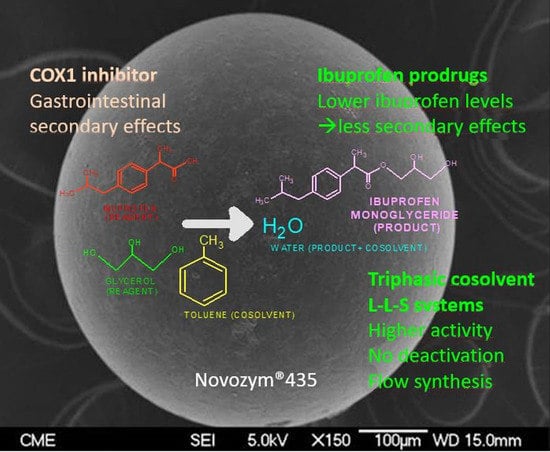
Synthesis of Ibuprofen Monoglyceride Using Novozym®435: Biocatalyst Activation and Stabilization in Multiphasic Systems
Abstract
:1. Introduction
2. Results and Discussion
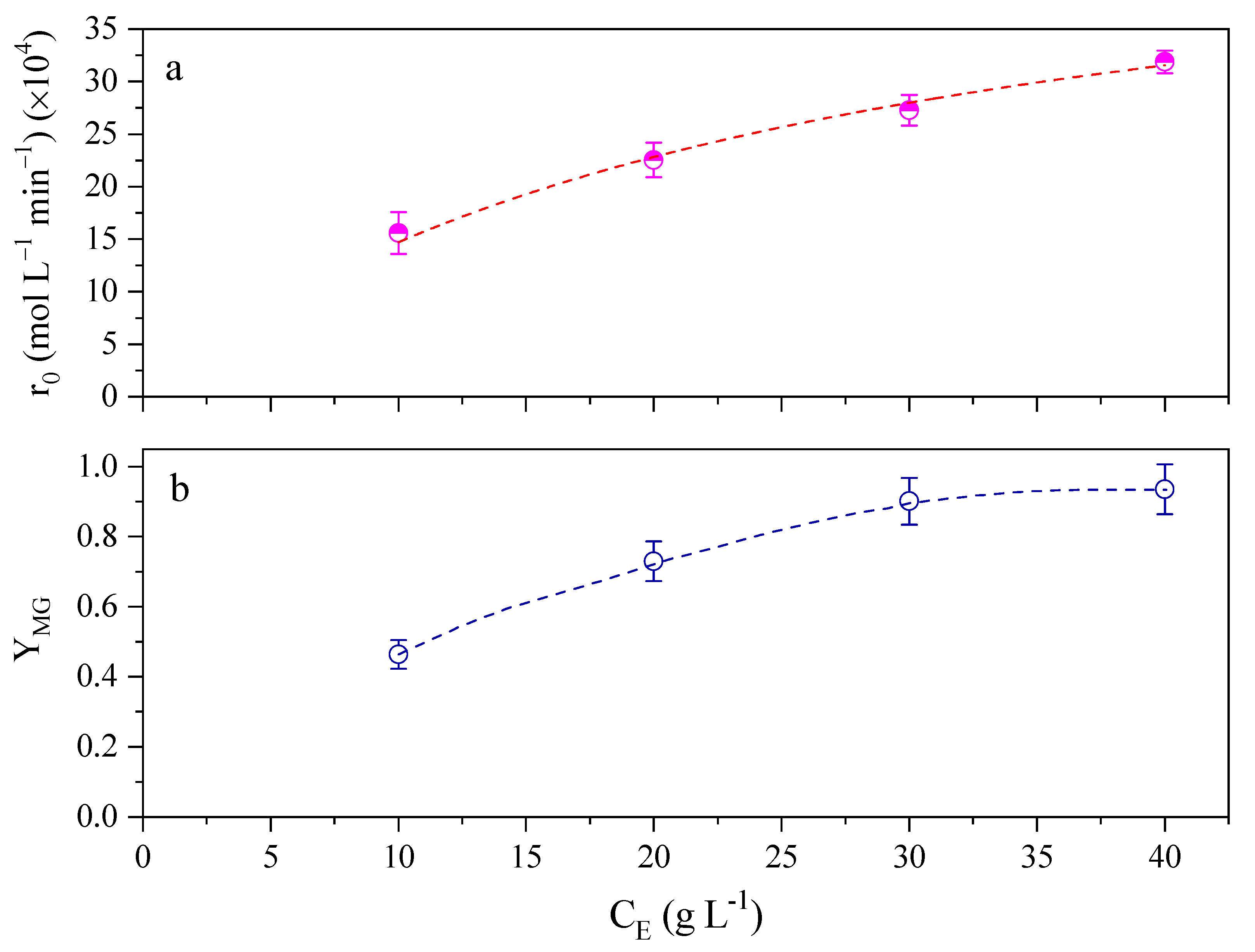
2.1. Effect of Enzyme Concentration
2.2. Effect of the Volume Ratio Glycerol: Toluene
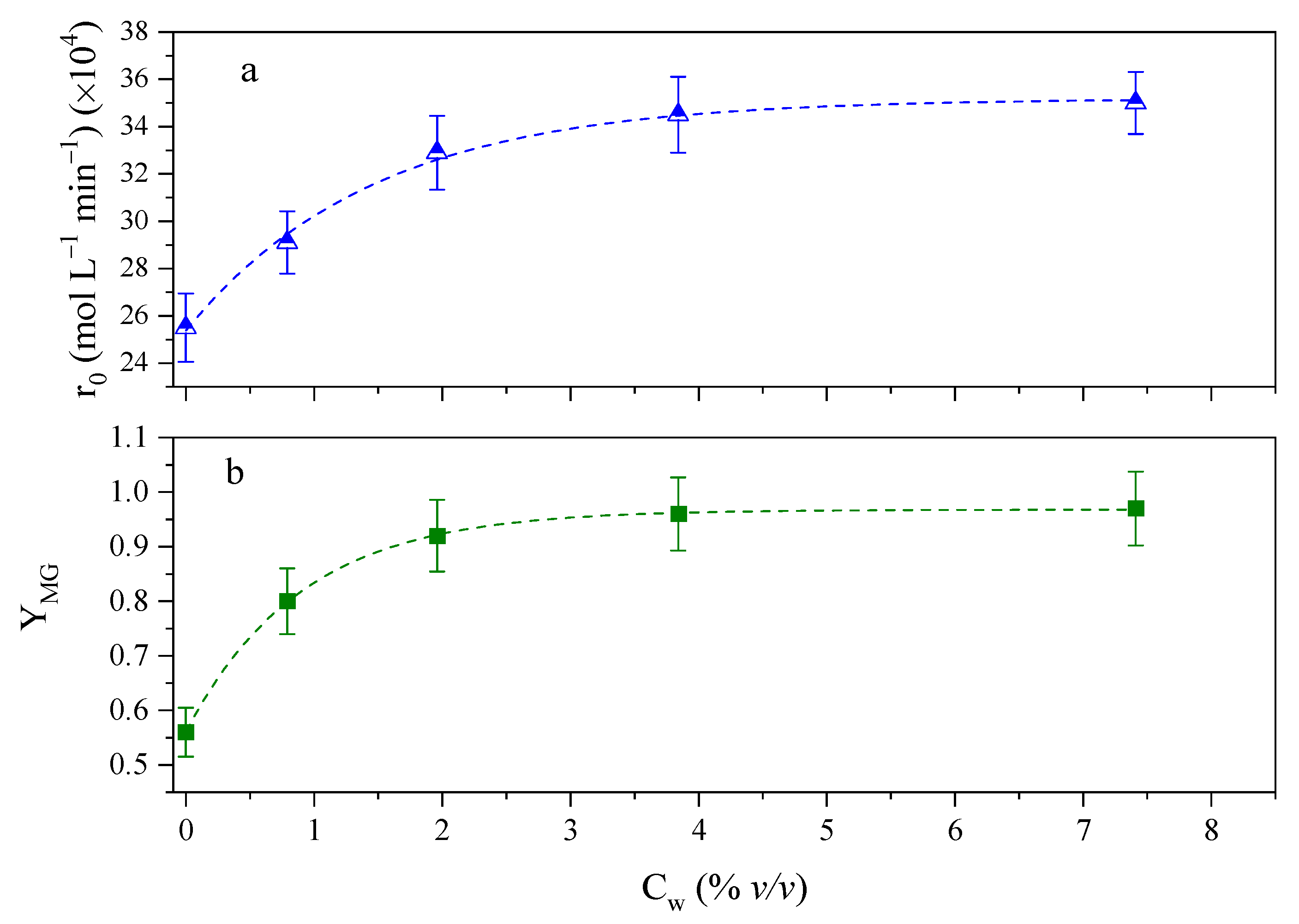
2.3. Influence of the Addition of Water as a Polar Cosolvent
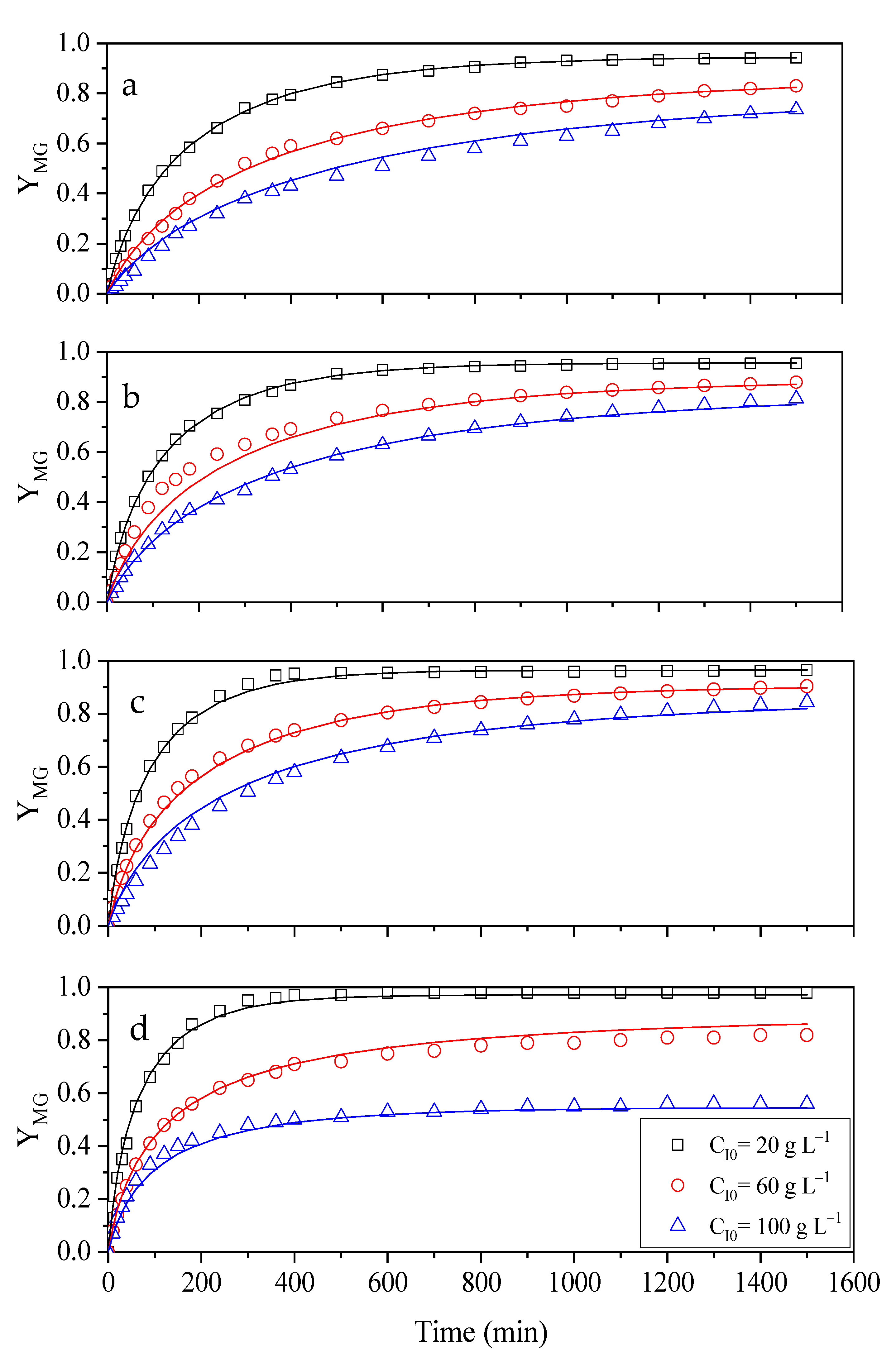
2.4. Influence of Temperature and Initial Concentration of Ibuprofen on Activity and Stability
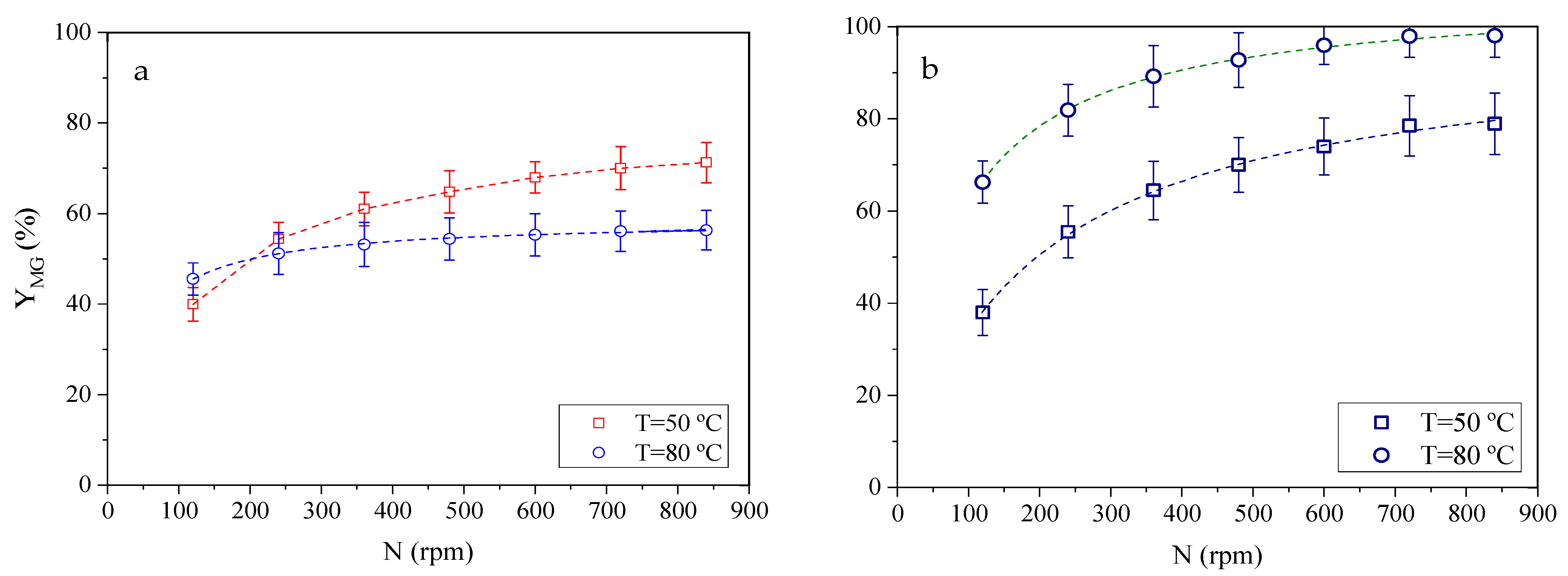
2.5. Influence of Mass Transfer
2.5.1. External Mass Transfer
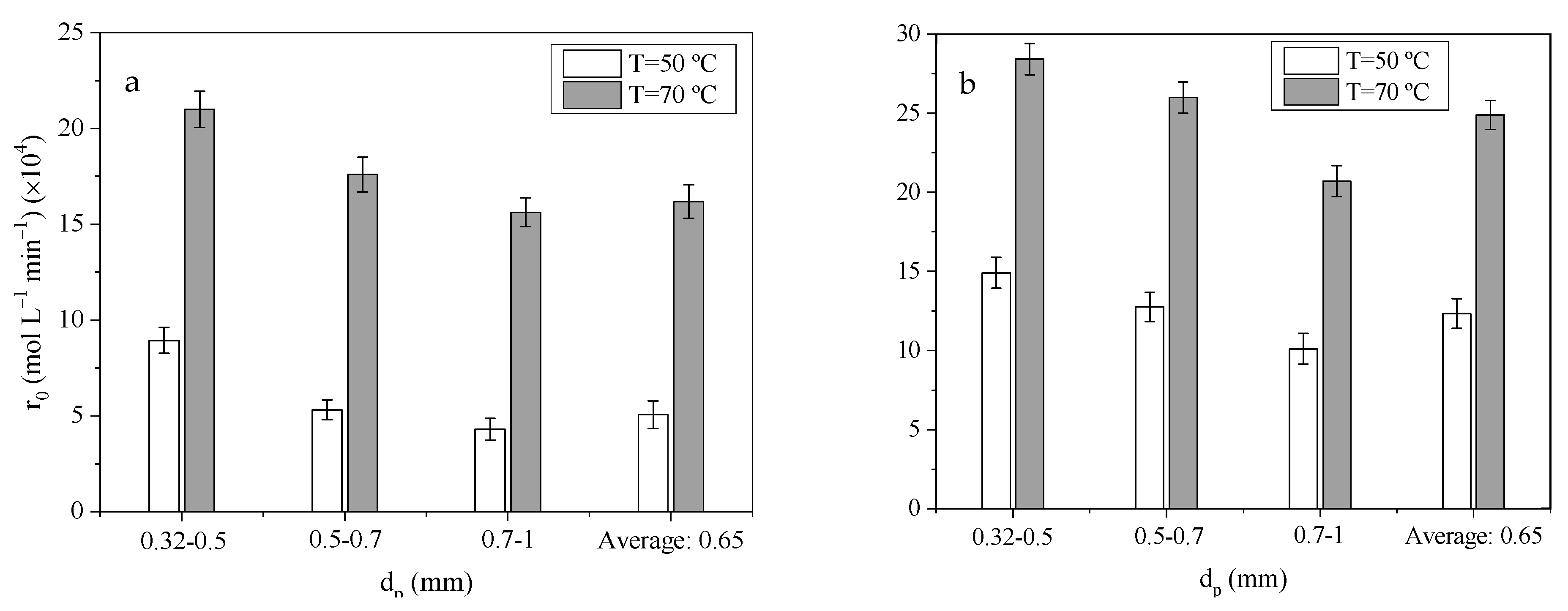
2.5.2. Internal Mass Transfer
2.6. Kinetic Modelling
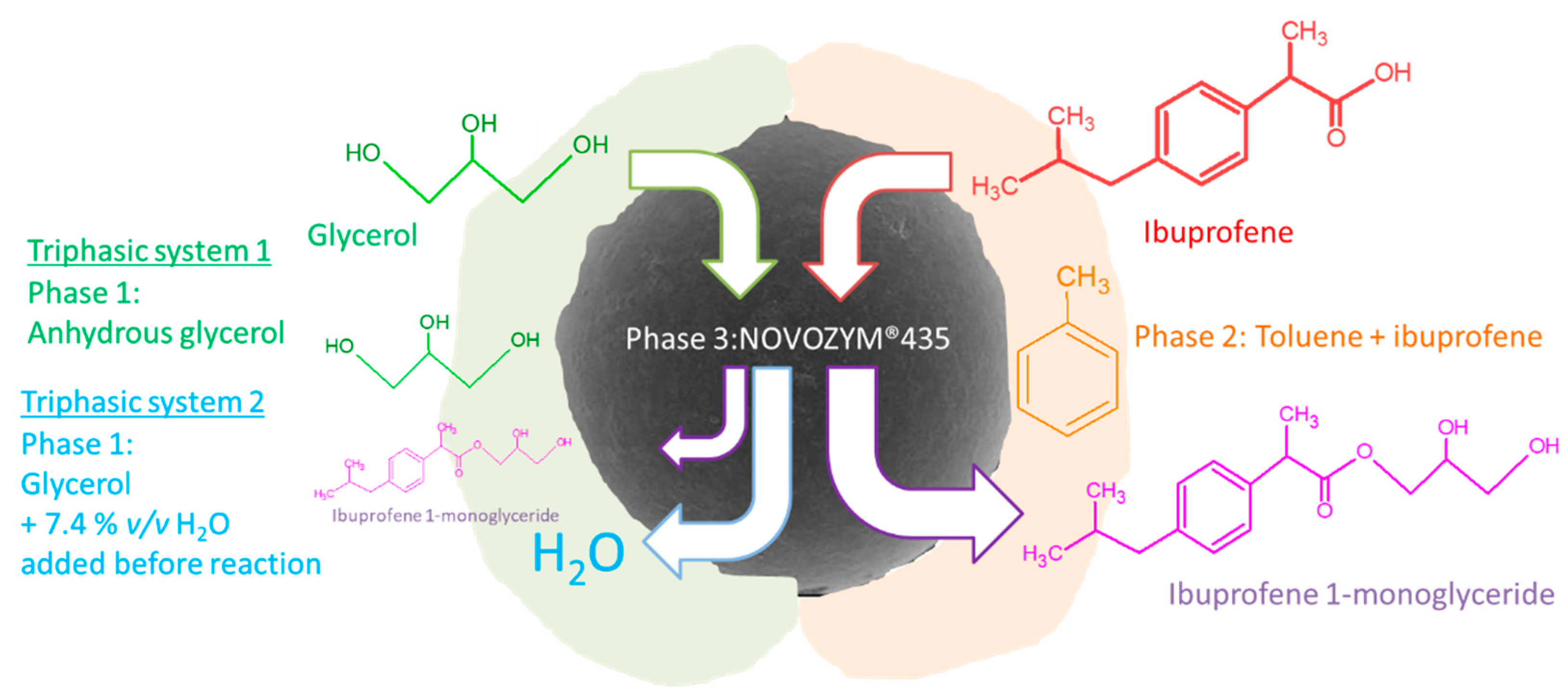
2.6.1. Triphasic L-L-S System Glycerol-toluene(+ibuprofene)-N435
2.6.2. Triphasic L-L-S System Glycerol(+water)-toluene(+ibuprofene)-N435
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Enzymatic Esterification of Ibuprofen Ester
3.2.2. Analytical Methods
- -
- Column: Teknokroma “Mediterranea Sea” C-18 column 25 × 0.46 cm dp 5 μm.
- -
- Mobile phase: Mixture: 83% methanol, 17% acidified water (the eluents used are milli-Q water acidified with sulfuric acid to pH 2.2 and high purity methanol).
- -
- Column temperature: 30 °C.
- -
- Flow rate: 0.8 mL/min.
3.2.3. Statistical Non-Linear Regression Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.M.; Han, R.; Wang, C.; Yu, B.; Liang, Q.M.; Yuan, X.C.; Chang, J.; Zhao, Q.; Liao, H.; Tang, B.; et al. Self-Preservation Strategy for Approaching Global Warming Targets in the Post-Paris Agreement Era. Nat. Commun. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of Energy Storage Systems in Energy Transition from Fossil Fuels to Renewables. Energy Storage 2021, 3, e135. [Google Scholar] [CrossRef] [Green Version]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of Crude Glycerol to Value-Added Products: Perspectives of Process Technology, Economics and Environmental Issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial Lipases and Their Industrial Applications: A Comprehensive Review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- José, C.; Toledo, M.V.; Nicolás, P.; Lasalle, V.; Ferreira, M.L.; Briand, L.E. Influence of the Nature of the Support on the Catalytic Performance of CALB: Experimental and Theoretical Evidence. Catal. Sci. Technol. 2018, 8, 3513–3526. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “Perfect” Lipase Immobilized Biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Toledo, M.V.; Briand, L.E.; Ferreira, M.L. A Simple Molecular Model to Study the Substrate Diffusion into the Active Site of a Lipase-Catalyzed Esterification of Ibuprofen and Ketoprofen with Glycerol. Top. Catal. 2022, 65, 944–956. [Google Scholar] [CrossRef]
- Zappaterra, F.; Tupini, C.; Summa, D.; Cristofori, V.; Costa, S.; Trapella, C.; Lampronti, I.; Tamburini, E. Xylitol as a Hydrophilization Moiety for a Biocatalytically Synthesized Ibuprofen Prodrug. Int. J. Mol. Sci. 2022, 23, 2026. [Google Scholar] [CrossRef]
- Zappaterra, F.; Summa, D.; Semeraro, B.; Buzzi, R.; Trapella, C.; Ladero, M.; Costa, S.; Tamburini, E. Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative. Fermentation 2020, 6, 96. [Google Scholar] [CrossRef]
- Ravelo, M.; Wojtusik, M.; Ladero, M.; García-Ochoa, F. Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis. Catalysts 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.R.; Baek, K.H. Lipase Immobilization with Support Materials, Preparation Techniques, and Applications: Present and Future Aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of Glutaraldehyde to Immobilize Lipases: Effect of the Immobilization Protocol on the Properties of Lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of Divinylsulfone Supports Permits the Tuning of CALB Properties during Its Immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.C.; Negra, M.D.; Kaiser, A.; Pinelo, M. Enzyme Immobilization on Inorganic Surfaces for Membrane Reactor Applications: Mass Transfer Challenges, Enzyme Leakage and Reuse of Materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Verma, S.; Meghwanshi, G.K.; Kumar, R. Current Perspectives for Microbial Lipases from Extremophiles and Metagenomics. Biochimie 2021, 182, 23–36. [Google Scholar] [CrossRef]
- Zhao, H. What Do We Learn from Enzyme Behaviors in Organic Solvents?—Structural Functionalization of Ionic Liquids for Enzyme Activation and Stabilization. Biotechnol. Adv. 2020, 45. [Google Scholar] [CrossRef]
- Lozano, P. Enzymes in Neoteric Solvents: From One-Phase to Multiphase Systems. Green Chem. 2010, 12, 555–556. [Google Scholar] [CrossRef]
- Van Dorp, D.A.; Beerthuis, R.K.; Nugteren, D.H.; Vonkeman, H. The Biosynthesis of Prostaglandins. BBA-Gen. Subj. 1964, 90, 204–207. [Google Scholar] [CrossRef]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible Isoforms of Cyclooxygenase and Nitric-Oxide Synthase in Inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef] [Green Version]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and Functional Insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Crofford, L.J. COX-1 and COX-2 Tissue Expression: Implications and Predictions. J. Rheumatol. 1997, 24, 15–19. [Google Scholar]
- Chandrasekharan, N.V.; Simmons, D.L. The Cyclooxygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; et al. Ibuprofen Suppresses Plaque Pathology and Inflammation in a Mouse Model for Alzheimer’s Disease. J. Neurosci. 2000, 20, 5709–5714. [Google Scholar] [CrossRef] [Green Version]
- Bonelli, P.; Tuccillo, F.M.; Calemma, R.; Pezzetti, F.; Borrelli, A.; Martinelli, R.; De Rosa, A.; Esposito, D.; Palaia, R.; Castello, G. Changes in the Gene Expression Profile of Gastric Cancer Cells in Response to Ibuprofen: A Gene Pathway Analysis. Pharm. J. 2011, 11, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Kulesza, A.; Zielniok, K.; Hawryluk, J.; Paczek, L.; Burdzinska, A. Ibuprofen in Therapeutic Concentrations Affects the Secretion of Human Bone Marrow Mesenchymal Stromal Cells, but Not Their Proliferative and Migratory Capacity. Biomolecules 2022, 12, 287. [Google Scholar] [CrossRef]
- Kofidis, T.; Lebl, D.R.; Swijnenburg, R.J.; Greeve, J.M.; Klima, U.; Gold, J.; Xu, C.; Robbins, R.C. Allopurinol/Uricase and Ibuprofen Enhance Engraftment of Cardiomyocyte-Enriched Human Embryonic Stem Cells and Improve Cardiac Function Following Myocardial Injury. Eur. J. Cardio-Thorac. Surg. 2006, 29, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Amanullah, A.; Joshi, V.; Dhiman, R.; Prajapati, V.K.; Poluri, K.M.; Mishra, A. Ibuprofen-Based Advanced Therapeutics: Breaking the Inflammatory Link in Cancer, Neurodegeneration, and Diseases. Drug Metab. Rev. 2021, 53, 100–121. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, P.; Singh, S. Neuroinflammatory Responses in Parkinson’s Disease: Relevance of Ibuprofen in Therapeutics. Inflammopharmacology 2021, 29, 5–14. [Google Scholar] [CrossRef]
- Griffin, M.R. Epidemiology of Nonsteroidal Anti-Inflammatory Drug-Associated Gastrointestinal Injury. Am. J. Med. 1998, 104, 23S–29S. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Sánchez-López, E. Dexibuprofen Therapeutic Advances: Prodrugs and Nanotechnological Formulations. Pharmaceutics 2021, 13, 414. [Google Scholar] [CrossRef]
- Ravelo, M.; Fuente, E.; Blanco, Á.; Ladero, M.; García-Ochoa, F. Esterification of Glycerol and Ibuprofen in Solventless Media Catalyzed by Free CALB: Kinetic Modelling. Biochem. Eng. J. 2015, 101, 228–236. [Google Scholar] [CrossRef]
- Ravelo, M.; Esteban, J.; Ladero, M.; García-Ochoa, F. Enzymatic Synthesis of Ibuprofen Monoglycerides Catalyzed by Free: Candida antarctica Lipase B in a Toluene-Glycerol Biphasic Medium. RSC Adv. 2016, 6, 69658–69669. [Google Scholar] [CrossRef]
- Doust, A.M.; Rahimi, M.; Feyzi, M. Effects of Solvent Addition and Ultrasound Waves on Viscosity Reduction of Residue Fuel Oil. Chem. Eng. Process. Process Intensif. 2015, 95, 353–361. [Google Scholar] [CrossRef]
- Poojari, Y.; Clarson, S.J. Thermal stability of Candida antarctica lipase b immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Wu, C.; Böttcher, C.; Haag, R. Enzymatically crosslinked dendritic polyglycerol nanogels for encapsulation of catalytically active proteins. Soft Matter 2015, 11, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Kurtovic, I.; Nalder, T.D.; Cleaver, H.; Marshall, S.N. Immobilisation of Candida rugosa lipase on a highly hydrophobic support: A stable immobilised lipase suitable for non-aqueous synthesis. Biotechnol. Rep. 2020, 28, e00535. [Google Scholar] [CrossRef]
- Mei, Y.; Miller, L.; Gao, W.; Gross, R.A. Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy. Biomacromolecules 2003, 4, 70–74. [Google Scholar] [CrossRef]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.-C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef]




| Triphasic L-L-S System with No Added Water | ||
| T(°C) | Dij × 1011 (m2 s−1) | µm (kg m−1 s−1) |
| 50 | 4.50 | 0.053 |
| 60 | 8.24 | 0.030 |
| 70 | 14.8 | 0.017 |
| 80 | 21.5 | 0.012 |
| Triphasic L-L-S System with 7.4% v/v Added Water | ||
| 50 | 6.27 | 0.038 |
| 60 | 18.3 | 0.014 |
| 70 | 28.9 | 0.009 |
| 80 | 36.4 | 0.007 |
| Triphasic Systems | With No Added Water | With 7.4% v/v Added Water | |||||
|---|---|---|---|---|---|---|---|
| T (°C) | N (rpm) | robs × 104 (mol L−1min−1 ) | kL·a·CI0 (mol L−1min−1) | Me (×104) | robs × 104 (mol L−1min−1 ) | kL·a·CI0 (mol L−1min−1) | Me (×104) |
| 50 | 600 | 7.79 | 2.01 | 1.94 | 17.7 | 2.65 | 3.33 |
| 720 | 8.76 | 2.20 | 1.99 | 19.5 | 2.90 | 3.36 | |
| 840 | 9.08 | 2.38 | 1.91 | 19.8 | 3.14 | 3.18 | |
| 80 | 600 | 23.9 | 7.36 | 1.62 | 48.6 | 11.5 | 2.12 |
| 720 | 25.5 | 8.06 | 1.58 | 52.6 | 12.5 | 2.10 | |
| 840 | 25.6 | 8.70 | 1.47 | 53.8 | 13.6 | 1.98 | |
| T (°C) | Dij × 1011 (m2 s−1) | De × 1013 (m2 s−1) | Size Fraction % w/w | Size Particle (mm) | robs × 104 Novozym®435 (mol L−1 min−1 ) | η | We-Pt |
|---|---|---|---|---|---|---|---|
| Triphasic glycerol-toluene-N435 (L-L-S) system with no added water at zero time | |||||||
| 50 | 4.50 | 6.75 | 13 | 0.32–0.5 | 11.6 | 0.23 | 4.0 |
| 52 | 0.5–0.7 | 6.92 | 0.15 | 5.4 | |||
| 35 | 0.7–1 | 5.65 | 0.12 | 8.0 | |||
| Average | 0.3–1 | 6.60 | 0.14 | 4.5 | |||
| 70 | 14.80 | 22.20 | 13 | 0.32–0.5 | 21.0 | 0.18 | 2.3 |
| 52 | 0.5–0.7 | 17.6 | 0.15 | 4.2 | |||
| 35 | 0.7–1 | 15.6 | 0.13 | 7.5 | |||
| Average | 0.3–1 | 16.2 | 0.14 | 4.4 | |||
| Triphasic glycerol-toluene-N435 (L-L-S) system with 7.4% v/v added water at zero time | |||||||
| 50 | 6.27 | 3.13 | 13 | 0.32–0.5 | 14.9 | 0.66 | 1.15 |
| 52 | 0.5–0.7 | 12.8 | 0.57 | 2.14 | |||
| 35 | 0.7–1 | 10.1 | 0.45 | 3.42 | |||
| Average | 0.3–1 | 12.3 | 0.55 | 2.38 | |||
| 70 | 28.9 | 14.5 | 13 | 0.32–0.5 | 28.4 | 0.45 | 0.47 |
| 52 | 0.5–0.7 | 26.0 | 0.41 | 0.94 | |||
| 35 | 0.7–1 | 20.7 | 0.32 | 1.52 | |||
| Average | 0.3–1 | 24.9 | 0.40 | 1.04 | |||
| Model | Kinetic Equation(s) |
|---|---|
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| Model | Kinetic Parameter | Value | Standard Error |
|---|---|---|---|
| 1 | lnk′10 | 16.67 | 4.25 |
| Eak′1/R | 3884 | 162 | |
| lnk′20 | 96.88 | 21.51 | |
| Eak′2/R | 21,329 | 2175 | |
| KI | 5.06 | 0.51 | |
| KMG | 54.87 | 6.20 | |
| 2 | lnk′10 | 4.57 | 0.28 |
| Eak′1/R | 3888 | 93 | |
| lnk′20 | −3.94 | 1.91 | |
| Eak′2/R | 1296 | 637 | |
| lnkd0 | 72.86 | 7.90 | |
| Eakd/R | 27,207 | 2795 | |
| KI | 9.90 | 0.89 | |
| KMG | 44.36 | 3.90 | |
| 3 | lnk′10 | 8.91 | 1.34 |
| Eak′1/R | 4124 | 110 | |
| lnkd0 | 71.40 | 8.64 | |
| Eakd/R | 22,738 | 1655 | |
| KI | 5.45 | 0.32 | |
| KMG | 60.20 | 4.67 | |
| 4 | lnk′10 | 4.00 | 0.31 |
| Eak′1/R | 3754 | 103 | |
| lnkd0 | 104.89 | 21.86 | |
| Eakd/R | 38,591 | 7730 | |
| lnkdT0 | 0.29 | 1.42 | |
| EakdT/R | 2406 | 475 | |
| KI | 8.46 | 0.60 | |
| KMG | 29.48 | 2.85 |
| Model | F-Fisher | Ndexp | K | SQR | N/K | AICc | BIC | RMSE | VE(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12,578 | 300 | 6 | 0.481 | 50 | −1918 | −1930 | 0.040 | 98.26 |
| 2 | 38,313 | 300 | 8 | 0.118 | 37.5 | −2335 | −2351 | 0.020 | 99.57 |
| 3 | 29,399 | 300 | 6 | 0.211 | 50 | −2165 | −2177 | 0.027 | 99.24 |
| 4 | 36,180 | 300 | 8 | 0.127 | 37.5 | −2314 | −2331 | 0.205 | 99.54 |
| Model | Kinetic Equation(s) |
|---|---|
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| Model | Kinetic Parameter | Value | Standard Error |
|---|---|---|---|
| 5 | lnk′10 | 6.69 | 0.38 |
| Eak′1/R | 4766 | 129 | |
| lnk′20 | −5.00 | 1.73 | |
| Eak′2/R | 1689 | 583 | |
| KI | 1.55 | 0.31 | |
| KMG | 14.70 | 0.98 | |
| 6 | lnk′10 | 7.04 | 0.43 |
| Eak′1/R | 4889 | 146 | |
| lnk′20 | −5.10 | 1.75 | |
| Eak′2/R | 1695 | 589 | |
| lnkd0 | 225 | 9097 | |
| Eakd/R | 81,550 | 3,212,980 | |
| KI | 1.56 | 0.31 | |
| KMG | 14.00 | 1.10 | |
| 7 | lnk′10 | 7.35 | 0.45 |
| Eak′1/R | 5032 | 150 | |
| KI | −0.39 | 0.32 | |
| KMG | 7.35 | 0.45 | |
| 8 | lnk′10 | 7.82 | 0.54 |
| Eak′1/R | 5194 | 184 | |
| lnkd0 | 104 | 274 | |
| Eakd/R | 38,668 | 96,710 | |
| KI | −0.32 | 0.31 | |
| KMG | 18.27 | 1.66 |
| Model | F-Fisher | Ndexp | K | SQR | N/K | AICc | BIC | RMSE | VE(%) |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 28,318 | 300 | 6 | 0.268 | −2093 | −2106 | 0.040 | 99.03 | 28318 |
| 8 | 21,436 | 300 | 8 | 0.030 | −2749 | −2765 | 0.205 | 99.05 | 21436 |
| Enzyme | Water a t = 0 | ln k′10 | Eak′1/R | ln k′20 | Eak′2/R | ln kdo | Eakd/R | KI | KMG |
|---|---|---|---|---|---|---|---|---|---|
| N435 | 0% v/v | 4.57 | 3888 | −3.94 | 1296 | 72.86 | 27.21 | 9.90 | 44.36 |
| 7.4% v/v | 6.69 | 4766 | −5.00 | 1689 | - | - | 1.55 | 14.70 | |
| Lipozyme CALB-L | 7.4.% v/v | 11.42 | 5274 | 2.30 | 2468 | - | - | 0.86 | 16.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravelo, M.; Gallardo, M.E.; Ladero, M.; Garcia-Ochoa, F. Synthesis of Ibuprofen Monoglyceride Using Novozym®435: Biocatalyst Activation and Stabilization in Multiphasic Systems. Catalysts 2022, 12, 1531. https://doi.org/10.3390/catal12121531
Ravelo M, Gallardo ME, Ladero M, Garcia-Ochoa F. Synthesis of Ibuprofen Monoglyceride Using Novozym®435: Biocatalyst Activation and Stabilization in Multiphasic Systems. Catalysts. 2022; 12(12):1531. https://doi.org/10.3390/catal12121531
Chicago/Turabian StyleRavelo, Marianela, M. Esther Gallardo, Miguel Ladero, and Felix Garcia-Ochoa. 2022. "Synthesis of Ibuprofen Monoglyceride Using Novozym®435: Biocatalyst Activation and Stabilization in Multiphasic Systems" Catalysts 12, no. 12: 1531. https://doi.org/10.3390/catal12121531
APA StyleRavelo, M., Gallardo, M. E., Ladero, M., & Garcia-Ochoa, F. (2022). Synthesis of Ibuprofen Monoglyceride Using Novozym®435: Biocatalyst Activation and Stabilization in Multiphasic Systems. Catalysts, 12(12), 1531. https://doi.org/10.3390/catal12121531