A Review on Pulsed Laser Preparation of Nanocomposites in Liquids and Their Applications in Photocatalysis
Abstract
:1. Introduction
2. Pulsed Laser Heating in a Liquid Medium
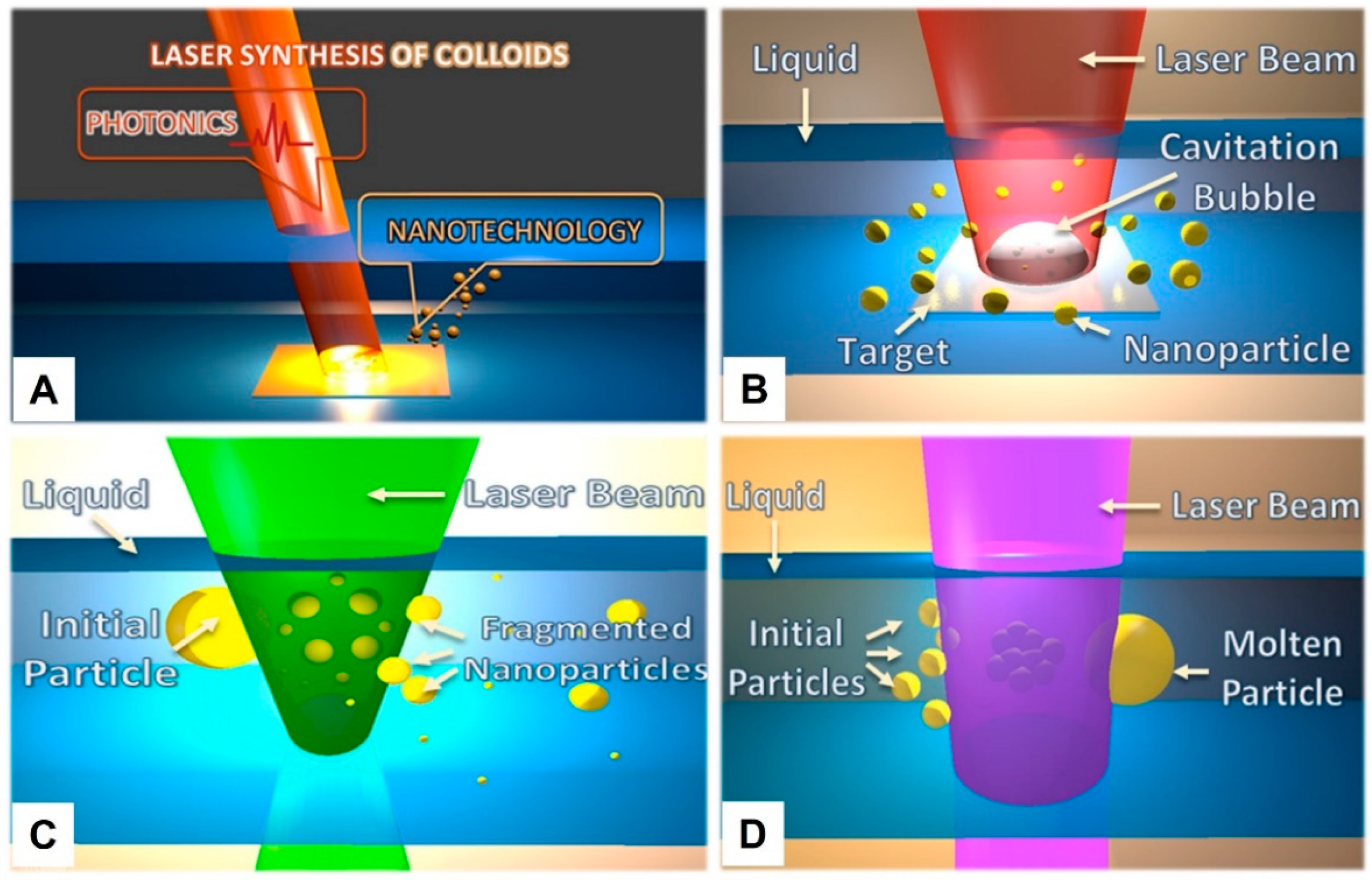
2.1. Classification of Pulsed Laser Synthesis of Colloids
- (1)
- PLAL is a technique using a focused or high-power density laser beam (exceeding 1 × 109 W/cm2) to generate the cavitation bubble of plasma from the surface of a bulk target; the plasma can be constrained and rapidly quenched by the liquid medium to form the desired nanomaterials [29]. During the formation of nanoparticles, because the laser fluence is a gradient form, it induces a wide size distribution, so this method is only suitable for the preliminary fabrication of nanoparticles.
- (2)
- PLFL is derived from PLAL, the laser energy directly interacting with the dispersed colloids in a liquid medium, and the initial particles with a bigger size can be fragmented into smaller nanoparticles under high-density laser irradiation. This improvement was first proposed by the P. V. Kamat group [30]. They used a high-density laser beam (355 nm, 6 ns) to irradiate the colloidal silver particles of bigger sizes (40–60 nm), and the colloids were transformed into smaller nanoparticles (5–20 nm) under the heat effect of the laser. In this way, particle size can be tailored by the tunability of laser fluence, but the morphology and phase may also be shifted under such a powerful laser density [29].
- (3)
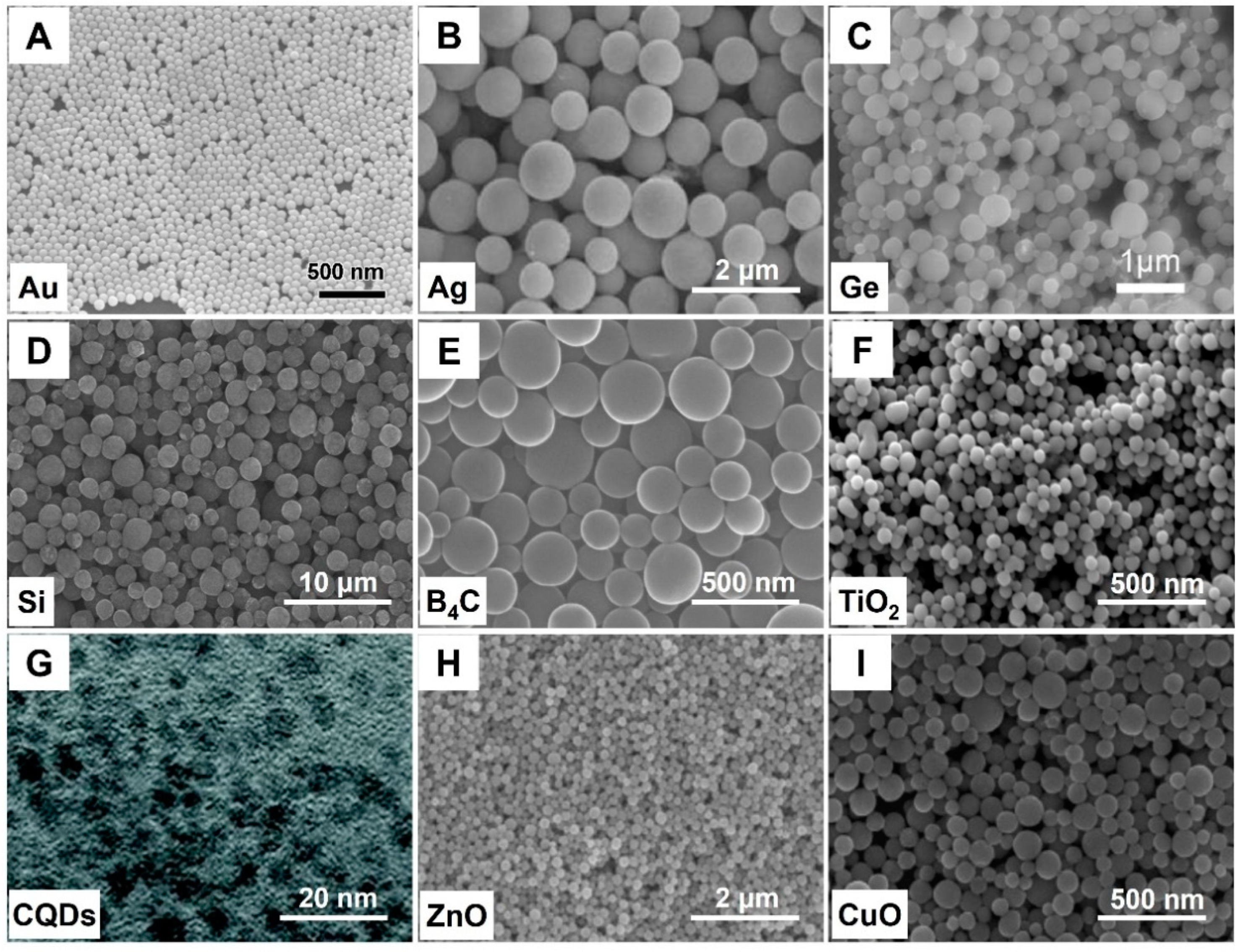
- PLML is contrary to PLFL, the laser beam is unfocused and the laser fluence is relatively modest. The smaller colloids can be melted and composited into a bigger size. This method was first demonstrated by the N. Koshizaki group [31]. The raw CuO nanoparticles with an average size of 34 nm were transformed into 300 nm under pulsed laser irradiation in acetone (355 nm, 66 mJ·pulse−1·cm−2, 30 min). Using this method, the irregularly shaped particles can also be spherically reshaped.
2.2. Characteristics of Pulsed Laser Synthesis of Colloids
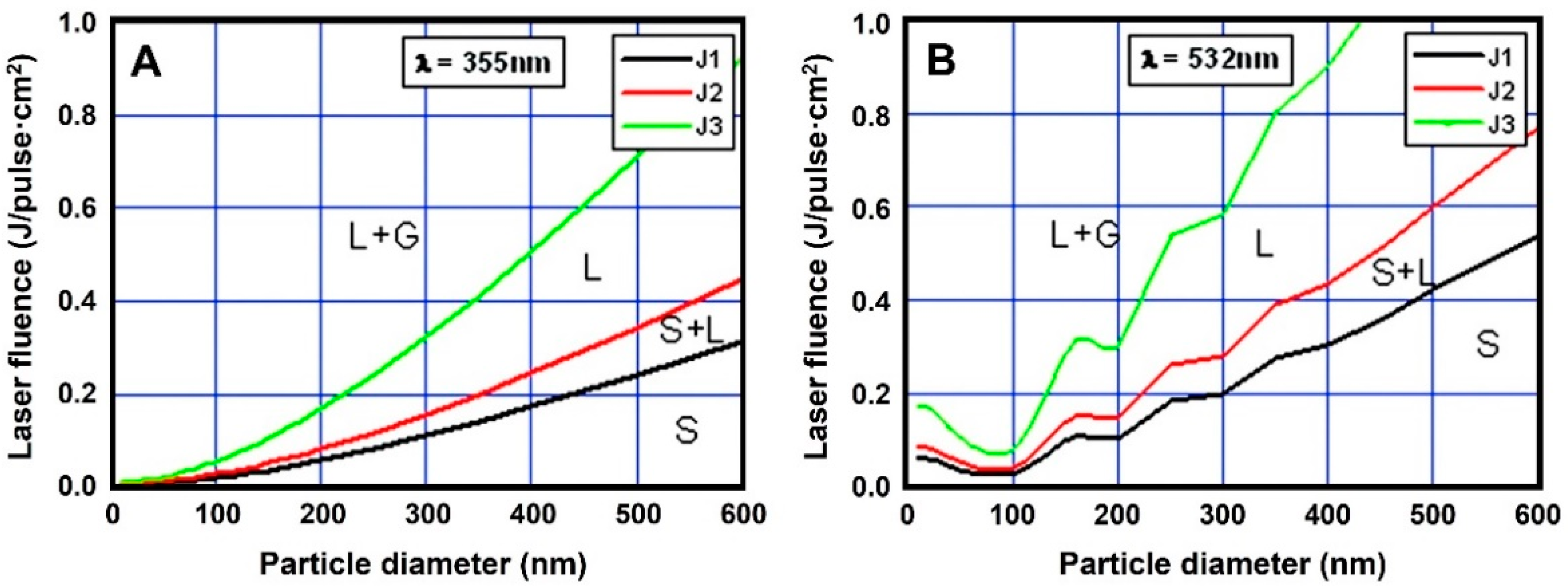
2.3. Parameter Effects on Pulsed Laser Synthesis of Colloids
3. Pulsed Laser Preparation of Nanocomposites for Photocatalysis
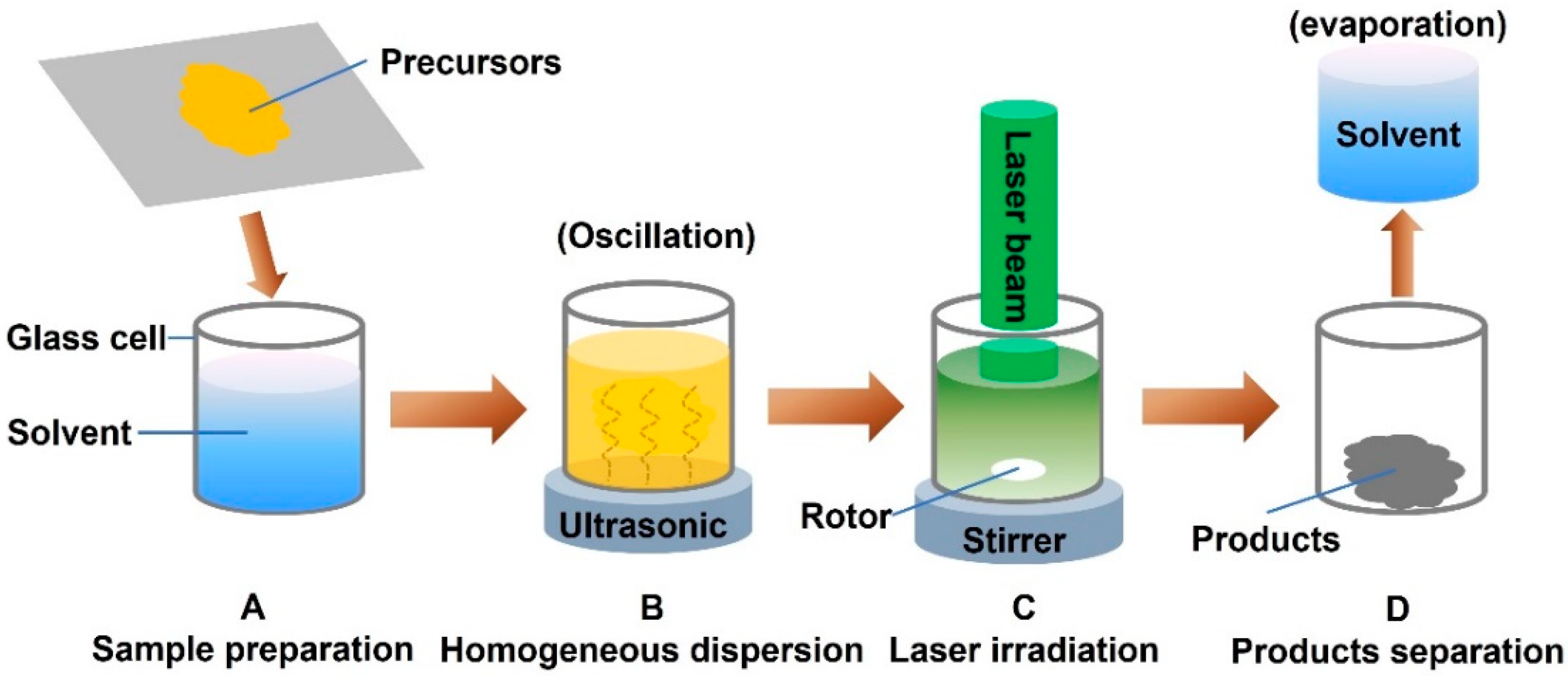
3.1. Pulsed Laser Preparation of Nanocomposites
3.2. Laser-Prepared Nanocomposites for Photocatalysis
3.2.1. Degradation of Organic Pollutants
3.2.2. Photocatalytic Bacteria Inactivation
3.2.3. Photocatalysts for Water Splitting
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts, Chinese. J. Catal. 2017, 38, 1936–1955. [Google Scholar]
- Li, Y.; Li, S.; Li, J.; Liu, K.; Tang, Y.; Li, X.; Zeng, X. Preparation of spherical silver and tin dioxide nanocomposites with the high photocatalytic performance by laser-induced deposition in liquid medium. J. Alloys Compd. 2022, 900, 163522. [Google Scholar] [CrossRef]
- He, E.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chinese. J. Catal. 2020, 41, 9–20. [Google Scholar]
- Masood, Z.; Ikhlaq, A.; Akram, A.; Qazi, U.Y.; Rizvi, O.S.; Javaid, R.; Alazmi, A.; Madkour, M.; Qi, F. Application of nanocrystals in advanced oxidation processes for wastewater purification: Challenges and future prospects. Catalysts 2022, 12, 741. [Google Scholar] [CrossRef]
- Chen, R.; Ren, Z.; Liang, Y.; Zhang, G.; Dittrich, T.; Liu, R.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef]
- Vallejo, M.; Ortiz, M.F.S.R.I.; Irabien, A. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (AOPs) to waste water treatment. Chemosphere 2015, 118, 44–56. [Google Scholar] [CrossRef]
- Thanavel, M.; Bankole, P.O.; Selvam, R.; Govindwar, S.P.; Sadasivam, S.K. Synergistic effect of biological and advanced oxidation process treatment in the biodegradation of removal yellow RR dye. Sci. Rep. 2020, 10, 20234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Benaissa, M.; Abbas, N.; Arni, S.A.; Elboughdiri, N.; Moumen, A.; Hamdy, M.S.; Abd-Rabboh, H.S.M.; Galal, A.H.; Al-Metwaly, M.G.; Ahmed, M.A. BiVO3/g-C3N4 S-scheme heterojunction nanocomposite photocatalyst for hydrogen production and amaranth dye removal. Opt. Mater. 2021, 118, 111237. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, M.A.; Ahmed, J.; Alsaiari, M.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A. Au nanoparticles decorated polypyrrole-carbon black/g-C3N4 nanocomposite as ultrafast and efficient visible light photocatalyst. Chemosphere 2022, 287, 131984. [Google Scholar] [CrossRef]
- Leeladevi, K.; Arunpandian, M.; Kumar, J.V.; Chellapandi, T.; Mathumitha, G.; Lee, J.W.; Nagarajan, E.R. CoWO4 decorated ZnO nanocomposite: Efficient visible-light activated photocatalyst for mitigation of noxious pollutants. Physica B-Condensed Matter. 2022, 626, 413493. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Zhang, J.Y.Y. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Darsara, S.A.; Seifi, M.; Askari, M.B. One-step hydrothermal synthesis of MoS2/CdS nanocomposite and study of structural, photocatalytic, and optical properties of this nanocomposite. Optik 2018, 169, 249–256. [Google Scholar] [CrossRef]
- Li, Y.T.; Xu, J.M.; Tang, Z.J.; Xu, T.T.; Li, X.J. Nearly white light photoluminescence from ZnO/rGO nanocomposite prepared by a one-step hydrothermal method. J. Alloys Compd. 2017, 715, 122–128. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Zhang, Z.; Xie, M. Hydrothermal synthesis of a graphene/magnetic/montmorillonite nanocomposite and its ultrasonically assisted methylene blue adsorption. J. Mater. Sci. 2019, 54, 11037–11055. [Google Scholar] [CrossRef]
- Xia, C.; Tao, S.; Zhu, S.; Song, Y.; Feng, T.; Zeng, Q.; Liu, J.; Yang, B. Hydrothermal addition polymerization for ultrahigh-yield carbonized polymer dots with room temperature phosphorescence via nanocomposite. Chem. Eur. J. 2018, 24, 11303–11308. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Yang, Y.; Li, X. Laser fabricated carbon quantum dots in anti-solvent for highly efficient carbon-based perovskite solar cells. J. Colloid. Interf. Sci. 2021, 600, 691–700. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, W.; Ren, L.; Wang, H.; Guo, P.; Yang, X.; Ye, Q.; Shchukin, D.; Du, Y.; Dou, S.; et al. Laser-generated supranano liquid metal as efficient electron mediator in hybrid perovskite solar cells. Adv. Mater. 2020, 32, 2001571. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Yang, Y.; Li, X. Laser induced core-shell liquid metal quantum dots for high-efficiency carbon-based perovskite solar cells. Appl. Surf. Sci. 2021, 565, 150470. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, K.; Chen, M.; Peng, W.; Zhang, C.; Yang, Y.; Li, X. Laser generated WS2 quantum dots for effective charge transport in high-performance carbon-based perovskite solar cells. J. Power. Sources 2022, 518, 230766. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed laser in liquids made nanomaterials for catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.J.; Shwetharani, R.; Kim, H.S.; Pasha, S.K.K.; Ashokkumar, M.; Choi, M.M. Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo- and electrocatalytic applications. Light-Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; Giacomo, A.D.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles engineering by pulsed laser ablation in liquids: Concept and applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Z.; Li, Z.; Chen, Y. High-power instant-synthesis technology of carbon nanomaterials and nanocomposites. Nano Energy 2021, 80, 105500. [Google Scholar] [CrossRef]
- Patil, P.P.; Phase, D.M.; Kulkarni, S.A.; Ghaisas, S.V.; Kulkarni, S.K.; Kanetkar, S.M.; Ogale, S.B.; Bhide, V.G. Pulsed-laser-induced reactive quenching at liquid-solid interface: Aqueous oxidation of iron. Phys. Rev. Lett. 1987, 58, 238–241. [Google Scholar] [CrossRef]
- Fojtik, A.; Henglein, A. Laser ablation of films and suspended particles in a solvent: Formation of cluster and colloid solution. Ber. Der Bunsenges. Für Phys. Chem. 1993, 97, 252–254. [Google Scholar]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room temperature laser synthesis in liquid of oxide, metal-oxide core-shell and doped oxide nanoparticles. Chem. Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and application. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Hartland, G.V. Picosecond dynamics of silver nanoclusters photoejection of electrons and fragmentation. J. Phys. Chem. B 1998, 102, 3123–3128. [Google Scholar] [CrossRef]
- Wang, H.; Pyatenko, A.; Kawaguchi, K.; Li, X.; Swiatkowska-Warkocka, Z.; Koshizaki, N. Selective pulsed laser heating for the synthesis of semiconductor and metal submicrometer sphere. Angew. Chem. Int. Ed. 2010, 49, 6361–6364. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhou, F.; Zhang, T.; Zhang, H.; Li, X.; Duan, G.; Cai, W.; Li, Y. Rapid synthesis of monodisperse Au nanospheres through a laser irradiation-induced shape conversion, self-assembly and their electromagnetic coupling SERS enhancement. Sci. Rep. 2015, 5, 7686–7695. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Koshizaki, N.; Pyatenko, A.; Shimizu, Y.; Wang, H.; Liu, J.; Wang, X.; Gao, M.; Wang, Z.; Zeng, X. Preparation of silver spheres by selective laser heating in silver-containing precursor solution. Opt. Express. 2011, 19, 2846–2851. [Google Scholar] [CrossRef]
- Zhang, D.; Lau, M.; Lu, S.; Barcikowski, S.; Gökce, B. Germanium submicrospheres synthesized by picosecond pulsed laser melting in liquids: Educt size effect. Sci. Rep. 2017, 7, 40355. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pyatenko, A.; Shimizu, Y.; Wang, H.; Koga, K.; Koshizaki, N. Fabrication of crystalline silicon spheres by selective laser heating in liquid medium. Langmuir 2011, 27, 5076–5080. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Boron carbide spherical particles encapsulated in graphite prepared by pulsed laser irradiation of boron in liquid medium. Appl. Phys. Lett. 2007, 91, 161110. [Google Scholar] [CrossRef]
- Li, H.Y.X.; Guo, L.; Zhou, Z.H.R.; Zeng, X. Preparation and formation mechanism of phase-controlled titanium dioxide microspheres by selective laser heating in liquid medium. RSC Adv. 2016, 6, 110911–110915. [Google Scholar]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of carbon dots by non-focusing pulsed laser irradiation in toluene. Chem. Commun. 2016, 52, 819–822. [Google Scholar] [CrossRef]
- Wang, H.; Koshizaki, N.; Li, L.; Jia, L.; Kawaguchi, K.; Li, X.; Pyatenko, A.; Swiatkowska-Warkocka, Z.; Bando, Y.; Golberg, D. Size-tailored ZnO submicrometer spheres: Bottom-up construction, size-related optical extinction, and selective aniline trapping. Adv. Mater. 2011, 23, 1865–1870. [Google Scholar] [CrossRef]
- Wang, H.; Kawaguchi, K.; Pyatenko, A.; Li, X.; Swiatkowska-Warkocka, Z.; Katou, Y.; Koshizaki, N. General bottom-up construction of spherical particles by pulsed laser irradiation of colloidal nanocomposites: A case study on CuO, Chem. Eur. J. 2012, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Takami, A.; Kurita, H.; Kosa, S. Laser-induced size reduction of noble metal particles. J. Phys. Chem. B 1999, 103, 1226–1232. [Google Scholar] [CrossRef]
- Pyatenko, A.; Wang, H.; Koshizaki, N.; Tsuji, T. Mechanism of pulsed laser interaction with colloidal nanoparticles. Laser Photon-Rev. 2013, 7, 596–604. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; He, C.; Zhu, C.; Li, Q.; Li, X.; Liu, K.; Zeng, X. Selective laser-induced preparation of metal-semiconductor nanocomposites and application for enhanced photocatalytic performance in the degradation of organic pollutants. J. Alloys Compd. 2021, 867, 159062. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhu, Z.; Li, X.; Li, J.; Zhang, Q. Constructing a hybrid high-performance photocatalyst by selective laser precisely heating in nanoscale. Appl. Surf. Sci. 2022, 588, 152946. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Zhu, Y.; Hao, Z.; Zeng, X. Investigation on the formation mechanism of hollow spheres prepared by pulsed laser selective heating colloidal nanoparticles in solution. J. Phys. Chem. C 2017, 121, 12469–12475. [Google Scholar] [CrossRef]
- Ma, R.; Islam, M.J.; Reddy, D.A.; Kim, T.K. Transformation of CeO2 into a mixed phase CeO2/Ce2O3 nanohybrid by liquid phase pulsed laser ablation for enhanced photocatalytic activity through Z-scheme patten. Ceram. Int. 2016, 42, 18495–18502. [Google Scholar] [CrossRef]
- Llyas, A.-M.; Gondal, M.A.; Yamani, Z.H.; Baig, U. Facile synthesis of titanium dioxide-cadmiun sulfide nanocomposite using pulsed laser ablation in liquid and its performance in photovoltaic and photocatalytic applications. Int. J. Energy. Res. 2017, 41, 1422–1435. [Google Scholar]
- Guo, J.L.; Chiou, Y.D.; Liang, W.I.; Liu, H.J.; Chen, Y.J.; Kuo, W.C.; Tsai, C.Y.; Tsai, K.A.; Kuo, H.H.; Hsieh, W.F.; et al. Complex oxide-noble metal conjugated nanoparticles. Adv. Mater. 2013, 25, 2040–2044. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO supported Au/Pd bimetallic nanocomposites for plasmon improved photocatalytic activity for methylene blue degradation under visible light irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Balati, A.; Wagle, D.; Nash, K.L.; Shipley, H.J. Heterojunction of TiO2 nanoparticle embedded into ZSM5 to 2D and 3D layered-structures of MoS2 nanosheets fabricated by pulsed laser ablation and microwave technique in deionized water: Structurally enhanced photocatalytic performance. Appl. Nanosci. 2019, 9, 19–32. [Google Scholar] [CrossRef]
- Balati, A.; Matta, A.; Nash, K.; Shipley, H.J. Heterojunction of vertically aligned MoS2 layers to hydrogenated black TiO2 and rutile based inorganic hollow microspheres for the highly enhanced visible light arsenic photooxidation. Compos. Part. B-Eng. 2022, 185, 107785. [Google Scholar] [CrossRef]
- Ma, R.; Kim, Y.J.; Reddy, D.A.; Kim, T.K. Synthesis of CeO2/Pd nanocomposites by pulsed laser ablation in liquids for the reduction of 4-nitrophenol to 4-aminophenol. Ceram. Int. 2015, 41, 12432–12438. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ma, R.; Reddy, D.A.; Kim, T.K. Liquid-phase pulsed laser ablation synthesis of graphitized carbon-encapsulated palladium core-shell nanospheres for catalytic reduction of nitrobenzene to aniline. Appl. Surf. Sci. 2015, 357, 2112–2120. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.H.Y.; Wang, X.; Cai, S.; Wang, C.; Yang, R. Green and facile synthesis of Rh/GO nanocomposites for high catalytic performance. Appl. Surf. Sci. 2019, 471, 929–934. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ghiaci, M.; Kulinich, S.A.; Wunderlich, W.; Farrokhpour, H.; Saraji, M.; Shahvar, A. Au-Pd@g-C3N4 as an efficient photocatalyst for visible-light oxidation of benzene to phenol: Experimental and mechanistic study. J. Phys. Chem. C 2018, 122, 27477–27485. [Google Scholar] [CrossRef]
- Shahzeydi, A.; Ghiaci, M.; Farrokhpour, H.; Shahvar, A.; Sun, M.; Saraji, M. Facile and green synthesis of copper nanoparticles loaded on the amorphous carbon nitride for the oxidation of cyclohexane. Chem. Eng. J. 2019, 370, 1310–1321. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Nizai, M.B.K.; Zubair, M.; Almatroudi, A.; Khurshid, M.; Tarip, F.; Mumtaz, R.; Li, B. Bioprospecting a native silver-resistance bacillus safensis strain for green synthesis and subsequent antibacterial and anticancer activities of silver nanoparticles. J. Adv. Res. 2020, 24, 475–483. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhai, X.; Luo, F.; Du, Y.; Yan, C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China Mater. 2019, 62, 1727–1739. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, S.; Zhou, R.; Li, G.; Li, X. Selective laser welding in liquid: A strategy for preparation of high-antibacterial activity nanozyme against staphylococcus aureus. J. Adv. Res. 2022. [Google Scholar] [CrossRef]
- Baig, U.; Hawsawi, A.; Ansari, M.A.; Gondal, M.A.; Dastageer, M.A.; Falath, W.S. Synthesis, characterization and evaluation of visible light activity cadmium sulfide-graphitic carbon nitride nanocomposite: A prospective solar light harvesting photocatalyst for the deactivation of waterborne pathogen. J. Photochem. Photobiol. B 2020, 204, 111783. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.M.; Blakemore, J.D.; Deimund, M.; Gray, H.B.; Winkler, J.R.; Müller, A.M. Highly activity mixed-metal nanosheets water oxidation catalysts made by pulsed-laser ablation in liquids. J. Am. Chem. Soc. 2014, 136, 13118–13121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Reddy, D.A.; Kim, Y.; Chun, S.Y.; Ma, R.; Kumar, D.P.; Song, J.K.; Kim, T.K. Drastic improvement of 1D-CdS solar-driven photocatalytic hydrogen evolution rate by integrating with NiFe layered double hydroxide nanosheets synthesized by liquid-phase pulsed-laser ablation. ACS Sustain. Chem. Eng. 2018, 6, 16734–16743. [Google Scholar] [CrossRef]
- Lee, H.; Reddy, D.A.; Kumar, D.P.; Lim, M.; Kim, T.K. Ultra-small cobalt nanocrystals embedded in 2D-MoS2 nano-sheets as efficient co-catalyst for solar-driven hydrogen production: Study of evolution rate dependence on cobalt nanocrystal size. Appl. Surf. Sci. 2019, 494, 239–248. [Google Scholar] [CrossRef]
- Baig, U.; Khan, A.; Gondal, M.A.; Dastageer, A.A.; Akhtar, S. Single-step synthesis of silicon carbon anchored graphitic carbon nitride nanocomposite photo-catalyst for efficient photoelectrochemical water splitting under visible-light irradiation. Colloids Surf. A 2021, 611, 125886. [Google Scholar] [CrossRef]
- Dramosh, Q.A.; Alade, I.O.; Alkanad, K.; Alnaggar, G.; Khan, A.; Khan, M.Y.; Elsayed, K.A.; Manda, A.A.; Hossain, M.K. WO3/BP/g-C3N4-based cauliflower nanocomposite fabricated by pulsed laser ablation for overall water splitting. Opt. Laser. Technol. 2022, 151, 108014. [Google Scholar] [CrossRef]
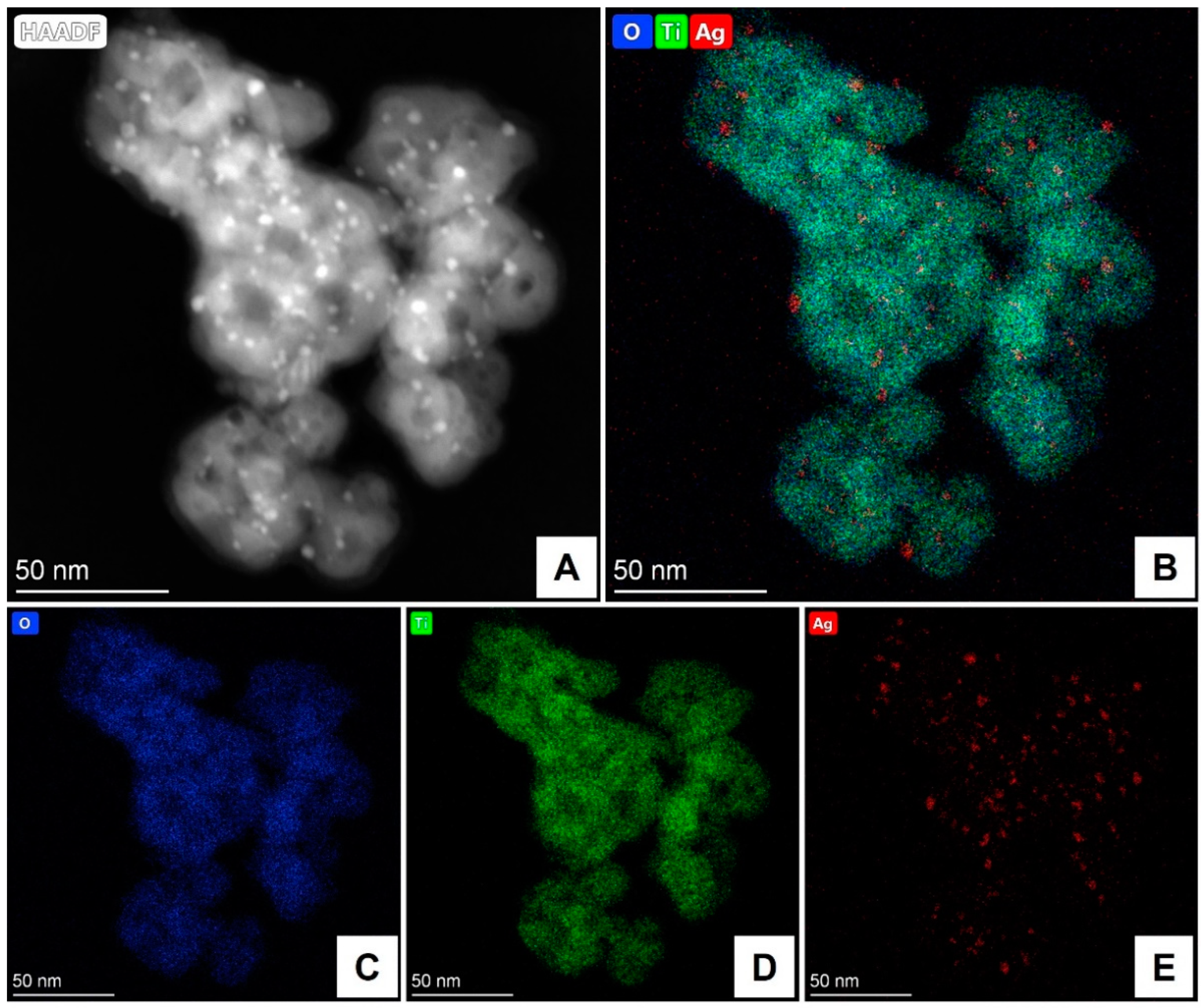

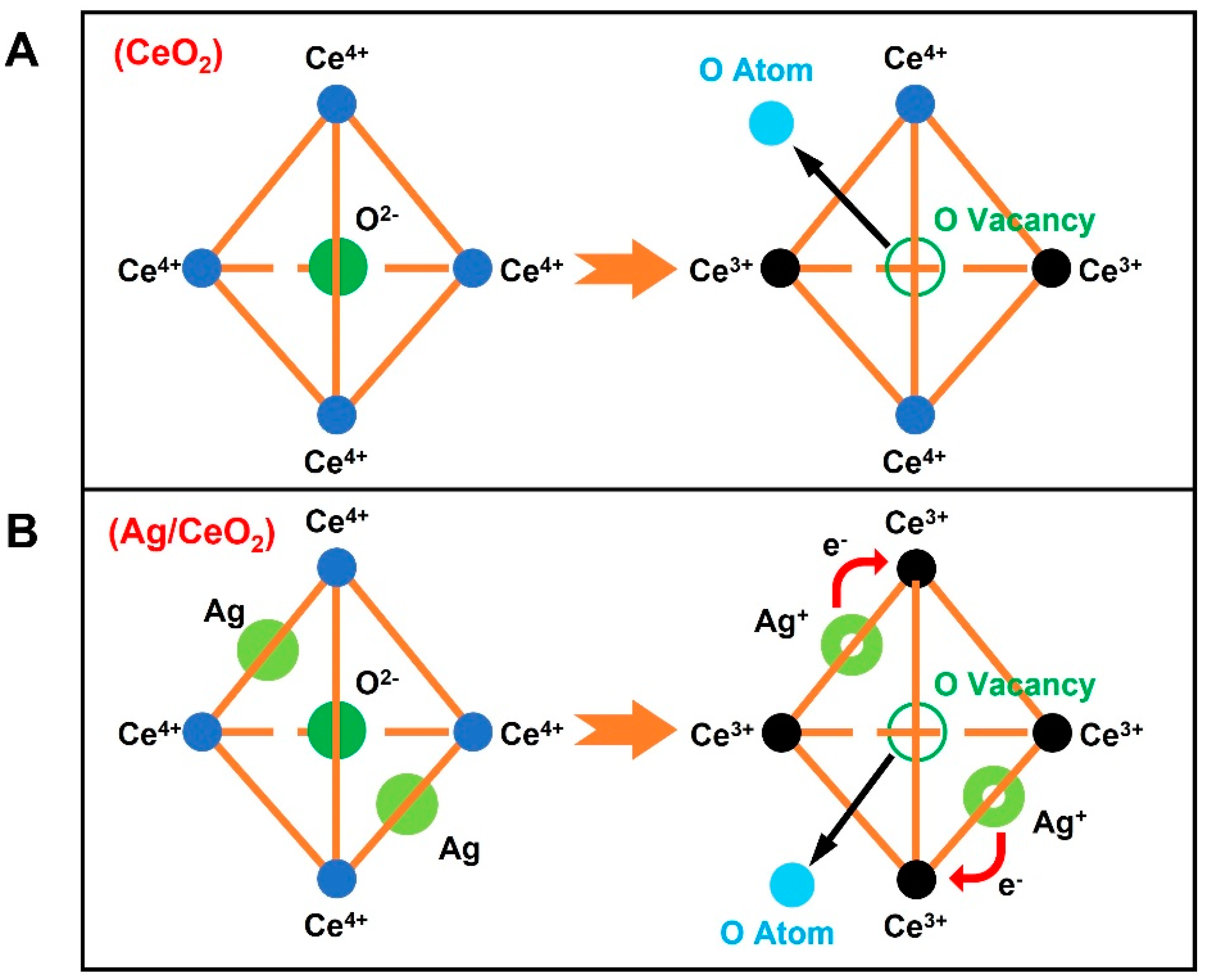

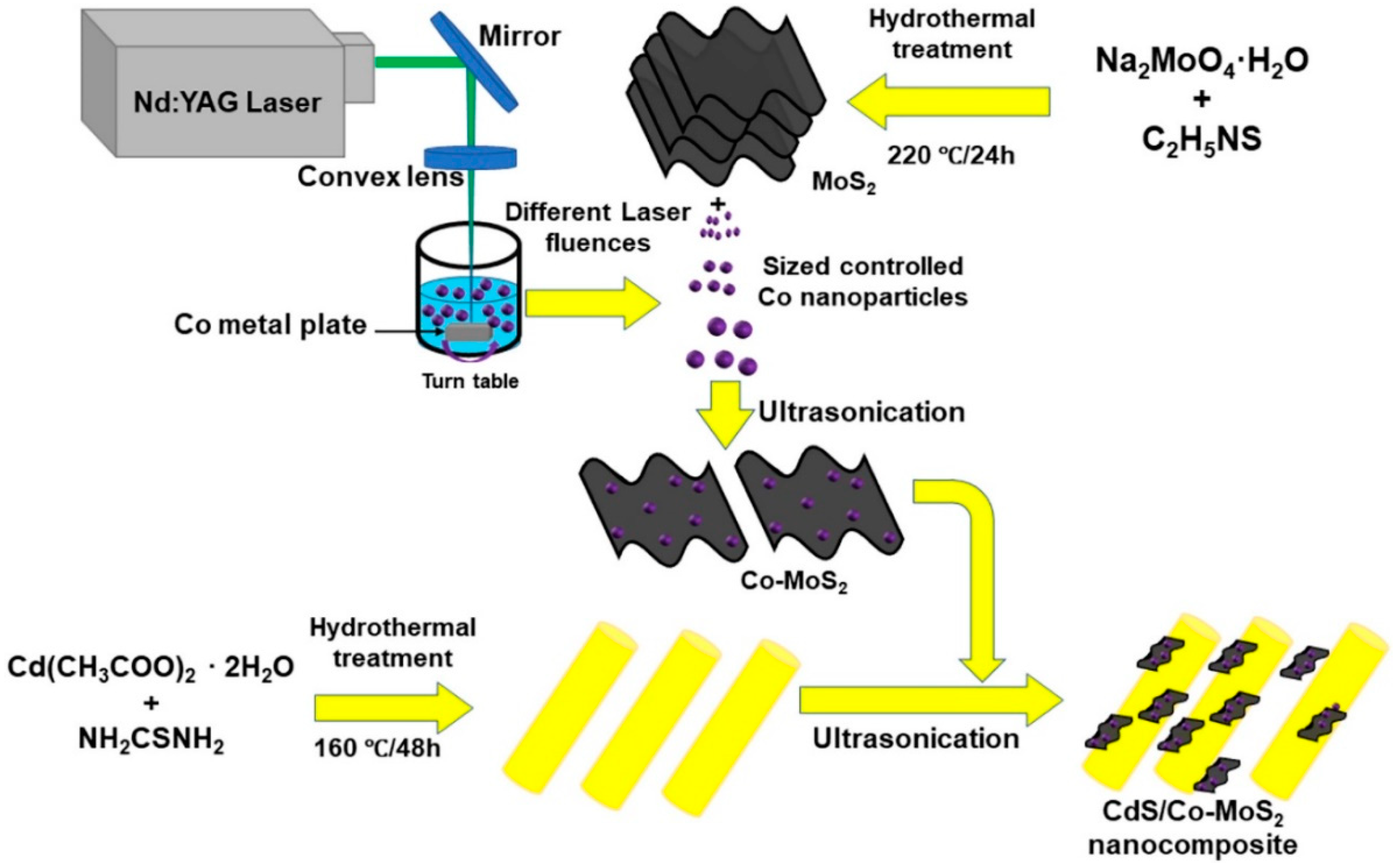
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zheng, Z.; Yan, J.; Lu, B.; Li, X. A Review on Pulsed Laser Preparation of Nanocomposites in Liquids and Their Applications in Photocatalysis. Catalysts 2022, 12, 1532. https://doi.org/10.3390/catal12121532
Li Y, Zheng Z, Yan J, Lu B, Li X. A Review on Pulsed Laser Preparation of Nanocomposites in Liquids and Their Applications in Photocatalysis. Catalysts. 2022; 12(12):1532. https://doi.org/10.3390/catal12121532
Chicago/Turabian StyleLi, Yang, Zhong Zheng, Jiujiang Yan, Bing Lu, and Xiangyou Li. 2022. "A Review on Pulsed Laser Preparation of Nanocomposites in Liquids and Their Applications in Photocatalysis" Catalysts 12, no. 12: 1532. https://doi.org/10.3390/catal12121532
APA StyleLi, Y., Zheng, Z., Yan, J., Lu, B., & Li, X. (2022). A Review on Pulsed Laser Preparation of Nanocomposites in Liquids and Their Applications in Photocatalysis. Catalysts, 12(12), 1532. https://doi.org/10.3390/catal12121532





