Emission Spectroscopy of CH4/CO2 Mixtures Processed in a Non-Thermal Plasma Augmented Burner
Abstract
:1. Introduction
2. Results and Discussions
2.1. Emission Spectroscopy of Air during Plasma Discharge
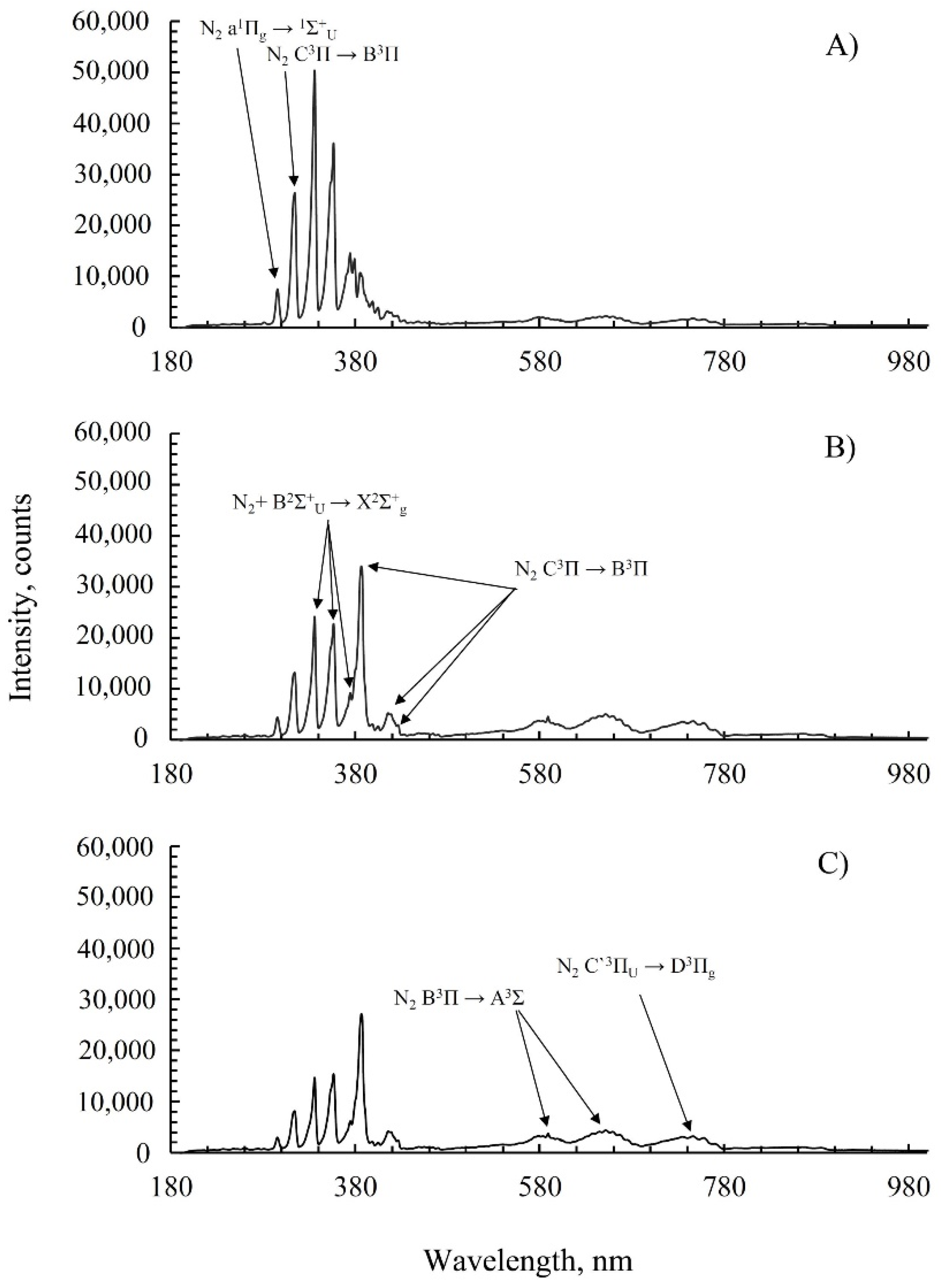
2.2. Emission Spectroscopy of Nitrogen during Plasma Discharge
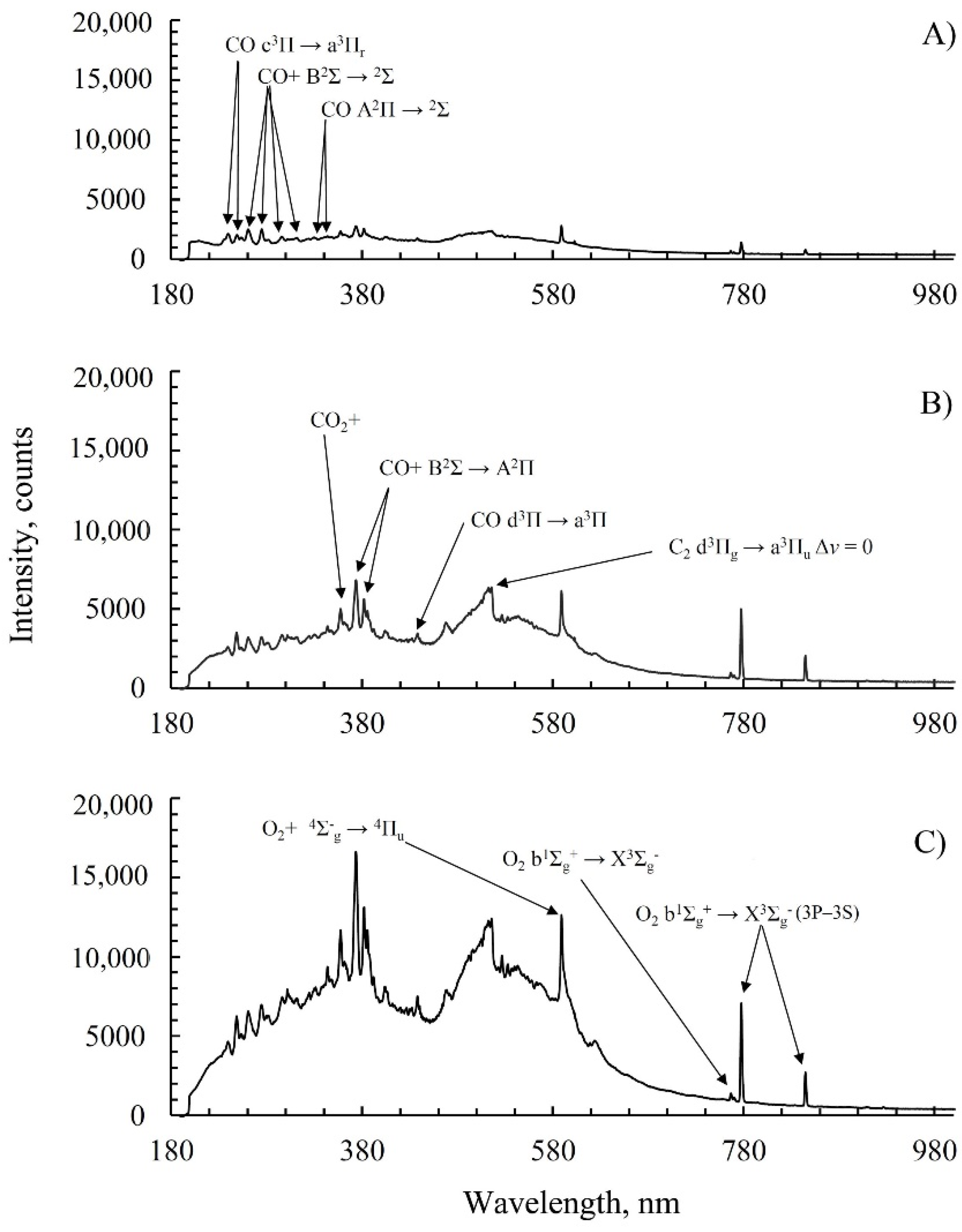
2.3. Emission Spectroscopy of Carbon Dioxide during Plasma Discharge
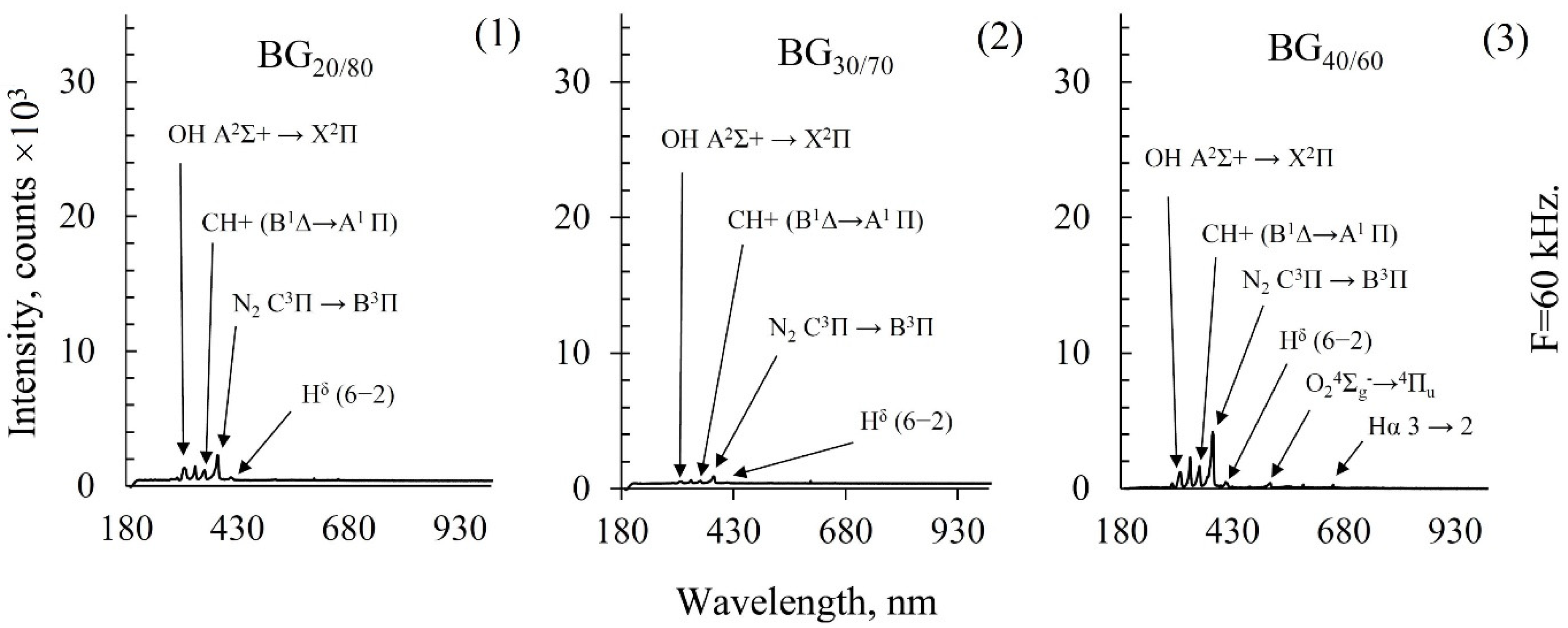
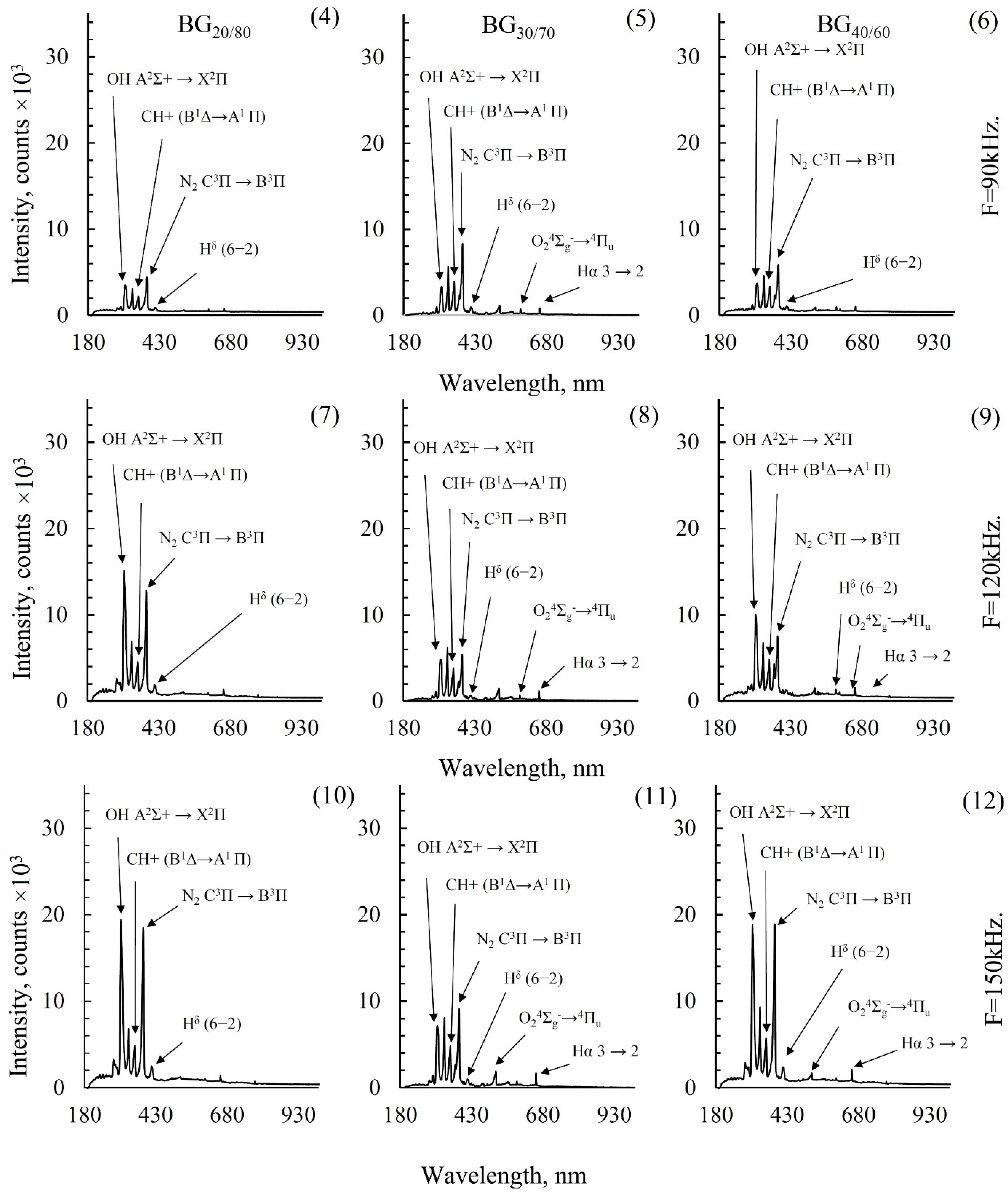
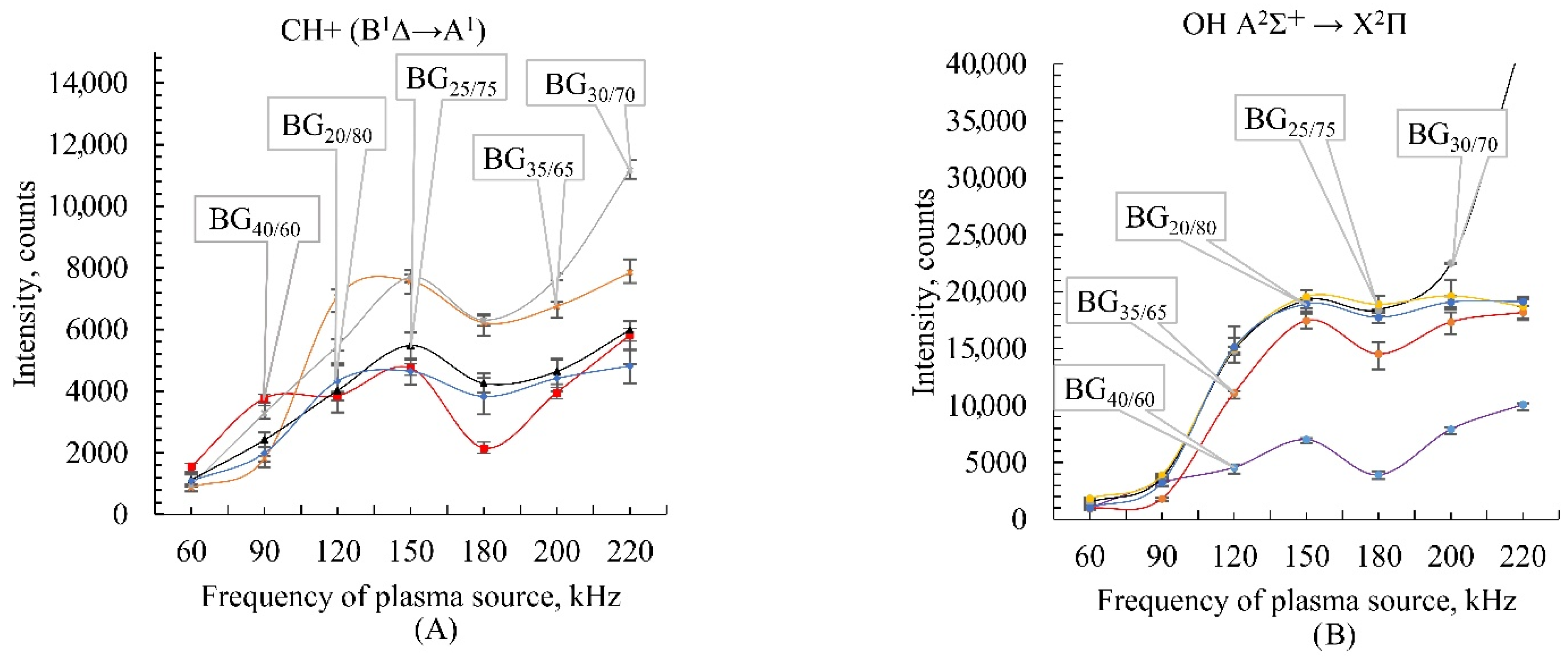
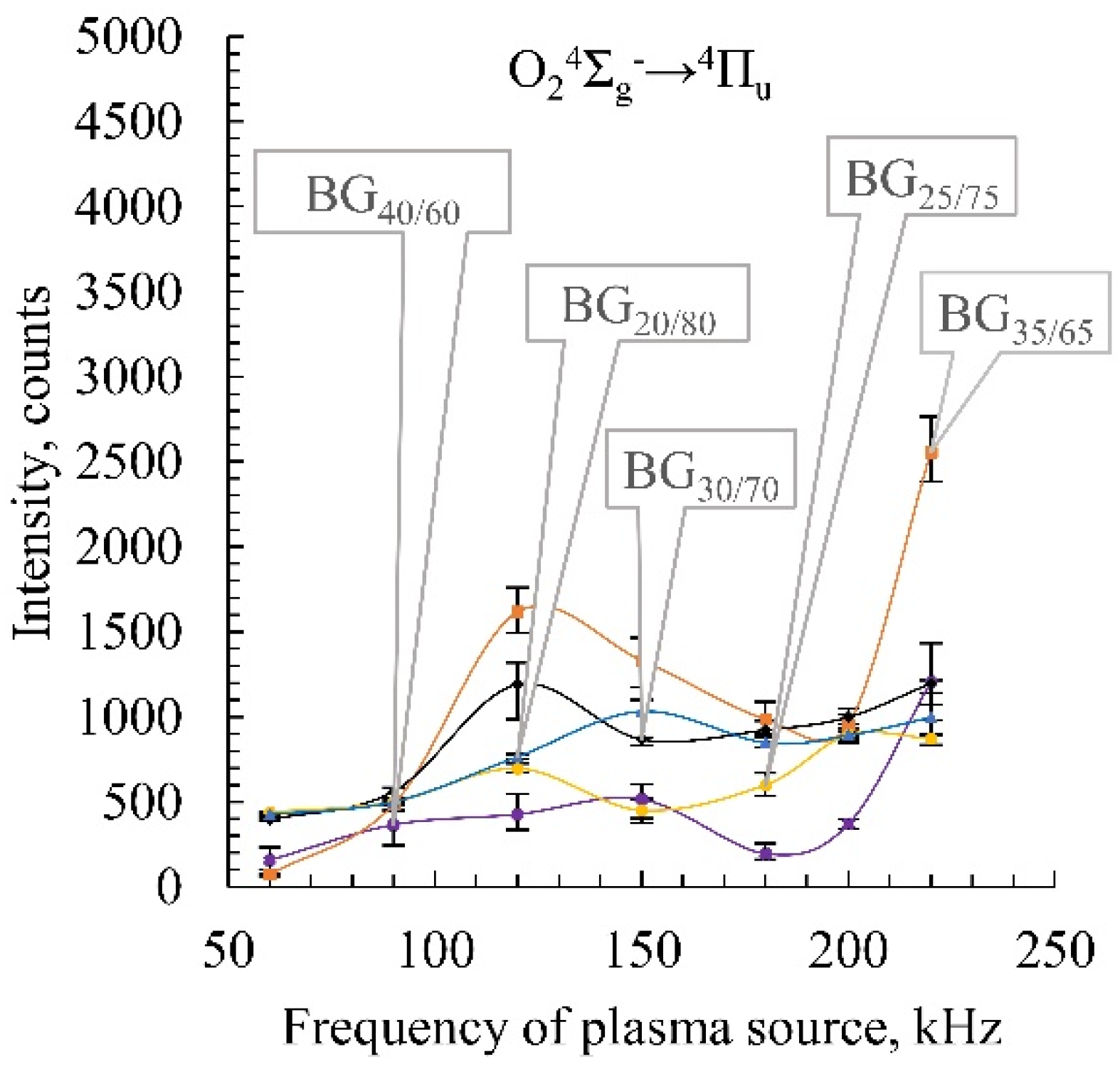
2.4. Emission Spectroscopy of Biogas
2.5. Gas Chromatography Analysis of Biogas
3. Materials and Methods
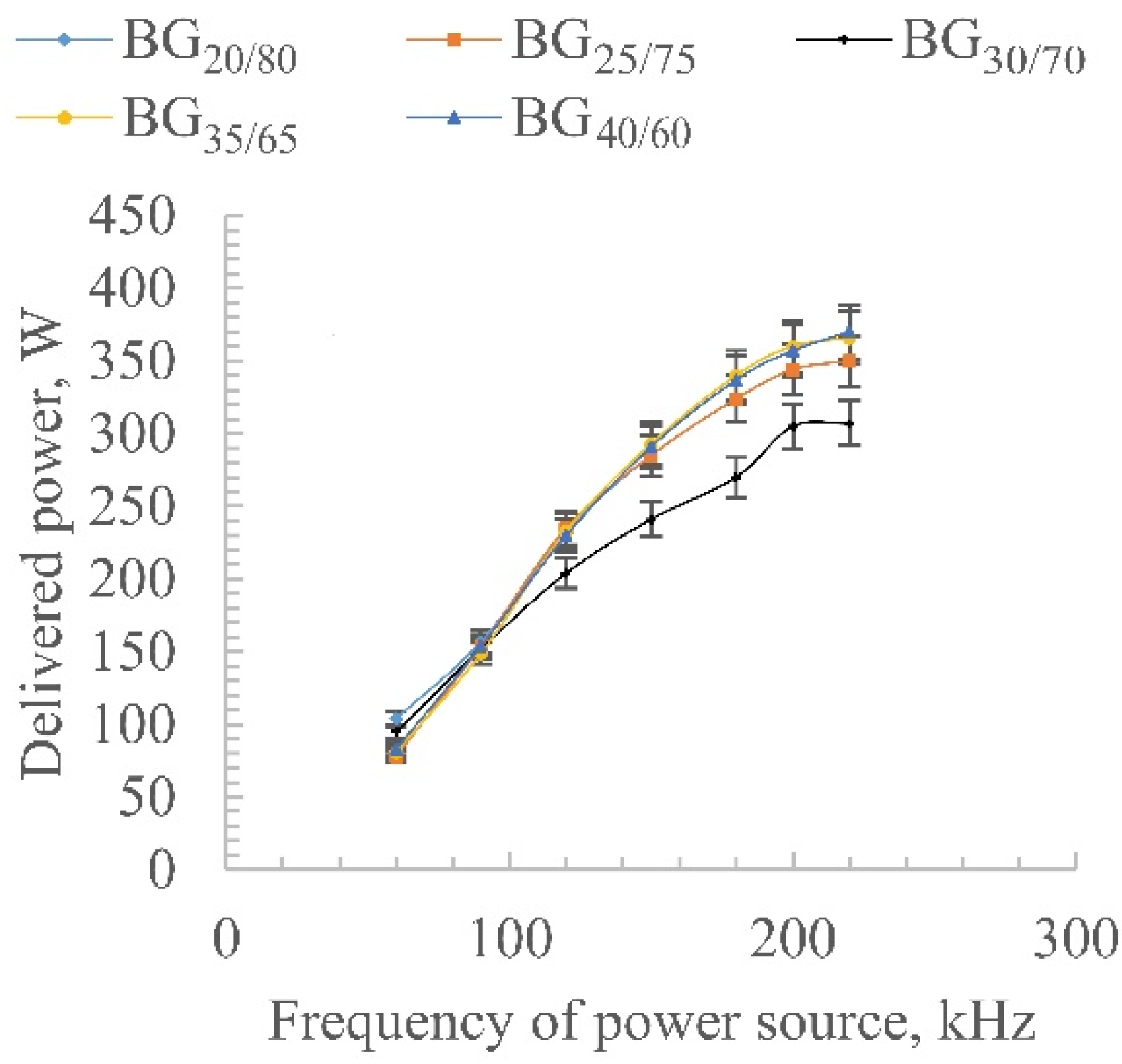
3.1. Experimental Setup
3.2. Emission Spectroscopy and Gas Chromatography
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhan, C.; Verma, L.; Singh, J. Alternative Fuels for Sustainable Development Environmental Concerns and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 317–331. [Google Scholar]
- Beychok, M.R. NOX emission from fuel combustion controlled. Oil Gas J. 1973, 1, 53–56. [Google Scholar]
- Starikovskaia, S.M.; Kukaev, E.N.; Kuksin, A.Y.; Nudnova, M.M.; Starikovskii, A.Y. Analysis of the spatial uniformity of the combustion of a gaseous mixture initiated by a nanosecond discharge Combust. Flame 2004, 139, 177–187. [Google Scholar]
- Cupitt, L.T.; Takacs, G.A.; Glass, G.P. Reaction of hydrogen atoms and O2(a 1g). Int. J. Chem. Kinet. 1982, 14, 487–497. [Google Scholar]
- Light, G.C. The effect of vibrational excitation on the reaction of O(3P) with H2 and the distribution of vibrational energy in the product OH. J. Chem. Phys. 1978, 68, 2831–2843. [Google Scholar] [CrossRef]
- Mao, X.; Chen, Q.; Guo, C. Methane pyrolysis with N2/Ar/He diluents in a repetitively-pulsed nanosecond discharge: Kinetics development for plasma assisted combustion and fuel reforming. Energy Convers. Manag. 2019, 200, 112018. [Google Scholar] [CrossRef]
- Zhong, H.; Mao, X.; Rousso, A.C.; Patrick, C.L.; Yan, C.; Xu, W.; Chen, Q.; Wysocki, G.; Ju, Y. Numerical modelling of ignition enhancement of CH4/O2/He mixtures using a hybrid repetitive nanosecond and DC discharge. Proc. Combust. Inst. 2019, 37, 5545–5552. [Google Scholar]
- Mione, M.A.; Engeln, R.; Vandalon, V.; Kessels, W.M.; Roozeboom, F. Infrared and optical emission spectroscopy study of atmospheric pressure plasma-enhanced spatial ALD of Al2O3. Appl. Phys. Lett. 2019, 115, 083101. [Google Scholar] [CrossRef]
- Mione, M.A.; Mameli, A.; Vandalon, V.; Kessels, W.M.M.; Roozeboom, F. Atmospheric-Pressure Plasma-Enhanced Spatial ALD of SiO 2 Studied by Gas-Phase Infrared and Optical Emission Spectroscopy. Appl. Phys. Lett. 2021, 115, 083101. [Google Scholar] [CrossRef]
- Liu, P.; He, L.; Zhao, B. Discharge and Optical Emission Spectrum Characteristics of a Coaxial Dielectric Barrier Discharge Plasma-Assisted Combustion Actuator. J. Spectrosc. 2020, 2020, 6034848. [Google Scholar] [CrossRef]
- Lin, Q.; Jiang, Y.; Liu, C.; Chen, L. Controllable NO emission and high flame performance of ammonia combustion assisted by non-equilibrium plasma. J. Fuel 2022, 319, 123818. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions The European Green Deal. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 15 June 2022).
- Rachdi, L.; Sushkov, V.; Hofmann, M. Optical emission spectroscopy diagnostics for plasma parameters investigation in a Duo-Plasmaline surface-wave sustained discharge. Spectrochim. Acta Part B At. Spectrosc. 2022, 19, 106432. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Bai, H.; Zhang, C.; Shao, T. Optical emission spectroscopy measurement of plasma parameters in a nanosecond pulsed spark discharge for CO2/CH4 dry reforming. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120590. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Gorbanev, Y.; Chys, M.; Nikiforov, A.; Van Hulle, S.W.; Cos, P.; Bogaerts, A.; De Geyter, N. Investigation of plasma-induced chemistry in organic solutions for enhanced electrospun PLA nanofibers. Plasma Processes Polym. 2018, 15, 1700226. [Google Scholar] [CrossRef]
- Zhu, J.; Kusano, Y.; Li, Z. Optical diagnostics of a gliding arc discharge at atmospheric pressure. In Atmospheric Pressure Plasmas: Processes, Technology and Applications; Nova Science Publishers: New York, NY, USA, 2016. [Google Scholar]
- Matveev, I. Plasma Assisted Combustion Gasification and Pollution Control: Volume 1. Methods of Plasma Generation for Pac; Outskirts: Denver, CO, USA, 2013; 540p, ISBN 9781432786885. [Google Scholar]
- Smirnov, V.V.; Stelmakh, O.M.; Fabelinsky, V.I.; Kozlov, D.N.; Starik, A.M.; Titova, N.S. On the influence of electronically excited oxygen molecules on combustion of hydrogen–oxygen mixture. J. Phys. D Appl. Phys. 2008, 41, 192001. [Google Scholar] [CrossRef]
- Starik, A.M.; Lukhovitskii, B.I.; Naumov, V.V.; Titova, N.S. On combustion intensification mechanisms in the case of electrical-discharge-excited oxygen molecules. Tech. Phys. 2007, 52, 1281–1290. [Google Scholar] [CrossRef]
- Dieke, G.H.; Babcock, H.D. The Structure of the Atmospheric Absorption Bands of Oxygen. Proc. Nat. Acad. Sci. USA 1927, 13, 670. [Google Scholar] [CrossRef] [Green Version]
- Hornbeck, G.A.; Hopfield, H.S. Spectroscopic Investigation of the Carbon Monoxide-Oxygen Reaction. J. Chem. Phys. 1949, 17, 982. [Google Scholar] [CrossRef]
- Yankovsky, V.; Vorobeva, E.; Manuilova, R. New techniques for retrieving the [O(3P)], [O3] and [CO2] altitude profiles from dayglow oxygen emissions: Uncertainty analysis by the Monte Carlo method. Adv. Space Res. 2019, 64, 1948–1967. [Google Scholar] [CrossRef]
- Starik, A.M.; Titova, N.S. Kinetics of detonation initiation in the supersonic flow of the H2 + O2 (air) mixture in O2 molecule excitation by resonance laser radiation. Kinet. Catal. 2003, 44, 28–39. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOx treatment: A review Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, J.R.F.; Kerr, J.A.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry. J. Phys. Chem. Ref. 1989, 18, S881–S1097. [Google Scholar]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen–oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207–220. [Google Scholar] [CrossRef]
- Sathiamoorthy, G.; Kalyana, S.; Finney, W.C.; Clark, R.J.; Locke, B.R. Chemical reaction kinetics and reactor modelling of NOx removal in a pulsed streamer corona discharge reactor. Ind. Eng. Chem. Res. 1999, 38, 1844–1855. [Google Scholar] [CrossRef]
- Sabu, T.; Miran, M.; Uroš, C.; Petrm, Š.; Praveen, K. Non-Thermal Plasma Technology for Polymeric Materials, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-813152-7. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Eliasson, B. Nonequilibrium volume plasma chemical processing. IEEE Trans. Plasma Sci. 1991, 19, 1063–1077. [Google Scholar] [CrossRef]
- Kim, W.; Godfrey, M.; Cappelli, M. The role of in situ reforming in plasma enhanced ultra-lean premixed methane/air flames. Combust Flame 2010, 157, 374–383. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Primc, G.; Vesel, A. Optical Emission Spectroscopy as a Diagnostic Tool for Characterization of Atmospheric Plasma Jets. Appl. Sci. 2021, 11, 2275. [Google Scholar] [CrossRef]
- Dang, J.; Chung, K.; Hwang, Y.S. A simple spectroscopic method to determine the degree of dissociation in hydrogen plasmas with wide-range spectrometer. Rev. Sci. Instrum. 2016, 87, 053503. [Google Scholar] [CrossRef]





| Type of Biogas | CH4 L/min | CO2 L/min | N2 L/min |
|---|---|---|---|
| BG20/80 | 0.69 | 2.76 | 6.57 |
| BG25/75 | 0.74 | 2.22 | 7.05 |
| BG30/70 | 0.78 | 1.82 | 7.43 |
| BG35/65 | 0.81 | 1.5 | 7.71 |
| BG40/60 | 0.84 | 1.26 | 8.0 |
| Frequency | BG20/80, MJ/m3 | BG25/75, MJ/m3 | BG30/70, MJ/m3 | BG35/65, MJ/m3 | BG40/60, MJ/m3 |
|---|---|---|---|---|---|
| No plasma | 2.24 | 2.49 | 2.71 | 2.91 | 2.99 |
| 60 | 6.68 | 6.11 | 10.58 | 8.42 | 12.82 |
| 90 | 7.71 | 8.08 | 11.02 | 10.9 | 12.98 |
| 120 | 8.44 | 8.29 | 13.80 | 11.56 | 13.45 |
| 150 | 7.72 | 9.15 | 12.24 | 11.46 | 12.51 |
| 180 | 6.56 | 7.50 | 11.71 | 13.37 | 10.59 |
| 200 | 7.76 | 7.88 | 12.55 | 12.16 | 12.05 |
| 220 | 7.51 | 8.06 | 9.45 | 13.25 | 13.46 |
| Voltage of Plasma Source | Frequency of Plasma Source |
|---|---|
| 8.33 kV | 60 kHz |
| 90 kHz | |
| 120 kHz | |
| 150 kHz | |
| 180 kHz | |
| 200 kHz | |
| 220 kHz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykov, E.; Striūgas, N.; Paulauskas, R. Emission Spectroscopy of CH4/CO2 Mixtures Processed in a Non-Thermal Plasma Augmented Burner. Catalysts 2022, 12, 1540. https://doi.org/10.3390/catal12121540
Bykov E, Striūgas N, Paulauskas R. Emission Spectroscopy of CH4/CO2 Mixtures Processed in a Non-Thermal Plasma Augmented Burner. Catalysts. 2022; 12(12):1540. https://doi.org/10.3390/catal12121540
Chicago/Turabian StyleBykov, Ernest, Nerijus Striūgas, and Rolandas Paulauskas. 2022. "Emission Spectroscopy of CH4/CO2 Mixtures Processed in a Non-Thermal Plasma Augmented Burner" Catalysts 12, no. 12: 1540. https://doi.org/10.3390/catal12121540
APA StyleBykov, E., Striūgas, N., & Paulauskas, R. (2022). Emission Spectroscopy of CH4/CO2 Mixtures Processed in a Non-Thermal Plasma Augmented Burner. Catalysts, 12(12), 1540. https://doi.org/10.3390/catal12121540







