Promoting the Electrocatalytic Ethanol Oxidation Activity of Pt by Alloying with Cu
Abstract
:1. Introduction
2. Results and Discussion
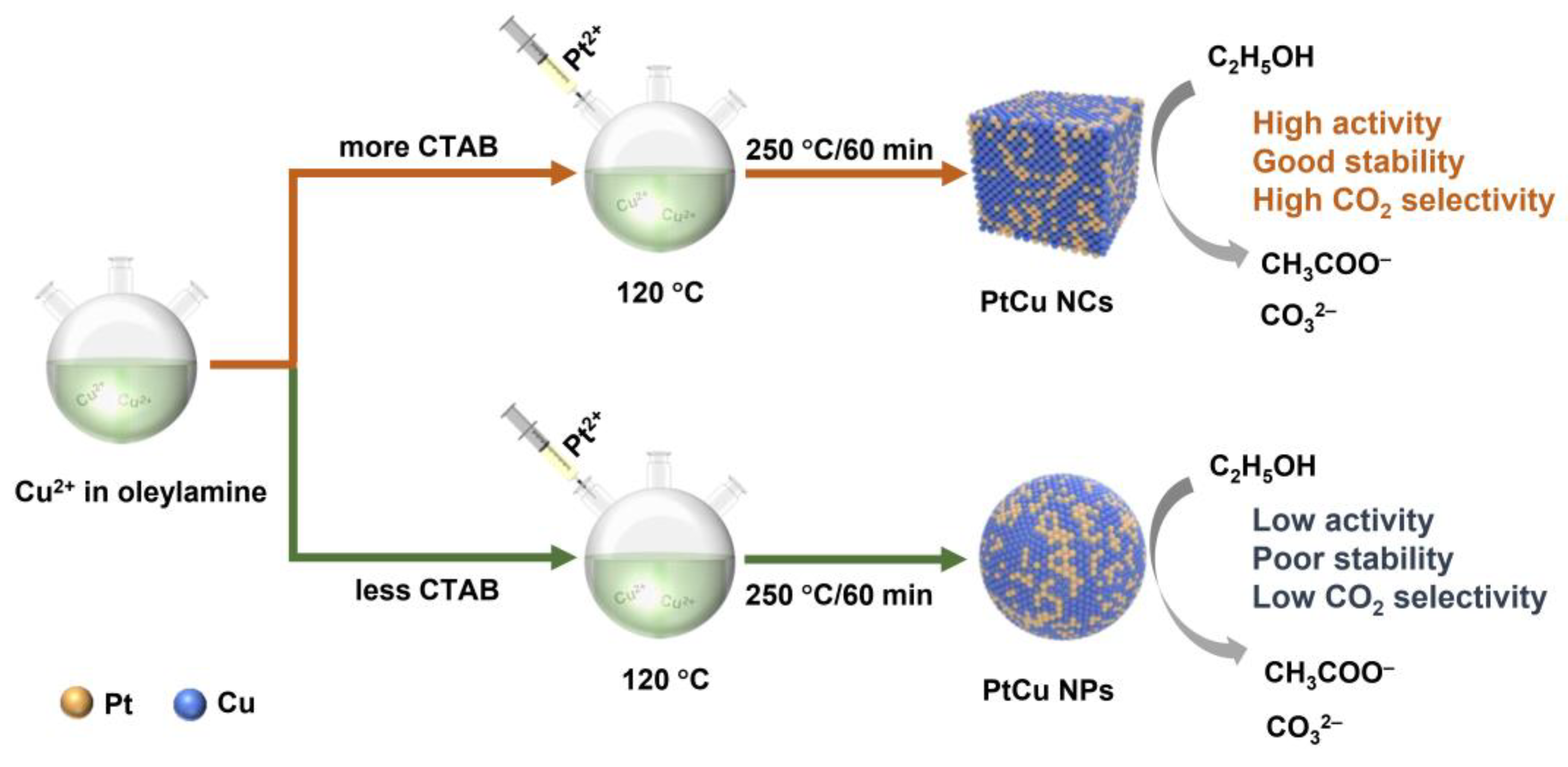
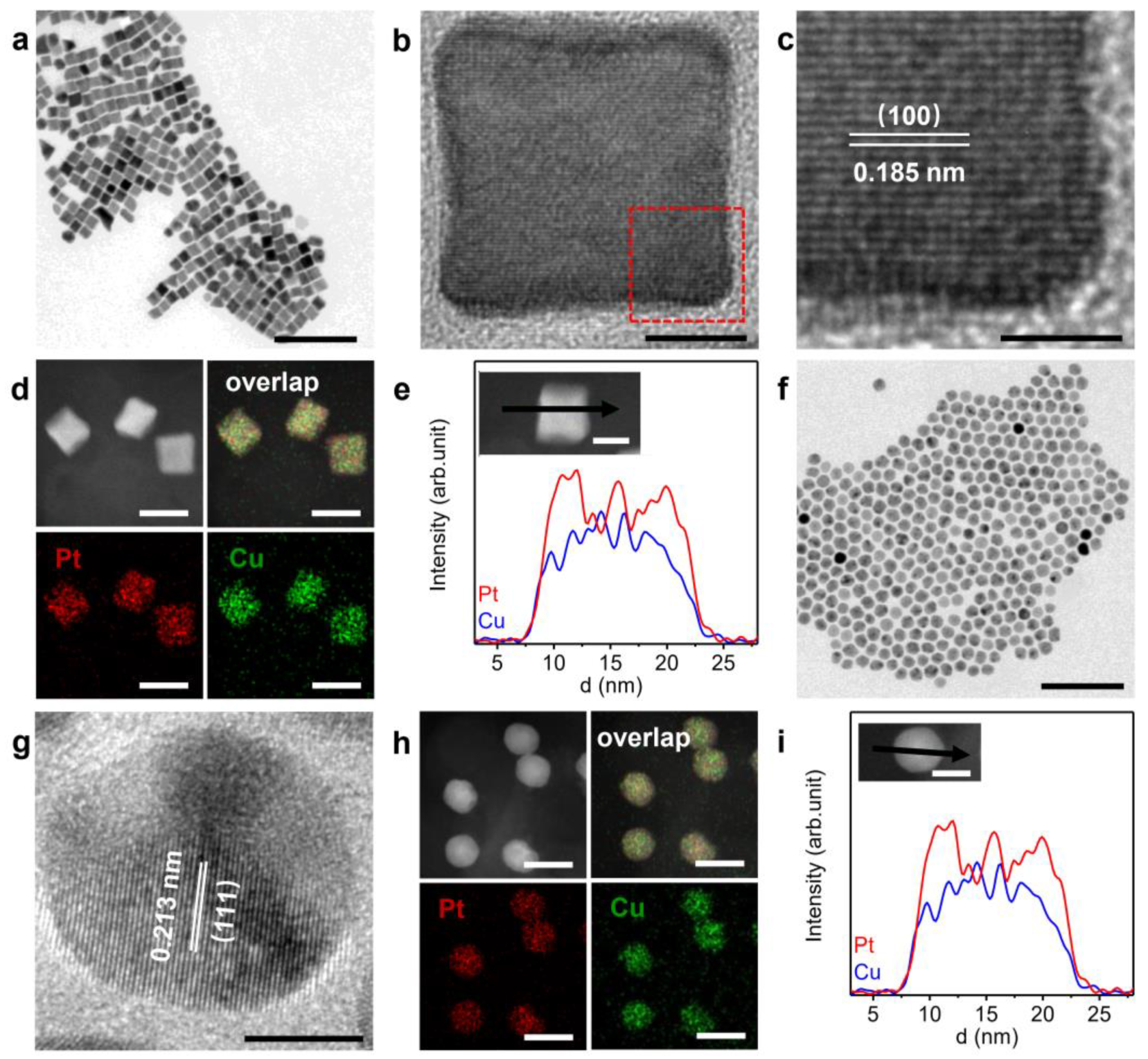
2.1. Catalyst Synthesis and Characterization
2.2. Surface Structures
2.3. CO-Stripping Experiments
2.4. In Situ FTIR
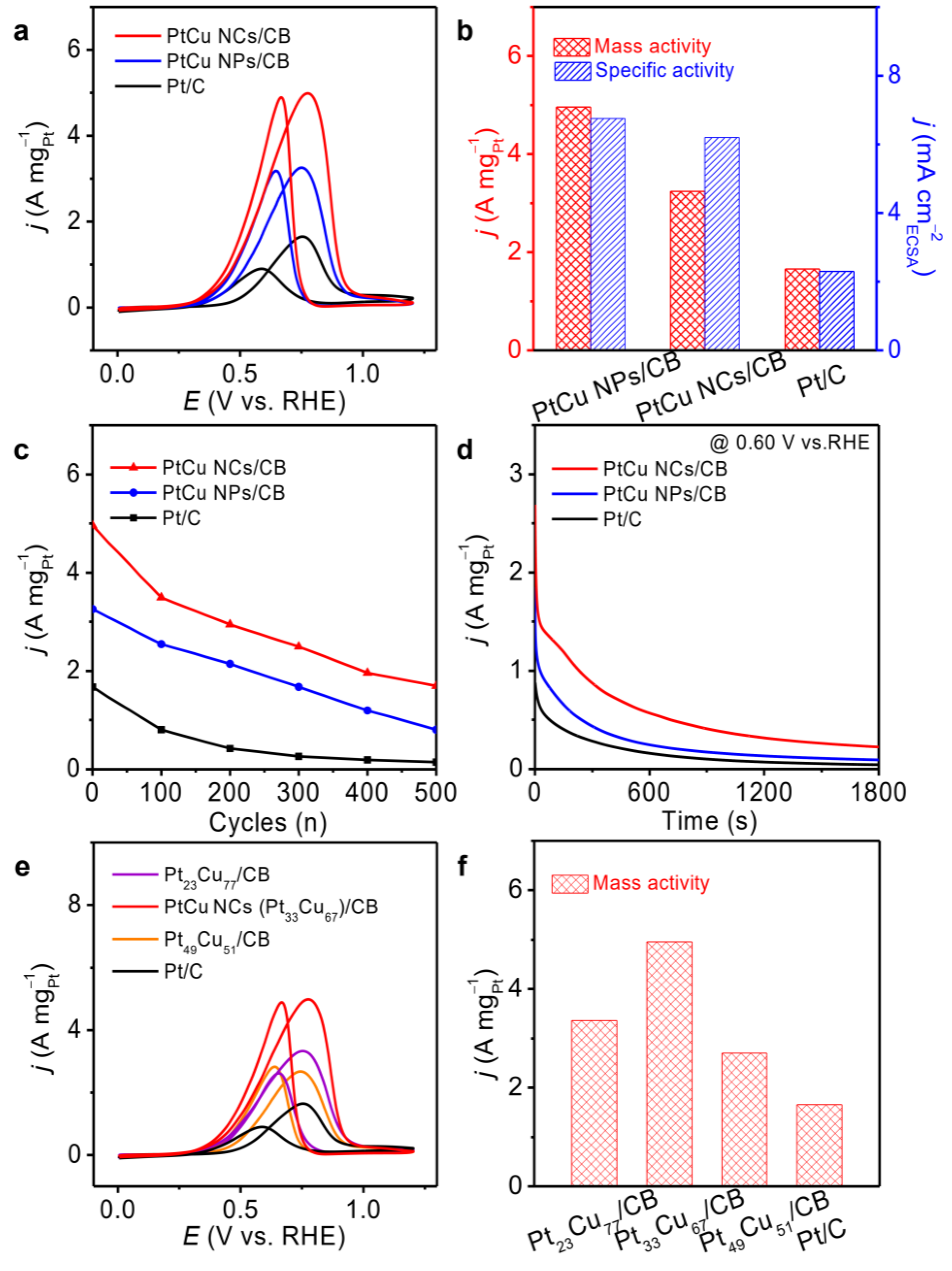
2.5. EOR Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pt-Based Catalysts
3.3. Loading Catalysts on Carbon Supports
3.4. Physicochemical Characterization
3.5. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majlan, E.H.; Rohendi, D.; Daud, W.R.W.; Husaini, T.; Haque, M.A. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 89, 117–134. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Goldemberg, J. Ethanol for a sustainable energy future. Science 2007, 315, 808–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Akhairi, M.A.F.; Kamarudin, S.K. Catalysts in direct ethanol fuel cell (DEFC): An overview. Int. J. Hydrogen Energy 2016, 41, 4214–4228. [Google Scholar] [CrossRef]
- Luo, M.C.; Wang, Z.Y.; Li, Y.G.C.; Li, J.; Li, F.W.; Lum, Y.W.; Nam, D.H.; Chen, B.; Wicks, J.; Xu, A.; et al. Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen. Nat. Commun. 2019, 10, 5814. [Google Scholar] [CrossRef] [Green Version]
- Karapinar, D.; Creissen, C.E.; Rivera de la Cruz, J.G.; Schreiber, M.W.; Fontecave, M. Electrochemical CO2 reduction to ethanol with copper-based Catalysts. ACS Energy Lett. 2021, 6, 694–706. [Google Scholar] [CrossRef]
- Kim, I.; Han, O.H.; Chae, S.A.; Paik, Y.; Kwon, S.H.; Lee, K.S.; Sung, Y.E.; Kim, H. Catalytic reactions in direct ethanol fuel cells. Angew. Chem. Int. Ed. 2011, 50, 2270–2274. [Google Scholar] [CrossRef]
- Menshikov, V.S.; Novomlinsky, I.N.; Belenov, S.V.; Alekseenko, A.A.; Safronenko, O.I.; Guterman, V.E. Methanol, ethanol, and formic acid oxidation on new platinum-containing catalysts. Catalysts 2021, 11, 158. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, X.J.; Cheng, X.; Cheng, K.; Dai, Z.F.; Liu, Z.H. Advanced catalytic materials for ethanol oxidation in direct ethanol fuel cells. Catalysts 2020, 10, 166. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, X.; Xue, Y.Y.; Jiang, J.X.; Zeng, J.H.; Li, X.F.; Chen, Y. Bimetallic platinum–rhodium alloy nanodendrites as highly active electrocatalyst for the ethanol oxidation reaction. ACS Appl. Mater. Interfaces 2018, 10, 19755–19763. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, S.Z.; Cai, W.B. Recent advances on electro-oxidation of ethanol on Pt- and Pd-based catalysts: From reaction mechanisms to catalytic materials. Catalysts 2015, 5, 1507–1534. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Zhang, Y.P.; Ren, F.F.; Song, T.X.; Du, Y.K. Tiny Ir doping of sub-one-nanometer PtMn nanowires: Highly active and stable catalysts for alcohol electrooxidation. Nanoscale 2020, 12, 12098–12105. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, D.; Yang, J.; Chen, Y. Nanocatalysts for electrocatalytic oxidation of ethanol. ChemSusChem 2019, 12, 2117–2132. [Google Scholar] [CrossRef]
- Kavanagh, R.; Cao, X.M.; Lin, W.F.; Hardacre, C.; Hu, P. Origin of low CO2 selectivity on platinum in the direct ethanol fuel cell. Angew. Chem. Int. Ed. 2012, 51, 1572–1575. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.W.; Lai, W.H.; Sheng, T.; Qu, X.M.; Wang, Y.X.; Ren, L.; Zhang, L.; Du, Y.; Jiang, Y.X.; Sun, S.G.; et al. Ordered platinum–bismuth intermetallic clusters with Pt-skin for a highly efficient electrochemical ethanol oxidation reaction. J. Mater. Chem. A 2019, 7, 5214–5220. [Google Scholar] [CrossRef]
- Hu, J.W.; Fang, C.; Jiang, X.M.; Zhang, D.L.; Cui, Z.Q. Ultrathin and porous 2D PtPdCu nanoalloys as high-performance multifunctional electrocatalysts for various alcohol oxidation reactions. Inorg. Chem. 2022, 61, 9352–9363. [Google Scholar] [CrossRef]
- Peng, H.C.; Ren, J.; Wang, Y.C.; Xiong, Y.; Wang, Q.C.; Li, Q.; Zhao, X.; Zhan, L.S.; Zheng, L.R.; Tang, Y.G.; et al. One-stone, two birds: Alloying effect and surface defects induced by Pt on Cu2−xSe nanowires to boost C–C bond cleavage for electrocatalytic ethanol oxidation. Nano Energy 2021, 88, 106307. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, S.F.; Wang, D.S.; He, W.; Li, Y.D. Syntheses of water-soluble octahedral, truncated octahedral, and cubic Pt–Ni nanocrystals and their structure-activity study in model hydrogenation reactions. J. Am. Chem. Soc. 2012, 134, 8975–8981. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Li, Y.R.; Ye, C.L.; Chen, J.; Ye, J.Y.; Zhang, Q.H.; Li, J.; Zhou, Z.Y.; Fu, X.Z.; et al. p-d orbital hybridization induced by a monodispersed Ga site on a Pt3Mn nanocatalyst boosts ethanol electrooxidation. Angew. Chem. Int. Ed. 2022, 61, e202115735. [Google Scholar]
- Liu, T.Y.; Wang, K.; Yuan, Q.; Shen, Z.B.; Wang, Y.; Zhang, Q.H.; Wang, X. Monodispersed sub-5.0 nm PtCu nanoalloys as enhanced bifunctional electrocatalysts for oxygen reduction reaction and ethanol oxidation reaction. Nanoscale 2017, 9, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Bliznakov, S.; Liu, Z.P.; Fang, J.Y.; Dimitrov, N. Composition-dependent electrocatalytic activity of Pt–Cu nanocube catalysts for formic acid oxidation. Angew. Chem. Int. Ed. 2010, 122, 1304–1307. [Google Scholar] [CrossRef]
- Yin, A.X.; Min, X.Q.; Zhu, W.; Liu, W.C.; Zhang, Y.W.; Yan, C.H. Pt–Cu and Pt–Pd–Cu concave nanocubes with high-index facets and superior electrocatalytic activity. Chem. Eur. J. 2012, 18, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lv, Q.Q.; Lu, S.Q.; Fang, J.J.; Zhang, Y.F.; Wang, X.D.; Xue, Y.R.; Zhu, W.; Zhuang, Z.B. IrCuNi deeply concave nanocubes as highly active oxygen evolution reaction electrocatalyst in acid electrolyte. Nano Lett. 2021, 21, 2809–2816. [Google Scholar] [CrossRef]
- Yu, T.; Kim, D.Y.; Zhang, H.; Xia, Y.N. Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 2773–2777. [Google Scholar] [CrossRef]
- Huang, M.H.; Jiang, Y.Y.; Jin, C.H.; Ren, J.; Zhou, Z.Y.; Guan, L.H. Pt–Cu alloy with high density of surface Pt defects for efficient catalysis of breaking C–C bond in ethanol. Electrochim. Acta 2014, 125, 29–37. [Google Scholar] [CrossRef]
- Dai, L.X.; Wang, X.Y.; Yang, S.S.; Zhang, T.; Ren, P.J.; Ye, J.Y.; Nan, B.; Wen, X.D.; Zhou, Z.Y.; Si, R.; et al. Intrinsic composition and electronic effects of multicomponent platinum nanocatalysts with high activity and selectivity for ethanol oxidation reaction. J. Mater. Chem. A 2018, 6, 11270–11280. [Google Scholar] [CrossRef]
- Lan, B.; Huang, M.; Wei, R.L.; Wang, C.N.; Wang, Q.L.; Yang, Y.Y. Ethanol electrooxidation on rhodium-lead catalysts in alkaline media: High mass activity, long-term durability, and considerable CO2 selectivity. Small 2020, 16, 2004380. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Q.L.; Ma, Z.X.; Wu, Y.J.; Jiang, X.L.; Jia, W.S.; Zhou, C.X.; Yang, Y.Y. Efficient electrochemical ethanol-to-CO2 conversion at rhodium and bismuth hydroxide interfaces. Appl. Catal. B 2022, 300, 120728. [Google Scholar] [CrossRef]
- Lee, Y.W.; Han, S.B.; Kim, D.Y.; Park, K.W. Monodispersed platinum nanocubes for enhanced electrocatalytic properties in alcohol electrooxidation. Chem. Commun. 2011, 47, 6296–6298. [Google Scholar] [CrossRef]
- Busó-Rogero, C.; Grozovski, V.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Herrero, E.; Feliu, J.M. Surface structure and anion effects in the oxidation of ethanol on platinum nanoparticles. J. Mater. Chem. A 2013, 1, 7068–7076. [Google Scholar] [CrossRef]
- Bai, S.X.; Xu, Y.; Cao, K.L.; Huang, X.Q. Selective ethanol oxidation reaction at the Rh–SnO2 interface. Adv. Mater. 2021, 33, 2005767. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Duanmu, K.; Wan, C.Z.; Cheng, T.; Zhang, L.; Dai, S.; Chen, W.X.; Zhao, Z.P.; Li, P.; Fei, H.L.; et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2019, 2, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Erini, N.; Beermann, V.; Gocyla, M.; Gliech, M.; Heggen, M.; Dunin-Borkowski, R.E.; Strasser, P. The Effect of surface site ensembles on the activity and selectivity of ethanol electrooxidation by octahedral PtNiRh nanoparticles. Angew. Chem. Int. Ed. 2017, 56, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Liu, H.M.; Chen, P.; Jiang, J.X.; Chen, Y. Porous trimetallic PtRhCu cubic nanoboxes for ethanol electrooxidation. Adv. Energy Mater. 2018, 8, 1801326. [Google Scholar] [CrossRef]
- Chen, L.G.; Liang, X.; Li, X.T.; Pei, J.J.; Lin, H.; Jia, D.Z.; Chen, W.X.; Wang, D.S.; Li, Y.D. Promoting electrocatalytic methanol oxidation of platinum nanoparticles by cerium modification. Nano Energy 2020, 73, 104784. [Google Scholar] [CrossRef]
- Wu, F.X.; Eid, K.; Abdullah, A.M.; Niu, W.X.; Wang, C.; Lan, Y.X.; Elzatahry, A.A.; Xu, G.B. Unveiling one-pot template-free fabrication of exquisite multidimensional PtNi multicube nanoarchitectonics for the efficient electrochemical oxidation of ethanol and methanol with a great tolerance for CO. ACS Appl. Mater. Interfaces 2020, 12, 31309–31318. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Gao, F.; Wang, C.Q.; Shiraishi, Y.; Du, Y.K. Engineering spiny PtFePd@PtFe/Pt core@multishell nanowires with enhanced performance for alcohol electrooxidation. ACS Appl. Mater. Interfaces 2019, 11, 30880–30886. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Liu, G.G.; Ling, C.Y.; Chen, B.; Huang, J.T.; Liu, X.Z.; Li, B.; Wang, A.L.; Hu, Z.N.; et al. Crystal phase-controlled growth of PtCu and PtCo alloys on 4H Au nanoribbons for electrocatalytic ethanol oxidation reaction. Nano Res. 2020, 13, 1970–1975. [Google Scholar] [CrossRef]
- Yuan, X.L.; Jiang, X.J.; Cao, M.H.; Chen, L.; Nie, K.Q.; Zhang, Y.; Xu, Y.; Sun, X.H.; Li, Y.G.; Zhang, Q. Intermetallic PtBi core/ultrathin Pt shell nanoplates for efficient and stable methanol and ethanol electro-oxidization. Nano Res. 2019, 12, 429–436. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, J.H.; Wang, L.J.; Tang, Y.W.; Xia, B.Y.; Chen, Y. Trimetallic PtRhNi alloy nanoassemblies as highly active electrocatalyst for ethanol electrooxidation. Nano Res 2017, 10, 3324–3332. [Google Scholar] [CrossRef]
- Eid, K.; Ahmad, Y.H.; Yu, H.J.; Li, Y.H.; Li, X.N.; AlQaradawi, S.Y.; Wang, H.J.; Wang, L. Rational one-step synthesis of porous PtPdRu nanodendrites for ethanol oxidation reaction with a superior tolerance for CO-poisoning. Nanoscale 2017, 9, 18881–18889. [Google Scholar] [CrossRef] [PubMed]
- Pech-Rodríguez, W.J.; González-Quijano, D.; Vargas-Gutiérrez, G.; Morais, C.; Napporn, T.W.; Rodríguez-Varela, F.J. Electrochemical and in situ FTIR study of the ethanol oxidation reaction on PtMo/C nanomaterials in alkaline media. Appl. Catal. B Environ. 2017, 203, 654–662. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, Y.; Wi, D.H.; Lee, S.; Lee, S.U.; Lee, Y.W.; Choi, S.I.; Han, S.W. Ultrathin free-standing ternary-alloy nanosheets. Angew. Chem. Int. Ed. 2016, 55, 2753–2758. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Huang, H.-Z.; Zhu, Z.; Li, J.; Chen, L.-W.; Jing, X.-T.; Yin, A.-X. Promoting the Electrocatalytic Ethanol Oxidation Activity of Pt by Alloying with Cu. Catalysts 2022, 12, 1562. https://doi.org/10.3390/catal12121562
Liu D, Huang H-Z, Zhu Z, Li J, Chen L-W, Jing X-T, Yin A-X. Promoting the Electrocatalytic Ethanol Oxidation Activity of Pt by Alloying with Cu. Catalysts. 2022; 12(12):1562. https://doi.org/10.3390/catal12121562
Chicago/Turabian StyleLiu, Di, Hui-Zi Huang, Zhejiaji Zhu, Jiani Li, Li-Wei Chen, Xiao-Ting Jing, and An-Xiang Yin. 2022. "Promoting the Electrocatalytic Ethanol Oxidation Activity of Pt by Alloying with Cu" Catalysts 12, no. 12: 1562. https://doi.org/10.3390/catal12121562



