Facile Fabrication of SiO2/Zr Assisted with EDTA Complexed-Impregnation and Templated Methods for Crude Palm Oil to Biofuels Conversion via Catalytic Hydrocracking
Abstract
:1. Introduction
2. Results and Discussion
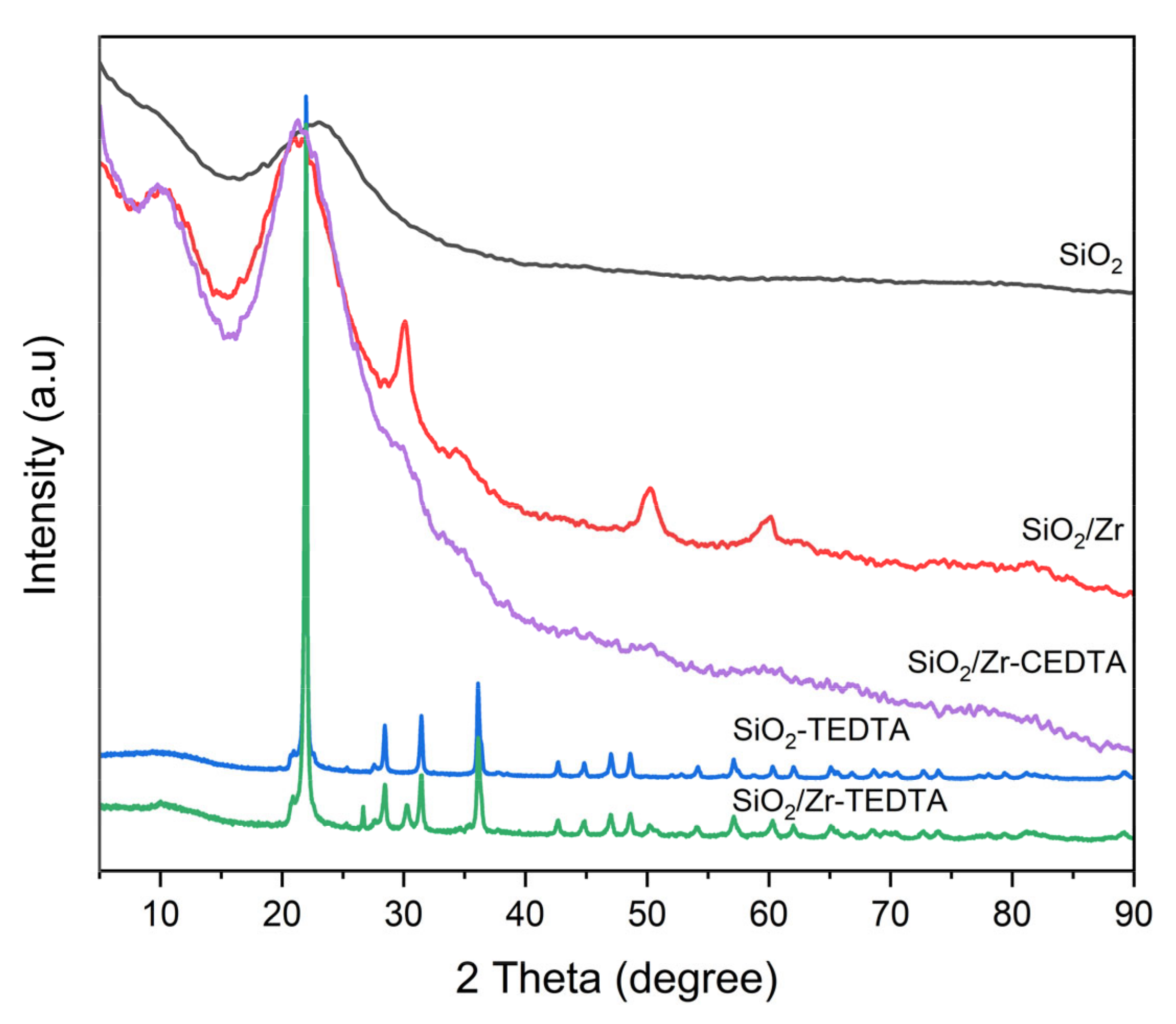
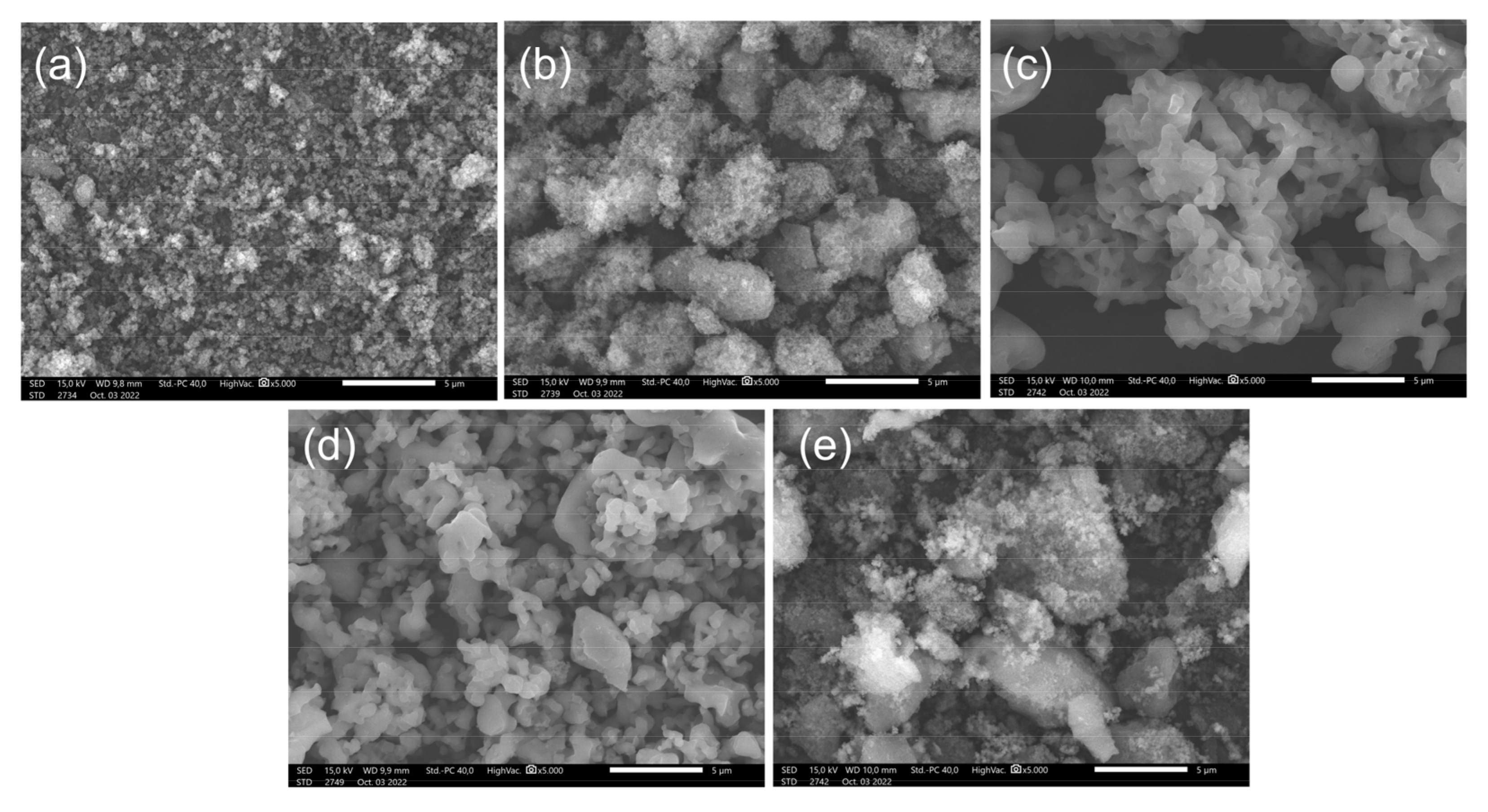
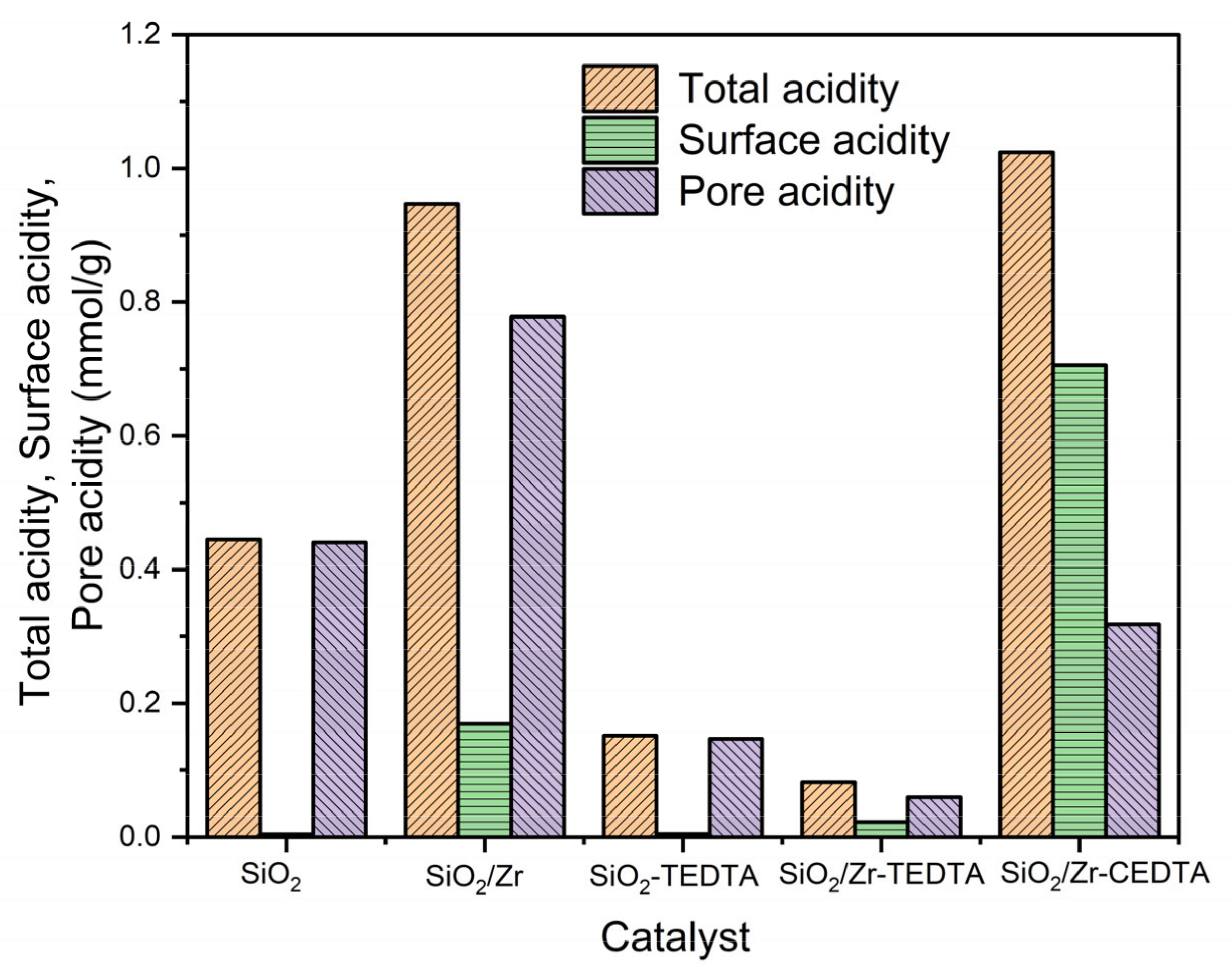
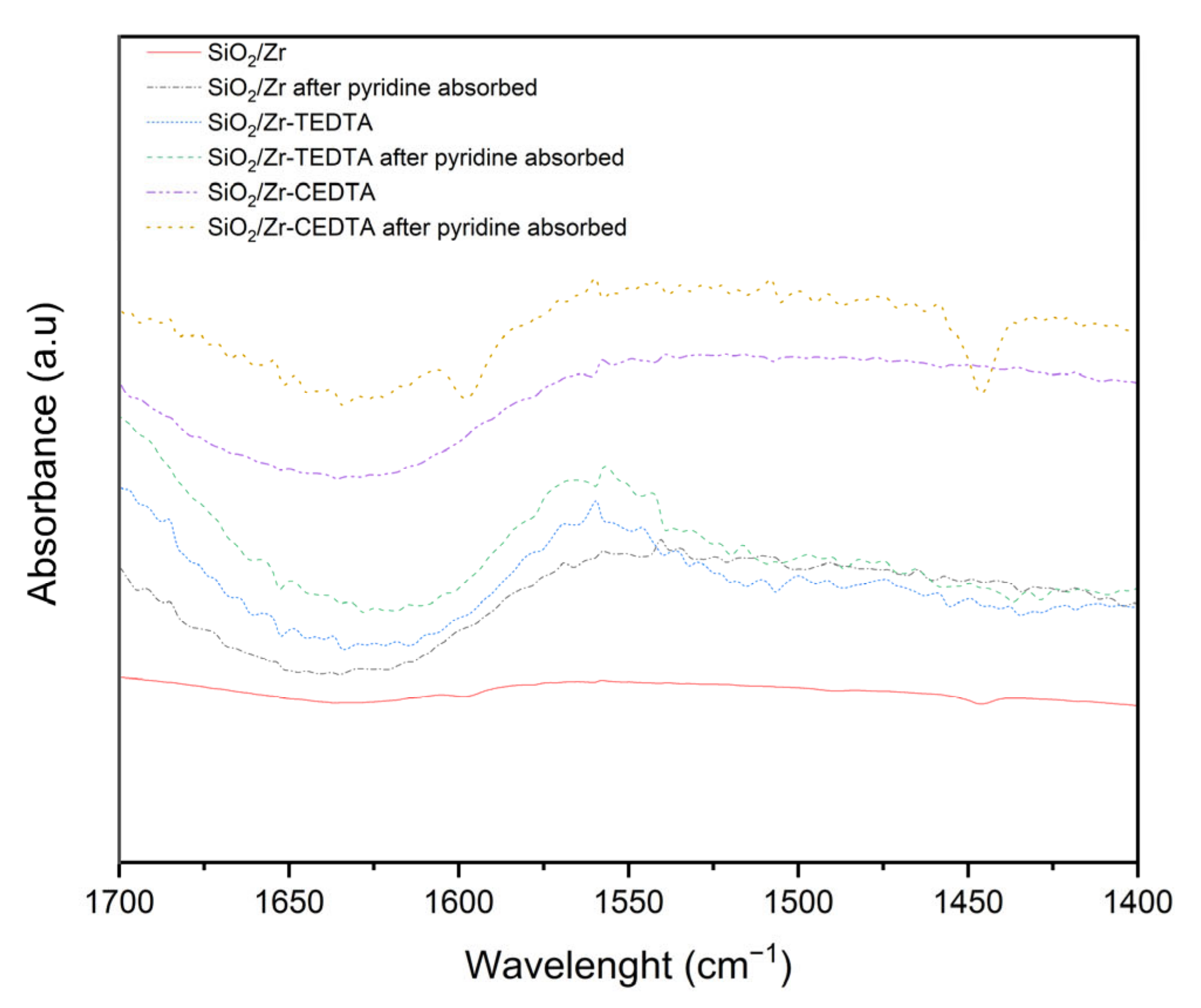
2.1. Characterization of Catalysts
2.2. Hydrocracking Test
3. Materials and Methods
3.1. Preparation of SiO2/Zr Using EDTA Template-Assisted
3.2. Preparation of SiO2/Zr Using EDTA Chelated-Assisted
3.3. Characterization of Catalyst
3.4. Catalytic Hydrocracking Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajesh, K.; Natarajan, M.P.; Devan, P.K.; Ponnuvel, S. Coconut Fatty Acid Distillate as Novel Feedstock for Biodiesel Production and Its Characterization as a Fuel for Diesel Engine. Renew. Energy 2021, 164, 1424–1435. [Google Scholar] [CrossRef]
- Sari, E.P.; Wijaya, K.; Trisunaryanti, W.; Syoufian, A.; Hasanudin, H.; Saputri, W.D. The Effective Combination of Zirconia Superacid and Zirconia-Impregnated CaO in Biodiesel Manufacturing: Utilization of Used Coconut Cooking Oil (UCCO). Int. J. Energy Environ. Eng. 2022, 13, 967–978. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Bazargan, A.; McKay, G.; Azelee, N.I.W.; Meili, L. Catalytic Deoxygenation of Palm Oil and Its Residue in Green Diesel Production: A Current Technological Review. Chem. Eng. Res. Des. 2021, 174, 158–187. [Google Scholar] [CrossRef]
- Hassan, S.N.; Sani, Y.M.; Abdul Aziz, A.R.; Sulaiman, N.M.N.; Daud, W.M.A.W. Biogasoline: An out-of-the-Box Solution to the Food-for-Fuel and Land-Use Competitions. Energy Convers. Manag. 2015, 89, 349–367. [Google Scholar] [CrossRef] [Green Version]
- Istadi, I.; Riyanto, T.; Buchori, L.; Anggoro, D.D.; Pakpahan, A.W.S.; Pakpahan, A.J. Biofuels Production from Catalytic Cracking of Palm Oil Using Modified Hy Zeolite Catalysts over a Continuous Fixed Bed Catalytic Reactor. Int. J. Renew. Energy Dev. 2021, 10, 149–156. [Google Scholar] [CrossRef]
- Melo-Banda, J.A. Catalytic Hydrocracking of Vegetable Oil for Agrofuels Production Using Ni-Mo, Ni-W, Pt and TFA Catalysts Supported on SBA-15. Catal. Today 2011, 166, 102–110. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Said, M.; Hidayati, P.T.; Purwaningrum, W.; Novia, N.; Wijaya, K. Hydrocracking Optimization of Palm Oil to Bio-Gasoline and Bio-Aviation Fuels Using Molybdenum Nitride-Bentonite Catalyst. RSC Adv. 2022, 12, 16431–16443. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of Jet Fuel and Green Diesel Range Biohydrocarbons by Hydroprocessing of Soybean Oil over Niobium Phosphate Catalyst. Fuel 2019, 245, 458–466. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic Hydrodeoxygenation of Triglycerides: An Approach to Clean Diesel Fuel Production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green Diesel Synthesis by Hydrodeoxygenation of Bio-Based Feedstocks: Strategies for Catalyst Design and Development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Z.; Cai, Q.; Guo, L. Catalytic Conversion of Carboxylic Acids in Bio-Oil for Liquid Hydrocarbons Production. Biomass Bioenergy 2012, 45, 138–143. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Zulaikha, I.S.; Ayu, C.; Rachmat, A.; Riyanti, F.; Hadiah, F.; Zainul, R.; Maryana, R. Hydrocracking of Crude Palm Oil to a Biofuel Using Zirconium Nitride and Zirconium Phosphide-Modified Bentonite. RSC Adv. 2022, 12, 21916–21925. [Google Scholar] [CrossRef] [PubMed]
- Srihanun, N.; Dujjanutat, P.; Muanruksa, P.; Kaewekannetra, P. Biofuels of Green Diesel–Kerosene–Gasoline Production from Palm Oil: Effect of Palladium Cooperated with Second Metal on Hydrocracking Reaction. Catalysts 2020, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Murata, K.; Inaba, M. Hydrocracking of Algae Oil to Aviation Fuel-Ranged Hydrocarbons over NiMo-Supported Catalysts. Catal. Today 2019, 332, 115–121. [Google Scholar] [CrossRef]
- Rasyid, R.; Malik, R.; Kusuma, H.S.; Roesyadi, A.; Mahfud, M. Triglycerides Hydrocracking Reaction of Nyamplung Oil with Non-Sulfided CoMo/γ-Al2O3 Catalysts. Bull. Chem. React. Eng. Catal. 2018, 13, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Cheng, J.; Huang, R.; Yang, W.; Zhou, J.; Cen, K. Hydrocracking of Palm Oil to Jet Biofuel over Different Zeolites. Int. J. Hydrogen Energy 2016, 41, 21883–21887. [Google Scholar] [CrossRef]
- Ishihara, A.; Fukui, N.; Nasu, H.; Hashimoto, T. Hydrocracking of Soybean Oil Using Zeolite-Alumina Composite Supported NiMo Catalysts. Fuel 2014, 134, 611–617. [Google Scholar] [CrossRef]
- Anand, M.; Farooqui, S.A.; Kumar, R.; Joshi, R.; Kumar, R.; Sibi, M.G.; Singh, H.; Sinha, A.K. Optimizing Renewable Oil Hydrocracking Conditions for Aviation Bio-Kerosene Production. Fuel Process. Technol. 2016, 151, 50–58. [Google Scholar] [CrossRef]
- Zandonai, C.H.; Yassue-Cordeiro, P.H.; Castellã-Pergher, S.B.; Scaliante, M.H.N.O.; Fernandes-Machado, N.R.C. Production of Petroleum-like Synthetic Fuel by Hydrocracking of Crude Soybean Oil over ZSM5 Zeolite-Improvement of Catalyst Lifetime by Ion Exchange. Fuel 2016, 172, 228–237. [Google Scholar] [CrossRef]
- Schmidt, J.; De Rosa, M. Certified Palm Oil Reduces Greenhouse Gas Emissions Compared to Non-Certified. J. Clean. Prod. 2020, 277, 124045. [Google Scholar] [CrossRef]
- Kuss, V.V.; Kuss, A.V.; Da Rosa, R.G.; Aranda, D.A.G.; Cruz, Y.R. Potential of Biodiesel Production from Palm Oil at Brazilian Amazon. Renew. Sustain. Energy Rev. 2015, 50, 1013–1020. [Google Scholar] [CrossRef]
- Purnomo, H.; Okarda, B.; Dermawan, A.; Ilham, Q.P.; Pacheco, P.; Nurfatriani, F.; Suhendang, E. Reconciling Oil Palm Economic Development and Environmental Conservation in Indonesia: A Value Chain Dynamic Approach. For. Policy Econ. 2020, 111, 102089. [Google Scholar] [CrossRef]
- Istadi, I.; Riyanto, T.; Khofiyanida, E.; Buchori, L.; Anggoro, D.D.; Sumantri, I.; Putro, B.H.S.; Firnanda, A.S. Low-Oxygenated Biofuels Production from Palm Oil through Hydrocracking Process Using the Enhanced Spent RFCC Catalysts. Bioresour. Technol. Rep. 2021, 14, 100677. [Google Scholar] [CrossRef]
- Šimáček, P.; Kubička, D. Hydrocracking of Petroleum Vacuum Distillate Containing Rapeseed Oil: Evaluation of Diesel Fuel. Fuel 2010, 89, 1508–1513. [Google Scholar] [CrossRef]
- Li, J.; Fang, X.; Bian, J.; Guo, Y.; Li, C. Microalgae Hydrothermal Liquefaction and Derived Biocrude Upgrading with Modified SBA-15 Catalysts. Bioresour. Technol. 2018, 266, 541–547. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Sadeghzadeh, M. Multi-Component Reaction on Free Nano-SiO2 Catalyst: Excellent Reactivity Combined with Facile Catalyst Recovery and Recyclability. J. Chem. Sci. 2013, 125, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Shi, J.; Xu, H.; Shen, W.; Hu, C. Low-Temperature CO2 Reforming of Methane on Zr-Promoted Ni/SiO2 Catalyst. Fuel Process. Technol. 2016, 144, 1–7. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chen, Y.W. Effects of Alkali Metal Oxide Additives on Cu/SiO2 Catalyst in the Dehydrogenation of Ethanol. Ind. Eng. Chem. Res. 2001, 40, 5889–5893. [Google Scholar] [CrossRef]
- Lomate, S.; Sultana, A.; Fujitani, T. Effect of SiO2 Support Properties on the Performance of Cu-SiO2 Catalysts for the Hydrogenation of Levulinic Acid to Gamma Valerolactone Using Formic Acid as a Hydrogen Source. Catal. Sci. Technol. 2017, 7, 3073–3083. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Chen, P.; Ma, L.; Zhang, Q.; Zhou, L.; Wang, C. Mild Hydrogenation of Lignin Depolymerization Products over Ni/SiO2 Catalyst. Energy Fuels 2017, 31, 7208–7213. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. A Novel Nano-Ni/SiO2 Catalyst for Hydrogen Production from Steam Reforming of Ethanol. Environ. Sci. Technol. 2010, 44, 5993–5998. [Google Scholar] [CrossRef]
- Giniyatova, S.; Dauletbekova, A.; Baimukhanov, Z.; Vlasukova, L.; Akilbekov, A.; Usseinov, A.; Kozlovskiy, A.; Akylbekova, A.; Seitbayev, A.; Karipbayev, Z. Structure, Electrical Properties and Luminescence of ZnO Nanocrystals Deposited in SiO2/Si Track Templates. Radiat. Meas. 2019, 125, 52–56. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Chen, D.; Yu, Q.; Yang, W.; Zhou, J.; Wu, S. Facile, Template-Free Synthesis of Macroporous SiO2 as Catalyst Support towards Highly Enhanced Catalytic Performance for Soot Combustion. Chem. Eng. J. 2019, 375, 121958. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, H.; Zhang, S.; Wang, G.; Zhu, X.; Li, C. Dehydrogenation of Isobutane over a Ni-P/SiO2 Catalyst: Effect of P Addition. Ind. Eng. Chem. Res. 2019, 58, 7834–7843. [Google Scholar] [CrossRef]
- Mashkin, M.; Tedeeva, M.; Fedorova, A.; Vasiliev, A.; Egorov, A.; Pribytkov, P.; Kalmykov, K.; Kapustin, G.; Morozov, I.; Kustov, L.; et al. CrOx/SiO2 Mesoporous Catalysts Prepared Using Beta-Cyclodextrin as a Template and Their Catalytic Properties in Propane Oxidative Dehydrogenation in the Presence of Carbon Dioxide. Microporous Mesoporous Mater. 2022, 338, 111967. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Parameters Affecting the Formation of 1,2-Propanediol from Glycerol over Ru/SiO2 Catalyst. Org. Process Res. Dev. 2011, 15, 925–931. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Yang, T.; Tong, Z.; Tang, Z.; Orita, A.; Qiu, R. Zirconium-Based Catalysts in Organic Synthesis. Top. Curr. Chem. 2022, 380, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Li, M.; Meng, Y.; Li, J.; Zhang, Z.; Zhang, H. Recyclable Zr/Hf-Containing Acid-Base Bifunctional Catalysts for Hydrogen Transfer Upgrading of Biofuranics: A Review. Front. Chem. 2021, 9, 1090. [Google Scholar] [CrossRef]
- Zhao, Y.; Sohn, H.; Hu, B.; Niklas, J.; Poluektov, O.G.; Tian, J.; Delferro, M.; Hock, A.S. Zirconium Modification Promotes Catalytic Activity of a Single-Site Cobalt Heterogeneous Catalyst for Propane Dehydrogenation. ACS Omega 2018, 3, 11117–11127. [Google Scholar] [CrossRef]
- Zhang, Q.; Long, K.; Wang, J.; Zhang, T.; Song, Z.; Lin, Q. A Novel Promoting Effect of Chelating Ligand on the Dispersion of Ni Species over Ni/SBA-15 Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2017, 42, 14103–14114. [Google Scholar] [CrossRef]
- Ren, H.P.; Hao, Q.Q.; Ding, S.Y.; Zhao, Y.Z.; Zhu, M.; Tian, S.P.; Ma, Q.; Song, W.Q.; Miao, Z.; Liu, Z.T. A High-Performance Ni/SiO2 Prepared by the Complexed-Impregnation Method with Citric Acid for Carbon Dioxide Reforming of Methane. Ind. Eng. Chem. Res. 2018, 57, 16257–16263. [Google Scholar] [CrossRef]
- Bian, Z.; Xin, Z.; Meng, X.; Tao, M.; Lv, Y.H.; Gu, J. Effect of Citric Acid on the Synthesis of CO Methanation Catalysts with High Activity and Excellent Stability. Ind. Eng. Chem. Res. 2017, 56, 2383–2392. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Ding, F.; Guo, X.; Wang, G. Syngas Production via Steam-CO2 Dual Reforming of Methane over LA-Ni/ZrO2 Catalyst Prepared by l -Arginine Ligand-Assisted Strategy: Enhanced Activity and Stability. ACS Sustain. Chem. Eng. 2015, 3, 3461–3476. [Google Scholar] [CrossRef]
- Ran, R.; Guo, Y.; Zheng, Y.; Wang, K.; Shao, Z. Well-Crystallized Mesoporous Samaria-Doped Ceria from EDTA-Citrate Complexing Process with in Situ Created NiO as Recyclable Template. J. Alloy. Compd. 2010, 491, 271–277. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Beaunier, P.; Che, M.; Train, C. Stabilization of Hexagonal Close-Packed Metallic Nickel for Alumina-Supported Systems Prepared from Ni(II) Glycinate. J. Solid State Chem. 2007, 180, 22–30. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Lunin, V.V. Template Synthesis of Porous Ceria-Based Catalysts for Environmental Application. Molecules 2020, 25, 4242. [Google Scholar] [CrossRef]
- Purwaningsih, H.; Ervianto, Y.; Pratiwi, V.M.; Susanti, D.; Purniawan, A. Effect of Cetyl Trimethyl Ammonium Bromide as Template of Mesoporous Silica MCM-41 from Rice Husk by Sol-Gel Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012051. [Google Scholar] [CrossRef]
- Rubab, R.; Ali, S.; Rehman, A.U.; Khan, S.A.; Khan, A.M. Templated Synthesis of NiO/SiO2 Nanocomposite for Dye Removal Applications: Adsorption Kinetics and Thermodynamic Properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126253. [Google Scholar] [CrossRef]
- Koizumi, N.; Ibi, Y.; Hongo, D.; Hamabe, Y.; Suzuki, S.; Hayasaka, Y.; Shindo, T.; Yamada, M. Mechanistic Aspects of the Role of Chelating Agents in Enhancing Fischer-Tropsch Synthesis Activity of Co/SiO2 Catalyst: Importance of Specific Interaction of Co with Chelate Complex during Calcination. J. Catal. 2012, 289, 151–163. [Google Scholar] [CrossRef]
- Wijaya, K.; Malau, L.L.M.; Utami, M.; Mulijani, S.; Patah, A.; Wibowo, A.C.; Chandrasekaran, M.; Rajabathar, J.R.; Al-Lohedan, H.A. Synthesis, Characterizations and Catalysis of Sulfated Silica and Nickel Modified Silica Catalysts for Diethyl Ether (DEE) Production from Ethanol towards Renewable Energy Applications. Catalysts 2021, 11, 1511. [Google Scholar] [CrossRef]
- Aneu, A.; Wijaya, K.; Syoufian, A. Silica-Based Solid Acid Catalyst with Different Concentration of H2SO4 and Calcination Temperature: Preparation and Characterization. Silicon 2021, 13, 2265–2270. [Google Scholar] [CrossRef]
- Kumar, S.; Bhunia, S.; Ojha, A.K. Effect of Calcination Temperature on Phase Transformation, Structural and Optical Properties of Sol-Gel Derived ZrO2 Nanostructures. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 66, 74–80. [Google Scholar] [CrossRef]
- Rauta, P.R.; Manivasakan, P.; Rajendran, V.; Sahu, B.B.; Panda, B.K.; Mohapatra, P. Phase Transformation of ZrO2 Nanoparticles Produced from Zircon. Phase Transit. 2012, 85, 13–26. [Google Scholar] [CrossRef]
- Utami, M.; Safitri, R.; Fajar Pradipta, M.; Wijaya, K.; Woong Chang, S.; Ravindran, B.; Ovi, D.; Rajabathar, J.R.; Poudineh, N.; Moonsamy Gengan, R. Enhanced Catalytic Conversion of Palm Oil into Biofuels by Cr-Incorporated Sulphated Zirconia. Mater. Lett. 2022, 309, 131472. [Google Scholar] [CrossRef]
- Wijaya, K.; Nadia, A.; Dinana, A.; Pratiwi, A.F.; Tikoalu, A.D.; Wibowo, A.C. Catalytic Hydrocracking of Fresh and Waste Frying Oil over Ni-and Mo-Based Catalysts Supported on Sulfated Silica for Biogasoline Production. Catalysts 2021, 11, 1150. [Google Scholar] [CrossRef]
- Nadia, A.; Wijaya, K.; Falah, I.I.; Sudiono, S.; Budiman, A. Self-Regeneration of Monodisperse Hierarchical Porous NiMo/Silica Catalyst Induced by NaHCO3 for Biofuel Production. Waste Biomass Valorization 2021, 13, 2335–2347. [Google Scholar] [CrossRef]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of Amorphous Silica and Sulfonic Acid Functionalized Silica Used as Reinforced Phase for Polymer Electrolyte Membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef] [Green Version]
- Gobara, H.M.; Hassan, S.A.; Betiha, M.A. The Interaction Characteristics Controlling Dispersion Mode-Catalytic Functionality Relationship of Silica–Modified Montmorillonite-Anchored Ni Nanoparticles in Petrochemical Processes. Mater. Chem. Phys. 2016, 181, 476–486. [Google Scholar] [CrossRef]
- Badoga, S.; Mouli, K.C.; Soni, K.K.; Dalai, A.K.; Adjaye, J. Beneficial Influence of EDTA on the Structure and Catalytic Properties of Sulfided NiMo/SBA-15 Catalysts for Hydrotreating of Light Gas Oil. Appl. Catal. B Environ. 2012, 125, 67–84. [Google Scholar] [CrossRef]
- Zaki, T.; Mukhtarova, G.S.; Al-Sabagh, A.M.; Soliman, F.S.; Betiha, M.A.; Mahmoud, T.; Abd El-Raouf, M.; Afandiyeva, N.K.; Alizade, A.; Hasanova, A.B.; et al. Slurry-Phase Catalytic Hydrocracking of Mazut (Heavy Residual Fuel Oil) Using Ni-Bentonite. Pet. Sci. Technol. 2018, 36, 1559–1567. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Andini, L.; Riyanti, F.; Mara, A.; Hadiah, F.; Fanani, Z. Enhanced Isopropyl Alcohol Conversion over Acidic Nickel Phosphate-Supported Zeolite Catalysts. ACS Omega 2022, 7, 38923–38932. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Putri, Q.U.; Fanani, Z.; Agustina, T.E.; Wijaya, K. Montmorillonite-Zirconium Phosphate Catalysts for Methanol Dehydration. Iran. J. Catal. 2022, 12, 389–397. [Google Scholar] [CrossRef]
- Wijaya, K.; Kurniawan, M.A.; Saputri, W.D.; Trisunaryanti, W.; Mirzan, M.; Hariani, P.L.; Tikoalu, A.D. Synthesis of Nickel Catalyst Supported on ZrO2/SO4 pillared Bentonite and Its Application for Conversion of Coconut Oil into Gasoline via Hydrocracking Process. J. Environ. Chem. Eng. 2021, 9, 105399. [Google Scholar] [CrossRef]
- Alisha, G.D.; Trisunaryanti, W.; Syoufian, A. Hydrocracking of Waste Palm Cooking Oil into Hydrocarbon Compounds over Mo Catalyst Impregnated on SBA-15. Silicon 2022, 14, 2309–2315. [Google Scholar] [CrossRef]
- Aziz, I.T.A.; Saputri, W.D.; Trisunaryanti, W.; Sudiono, S.; Syoufian, A.; Budiman, A.; Wijaya, K. Synthesis of Nickel-Loaded Sulfated Zirconia Catalyst and Its Application for Converting Used Palm Cooking Oil to Gasoline via Hydrocracking Process. Period. Polytech. Chem. Eng. 2022, 66, 101–113. [Google Scholar] [CrossRef]
- Hasanudin, H.; Rachmat, A.; Said, M.; Wijaya, K. Kinetic Model of Crude Palm Oil Hydrocracking over Ni/Mo ZrO2 –Pillared Bentonite Catalyst. Period. Polytech. Chem. Eng. 2020, 64, 238–247. [Google Scholar] [CrossRef]

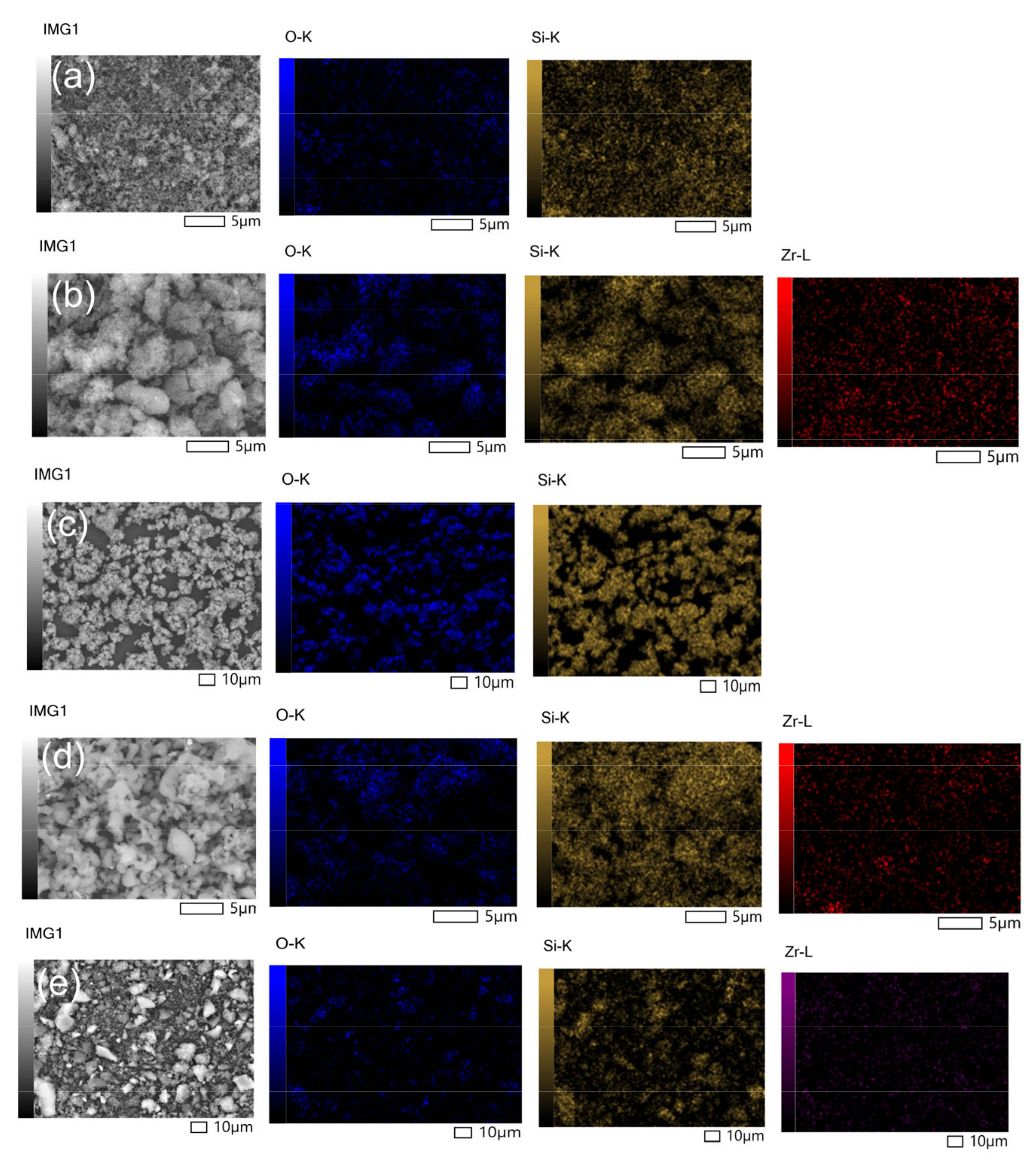


| Catalyst | Atomic (wt.%) | ||
|---|---|---|---|
| Si | O | Zr | |
| SiO2 | 18.68 | 81.33 | |
| SiO2/Zr | 21.6 | 78.14 | 0.26 |
| SiO2-TEDTA | 28.43 | 71.57 | |
| SiO2/Zr-TEDTA | 26.18 | 73.43 | 0.39 |
| SiO2/Zr-CEDTA | 21.6 | 77.85 | 0.55 |
| Catalyst | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Radius (Å) |
|---|---|---|---|
| SIO2 | 294 | 0.73 | 49.76 |
| SIO2/Zr | 76.43 | 0.78 | 20.48 |
| SIO2-TEDTA | 268 | 0.81 | 60.44 |
| SIO2/Zr-TEDTA | 220.5 | 0.83 | 75.37 |
| SIO2/Zr-CEDTA | 134 | 0.53 | 79.58 |
| Catalyst | Average Particle Size (μm) |
|---|---|
| SiO2 | 21.66 ± 0.32 |
| SiO2/Zr | 48.39 ± 0.05 |
| SiO2-TEDTA | 19.08 ± 0.07 |
| SiO2/Zr-TEDTA | 34.26 ± 0.16 |
| SiO2/Zr-CEDTA | 61.45 ± 0.07 |
| Catalyst | Conversion (%) | Yield (%.wt) | |||
|---|---|---|---|---|---|
| Liquid | Gas | Coke | Residue | ||
| SiO2 | 77.10 | 53.44 | 17.971 | 0.09 | 28.58 |
| SIO2/Zr | 82.75 | 45.93 | 40.23 | 0.21 | 13.63 |
| SIO2-TEDTA | 60.33 | 62.84 | 12.99 | 0.02 | 24.15 |
| SIO2/Zr-TEDTA | 62.17 | 47.45 | 38.43 | 0.12 | 14 |
| SIO2/Zr-CEDTA | 87.73 | 66.29 | 23.33 | 0.34 | 10.04 |
| Catalyst | Selectivity (%) | ||
|---|---|---|---|
| Bio-Gasoline | Bio-Jet | Bio-Diesel | |
| SiO2 | 23.13 | 31.64 | 45.23 |
| SiO2/Zr | 24.58 | 34.47 | 40.95 |
| SiO2-TEDTA | 27.23 | 44.67 | 28.1 |
| SiO2/Zr-TEDTA | 26.94 | 36.88 | 36.18 |
| SiO2/Zr-CEDTA | 26.80 | 41.77 | 31.43 |
| Catalyst | Conversion (%) | ||
|---|---|---|---|
| 1st Run | 2nd Run | 3rd Run | |
| SiO2/Zr-TEDTA | 61.25 | 56.12 | 48.21 |
| SiO2/Zr-CEDTA | 85.63 | 82.23 | 75.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanudin, H.; Asri, W.R.; Fanani, Z.; Adisti, S.J.; Hadiah, F.; Maryana, R.; Al Muttaqii, M.; Zhu, Z.; Machado, N.T. Facile Fabrication of SiO2/Zr Assisted with EDTA Complexed-Impregnation and Templated Methods for Crude Palm Oil to Biofuels Conversion via Catalytic Hydrocracking. Catalysts 2022, 12, 1522. https://doi.org/10.3390/catal12121522
Hasanudin H, Asri WR, Fanani Z, Adisti SJ, Hadiah F, Maryana R, Al Muttaqii M, Zhu Z, Machado NT. Facile Fabrication of SiO2/Zr Assisted with EDTA Complexed-Impregnation and Templated Methods for Crude Palm Oil to Biofuels Conversion via Catalytic Hydrocracking. Catalysts. 2022; 12(12):1522. https://doi.org/10.3390/catal12121522
Chicago/Turabian StyleHasanudin, Hasanudin, Wan Ryan Asri, Zainal Fanani, Selvi Julpani Adisti, Fitri Hadiah, Roni Maryana, Muhammad Al Muttaqii, Zongyuan Zhu, and Nelio Teixeira Machado. 2022. "Facile Fabrication of SiO2/Zr Assisted with EDTA Complexed-Impregnation and Templated Methods for Crude Palm Oil to Biofuels Conversion via Catalytic Hydrocracking" Catalysts 12, no. 12: 1522. https://doi.org/10.3390/catal12121522
APA StyleHasanudin, H., Asri, W. R., Fanani, Z., Adisti, S. J., Hadiah, F., Maryana, R., Al Muttaqii, M., Zhu, Z., & Machado, N. T. (2022). Facile Fabrication of SiO2/Zr Assisted with EDTA Complexed-Impregnation and Templated Methods for Crude Palm Oil to Biofuels Conversion via Catalytic Hydrocracking. Catalysts, 12(12), 1522. https://doi.org/10.3390/catal12121522







