Catalytic Wet Peroxide Oxidation of Anionic Pollutants over Fluorinated Fe3O4 Microspheres at Circumneutral pH Values
Abstract
:1. Introduction
2. Results and Discussion
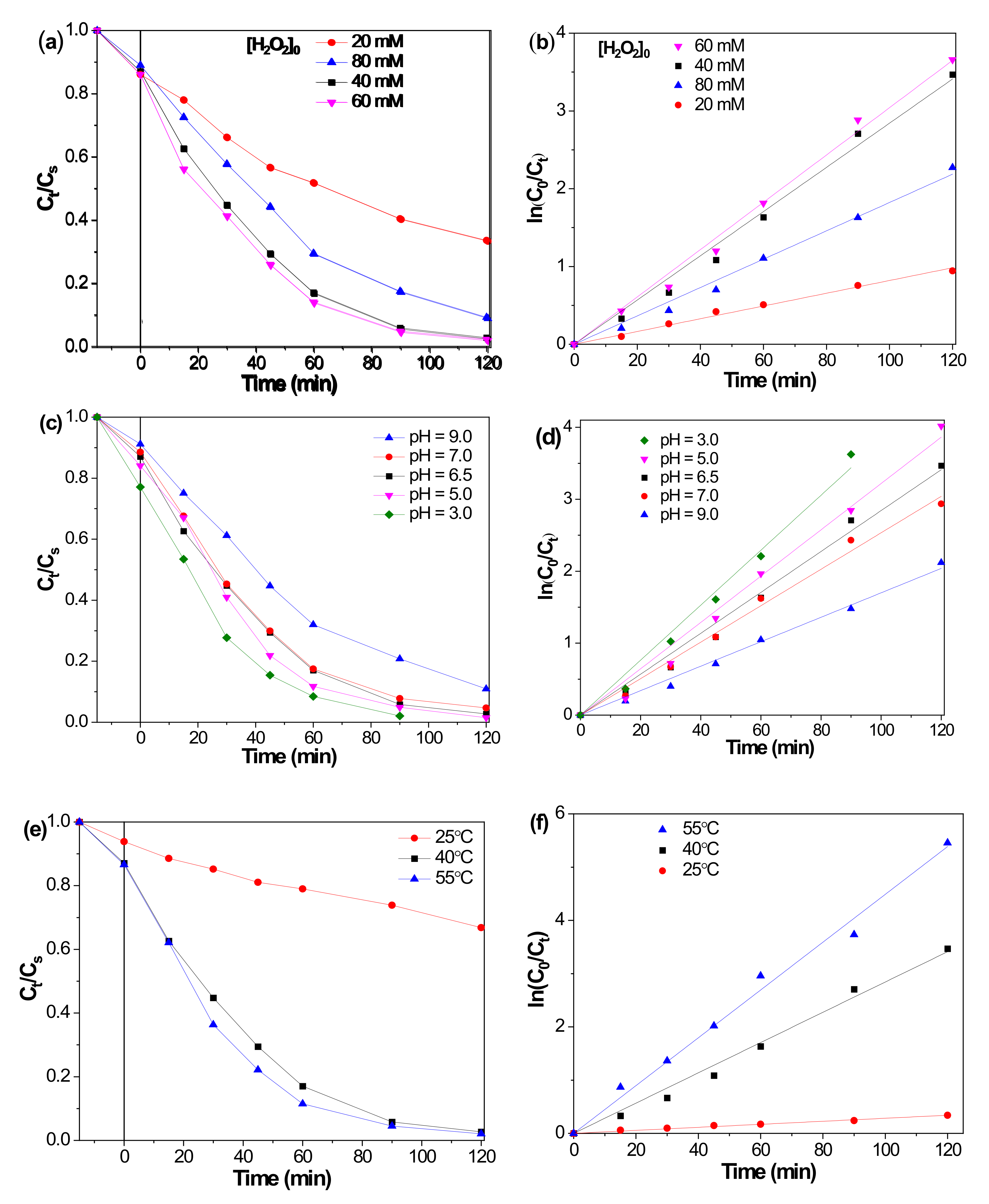
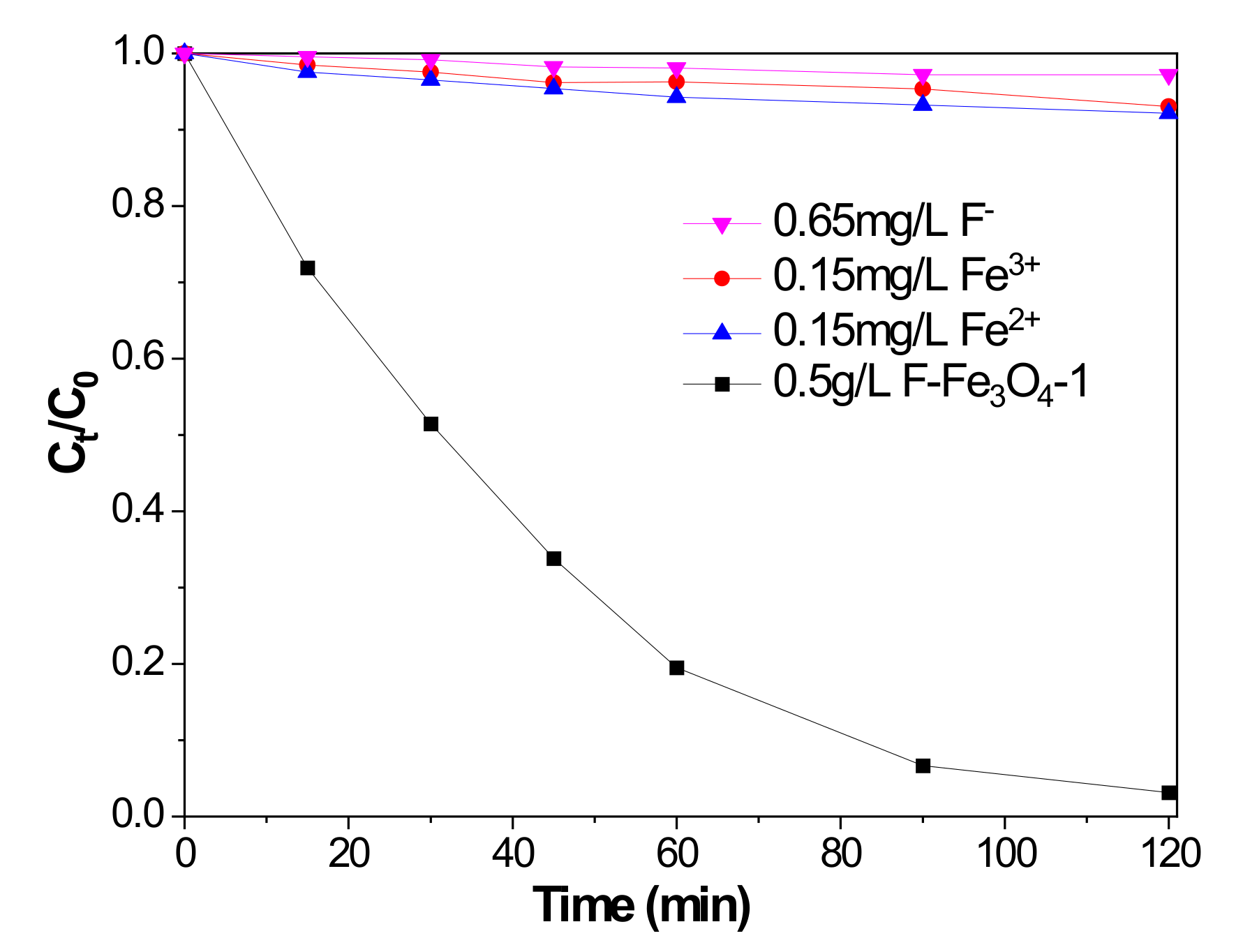
2.1. Catalytic Performances of F-Fe3O4-r Microspheres
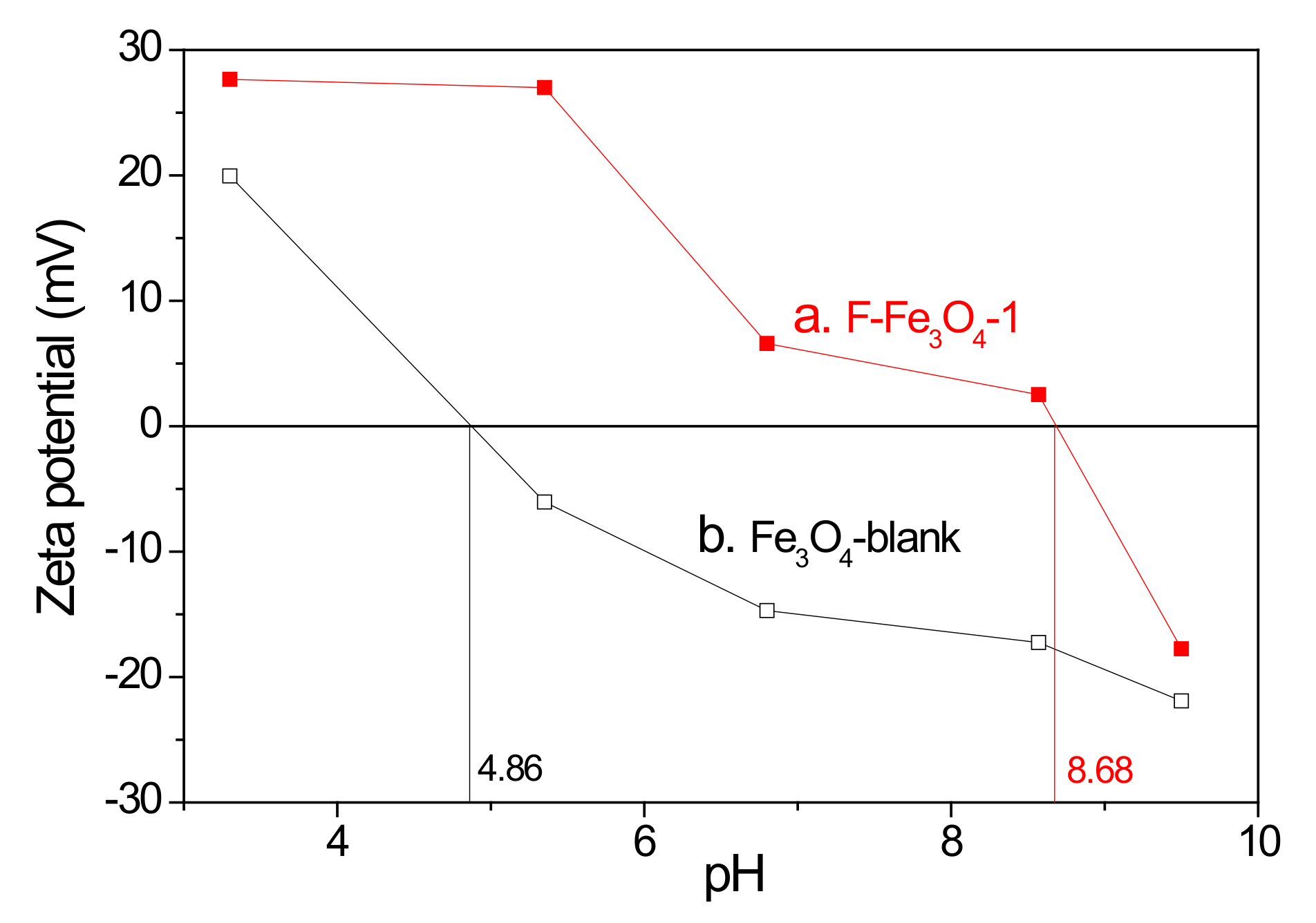
2.2. Characterization of F-Fe3O4-r Microspheres
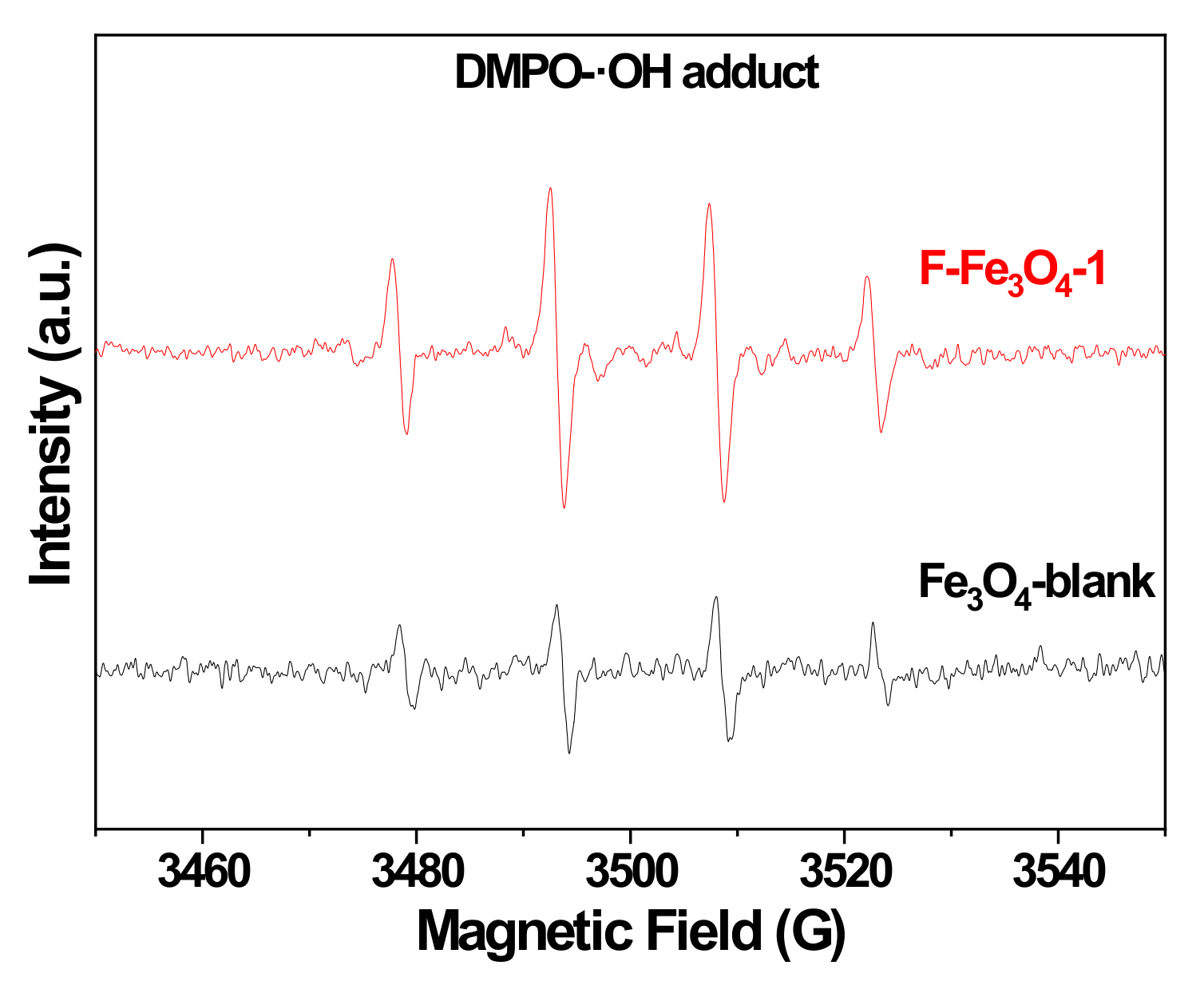
2.3. Mechanistic Investigation
3. Materials and Methods
3.1. Chemicals and Regents
3.2. Synthesis of Fluoride-Modified Fe3O4 Microspheres
3.3. CWPO of Organic Dyes
3.4. Physicochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Fang, Z.; Yan, X.; Cheng, W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]
- Pham, A.L.-T.; Lee, C.; Doyle, F.M.; Sedlak, D.L. A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ. Sci. Technol. 2009, 43, 8930–8935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Pouran, S.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, L.; Li, W.; Li, X.; Wu, H.; Ao, Z.; Tian, W.; Liu, S.; Wang, S.; Sun, H. Boosting Fenton-like reactions via single atom Fe catalysis. Environ. Sci. Technol. 2019, 53, 11391–11400. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Huang, X.; Qiu, X. Ionothermal synthesis of Fe3O4 magnetic nanoparticles as efficient heterogeneous Fenton-like catalysts for degradation of organic pollutants with H2O2. J. Hazard. Mater. 2017, 322, 152–162. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Ma, J.; Chen, F. Ionothermal synthesis of Cu-doped Fe3O4 magnetic nanoparticles with enhanced peroxidase-like activity for organic wastewater treatment. Adv. Powder Technol. 2018, 29, 796–803. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, J.; Huang, X.; Chen, F. One-step nonaqueous synthesis of modified magnetic Fe3O4 microspheres by using epichlorohydrin as functional solvent. Chem. Lett. 2019, 48, 22–25. [Google Scholar] [CrossRef]
- Huang, X.; Du, W.; Chen, R.; Chen, F. Adsorption-enhanced catalytic wet peroxide oxidation of aromatic compounds on ionothermally synthesised copper-doped magnetite magnetic nanoparticles. Environ. Chem. 2020, 17, 426–435. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, Y.; He, H.; Yuan, P.; Zhu, J.; Zhu, S.; Jiang, Z. The application of chromium substituted magnetite as heterogeneous Fenton catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2012, 191, 177–184. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interf. Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef]
- Cai, H.; Li, X.; Ma, D.; Feng, Q.; Wang, D.; Liu, Z.; Wei, X.; Chen, K.; Lin, H.; Qin, S.; et al. Stable Fe3O4 submicrospheres with SiO2 coating for heterogeneous Fenton-like reaction at alkaline condition. Sci. Total Environ. 2021, 764, 14420. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Chen, R.; Chen, F. New soft chemistry route to titanomagnetite magnetic nanoparticles with enhanced peroxidase-like activity. Powder Technol. 2020, 373, 39–45. [Google Scholar] [CrossRef]
- Du, W.; Huang, R.; Huang, X.; Chen, R.; Chen, F. Copper-promoted heterogeneous Fenton-like oxidation of Rhodamine B over Fe3O4 magnetic nanocatalysts at mild conditions. Environ. Sci. Pollut. Res. 2021, 28, 19959–19968. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.K.; Jiang, Z.; Zhang, L. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Kumar, V.; Govind, A.; Nagarajan, R. Optical and photocatalytic properties of heavily F−-doped SnO2 nanocrystals by a novel single-source precursor approach. Inorg. Chem. 2011, 50, 5637–5645. [Google Scholar] [CrossRef]
- Gasparotto, A.; Barreca, D.; Bekermann, D.; Devi, A.; Fischer, R.A.; Fornasiero, P.; Gombac, V.; Lebedev, O.I.; Maccato, C.; Montini, T.; et al. F-doped Co3O4 photocatalysts for sustainable H2 generation from water/ethanol. J. Am. Chem. Soc. 2011, 133, 19362–19365. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-P.; Wang, L.-L.; Bing, N.-C.; Huang, C.; Wang, L.-J.; Liao, G.-H. Porous fluorine-doped γ-Fe2O3 hollow spheres: Synthesis, growth mechanism, and their application in photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 12478–12487. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xiong, L.; Yu, Y. Cu2O homojunction solar cells: F-doped n-type thin film and highly improved efficiency. J. Phys. Chem. C 2015, 119, 22803–22811. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, S.; Xu, T.; Zhu, Y.; Chen, J. Photocatalytic degradation of RhB by fluorinated Bi2WO6 and distributions of the intermediate products. Environ. Sci. Technol. 2008, 42, 2085–2091. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Chen, F. Glycothermal synthesis of fluorinated Fe3O4 microspheres with distinct peroxidase-like activity. Adv. Powder Technol. 2019, 30, 999–1005. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient removal of organic pollutants with magnetic nanoscaled BiFeO3 as a reusable heterogeneous Fenton-like catalyst. Environ. Sci. Technol. 2010, 44, 1786–1791. [Google Scholar] [CrossRef]
- Xue, X.; Hanna, K.; Deng, N. Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J. Hazard. Mater. 2009, 166, 407–414. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Wang, N.; Zhu, L.; Wang, M.; Wang, D.; Tang, H. Sono-enhanced degradation of dye pollutants with the use of H2O2 activated by Fe3O4 magnetic nanoparticles as peroxidase mimetic. Ultrason. Sonochem. 2010, 17, 78–83. [Google Scholar] [CrossRef]
- Lin, C.-C.; Ho, J.-M.; Hsieh, H.-L. Feasibility of using a rotating packed bed in preparing Fe3O4 nanoparticles. Chem. Eng. J. 2012, 203, 88–94. [Google Scholar] [CrossRef]
- Anandkumar, J.; Mandal, B. Adsorption of chromium(VI) and Rhodamine B by surface modified tannery waste: Kinetic, mechanistic and thermodynamic studies. J. Hazard. Mater. 2011, 186, 1088–1096. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dye. Pigment. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yang, W.; Fida, H.; You, L.; Zhou, K. Scalable synthesis of Ca-doped α-Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl. Catal. B Environ. 2020, 262, 118250. [Google Scholar] [CrossRef]
- Lin, S.-S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Hanna, K.; Kone, T.; Medjahdi, G. Synthesis of the mixed oxides of iron and quartz and their catalytic activities for the Fenton-like oxidation. Catal. Commun. 2008, 9, 955–959. [Google Scholar] [CrossRef]
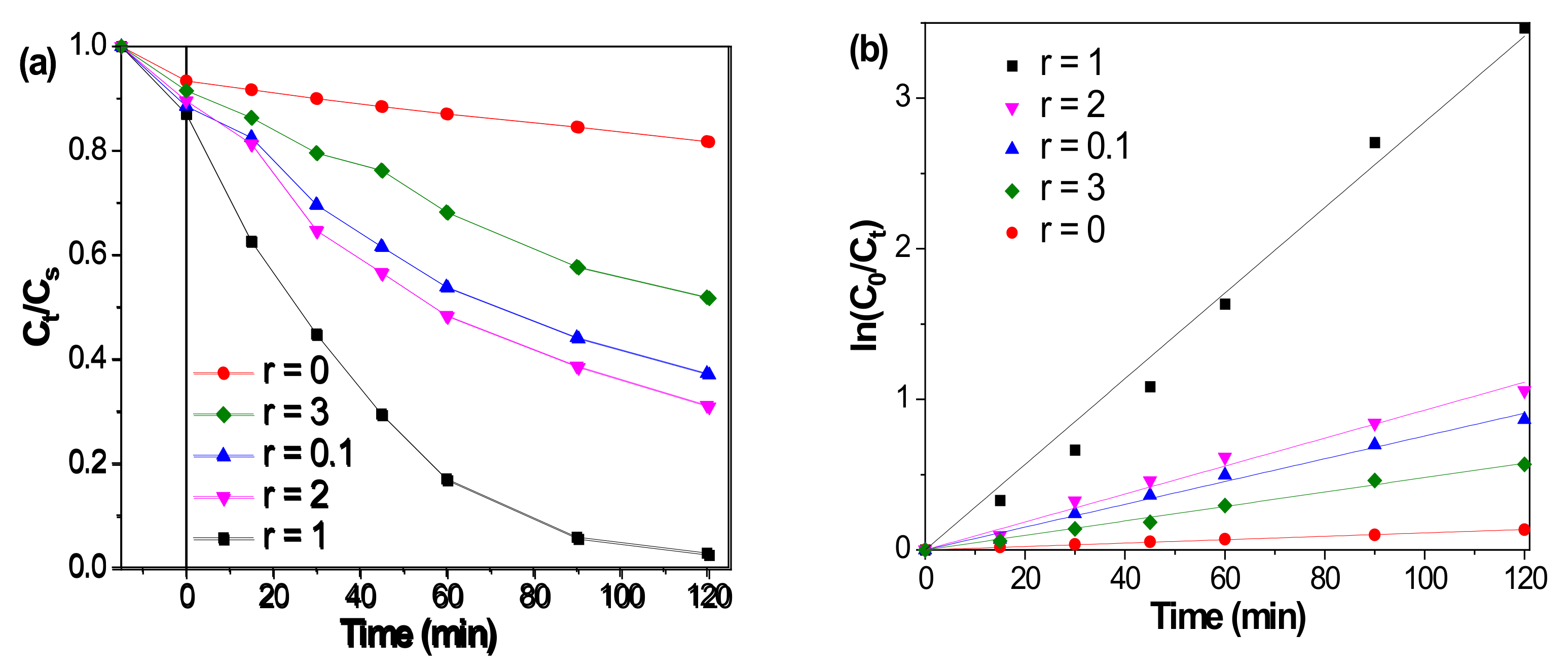




| Entry | r | 15-min Adsorption (%) | 2-h DR (%) | ks1/min−1 (R2) |
|---|---|---|---|---|
| 1 | 0 | 5.9 | 17.6 | 0.0011 (0.997) |
| 2 | 0.1 | 10.8 | 62.2 | 0.0070 (0.990) |
| 3 | 1 | 12.3 | 96.8 | 0.0284 (0.991) |
| 4 | 2 | 9.8 | 68.4 | 0.0092 (0.994) |
| 5 | 3 | 7.8 | 47.5 | 0.0045 (0.991) |
| 6 2 | 1 | 8.3 | 35.2 | 0.0026 (0.996) |
| Organic Dyes | r | 15-min Adsorption (%) | Degradation Rate (%) | ks1/min−1 (R2) |
|---|---|---|---|---|
| CR (100 mg/L, pH 6.8) | 0 | 5.2 | 19.2 | 0.0012 (0.975) |
| 1 | 34.3 | 91.7 | 0.024 (0.976) | |
| MB (0.01 mM, pH 6.5) | 0 | 11.6 | 44.2 | 0.0036 (0.989) |
| 1 | 7.3 | 42.4 | 0.0040 (0.990) | |
| RhB (0.01 mM, pH 6.6) | 0 | 17.4 | 49.3 | 0.0039 (0.984) |
| 1 | 16.6 | 67.1 | 0.0080 (0.993) |
| Run | 2-h DR (%) | ks1/min−1 (R2) | COD Reduction Rate (%) |
|---|---|---|---|
| 1 | 96.8 | 0.0284 (0.988) | 83.2 |
| 2 | 92.7 | 0.0191 (0.978) | 80.1 |
| 3 | 89.6 | 0.0178 (0.990) | 75.5 |
| 4 | 89.1 | 0.0170 (0.988) | 70.4 |
| 5 | 87.1 | 0.0163 (0.985) | 68.6 |
| 6 | 86.4 | 0.0160 (0.990) | 66.4 |
| 7 | 85.9 | 0.0157 (0.987) | 66.1 |
| 8 | 84.9 | 0.0151 (0.987) | 65.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Lv, H.; Chen, W.; Chen, R. Catalytic Wet Peroxide Oxidation of Anionic Pollutants over Fluorinated Fe3O4 Microspheres at Circumneutral pH Values. Catalysts 2022, 12, 1564. https://doi.org/10.3390/catal12121564
Chen F, Lv H, Chen W, Chen R. Catalytic Wet Peroxide Oxidation of Anionic Pollutants over Fluorinated Fe3O4 Microspheres at Circumneutral pH Values. Catalysts. 2022; 12(12):1564. https://doi.org/10.3390/catal12121564
Chicago/Turabian StyleChen, Fengxi, Huaixiang Lv, Wu Chen, and Rong Chen. 2022. "Catalytic Wet Peroxide Oxidation of Anionic Pollutants over Fluorinated Fe3O4 Microspheres at Circumneutral pH Values" Catalysts 12, no. 12: 1564. https://doi.org/10.3390/catal12121564