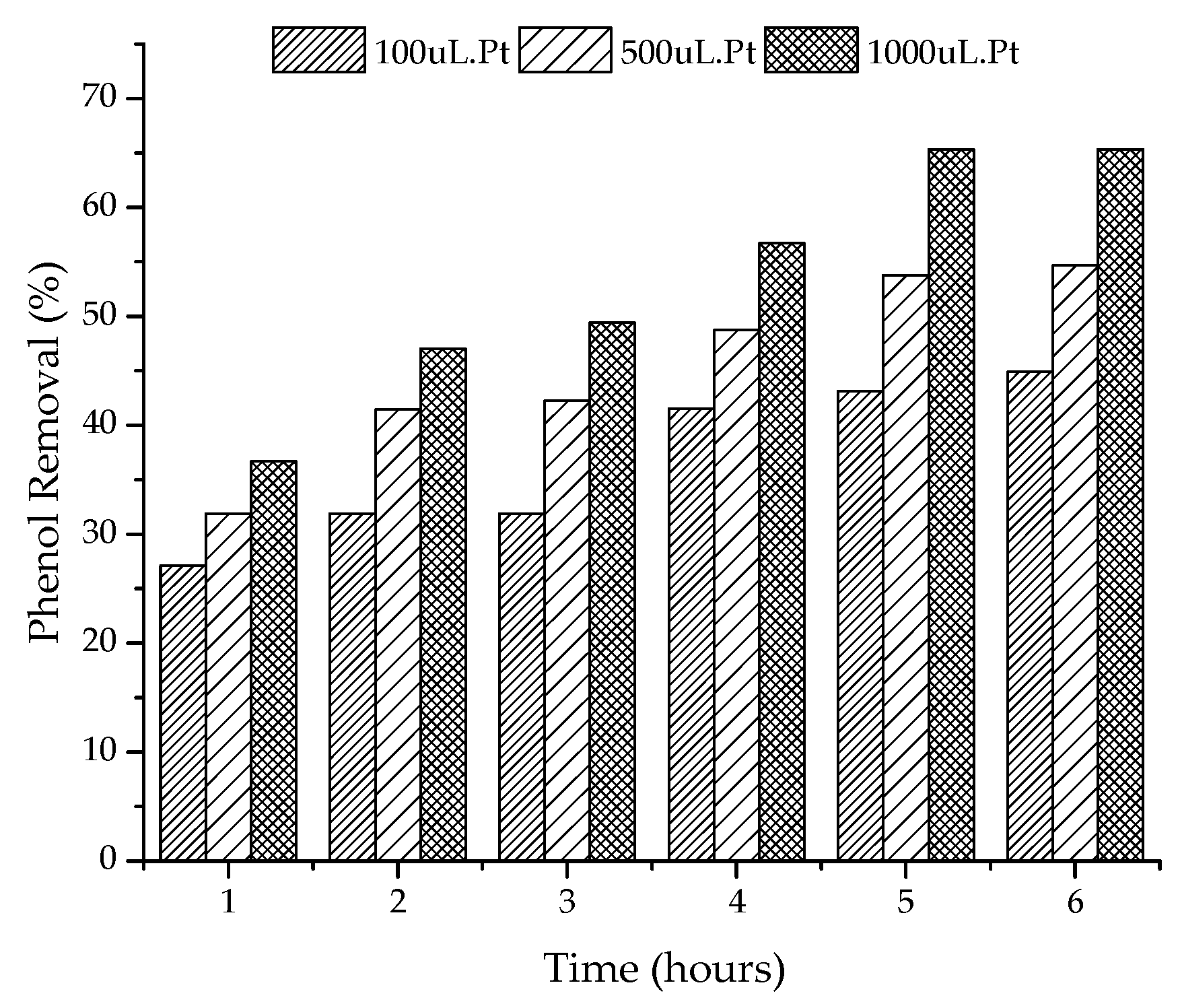
2.2. Photocatalytic Tests
2.2.1. Variation of pH of the Phenolic Solution
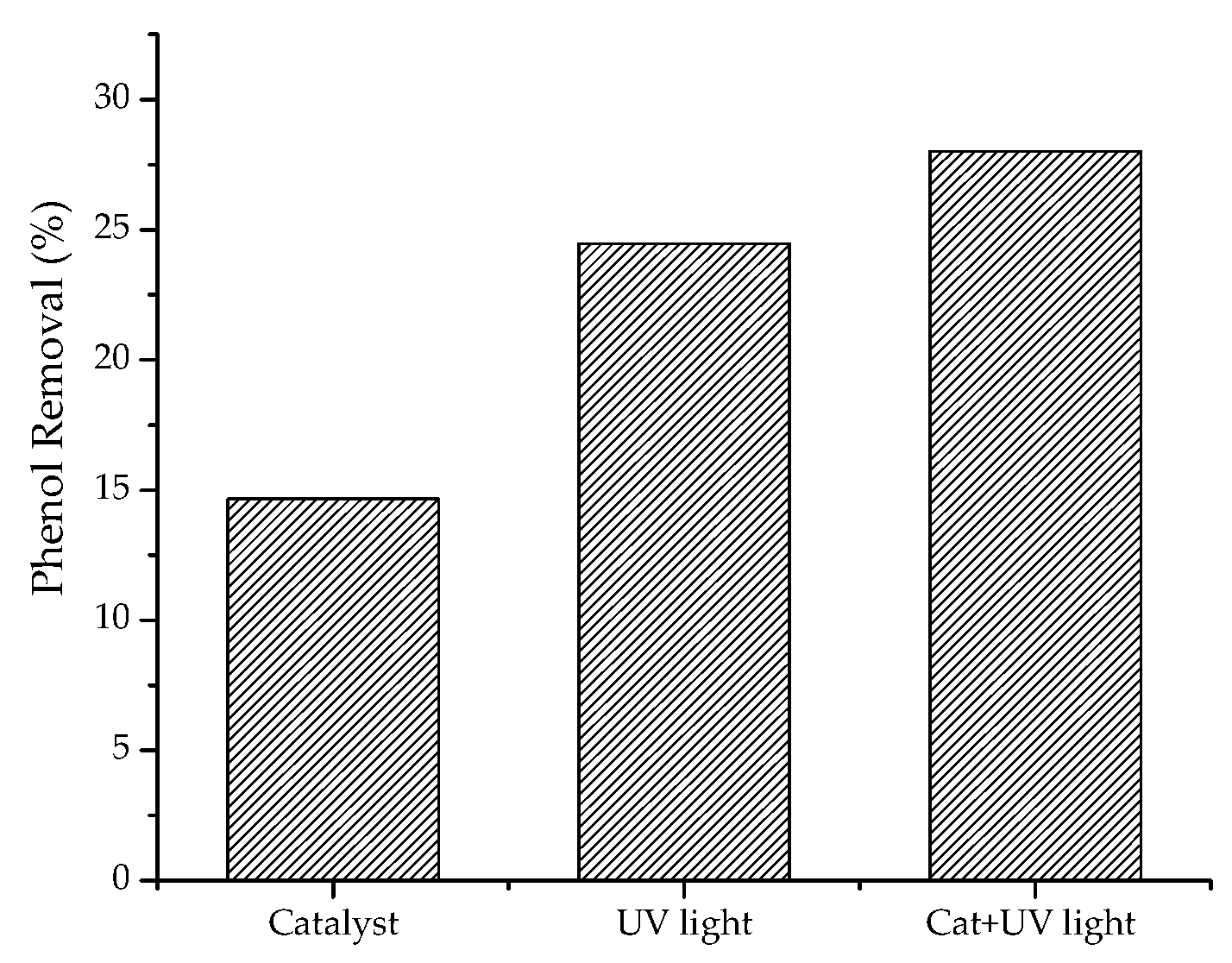
2.2.2. Oxidizing Species
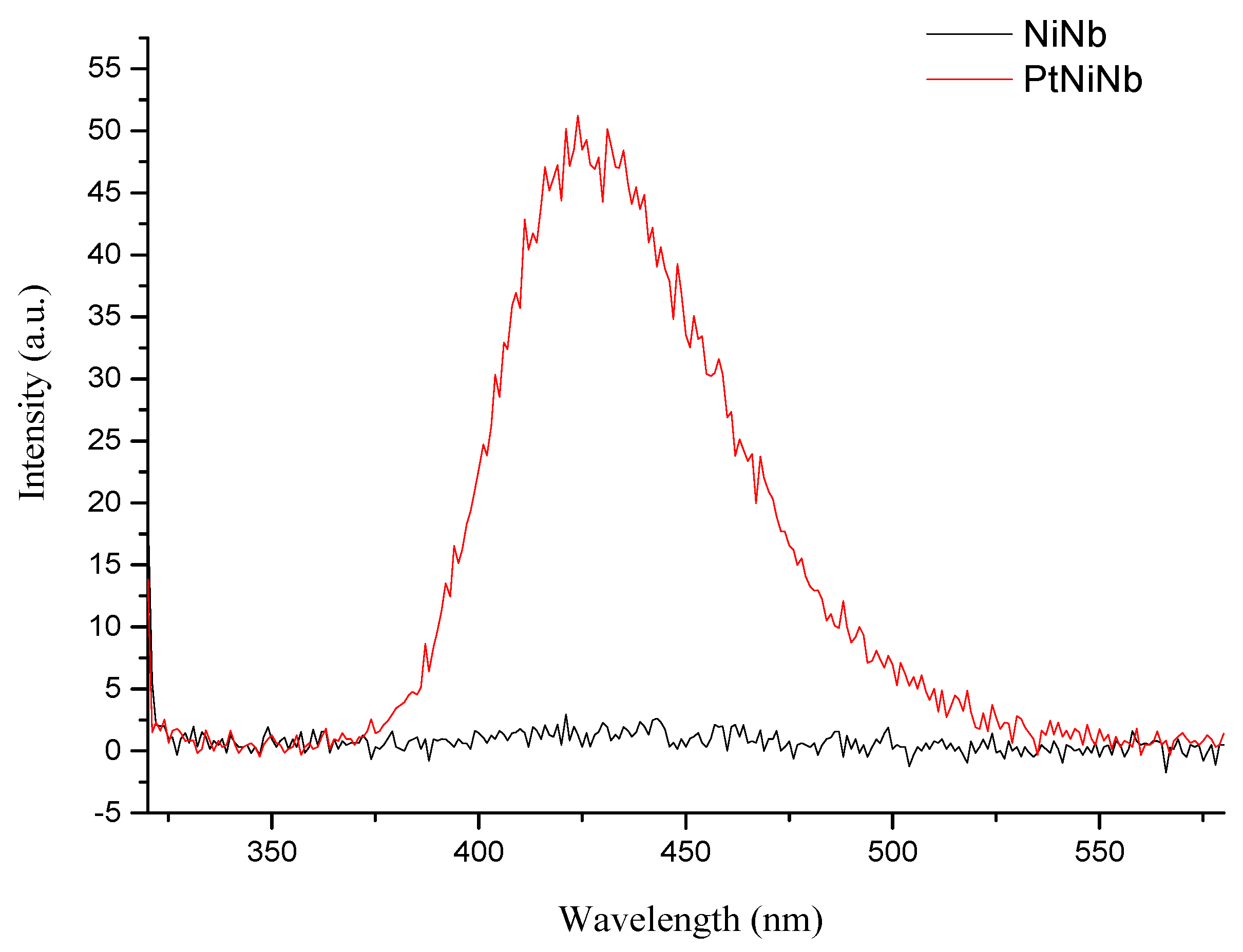
2.2.3. Hydroxyl Radical (●OH) Detection Experiments
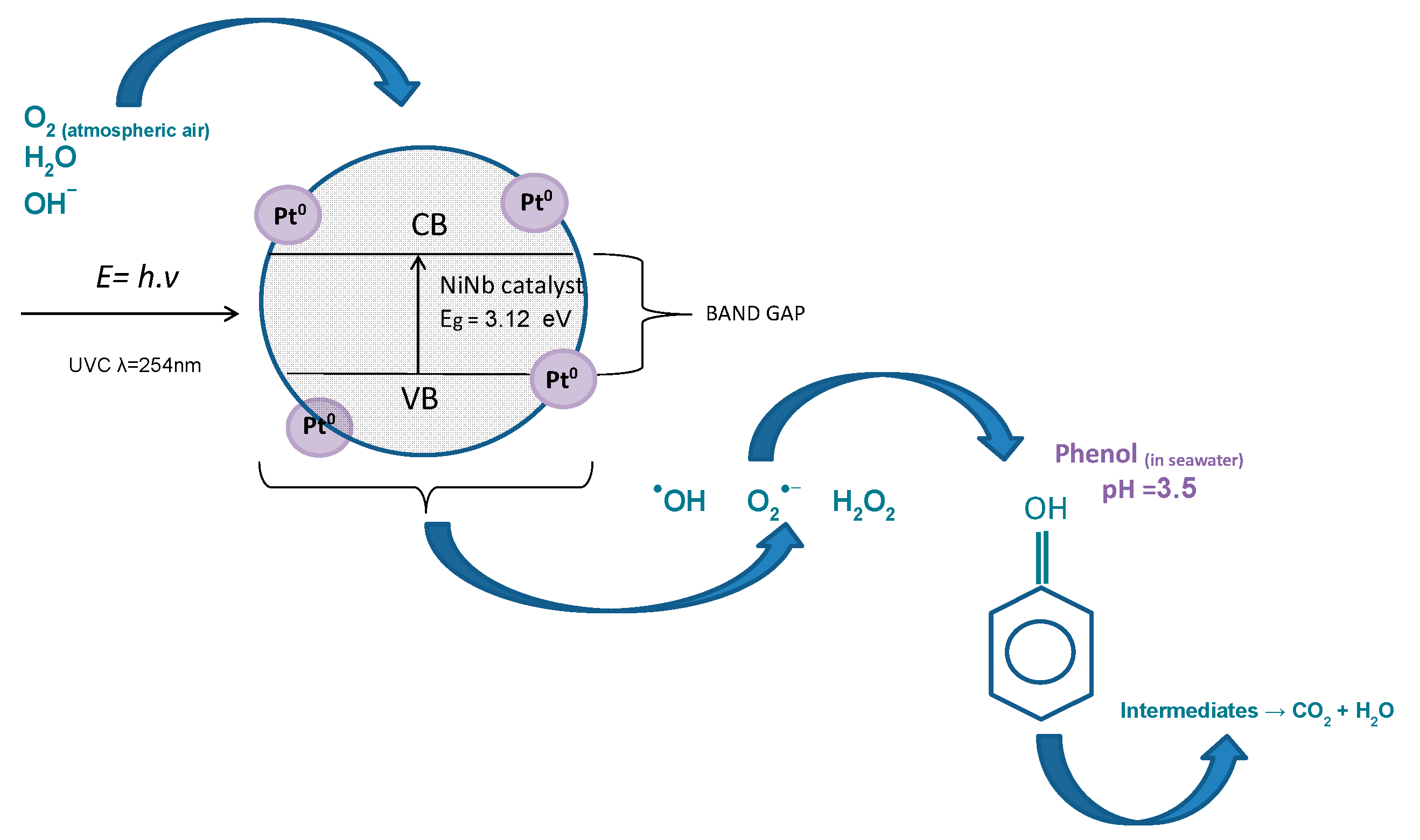
2.2.4. Proposed Mechanism
2.2.5. Catalyst Reuse
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Photocatalytic Tests
3.3.1. Variation of pH of the Solution
3.3.2. Verification of Oxidizing Species
3.3.3. Hydroxyl Radical Measurement Experiments (●OH)
3.3.4. Catalyst Reuse
3.3.5. Comparison with Other Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MSDS—Chemical Product Safety Data Sheet—Phenol. Sigma-Aldrich. 2011. Available online: https://sites.ffclrp.usp.br/cipa/fispq/Fenol.pdf (accessed on 20 November 2022).
- Neff, J.M. Bioaccumulation in Marine Organisms Effect of Contaminants From Oil Well Produced Water; Elsevier Science: Amsterdam, The Netherlands, 2002; p. 468. [Google Scholar]
- Motta, A.R.P.; Borges, C.P.; Kiperstok, A.; Esquerre, K.P.; Araujo, P.M. Produced water treatment for oil removal by membrane separation process: Review: Review. Eng. Sanit. Ambient 2013, 18, 15–26. [Google Scholar] [CrossRef]
- ANP- Monthly Bulletin of Oil and Natural Gas Production. The National Agency of Petroleum, Natural Gas and Biofuels. 2021. Available online: https://www.gov.br/anp/pt-br/centrais-de-conteudo/publicacoes/bulletins-anp/bulletin-mensal-da-producao-de-petroleo-e-gas-natural (accessed on 20 November 2022).
- Jing, S.; Li, T.; L1, X.; Xu, Q.; Hu, J.; Li, R. Phenolic foams modified by cardanol through bisphenol modification. J. Appl. Polym. Sci. 2014, 131, 39942. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bessa, E.; Santanna, J.; Dezotti, M. Photocatalytic/H2O2 treatment of oil field produced waters. Appl. Catal. B Environ. 2001, 29, 125–134. [Google Scholar] [CrossRef]
- Braga, U. Use of Clay Minerals and Diatomite as an Adsorbent for Phenols in Water Produced in the Oil Industry. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2018. Available online: https://repositorio.ufrn.br/handle/123456789/12884 (accessed on 20 November 2022).
- Asencios, Y.; Lourenço, V.; Carvalho, W. Removal of phenol in seawater by heterogeneous photocatalysis using activated carbon materials modified with TiO2. Catal. Today 2022, 388–389, 247–258. [Google Scholar] [CrossRef]
- Chamara, L.S.; Ravinder, K.; Garlapalli, J.P.T.; Trembly, J.P. Removal of phenol from oil/gas produced water using supercritical water treatment with TiO2 supported MnO2 catalyst. J. Environ. Chem. Eng. 2017, 5, 488–493. [Google Scholar]
- CONAMA—National Environment Council of Brazil. Resolution No. 430, 05/13/2011. Available online: https://www.braziliannr.com/brazilian-environmental-legislation/conama-resolution-43011/ (accessed on 20 November 2022).
- Asencios, Y.J.O.; Quijo, V.; Francielle, M.; Nogueira, A.; Rocca, R.; Assaf, E.M. Photocatalytic activity of Nb heterostructure (NaNbO3/Na2Nb4O11) and Nb/clay materials in the degradation of organic compounds. Solar Energy 2019, 194, 37–46. [Google Scholar] [CrossRef]
- Choi, J. Development of Visible- Light-Active Photocatalyst for Hydrogen Production and Environmental Application. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 2010. [Google Scholar]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Kim, J.; Choi, W. Hydrogen producing water treatment through solar photocatalysis. Energy Environ. Sci. 2010, 3, 1042–1045. [Google Scholar] [CrossRef]
- Ferraz, N.P.; Marcos, C.F.; Nogueira, A.E.; Martins, A.S.; Assaf, E.M.; Asencios, Y.J.O. Hexagonal-Nb2O5/Anatase-TiO2 mixtures and their applications in the removal of Methylene Blue dye under various conditions. Mater. Chem. Phys. 2017, 198, 331–340. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Zhang, L.; Liu, Q.; Dai, W. Facile synthesis of highly efficient Pt/N-rGO/N-NaNbO3 nanorods toward photocatalytic hydrogen production. Appl. Catal. B Environ. 2019, 257, 117901. [Google Scholar] [CrossRef]
- Agostini, G.; Lamberti, C. Characterization of Semiconductor Heterostructures and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Fan, M.; Hu, B.; Yan, X.; Song, C.; Chen, T.; Feng, Y.; Shi, W. Excellent visible-light driven photocatalytic performance of Cu2O sensitized NaNbO3 heterostructures. New J. Chem. 2015, 39, 6171–6177. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Yang, N.; Zhang, W.F. Composition dependence of AgSbO3/ NaNbO3 composite on surface photovoltaic and visible-light photocatalytic properties. App. Phys. A 2011, 103, 251–256. [Google Scholar] [CrossRef]
- Kumar, S.; Khanchandani, S.; Thirumal, M.; Ganguli, A.K. Achieving enhanced visible-light-driven photocatalysis using type-II NaNbO3/CdS core/shell heterostructures. ACS Appl. Mother Interf. 2014, 6, 13221–13233. [Google Scholar] [CrossRef]
- Zhou, W.; Yao, M.; Guo, L.; Li, Y.; Li, J.; Yang, S. Hydrazine-Linked Convergent Self-Assembly of Sophisticated Concave Polyhedrons of β-Ni(OH)2 and NiO from Nanoplate Building Blocks. J. Am. Chem. Soc. 2009, 131, 2959. [Google Scholar] [CrossRef]
- Joshi, M.; Labhsetwar, K.; Parwate, D.V. Efficient photocatalytic hydrogen generation by silica supported and platinum promoted titanium dioxide. Mater. Res. Bull. 2013, 48, 3545–3552. [Google Scholar] [CrossRef]
- Silva, P.C.; Ferraz, N.P.; Perpetuo, E.A.; Asencios, Y.J.O. Oil produced water treatment using advanced oxidative processes: Heterogeneous-photocatalysis and photofenton. J. Sediment. Environ. 2019, 4, 99–107. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.R.; Pan, J.H.; Wu, L.G.; Wang, Z.Q.; Wang, Z.H.; Jiang, B.Q. Deposition of quantum-sized Ag on TiO2 through adsorbed-layer nanoreactor synthesis and its performance for photodegrading phenol in seawater under visible-light irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 555, 448–456. [Google Scholar] [CrossRef]
- George, R.T.; Dar, T.A.; Joshi, D.C.; Sathe, V.; Rathore, A.K.; Thota, S. Thermal hysteresis and vibrational excitations in NiO containing NaNbO3. J. Phys. D Appl. Phys. 2019, 52, 115301. [Google Scholar] [CrossRef]
- George, R.T.; Joshi, D.C.; Nayak, S.; Tiwari, N.; Chauhan, R.N.; Pramanik, P.; Dar, T.A.; Ghosh, S.; Thota, S. Effect of NiO substitution on the structural and dielectric behavior of NaNbO3. J. Appl. Phys. 2018, 123, 054101. [Google Scholar] [CrossRef]
- Zielińska, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Preparation, characterization and photocatalytic activity of metal-loaded NaNbO3. J. Phys. Chem. Solids 2011, 72, 117–123. [Google Scholar] [CrossRef]
- Iwase, A.; Saito, K.; Kudo, A. Sensitization of NaMO3(M: Nb and Ta) Photocatalysts with Wide Band Gaps to Visible Light by Ir Doping. Bull. Chem. Soc. Jpn. 2011, 82, 514–518. [Google Scholar] [CrossRef]
- Kioka, K.; Honma, T.; Oh-ishi, K.; Reibstein, S.; Da, N.; Wondraczek, L.; Komatsu, T. Effect of Al2O3 addition on the formation of perovskite-type NaNbO3 nanocrystals in silicate-based glasses. J. Non-Cryst. Solids 2012, 358, 1523–1529. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Souza, J.K.D. Photocatalytic Evaluation of Lamellar Niobates: Influence of Synthesis Methods, Theoretical and Experimental Aspects. Ph.D. Thesis, Universidade Federal da Paraíba, Paraíba, Brazil, 2018. Available online: https://repositorio.ufpb.br/jspui/handle/123456789/14841 (accessed on 20 November 2022).
- Bhatkhande, D.S.; Kamble, S.P.; Sawant, S.B.; Pangarkar, V.G. Photocatalytic and photochemical degradation of nitrobenzene using artificial ultraviolet light. Chem. Eng. J. 2004, 102, 283–290. [Google Scholar] [CrossRef]
- Almeida, T.P.; Fay, M.W.; Hansen, T.W.; Zhu, Y.; Brown, P.D. Insights from in situ and environmental TEM on the oriented attachment of α-Fe2O3 nanoparticles during α-Fe2O3 nanorod formation. Cryst. Eng. Comm. 2014, 16, 1540–1546. [Google Scholar] [CrossRef]
- Lourenço, V.S.; Boto, S.M.; Carvalho, W.A.; Asencios, Y.J. Peruíbe black mud based catalysts for the removal of organic pollutants in water. J. Sediment. Environ. 2020, 5, 293–305. [Google Scholar] [CrossRef]
- Pinheiro, N.D.A. Lamellar Niobates (KNb3O8 and K4Nb6O17) Synthesized by the Polymeric Precursor Method and Applied in the Photodegradation of the Remazol Yellow Gold Dye. Master’s Thesis, Universidade Federal da Paraíba, Paraíba, Brazil, 2020. Available online: https://repositorio.ufpb.br/jspui/handle/123456789/18196 (accessed on 20 November 2022).
- Praxedes, F.R. Sodium and Potassium Niobates Obtained by the Spray Pyrolysis Method: Characterization and Photocatalytic Activity for the Decolorization of Aqueous Solutions of Dyes. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2019. Available online: https://repositorio.unesp.br/handle/11449/181471 (accessed on 20 November 2022).
- Ferreira, I.V.L.; Daniel, L.A. Heterogeneous photocatalysis with TiO2 applied to the treatment of secondary sanitary sewage. Eng. Sanit. Ambient. 2004, 9, 335–342. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Gonzalez, E.R. Correlation between catalytic activity and particle size of Pt/C prepared by different methods. Eclet. Quím. Online. 2003, 28, 77–85. [Google Scholar] [CrossRef]
- Ghorai, T.K.; Dhak, P. Niobium Oxides: An overview of the synthesis of Nb2O5 and its application in Heterogeneous Photocatalysis. Adv. Mater. Lett. 2013, 4, 121–130. [Google Scholar]
- Assis, R.B. Synthesis of Sodium and Potassium Niobate (K1-xNaxNbO3) with Photocatalytic and Photoluminescent Properties. Ph.D. Thesis, Federal University of Rio Grande do Norte, Natal, Brazil, 2017. Available online: https://repositorio.ufrn.br/handle/123456789/45428 (accessed on 20 November 2022).
- Wu, J.; Xue, D. Advances in Chemical Synthesis of Nb-Containing Oxides. J. Nanoeng. Nanomanuf. 2011, 2, 136–154. [Google Scholar] [CrossRef]
- Shishido, T.; Miyatake, T.; Teramura, K.; Hitomi, Y.; Yamashita, H.; Tanaka, T. Mechanism of photooxidation of alcohol over Nb2O5. J. Phys. Chem. C 2009, 113, 18713–18718. [Google Scholar] [CrossRef]
- Gonçalves, M.C.P.; Padilha, S.R.R.; Lopes, M.F.; Paris, E.C. Application of commercial Nb2O5 for decolorization of methylene blue dye by photocatalysis process. Rev. Eng. Tecnol. 2017, 9, 95–105. Available online: https://revistas.uepg.br/index.php/ret/article/view/11269 (accessed on 20 November 2022).
- APHA. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- EPA (US Environmental Protection Agency). Method 604, Methods for Organic Chemical Analysis of Municipal and Industrial Wastewater; Part VIII, 40 CFR Part 136. Appendix A; Environmental Protection Agency EPA: Washington, DC, USA, 1984.
- Ziolli, W.; Jardim, F. Photocatalytic decomposition of seawater-soluble crude-oil fractions using high surface area colloid nanoparticles of TiO2. J. Photochem. Photobiol. A Chem. 2002, 147, 205–212. [Google Scholar] [CrossRef]
- Shaban, Y.A.; El Sayed, M.A.; El Maradny, A.A.; Al Farawati, R.K.; Al Zobidi, M.I. Photocatalytic degradation of phenol in natural seawater using visible light active carbon modified (CM)-n-TiO2 nanoparticles under UV light and natural sunlight illuminations. Chemosphere 2013, 91, 307–313. [Google Scholar] [CrossRef]
- Hippargi, G.; Mangrulkar, P.; Chilkalwar, A.; Labhsetwar, N.; Rayalu, S. Chloride ion: A promising hole scavenger for photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2018, 43, 6815–6823. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Machado, V.A. Photodegradation of Organic Pollutants in Seawater and Hydrogen Production via Methanol Photoreforming with Hydrated Niobium Pentoxide Catalysts. Sustain. Chem. 2022, 3, 172–191. [Google Scholar] [CrossRef]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Effect of chloride ion as a hole scavenger on the photocatalytic conversion of CO2 in an aqueous solution over Ni−Al layered double hydroxides. Phys. Chem. 2015, 17, 17995–18003. [Google Scholar] [CrossRef]
- Kinney, L.C.; Ivanuski, V.R. Photolysis Mechanisms for Pollution Abatement, Available on U.S. Department of the Interior; Federal Water Pollution Control Administration: Cincinnati, OH, USA, 1969.
- Beker, U.; Ganbold, B.; Dertli, H.; Gülbayir, D.D. Adsorption of phenol by activated carbon: Influence of activation methods and solution pH. Energy Convers. Manag. 2010, 51, 235–240. [Google Scholar] [CrossRef]
- Lopes, W.S.; Azevedo, M.G.; Leite, V.D.; Sousa, J.T.; Buriti, J.S. Degradation of 17α-ethinylestradiol in water by heterogeneous photocatalysis. Rev. Ambient. Água. 2015, 10, 728–736. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Satoshi, I.; Abdullah, A.Z.; Mohamed, A.R. Enhanced sunlight photocatalytic performance over Nb2O5/ZnO nanorod composites and the mechanism study. Appl. Catal. A Gen. 2014, 471, 126–135. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, J.; Chen, F.; Anpo, M. Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Appl. Catal. B Environ. 2009, 85, 148–154. [Google Scholar] [CrossRef]
- Rosli, M.; Lam, N.I.; Sin, S.M.; Satoshi, J.C.; Mohamed, A.R. Photocatalytic Performance of ZnO/gC3N4 for Removal of Phenol under Simulated Sunlight Irradiation. J. Environ. Eng. 2018, 144, 04017091. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, H.; Chen, J.; Jin, D.; Cheng, J. The photocatalysis of BiFeO3 disks under visible light irradiation. Catal. Commun. 2016, 87, 23–26. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Shi, Z. Immobilization of TiO2 nanoparticles on activated carbon fiber and its photodegradation performance for organic pollutants. Particuology 2010, 8, 272–278. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Haque, M.M.; Muneer, M. Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. J. Hazard. Mater. 2007, 145, 51–57. [Google Scholar] [CrossRef]
- Carvalho, K.T.G.; Silva, A.C.; Oliveira, L.C.A.; Goncalves, M.; Magriotis, Z.M. Modified synthetic niobia as catalyst in the oxidation of organic dye: Utilization of H2O2 and atmospheric O2 as oxidants. Química Nova 2009, 32, 1373–1377. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Oliveira, L.C.; Ramalho, T.C. Photocatalytic degradation of organic compound in water using synthetic niobia: Experimental and theoretical studies. Top. Catal. 2011, 54, 270–276. [Google Scholar] [CrossRef]
- Wood, D.L.; Tauc, J. Weak Absorption Tails in Amorphous Semiconductors. Phys. Rev. B. 1972, 5, 3144–3151. [Google Scholar] [CrossRef]
- Deng, P.C.; Hu, J.Z.; Wang, H.Z. Photodegradation of Phenol on Halogen-Doping TiO2 Nanoparticles Composites in Seawater. Mater. Sci. Forum 2011, 694, 355–359. [Google Scholar]
- Wang, T.; Xu, Z.Y.; Wu, L.G.; Li, B.R.; Chen, M.X.; Xue, S.Y.; Zhu, Y.C.; Cai, J. Enhanced photocatalytic activity for degrading phenol in seawater by TiO2-based catalysts under weak light irradiation. RSC Adv. 2017, 7, 31921–31929. [Google Scholar] [CrossRef]
- L’Amour, R.J.A.; Azevedo, E.B.; Leite, S.G.F.; Dezotti, M. Removal of phenol in high salinity media by a hybrid process (activated sludge + photocatalysis). Sep. Purif. Technol. 2008, 60, 142–146. [Google Scholar]
- Scott, T.; Zhao, H.; Deng, W.; Feng, X.; Li, Y. Photocatalytic degradation of phenol in water under simulated sunlight by an ultrathin MgO coated Ag/TiO2 nanocomposite. Chemosphere 2019, 216, 1–8. [Google Scholar] [CrossRef]


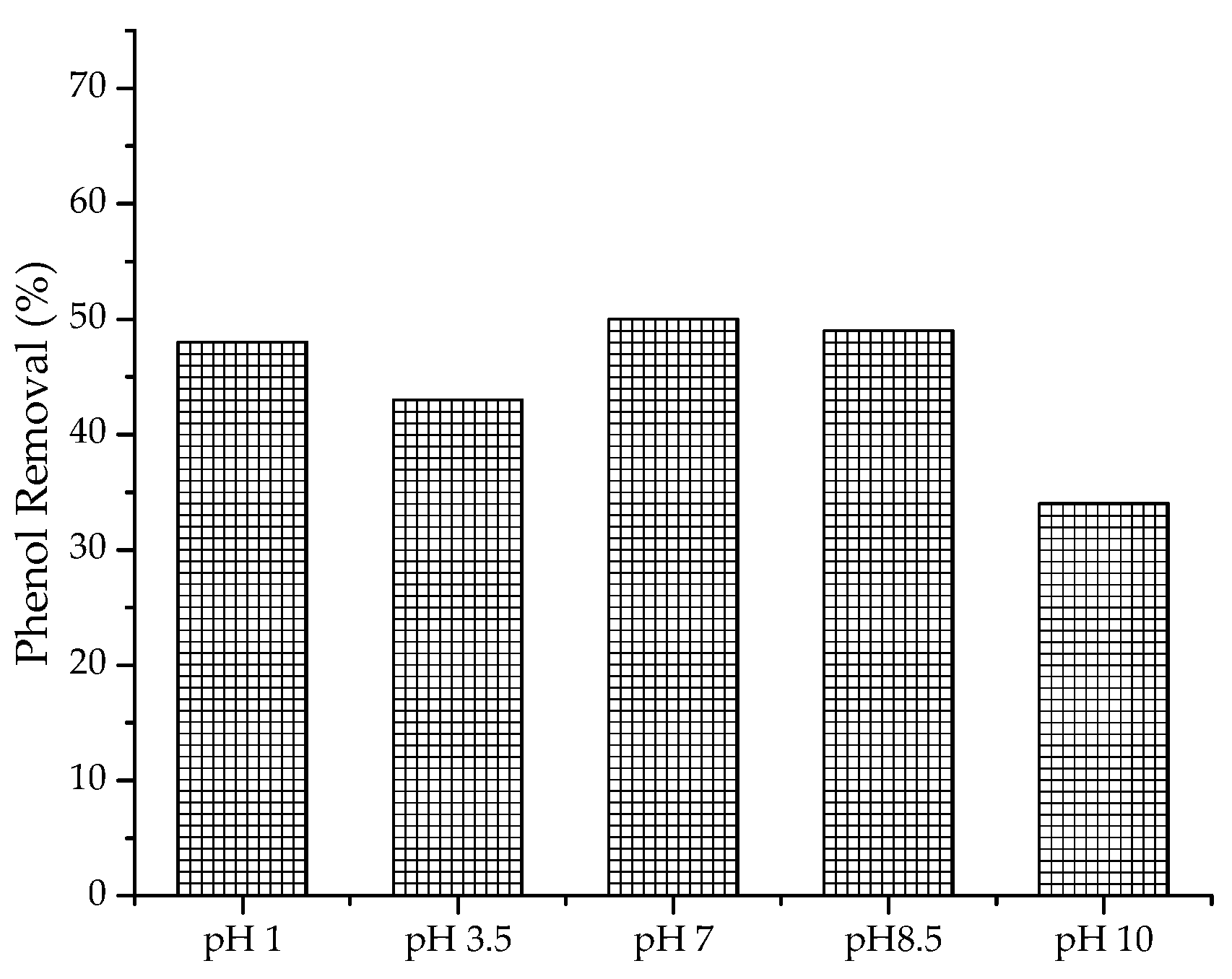


| Weight % | Weight % | |
|---|---|---|
| Element | NiNb | NiNb/Pt |
| P | -- | 0.0137 |
| S | -- | 3.6281 |
| Cl | -- | 40.0911 |
| K | 0.011 | 0.8279 |
| Ca | 0.0091 | 1.4181 |
| Ti | 0.0017 | 0.0037 |
| Fe | 0.0026 | 0.0046 |
| Cr | -- | 0.023 |
| Ni | 1.8689 | 0.762 |
| Cu | 0.0049 | -- |
| Zn | 0.0023 | 0.0022 |
| Nb | 98.099 | 53.0232 |
| Nd | 0.0004 | -- |
| Pt | -- | 0.2023 |
| NiNb | |
|---|---|
| Element | wt % |
| O | 47.87 |
| Na | 23.99 |
| Ni | 3.32 |
| Nb | 24.82 |
| Phenol Removal (%) | Phenol Initial Concentration (mg/L) | Application | Catalyst | Catalyst Concentration (g/L) | Degradation Time (h) | Reference |
|---|---|---|---|---|---|---|
| 65 | 40 | Seawater | (NaNbO3/NaNb3O8)/NiO + 3.2% Pt | 0.5 | 5 | This paper |
| 60 | 50 | Seawater | I/TiO2 | 0.5 | 3 | Deng et al. [65] |
| 50 | 25 | Seawater | La3+-doped Red–GO–TiO2 | 0.5 | 5 | Wang et al. [66] |
| 98 | 200 | Seawater | TiO2 P25 | 0.5 | 25 * Hybrid process | L’Amour et al. [67] |
| * 24 h activated sludge + 1 h Photocatalysis | ||||||
| 76 | 15 | distilled water | Ag_TiO2 | 0.2 | 2 | Scott et al. [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, K.G.; Asencios, Y.J.O. Development of a Solid Catalyst Based on Pt Supported on Heterostructure (NaNbO3/NaNb3O8/NiO) Applied to the Photodegradation of Phenol in Seawater. Catalysts 2022, 12, 1565. https://doi.org/10.3390/catal12121565
Costa KG, Asencios YJO. Development of a Solid Catalyst Based on Pt Supported on Heterostructure (NaNbO3/NaNb3O8/NiO) Applied to the Photodegradation of Phenol in Seawater. Catalysts. 2022; 12(12):1565. https://doi.org/10.3390/catal12121565
Chicago/Turabian StyleCosta, Kimberly G., and Yvan J. O. Asencios. 2022. "Development of a Solid Catalyst Based on Pt Supported on Heterostructure (NaNbO3/NaNb3O8/NiO) Applied to the Photodegradation of Phenol in Seawater" Catalysts 12, no. 12: 1565. https://doi.org/10.3390/catal12121565
APA StyleCosta, K. G., & Asencios, Y. J. O. (2022). Development of a Solid Catalyst Based on Pt Supported on Heterostructure (NaNbO3/NaNb3O8/NiO) Applied to the Photodegradation of Phenol in Seawater. Catalysts, 12(12), 1565. https://doi.org/10.3390/catal12121565






