Photocatalytic Degradation of 4-tert-butylphenol Using Solar Light Responsive Ag2CO3
Abstract
1. Introduction
2. Results and Discussion
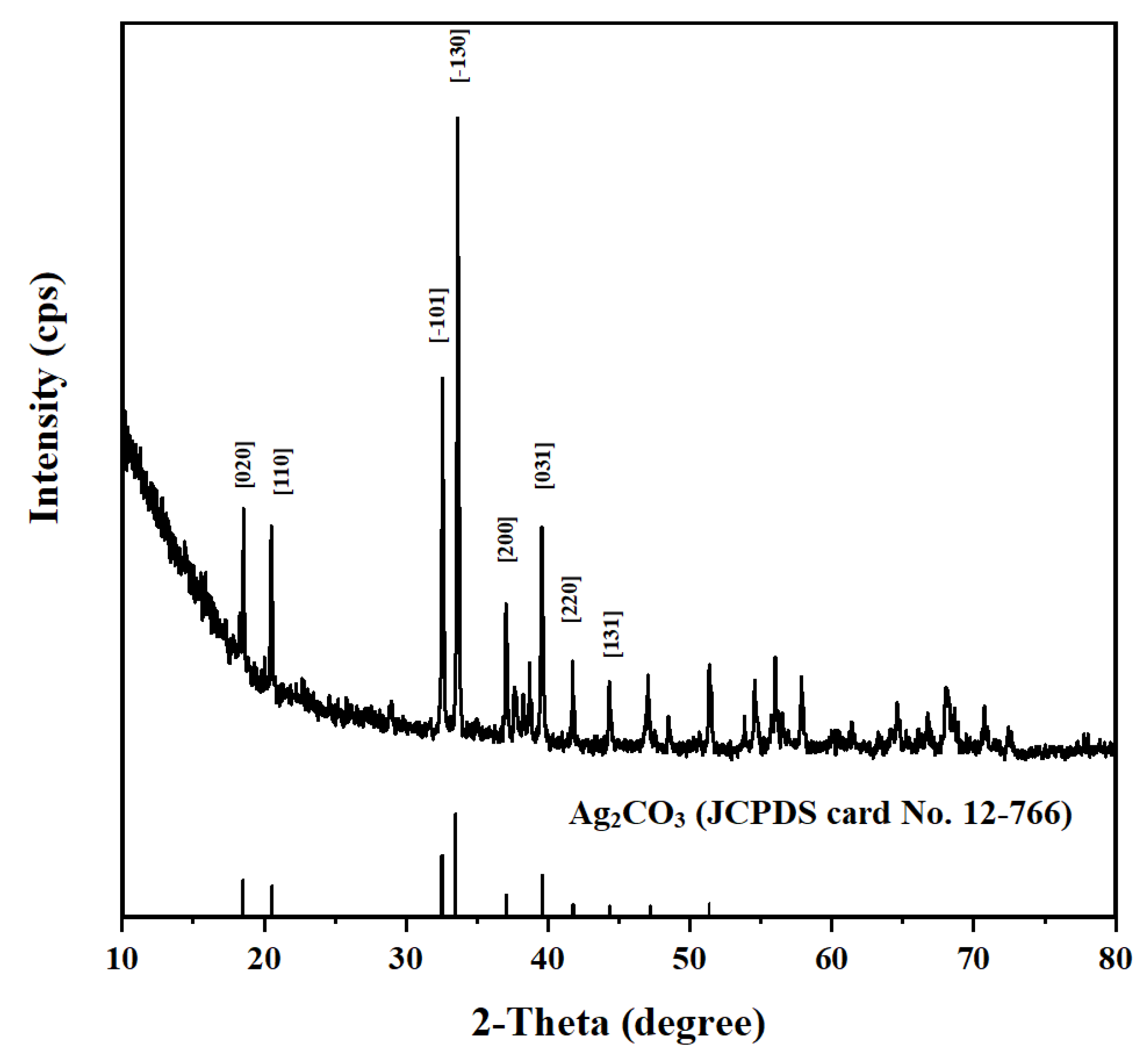
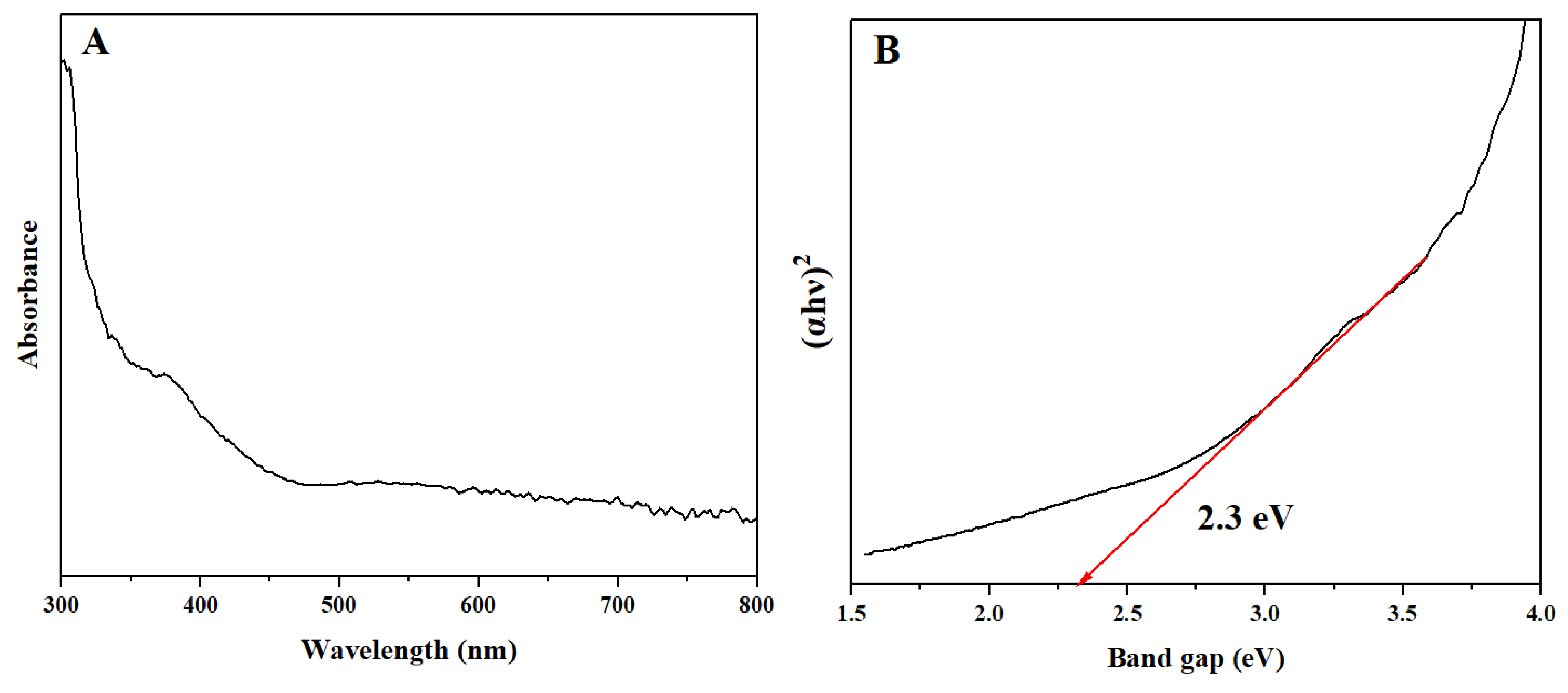
2.1. Characterization
2.2. Photocatalytic Activity of Ag2CO3
2.2.1. Effect of 4-t-BP Initial Concentration and Catalyst Dosage
2.2.2. Effect of Lamp Type
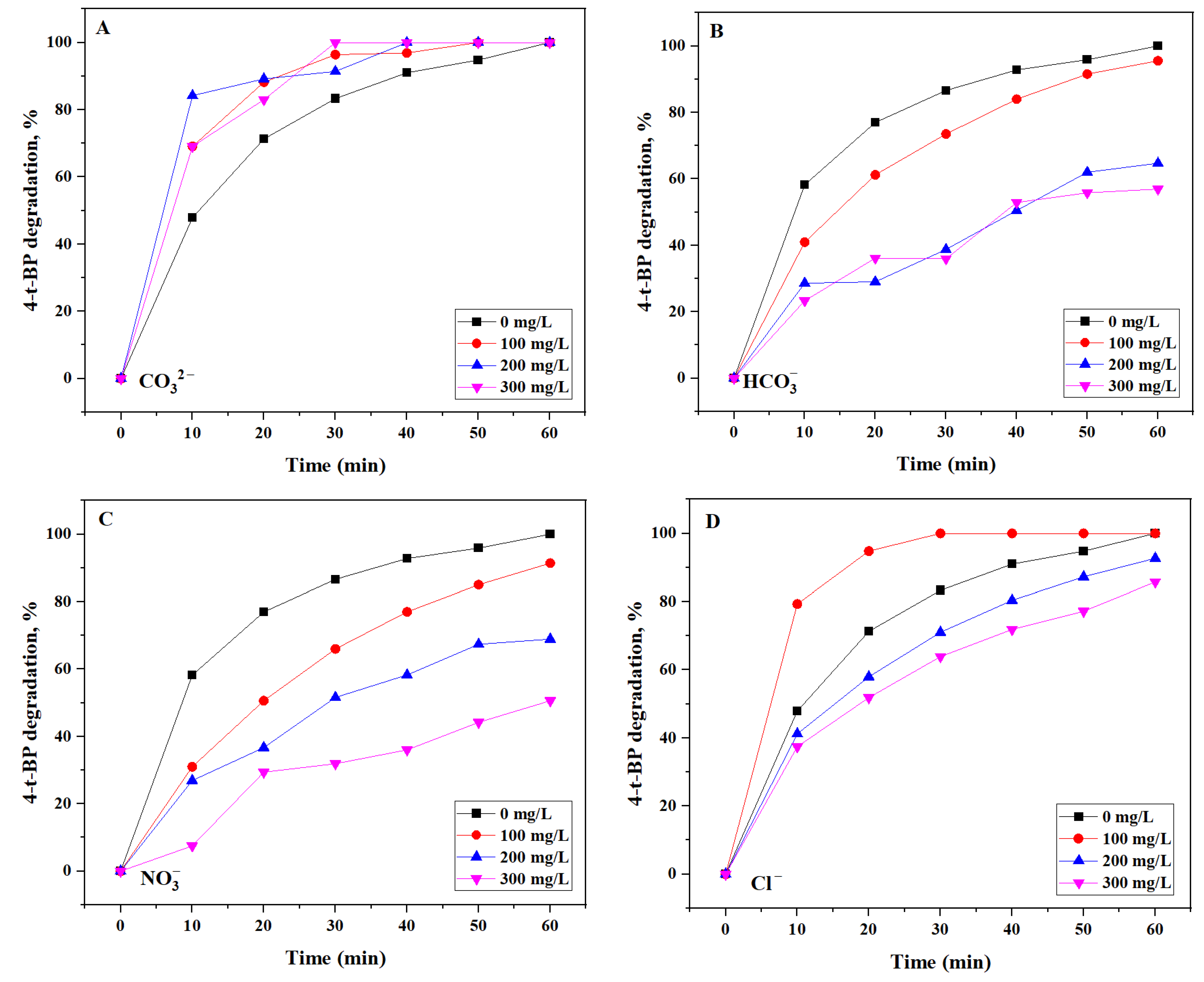
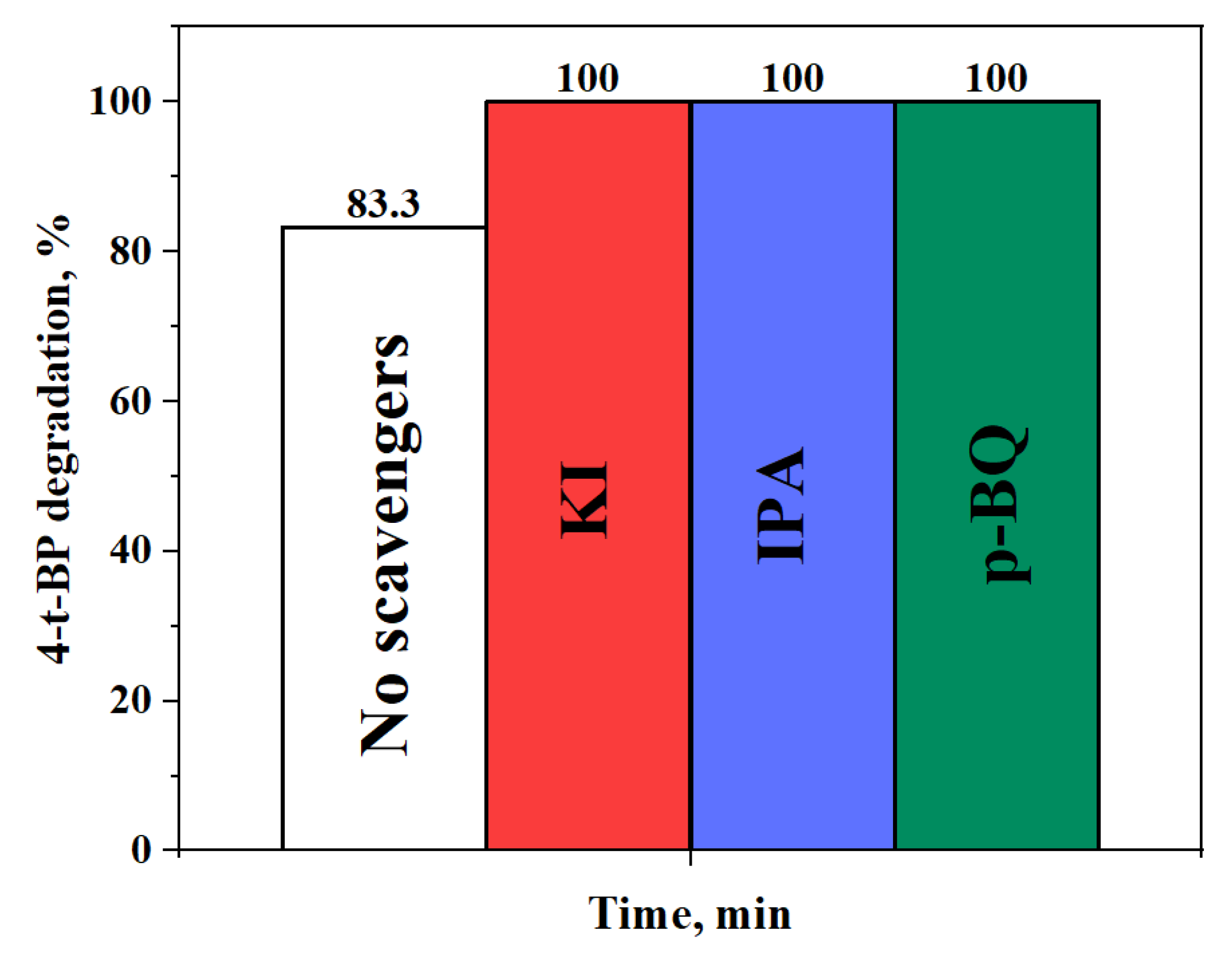
2.2.3. Effect of Water Matrix
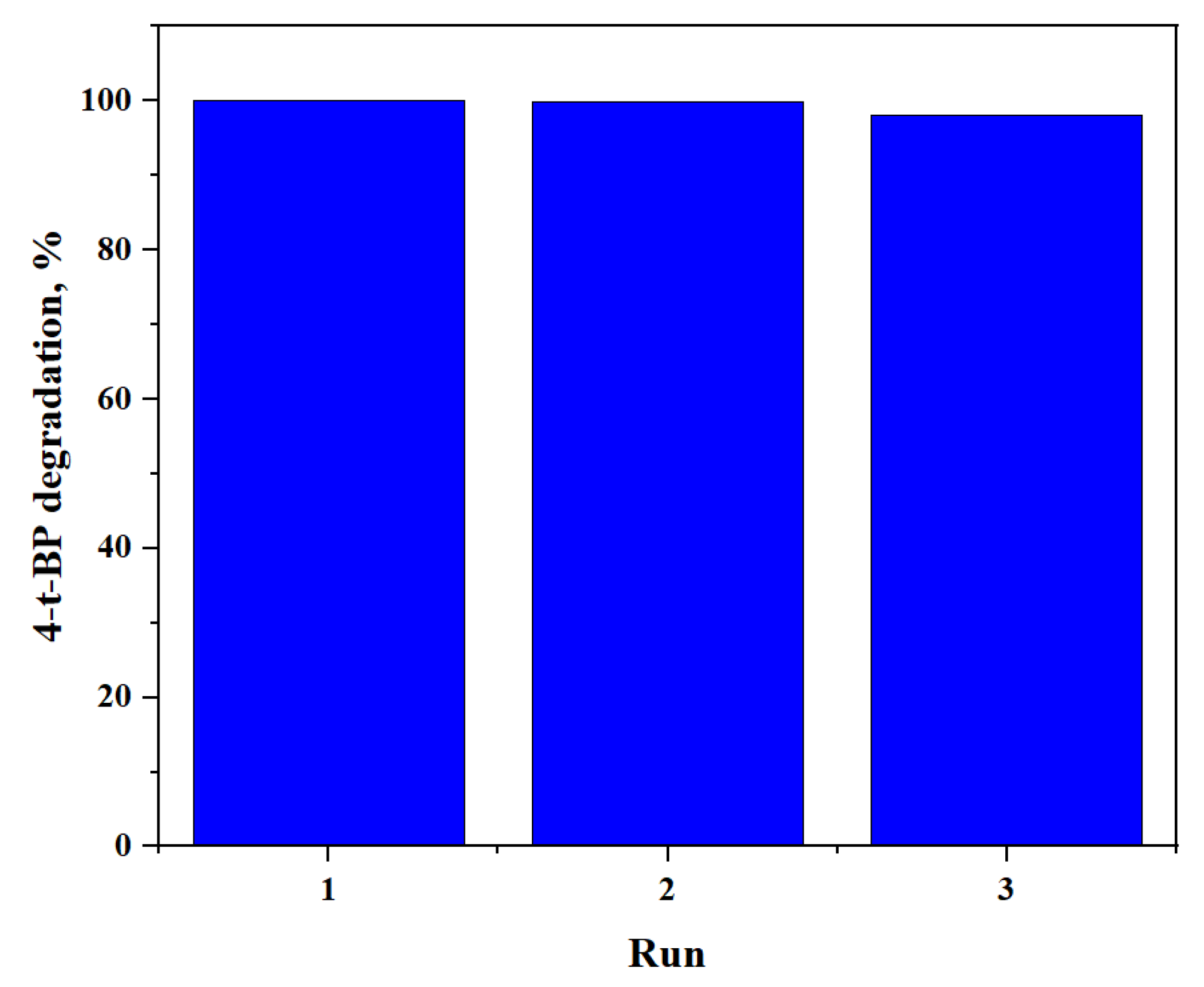
2.2.4. Reusability and Stability of Ag2CO3
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ag2CO3
3.3. Characterization of the Prepared Catalyst
3.4. Photocatalytic Degradation of 4-t-BP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, A.M.; Baier, R.; Kocher, B.; Reifferscheid, G.; Buchinger, S.; Ternes, T. Ecotoxicological Characterization of Emissions from Steel Coatings in Contact with Water. Water Res. 2020, 173, 115525. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Momotani, N.; Ogata, Y.; Miyamori, Y.; Inoue, D.; Sei, K.; Mori, K.; Kikuchi, S.; Ike, M. Isolation and Characterization of 4-Tert-Butylphenol-Utilizing Sphingobium Fuliginis Strains from Phragmites Australis Rhizosphere Sediment. Appl. Environ. Microbiol. 2010, 76, 6733–6740. [Google Scholar] [CrossRef]
- Gao, X.; Huang, P.; Huang, Q.; Rao, K.; Lu, Z.; Xu, Y.; Gabrielsen, G.W.; Hallanger, I.; Ma, M.; Wang, Z. Organophosphorus Flame Retardants and Persistent, Bioaccumulative, and Toxic Contaminants in Arctic Seawaters: On-Board Passive Sampling Coupled with Target and Non-Target Analysis. Environ. Pollut. 2019, 253, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, J.; Guo, M.; Xu, H.; Zhang, S.; Shi, L.; Yao, C. Occurrence, Distribution, and Risk Assessment of Alkylphenols, Bisphenol A, and Tetrabromobisphenol A in Surface Water, Suspended Particulate Matter, and Sediment in Taihu Lake and Its Tributaries. Mar. Pollut. Bull. 2016, 112, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Zhang, S.-H.; Ji, G.-X.; Wu, S.-M.; Guo, R.-X.; Cheng, J.; Yan, Z.-Y.; Chen, J.-Q. Occurrence, Distribution and Risk Assessment of Suspected Endocrine-Disrupting Chemicals in Surface Water and Suspended Particulate Matter of Yangtze River (Nanjing Section). Ecotoxicol. Environ. Saf. 2017, 135, 90–97. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, J.; Zhang, Q.; Ye, C.; Zheng, G.; Shan, Q.; Li, L.; Shao, X. Occurrence, Distribution and Bioaccumulation of Alkylphenols in the Pearl River Networks, South China. Ecol. Indic. 2020, 110, 105847. [Google Scholar] [CrossRef]
- Kojima, M.; Tsunoi, S.; Tanaka, M. Determination of 4-Alkylphenols by Novel Derivatization and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2003, 984, 237–243. [Google Scholar] [CrossRef]
- Colin, A.; Bach, C.; Rosin, C.; Munoz, J.; Dauchy, X. Is Drinking Water a Major Route of Human Exposure to Alkylphenol and Bisphenol Contaminants in France? Arch. Environ. Contam. Toxicol. 2013, 66, 86–99. [Google Scholar] [CrossRef]
- Wu, Y.; Brigante, M.; Dong, W.; de Sainte-Claire, P.; Mailhot, G. Toward a Better Understanding of Fe(III)–EDDS Photochemistry: Theoretical Stability Calculation and Experimental Investigation of 4-Tert-Butylphenol Degradation. J. Phys. Chem. A 2014, 118, 396–403. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Poulopoulos, S.G. Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-Tert-Butylphenol. Processes 2022, 10, 8. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal Synthesis of Novel Hierarchical Bi4O5I2 Nanoflakes with Highly Visible Light Photocatalytic Performance for the Degradation of 4-Tert-Butylphenol. Appl. Catal. B Environ. 2014, 148–149, 154–163. [Google Scholar] [CrossRef]
- del Rio, M.; Grimalt Escarabajal, J.C.; Turnes Palomino, G.; Palomino Cabello, C. Zinc/Iron Mixed-Metal MOF-74 Derived Magnetic Carbon Nanorods for the Enhanced Removal of Organic Pollutants from Water. Chem. Eng. J. 2022, 428, 131147. [Google Scholar] [CrossRef]
- Ogata, Y.; Toyama, T.; Yu, N.; Wang, X.; Sei, K.; Ike, M. Occurrence of 4-Tert-Butylphenol (4-t-BP) Biodegradation in an Aquatic Sample Caused by the Presence of Spirodela Polyrrhiza and Isolation of a 4-t-BP-Utilizing Bacterium. Biodegradation 2013, 24, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Kanafin, Y.; Makhatova, A.; Arkhangelsky, E.; Poulopoulos, S. Photochemical Treatment of an Actual Municipal Wastewater by Means of UV, Potassium Persulfate and Iron. IOP Conf. Ser. Earth Environ. Sci. 2021, 899, 012067. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Ashir, A.; Yergali, B.; Ulykbanova, G.; Poulopoulos, S. Modified TiO2 for Photocatalytic Removal of Organic Pollutants in Water: 2nd International Conference on Environmental Design, ICED 2021. IOP Conf. Ser. Earth Environ. Sci. 2021, 899, 012068. [Google Scholar] [CrossRef]
- Padovan, R.N.; de Carvalho, L.S.; de Souza Bergo, P.L.; Xavier, C.; Leitão, A.; dos Santos Neto, Á.J.; Lanças, F.M.; Azevedo, E.B. Degradation of Hormones in Tap Water by Heterogeneous Solar TiO2-Photocatalysis: Optimization, Degradation Products Identification, and Estrogenic Activity Removal. J. Environ. Chem. Eng. 2021, 9, 106442. [Google Scholar] [CrossRef]
- Taheri, M.E.; Petala, A.; Frontistis, Z.; Mantzavinos, D.; Kondarides, D.I. Fast Photocatalytic Degradation of Bisphenol A by Ag3PO4/TiO2 Composites under Solar Radiation. Catal. Today 2017, 280, 99–107. [Google Scholar] [CrossRef]
- Silva, T.F.C.V.; Peri, P.; Fajardo, A.S.; Paulista, L.O.; Soares, P.A.; Martínez-Huitle, C.A.; Vilar, V.J.P. Solar-Driven Heterogeneous Photocatalysis Using a Static Mixer as TiO2-P25 Support: Impact of Reflector Optics and Material. Chem. Eng. J. 2022, 435, 134831. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Li, H.; Duan, L.; Zu, L.; Zhai, Y.; Li, W.; Wang, L.; Fu, H.; Zhao, D. Visible-Light Responsive TiO2-Based Materials for Efficient Solar Energy Utilization. Adv. Energy Mater. 2021, 11, 2003303. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A Review of Solar and Visible Light Active Oxo-Bridged Materials for Energy and Environment. Catal. Sci. Technol. 2017, 7, 2153–2164. [Google Scholar] [CrossRef]
- He, G.; Xing, C.; Xiao, X.; Hu, R.; Zuo, X.; Nan, J. Facile Synthesis of Flower-like Bi12O17Cl2/β-Bi2O3 Composites with Enhanced Visible Light Photocatalytic Performance for the Degradation of 4-Tert-Butylphenol. Appl. Catal. B Environ. 2015, 170–171, 1–9. [Google Scholar] [CrossRef]
- Makhatova, A.; Ulykbanova, G.; Sadyk, S.; Sarsenbay, K.; Atabaev, T.S.; Inglezakis, V.J.; Poulopoulos, S.G. Degradation and Mineralization of 4-Tert-Butylphenol in Water Using Fe-Doped TiO2 Catalysts. Sci. Rep. 2019, 9, 19284. [Google Scholar] [CrossRef] [PubMed]
- Mergenbayeva, S.; Atabaev, T.S.; Poulopoulos, S.G. Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-Tert-Butylphenol in Water. Catalysts 2021, 11, 1379. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Kumarov, A.; Atabaev, T.S.; Hapeshi, E.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S.G. Degradation of 4-Tert-Butylphenol in Water Using Mono-Doped (M1: Mo, W) and Co-Doped (M2-M1: Cu, Co, Zn) Titania Catalysts. Nanomaterials 2022, 12, 2326. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, J.M.; Durán, A.; Chatzisymeon, E.; San Martín, I.; Naranjo, S. Solar Activation of TiO2 Intensified with Graphene for Degradation of Bisphenol-A in Water. Solar Energy 2018, 174, 1035–1043. [Google Scholar] [CrossRef]
- Garg, A.; Singhania, T.; Singh, A.; Sharma, S.; Rani, S.; Neogy, A.; Yadav, S.R.; Sangal, V.K.; Garg, N. Photocatalytic Degradation of Bisphenol-A Using N, Co Codoped TiO2 Catalyst under Solar Light. Sci. Rep. 2019, 9, 765. [Google Scholar] [CrossRef]
- de la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Synthesis and Characterization of Bimetallic TiO2/CNT/Pd-Cu for Efficient Remediation of Endocrine Disruptors under Solar Light. J. Environ. Chem. Eng. 2022, 10, 107245. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S. MoS2/WO3 Heterojunction with the Intensified Photocatalytic Performance for Decomposition of Organic Pollutants under the Broad Array of Solar Light. J. Clean. Prod. 2021, 324, 129290. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Li, S.; Wang, L.; Huang, F.; Guan, R.; Li, J.; Jiao, Y.; Sun, J. Rapidly Degradation of Di-(2-Ethylhexyl) Phthalate by Z-Scheme Bi2O3/TiO2@reduced Graphene Oxide Driven by Simulated Solar Radiation. Chemosphere 2021, 272, 129631. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, G.; Dong, F.; Wang, C.; Zhu, L. Glass Fiber Supported BiOI Thin-Film Fixed-Bed Photocatalytic Reactor for Water Decontamination under Solar Light Irradiation. J. Environ. Sci. 2019, 80, 277–286. [Google Scholar] [CrossRef]
- Deekshitha; Shetty, V.K. Solar Light Active Biogenic Titanium Dioxide Embedded Silver Oxide (AgO/Ag2O@TiO2) Nanocomposite Structures for Dye Degradation by Photocatalysis. Mater. Sci. Semicond. Process. 2021, 132, 105923. [Google Scholar] [CrossRef]
- Ran, R.; McEvoy, J.G.; Zhang, Z. Ag2O/Ag3VO4/Ag4V2O7 Heterogeneous Photocatalyst Prepared by a Facile Hydrothermal Synthesis with Enhanced Photocatalytic Performance under Visible Light Irradiation. Mater. Res. Bull. 2016, 74, 140–150. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Xu, J.; Shang, C.; Wang, Y. AgCl-Loaded Mesoporous Anatase TiO2 with Large Specific Surface Area for Enhancing Photocatalysis. Appl. Surf. Sci. 2015, 351, 416–424. [Google Scholar] [CrossRef]
- Du, J.; Ma, S.; Zhang, N.; Liu, W.; Lv, M.; Ni, T.; An, Z.; Li, K.; Bai, Y. Efficient Photocatalytic Organic Degradation and Disinfection Performance for Ag/AgFeO2/g-C3N4 Nanocomposites under Visible-Light: Insights into the Photocatalysis Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130094. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.; Gao, Y.; Liu, S.; Lv, D.; Zhao, S.; Gao, H.; Yang, G.; Li, N.; Ge, L. Ag-AgI/Bi3O4Cl for Efficient Visible Light Photocatalytic Degradation of Methyl Orange: The Surface Plasmon Resonance Effect of Ag and Mechanism Insight. Appl. Catal. B Environ. 2019, 246, 140–148. [Google Scholar] [CrossRef]
- Dai, G.; Yu, J.; Liu, G. A New Approach for Photocorrosion Inhibition of Ag2CO3 Photocatalyst with Highly Visible-Light-Responsive Reactivity. J. Phys. Chem. C 2012, 116, 15519–15524. [Google Scholar] [CrossRef]
- Petala, A.; Nasiou, A.; Mantzavinos, D.; Frontistis, Z. Photocatalytic Evaluation of Ag2CO3 for Ethylparaben Degradation in Different Water Matrices. Water 2020, 12, 1180. [Google Scholar] [CrossRef]
- Guo, S.; Bao, J.; Hu, T.; Zhang, L.; Yang, L.; Peng, J.; Jiang, C. Controllable Synthesis Porous Ag2CO3 Nanorods for Efficient Photocatalysis. Nanoscale Res. Lett. 2015, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Lončarević, D.; Vukoje, I.; Dostanić, J.; Bjelajac, A.; Đorđević, V.; Dimitrijević, S.; Nedeljković, J.M. Antimicrobial and Photocatalytic Abilities of Ag2CO3 Nano-Rods. ChemistrySelect 2017, 2, 2931–2938. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, L.; Talifu, D.; Abulizi, A. Sonochemical Fabrication of Ag2CO3 Nanomaterial and Influencing Factors on Photocatalytic Properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 167, 012032. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Huo, P.; Yan, Y.; Guan, Q. Preparation of Ag2O/Ag2CO3/MWNTs Composite Photocatalysts for Enhancement of Ciprofloxacin Degradation. Appl. Surf. Sci. 2016, 366, 1–8. [Google Scholar] [CrossRef]
- Yu, C.; Wei, L.; Chen, J.; Xie, Y.; Zhou, W.; Fan, Q. Enhancing the Photocatalytic Performance of Commercial TiO2 Crystals by Coupling with Trace Narrow-Band-Gap Ag2CO3. Ind. Eng. Chem. Res. 2014, 53, 5759–5766. [Google Scholar] [CrossRef]
- Si, M.; Wang, W.; Guan, Q.; Zhang, H.; Puttaswamy, M. Facile Fabrication of Highly Catalytic-Active Ag2CO3/AgBr/Graphene Oxide Ternary Composites towards the Photocatalytic Wastewater Treatment. Environ. Sci. Pollut. Res. 2021, 28, 4173–4183. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Zhang, H.; Qian, Y.; Guo, Z. Re-Investigation on Reduced Graphene Oxide/Ag2CO3 Composite Photocatalyst: An Insight into the Double-Edged Sword Role of RGO. Appl. Surf. Sci. 2017, 396, 102–109. [Google Scholar] [CrossRef]
- Li, T.; Hu, X.; Liu, C.; Tang, C.; Wang, X.; Luo, S. Efficient Photocatalytic Degradation of Organic Dyes and Reaction Mechanism with Ag2CO3/Bi2O2CO3 Photocatalyst under Visible Light Irradiation. J. Mol. Catal. A Chem. 2016, 425, 124–135. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Song, Y.; Zhu, T.; Zhao, W.; Song, Y.; Da, Z.; Liu, C.; Li, H. Fabrication of AgX-Loaded Ag2CO3 (X=Cl, I) Composites and Their Efficient Visible-Light-Driven Photocatalytic Activity. J. Alloy. Compd. 2015, 622, 347–357. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Song, Y.; Zhao, W.; Xu, Y.; Song, Y.; Ji, H.; Li, H. Ion-Exchange Preparation for Visible-Light-Driven Photocatalyst AgBr/Ag2CO3 and Its Photocatalytic Activity. RSC Adv. 2014, 4, 9139–9147. [Google Scholar] [CrossRef]
- Lei, X.; You, M.; Pan, F.; Liu, M.; Yang, P.; Xia, D.; Li, Q.; Wang, Y.; Fu, J. CuFe2O4@GO Nanocomposite as an Effective and Recoverable Catalyst of Peroxymonosulfate Activation for Degradation of Aqueous Dye Pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic Degradation of Pollutants in Petroleum Refinery Wastewater by TiO2- and ZnO-Based Photocatalysts: Recent Development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen Self-Doped g-C3N4 Nanosheets with Tunable Band Structures for Enhanced Photocatalytic Tetracycline Degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Y.; Geng, W.; Su, H.; Long, M. Efficient Bifunctional Piezocatalysis of Au/BiVO4 for Simultaneous Removal of 4-Chlorophenol and Cr(VI) in Water. Appl. Catal. B Environ. 2019, 259, 118084. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.; Zeng, G.; Wang, D.; Niu, C.; Zhao, J.; An, H.; Xie, T.; Deng, Y. Hierarchical Assembly of Graphene-Bridged Ag3PO4/Ag/BiVO4 (040) Z-Scheme Photocatalyst: An Efficient, Sustainable and Heterogeneous Catalyst with Enhanced Visible-Light Photoactivity towards Tetracycline Degradation under Visible Light Irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Wang, R.; Wang, Y.; Zhang, X. Microwave Catalytic Activities of Supported Perovskite Catalysts MOx/LaCo0.5Cu0.5O3@CM (M = Mg, Al) for Salicylic Acid Degradation. J. Colloid Interface Sci. 2020, 564, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sun, X.; Yu, Y.; Wang, H.; Wang, Y. Photocatalytic Degradation of Flumequine with B/N Codoped TiO2 Catalyst: Kinetics, Main Active Species, Intermediates and Pathways. Chem. Eng. J. 2019, 378, 122226. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Rajiv, P.; Mengelizadeh, N.; Mohammed, I.A.; Balarak, D. Development of Sonophotocatalytic Process for Degradation of Acid Orange 7 Dye by Using Titanium Dioxide Nanoparticles/Graphene Oxide Nanocomposite as a Catalyst. J. Environ. Manag. 2021, 292, 112777. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Z.; Zeng, D.; Yu, C.; Liu, X.; Yang, K.; Liu, H. The Preparation and Characterization of CaMg(CO3)2@Ag2CO3/Ag2S/NCQD Nanocomposites and Their Photocatalytic Performance in Phenol Degradation. J Nanopart Res 2018, 20, 182. [Google Scholar] [CrossRef]
- Shen, J.; Qian, L.; Huang, J.; Guo, Y.; Zhang, Z. Enhanced Degradation toward Levofloxacin under Visible Light with S-Scheme Heterojunction In2O3/Ag2CO3: Internal Electric Field, DFT Calculation and Degradation Mechanism. Sep. Purif. Technol. 2021, 275, 119239. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Al-Kahlawy, A.A.; Nada, A.A.; Zaki, T. Photocorrosion Resistant Ag2CO3@Fe2O3/TiO2-NT Nanocomposite for Efficient Visible Light Photocatalytic Degradation Activities. J. Hazard. Mater. 2018, 360, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Liang, C.; Niu, C.-G.; Huang, D.-W.; Du, Y.-B.; Guo, H.; Zhang, L.; Yang, Y.-Y.; Zeng, G.-M. Facile Assembly of G-C3N4/Ag2CO3/Graphene Oxide with a Novel Dual Z-Scheme System for Enhanced Photocatalytic Pollutant Degradation. Appl. Surf. Sci. 2019, 475, 421–434. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Harun, Z.; Ismail, A.F.; Aziz, F. Constructing a Compact Heterojunction Structure of Ag2CO3/Ag2O in-Situ Intermediate Phase Transformation Decorated on ZnO with Superior Photocatalytic Degradation of Ibuprofen. Sep. Purif. Technol. 2020, 251, 117391. [Google Scholar] [CrossRef]
- Alexopoulou, C.; Petala, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Copper Phosphide and Persulfate Salt: A Novel Catalytic System for the Degradation of Aqueous Phase Micro-Contaminants. Appl. Catal. B Environ. 2019, 244, 178–187. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Petala, A.; Zalaora, A.-A.; Mantzavinos, D.; Frontistis, Z. Solar Light-Induced Photocatalytic Degradation of Methylparaben by g-C3N4 in Different Water Matrices. J. Chem. Technol. Biotechnol. 2020, 95, 2811–2821. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of Inorganic Anions on the Performance of Advanced Oxidation Processes for Degradation of Organic Contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Kumar, A.; Chandel, M.; Sharma, A.; Thakur, M.; Kumar, A.; Pathania, D.; Singh, L. Robust Visible Light Active PANI/LaFeO3/CoFe2O4 Ternary Heterojunction for the Photo-Degradation and Mineralization of Pharmaceutical Effluent: Clozapine. J. Environ. Chem. Eng. 2021, 9, 106159. [Google Scholar] [CrossRef]
- Azarpira, H.; Sadani, M.; Abtahi, M.; Vaezi, N.; Rezaei, S.; Atafar, Z.; Mohseni, S.M.; Sarkhosh, M.; Ghaderpoori, M.; Keramati, H.; et al. Photo-Catalytic Degradation of Triclosan with UV/Iodide/ZnO Process: Performance, Kinetic, Degradation Pathway, Energy Consumption and Toxicology. J. Photochem. Photobiol. A Chem. 2019, 371, 423–432. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Yang, F.; Wu, L.; Li, W.; Ren, R.; Lv, Y. Uniform Flower-like BiOBr/BiOI Prepared by a New Method: Visible-Light Photocatalytic Degradation, Influencing Factors and Degradation Mechanism. New J. Chem. 2019, 43, 14829–14840. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-Radical-Dominated Catalytic Degradation of Bisphenol A by ZIF-67 Derived Nitrogen-Doped Carbon Nanotubes Frameworks in the Presence of Peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Du, Y.; Tong, T.; Zhang, L.; Andrew Lin, K.-Y.; Han, X. One-Step Synthesis of Novel Fe3C@nitrogen-Doped Carbon Nanotubes/Graphene Nanosheets for Catalytic Degradation of Bisphenol A in the Presence of Peroxymonosulfate. Chem. Eng. J. 2019, 356, 1022–1031. [Google Scholar] [CrossRef]
- Dehghan, S.; Kakavandi, B.; Kalantary, R.R. Heterogeneous Sonocatalytic Degradation of Amoxicillin Using ZnO@Fe3O4 Magnetic Nanocomposite: Influential Factors, Reusability and Mechanisms. J. Mol. Liq. 2018, 264, 98–109. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, H.; Jiang, F. Cobalt Modified Mesoporous Graphitic Carbon Nitride with Enhanced Visible-Light Photocatalytic Activity. RSC Adv. 2014, 4, 39969–39977. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Li, T.; Chen, Y.; Wu, Q.; Xie, T.; Lin, Y.; Dong, S. Visible-Light-Driven Photo-Fenton Reaction with α-Fe2O3/BiOI at near Neutral PH: Boosted Photogenerated Charge Separation, Optimum Operating Parameters and Mechanism Insight. J. Colloid Interface Sci. 2019, 554, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, G.; Kumar, S.; Yang, K.; Jin, R. Phase Transformation Synthesis of Novel Ag2O/Ag2CO3 Heterostructures with High Visible Light Efficiency in Photocatalytic Degradation of Pollutants. Adv. Mater. 2014, 26, 892–898. [Google Scholar] [CrossRef] [PubMed]





| Processes | Initial Pollutant Concentration (mg/L) | Catalyst Dosage (g/L) | Degradation Time (min) | Degradation Efficiency (%) | Reference | |
|---|---|---|---|---|---|---|
| 1 | Visible light/Bi4O5I2 nanoflakes | 60 | 1 | 90 | 99.8 | [11] |
| 2 | Visible light/Bi12O17Cl2/β-Bi2O3 heterojunction (Bi:Cl ratio 1:8) | 60 | 1 | 90 | 97 | [21] |
| 3 | UV(254 nm)/Fe-TiO2 | 30 | 1 | 60 | 92 | [22] |
| 4 | Solar light/Ti2O3/TiO2 | 5 | 0.2 | 150 | 89.8 | [23] |
| 5 | UV (365 nm)/Cu-Mo-TiO2 | 15 | 0.1 | 60 | 100 | [24] |
| Catalyst | Pollutant | Light Source | Degradation Time (min) | Degradation Efficiency (%) | Reference | |
|---|---|---|---|---|---|---|
| 1 | CaMg(CO3)2@Ag2CO3/Ag2S/NCQD | phenol | Simulated solar | 100 | 96.5 | [56] |
| 2 | Ag2O/Ag2CO3/MWNTs | ciprofloxacin | visible light | 60 | 76 | [41] |
| 3 | In2O3/Ag2CO3 S-scheme heterojunction | levofloxacin | visible light | 90 | 86.1 | [57] |
| 4 | Ag2CO3@Fe2O3/TiO2-NT | phenol | solar | 240 | 96.2 | [58] |
| 5 | g-C3N4/Ag2CO3/graphene oxide | tetracycline | visible | 60 | 81.6 | [59] |
| 6 | ZnO/Ag2CO3/Ag2O | ibuprofen | visible | 480 | 99.3 | [60] |
| 7 | Ag2CO3 microparticles | 4-tert-butylphenol | Simulated solar | 60 | 100 | this work |
| Light Source | EEO (kW m−3 order−1) |
|---|---|
| Hg lamp (365 nm) | 9.12 |
| Xe lamp (300–600 nm) | 8.29 |
| Xe lamp (solar light) | 0.98 |
| Properties | Value |
|---|---|
| Conductivity | 158.8 μS/cm |
| pH | 7.2 |
| Total organic carbon (TOC) | 1.02 mg/L |
| Total inorganic carbon | 16.72 mg/L |
| 1–15 mg/L | |
| 0–5 mg/L | |
| 10–45 mg/L | |
| 5–25 mg/L | |
| 50–200 mg/L | |
| 3–35 mg/L | |
| 1–30 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergenbayeva, S.; Atabaev, T.S.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S.G. Photocatalytic Degradation of 4-tert-butylphenol Using Solar Light Responsive Ag2CO3. Catalysts 2022, 12, 1523. https://doi.org/10.3390/catal12121523
Mergenbayeva S, Atabaev TS, Vakros J, Mantzavinos D, Poulopoulos SG. Photocatalytic Degradation of 4-tert-butylphenol Using Solar Light Responsive Ag2CO3. Catalysts. 2022; 12(12):1523. https://doi.org/10.3390/catal12121523
Chicago/Turabian StyleMergenbayeva, Saule, Timur Sh. Atabaev, John Vakros, Dionissios Mantzavinos, and Stavros G. Poulopoulos. 2022. "Photocatalytic Degradation of 4-tert-butylphenol Using Solar Light Responsive Ag2CO3" Catalysts 12, no. 12: 1523. https://doi.org/10.3390/catal12121523
APA StyleMergenbayeva, S., Atabaev, T. S., Vakros, J., Mantzavinos, D., & Poulopoulos, S. G. (2022). Photocatalytic Degradation of 4-tert-butylphenol Using Solar Light Responsive Ag2CO3. Catalysts, 12(12), 1523. https://doi.org/10.3390/catal12121523









