Materials Design for N2O Capture: Separation in Gas Mixtures
Abstract
:1. Introduction
2. N2O Capture
2.1. Kinetic and Thermodynamic Considerations
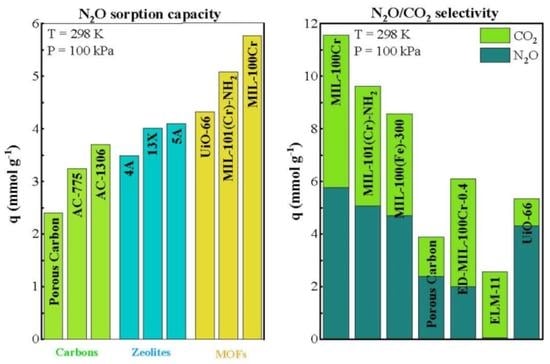
2.2. Separation Efficiency
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation Technologies for CO2 Utilisation: Current Status, Challenges and Future Prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Available online: https://Www.Epa.Gov/Ghgemissions/Overview-Greenhouse-Gases#CO2-References (accessed on 8 September 2022).
- Monthly Average Mauna Loa CO2. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 8 September 2022).
- Available online: https://Gml.Noaa.Gov/Ccgg/Trends_ch4/. (accessed on 9 September 2022).
- Available online: https://Gml.Noaa.Gov/Ccgg/Trends_n2o/ (accessed on 9 September 2022).
- Hanqin, T.; Rongting, X.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Ju, Y.; Kim, J.H.; Ahn, H.; Lee, C.H. Equilibrium and Kinetics of Nitrous Oxide, Oxygen and Nitrogen Adsorption on Activated Carbon and Carbon Molecular Sieve. Sep. Purif. Technol. 2019, 223, 63–80. [Google Scholar] [CrossRef] [Green Version]
- Ko, B.H.; Hasa, B.; Shin, H.; Zhao, Y.; Jiao, F. Electrochemical Reduction of Gaseous Nitrogen Oxides on Transition Metals at Ambient Conditions. J. Am. Chem. Soc. 2022, 144, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Tanaka, K.; Fujimori, M. Abatement Technologies for N2O Emissions in the Adipic Acid Industry. Chemosphere—Glob. Chang. Sci. 2000, 2, 425–434. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Shi, W.; Cheng, P. Highly Selective Sorption of CO2 and N2O and Strong Gas-Framework Interactions in a Nickel(II) Organic Material. J. Mater. Chem. A 2016, 4, 16198–16204. [Google Scholar] [CrossRef]
- Chen, D.L.; Wang, N.; Wang, F.F.; Xie, J.; Zhong, Y.; Zhu, W.; Johnson, J.K.; Krishna, R. Utilizing the Gate-Opening Mechanism in ZIF-7 for Adsorption Discrimination between N2O and CO2. J. Phys. Chem. C 2014, 118, 17831–17837. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, F.; Li, K.; Zhang, Y.; Song, Z.; Wang, L.; Yang, J.; Li, J. Improved N2O Capture Performance of Chromium Terephthalate MIL-101 via Substituent Engineering. J. Solid State Chem. 2022, 309, 122951. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Elliott, B.A.; Niehaus, A.M.S.; Yokozeki, A. Separation of N2O and CO2 Using Room-Temperature Ionic Liquid [Bmim][Ac]. Sep. Sci. Technol. 2012, 47, 411–421. [Google Scholar] [CrossRef]
- Pereira, L.M.C.; Oliveira, M.B.; Llovell, F.; Vega, L.F.; Coutinho, J.A.P. Assessing the N2O/CO2 High Pressure Separation Using Ionic Liquids with the Soft-SAFT EoS. J. Supercrit. Fluids 2014, 92, 231–241. [Google Scholar] [CrossRef]
- Park, D.; Hong, S.H.; Kim, K.M.; Lee, C.H. Adsorption Equilibria and Kinetics of Silica Gel for N2O, O2, N2, and CO2. Sep. Purif. Technol. 2020, 251, 117326. [Google Scholar] [CrossRef]
- Kloutse, F.A.; Gauthier, W.; Hourri, A.; Natarajan, S.; Benard, P.; Chahine, R. Study of Competitive Adsorption of the N2O-CO2-CH4-N2 Quaternary Mixture on CuBTC. Sep. Purif. Technol. 2020, 235, 116211. [Google Scholar] [CrossRef]
- Cornelissen, G.; Rutherford, D.W.; Arp, H.P.H.; Dörsch, P.; Kelly, C.N.; Rostad, C.E. Sorption of Pure N2O to Biochars and Other Organic and Inorganic Materials under Anhydrous Conditions. Environ. Sci. Technol. 2013, 47, 7704–7712. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of Organic Molecules on Silica Surface. Adv. Colloid Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.L.; Wang, Q. Adsorption Characteristics of the Silica Gels as Adsorbent for Gasoline Vapors Removal. IOP Conf. Ser. Earth Environ. Sci 2018, 153, 022010. [Google Scholar] [CrossRef] [Green Version]
- Wahshi, F.S.; Alqahtani, M.D.; Abdulla, M.; Ramachandran, T.; Hamed, F.; Thiemann, T. Adsorption of Model Dyes on Recycled Silica Gel. Proceedings 2020, 48, 10. [Google Scholar] [CrossRef] [Green Version]
- Chapter 9 Physical Methods of Gas Analysis. Compr. Anal. Chem. 1991, 28, 123–221. [CrossRef]
- Xia, K.; Gao, Q.; Wu, C.; Song, S.; Ruan, M. Activation, Characterization and Hydrogen Storage Properties of the Mesoporous Carbon CMK-3. Carbon 2007, 45, 1989–1996. [Google Scholar] [CrossRef]
- Centi, G.; Generali, P.; Dall’olio, L.; Perathoner, S.; Rak, Z. Removal of N2O from Industrial Gaseous Streams by Selective Adsorption over Metal-Exchanged Zeolites. Ind. Eng. Chem. Res. 2000, 39, 131–137. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal-Organic Frameworks—Prospective Industrial Applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Edubilli, S.; Mishra, A.; Ghoshal, A.K. CO2 Adsorption Kinetics on Mesoporous Silica under Wide Range of Pressure and Temperature. Chem. Eng. J. 2014, 256, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zhou, L.; Huang, D.; Zhou, Y. Adsorption of CO2, CH4 and N2 on Ordered Mesoporous Silica Molecular Sieve. Chem. Phys. Lett. 2005, 415, 198–201. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Modeling Adsorption of CO2 on Amine-Functionalized Mesoporous Silica. 2: Kinetics and Breakthrough Curves. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Modeling CO2 Adsorption on Amine-Functionalized Mesoporous Silica: 1. A Semi-Empirical Equilibrium Model. Chem. Eng. J. 2010, 161, 173–181. [Google Scholar] [CrossRef]
- Wu, T.; Shen, Y.; Feng, L.; Tang, Z.; Zhang, D. Adsorption Properties of N2O on Zeolite 5A, 13X, Activated Carbon, ZSM-5, and Silica Gel. J. Chem. Eng. Data 2019, 64, 3473–3482. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Xu, C.; Xiao, Q.; Zhong, Y.; Zhu, W. Adsorption of Nitrous Oxide on Activated Carbons. J. Chem. Eng. Data 2009, 54, 3079–3081. [Google Scholar] [CrossRef]
- Hernández Huesca, R.; Pérez Arcos, J.; Vargas Hernández, D.; Pérez Cruz, M.A. Adsorption Kinetics of N2O on Natural Zeolites. Int. J. Environ. Pollut. 2016, 32, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Jahandar Lashaki, M.; Fayaz, M.; Niknaddaf, S.; Hashisho, Z. Effect of the Adsorbate Kinetic Diameter on the Accuracy of the Dubinin-Radushkevich Equation for Modeling Adsorption of Organic Vapors on Activated Carbon. J. Hazard. Mater. 2012, 241–242, 154–163. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Adsorption Equilibrium, Kinetics, and Enthalpy of N2O on Zeolite 4A and 13X. J. Chem. Eng. Data 2010, 55, 3312–3317. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Adsorption Equilibrium and Kinetics of CO2, CH4, N2O, and NH3 on Ordered Mesoporous Carbon. J. Colloid Interface Sci. 2010, 345, 402–409. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Wang, C.; Li, Y.; Yang, J.; Li, L.; Li, J. Ethylenediamine-Functionalized Metal Organic Frameworks MIL-100(Cr) for Efficient CO2/N2O Separation. Sep. Purif. Technol. 2020, 235, 116219. [Google Scholar] [CrossRef]
- Groen, J.C.; Pérez-Ramírez, J.; Zhu, W. Adsorption of Nitrous Oxide on Silicalite-1. J. Chem. Eng. Data 2002, 47, 587–589. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Du, B.; Liu, J.; Krishna, R.; Zhang, F.; Zhou, W.; Wang, Y.; Li, J.; Chen, B. MIL-100Cr with Open Cr Sites for a Record N2O Capture. Chem. Commun. 2018, 54, 14061–14064. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, F.; Yang, J.; Li, L.; Li, J. The Efficient Separation of N2O/CO2 using Unsaturated Fe2+ sites in MIL-100Fe. Chem. Commun. 2021, 57, 6636–6639. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Wang, Y.; Yang, J.; Li, L.; Li, J. Research on CO2-N2O Separation Using Flexible Metal Organic Frameworks. Sep. Purif. Technol. 2020, 251, 117311. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S.; Yang, Z. Hydrogen Adsorption on Metal-Organic Framework (MOF-5) Synthesized by DMF Approach. J. Porous Mater. 2009, 16, 141–149. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T. Gas Adsorption and Storage in Metal-Organic Framework MOF-177. Langmuir 2007, 23, 12937–12944. [Google Scholar] [CrossRef]
- Culp, J.T.; Smith, M.R.; Bittner, E.; Bockrath, B. Hysteresis in the Physisorption of CO2 and N2 in a Flexible Pillared Layer Nickel Cyanide. J. Am. Chem. Soc. 2008, 130, 12427–12434. [Google Scholar] [CrossRef] [PubMed]
- Culp, J.T.; Goodman, A.L.; Chirdon, D.; Sankar, S.G.; Matranga, C. Mechanism for the Dynamic Adsorption of CO2 and CH4 in a Flexible Linear Chain Coordination Polymer as Determined from in Situ Infrared Spectroscopy. J. Phys. Chem. C 2010, 114, 2184–2191. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Strategies for Hydrogen Storage in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
| Material | Features |
|---|---|
| Silica gel | Possible to modulate the dimensions and morphologies of the pore, possible to functionalize, good surface area, and low cost [19,20,21,22] |
| AC | Hydrophobic, high surface area and micropore volume, and low cost [8,23] |
| Zeolites | High-volume pores and surface area, capacity to be modified by ion exchange [24] |
| MOFs | High specific surface high ordering and narrow microporosity, crystalline nature, possibility of modification of the pore network and functionalization, and high-cost synthesis [25]. |
| Adsorbent | T (K) | P (kPa) | BET (m2 g−1) | Vp (cm3 g−1) | q (mmol g−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Micro | Meso/ Macro | ||||||
| Silica gel | 293 | 102 | 775 | 0.350 | 0.103 | 1.01 | [16] |
| SiO2-205 | 293 | 100 | 205 | - | - | 0.38 | [18] |
| SiO2-598 | 293 | 100 | 598 | - | - | 1.53 | [18] |
| Silica gel | 298 | 100 | 759 | 0.485 | 0.79 | [30] | |
| Adsorbent | T (K) | P (kPa) | BET (m2 g−1) | Vp (cm3 g−1) | q (mmol g−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Micro | Meso/ Macro | ||||||
| AC-775 | 293 | 100 | 775 | - | - | 3.24 | [18] |
| AC-569 | 293 | 100 | 569 | - | - | 3.21 | [18] |
| AC | 298 | 100 | 904 | 0.502 | - | 2.39 | [30] |
| Porous carbon | 298 | 106 | 798 | - | 0.87 | 2.40 | [36] |
| AC | 293 | 100 | 1306 | 0.370 | - | 3.70 | [8] |
| CMS | 293 | 100 | 641 | 0.241 | 0.236 | 2.50 | [8] |
| Kureha | 293 | 101 | 1300 | 0.560 | - | 3.10 | [31] |
| Ovcls | 293 | 101 | 1055 | 0.397 | 0.104 | 3.60 | [31] |
| Vruf | 293 | 101 | 1330 | 0.040 | 0.770 | 2.10 | [31] |
| Adsorbent | T (K) | P (kPa) | BET (m2 g−1) | Vp (cm3 g−1) | q (mmol g−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Micro | Meso/ Macro | ||||||
| Silicalite-1 | 298 | 100 | 372 | 0.179 | - | 1.74 | [38] |
| 5A | 298 | 100 | 580 | - | 0.257 | 3.72 | [30] |
| 13X | 298 | 100 | 529 | 0.425 | - | 4.01 | [30] |
| ZSM-5 | 298 | 100 | 241 | 0.258 | - | 1.34 | [30] |
| 4A | 298 | 108 | - | x | - | 3.49 | [34] |
| 13X | 298 | 108 | - | x | - | 0.84 | [34] |
| 5A | 298 | 106 | - | x | - | 4.10 | [35] |
| Erionite | 293 | 100 | 426 | 0.188 | 0.032 | 2.10 | [32] |
| Clinoptilolite | 293 | 100 | 23 | 0.108 | 0.082 | 1.75 | [32] |
| Mordenite | 293 | 100 | 266 | 0.119 | 0.055 | 1.50 | [32] |
| Adsorbent | T (K) | P (kPa) | BET (m2 g−1) | Vp (cm3 g−1) | q (mmol g−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Micro | Meso/ Macro | ||||||
| MIL-100Cr-150 | 298 | 100 | 1764 | - | - | 1.95 | [40] |
| MIL-100Cr-250 | 298 | 100 | 2118 | - | - | 5.78 | [40] |
| MIL-100Cr-250 | 273 | 100 | 2118 | - | - | 8.25 | [40] |
| MIL-100Cr | 298 | 100 | 1574 | 0.81 | - | 5.77 | [37] |
| ED *-MIL-100Cr-0.2 | 298 | 100 | 1050 | 0.39 | - | 2.14 | [37] |
| ED *-MIL-100Cr-0.4 | 298 | 100 | 952 | 0.34 | - | 2.00 | [37] |
| ED *-MIL-100Cr-0.6 | 298 | 100 | 981 | 0.34 | - | 1.99 | [37] |
| MOF-5 | 298 | 106 | 900 | - | - | 0.90 | [35] |
| MOF-177 | 298 | 106 | 3500 | 1.60 | - | 0.07 | [35] |
| Ni-MOF | 298 | 100 | 447 | 0.094 | - | 2.81 | [11] |
| HKUST-1 | 298 | 100 | - | - | - | 3.91 | [37] |
| ZIF-7 | 298 | 100 | - | x | - | 2.50 | [12] |
| ZIF-8 | 298 | 100 | - | - | - | 1.39 | [37] |
| UiO-66 | 298 | 100 | - | - | - | 4.32 | [37] |
| MIL-100Fe-300 | 298 | 100 | - | x | x | 4.70 | [41] |
| MIL-101(Cr)-NO2 | 298 | 100 | 3255 | 1.14 | 0.76 | 2.38 | [13] |
| MIL-101(Cr)-Br | 298 | 100 | 1626 | 0.65 | 0.33 | 2.58 | [13] |
| MIL-101(Cr)-NH2 | 298 | 100 | 3768 | 1.42 | 0.82 | 5.08 | [13] |
| MIL-101(Cr)-H | 298 | 100 | 4156 | 2.03 | 0.31 | 5.44 | [13] |
| MIL-53Al | 298 | 100 | - | - | - | 2.70 | [42] |
| CuBTC | 297 | 122.8 | - | - | - | 5.38 | [17] |
| Adsorbent | T (K) | P (kPa) | q (mmol g−1) | Ref. | |
|---|---|---|---|---|---|
| N2O | CO2 | ||||
| Porous carbon | 298 | 106 | 2.40 | 1.49 | [36] |
| Ni-MOF | 298 | 100 | 2.81 | 3.24 | [11] |
| ZIF-7 | 298 | 100 | 2.50 | 2.29 | [12] |
| CuBTC | 297 | 112 | 3.70 | 3.10 | [17] |
| MOF-5 | 298 | 106 | 0.90 | 0.77 | [35] |
| ZIF-8 | 298 | 100 | 1.39 | 1.03 | [37] |
| UiO-66 | 298 | 100 | 4.32 | 1.03 | [37] |
| HKUST-1 | 298 | 100 | 3.91 | 3.66 | [37] |
| MIL-100Cr | 298 | 100 | 5.77 | 5.80 | [37] |
| ED *-MIL-100Cr-0.4 | 298 | 100 | 2.00 | 4.10 | [37] |
| MIL-100Fe-300 | 298 | 100 | 4.70 | 3.86 | [41] |
| MIL-101(Cr)-NO2 | 298 | 100 | 2.38 | 2.34 | [13] |
| MIL-101(Cr)-Br | 298 | 100 | 2.58 | 2.35 | [13] |
| MIL-101(Cr)-NH2 | 298 | 100 | 5.08 | 4.54 | [13] |
| MIL-101(Cr)-H | 298 | 100 | 5.44 | 5.38 | [13] |
| ELM-11 | 298 | 100 | 0.06 | 2.5 | [42] |
| ELM-12 | 298 | 100 | 0.86 | 0.75 | [42] |
| MIL-53Al | 298 | 100 | 2.70 | 2.71 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballesteros-Plata, D.; Cecilia, J.A.; Barroso-Martín, I.; Jiménez-Jiménez, J.; Infantes-Molina, A.; Rodríguez-Castellón, E. Materials Design for N2O Capture: Separation in Gas Mixtures. Catalysts 2022, 12, 1539. https://doi.org/10.3390/catal12121539
Ballesteros-Plata D, Cecilia JA, Barroso-Martín I, Jiménez-Jiménez J, Infantes-Molina A, Rodríguez-Castellón E. Materials Design for N2O Capture: Separation in Gas Mixtures. Catalysts. 2022; 12(12):1539. https://doi.org/10.3390/catal12121539
Chicago/Turabian StyleBallesteros-Plata, Daniel, Juan Antonio Cecilia, Isabel Barroso-Martín, José Jiménez-Jiménez, Antonia Infantes-Molina, and Enrique Rodríguez-Castellón. 2022. "Materials Design for N2O Capture: Separation in Gas Mixtures" Catalysts 12, no. 12: 1539. https://doi.org/10.3390/catal12121539
APA StyleBallesteros-Plata, D., Cecilia, J. A., Barroso-Martín, I., Jiménez-Jiménez, J., Infantes-Molina, A., & Rodríguez-Castellón, E. (2022). Materials Design for N2O Capture: Separation in Gas Mixtures. Catalysts, 12(12), 1539. https://doi.org/10.3390/catal12121539