Global-Local CNTs Conductive Network Couple with Co-Based Polyhedral Promotes the Electrocatalytic Reduction of Oxygen
Abstract
:1. Introduction
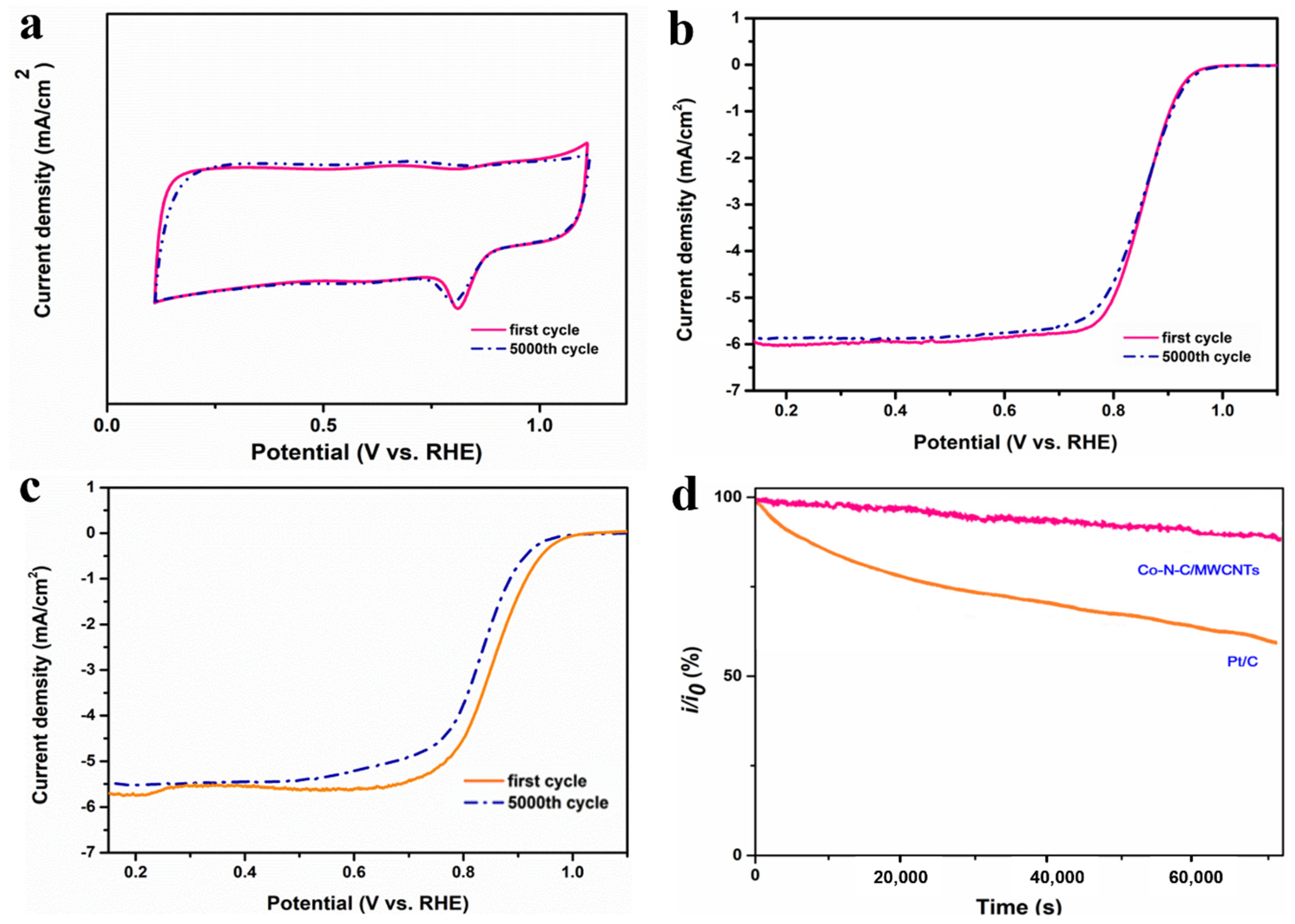
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Chemicals and Materials
4.2. Synthesis of gl-CNTs/Co@N-C
4.3. Electrochemical Measurements
4.4. Characterizations
Author Contributions
Funding
Conflicts of Interest
References
- Shi, H.C.; Fu, J.X.; Wu, Q.M.; Huang, M.; Jiang, L.H.; Cui, M.Q.; Idem, R.; Tontiwachwuthikul, P. Studies of the Coordination Effect of DEA-MEA Blended Amines (within 1+4 to 2+3 M) under Heterogeneous Catalysis by Means of Absorption and Desorption Parameters. Sep. Purif. Technol. 2020, 236, 116179. [Google Scholar] [CrossRef]
- Shi, H.; Peng, J.; Cheng, X.; Yang, X.; Jin, J.; Hu, J. The CO2 desorption analysis of tri-solvent MEA+BEA+DEEA with several commercial solid acid catalysts. Int. J. Greenh. Gas Control 2022, 116, 103647. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sheng, M.; Drisdell, W.S.; Yano, J.; Sun, Y. Bimetal-organic framework self-adjusted synthesis of support-free nonprecious electrocatalysts for efficient oxygen reduction. Acs Catal. 2015, 5, 7068–7076. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.R.; Yoo, H.; Choi, J.; Ahn, W.S. Electrocatalytic oxygen reduction over Co@Co3O4/n-doped porous carbon derived from pyrolysis of ZIF-8/67 on cellulose nanofibers. Cellulose 2020, 27, 2723–2735. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Zhou, S.; Li, X.; Li, L.; Yu, Z.; Gu, L. ZIF-8/ZIF-67-derived Co-Nx-embedded 1D porous carbon nanofibers with graphitic carbon-encased Co nanoparticles as an efficient bifunctional electrocatalyst. Small 2018, 14, 1800423. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct methanol fuel cells system-a review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Hu, C.G.; Dai, L.M. Carbon-based metal-free catalysts for electrocatalysis beyond the ORR. Angew. Chem. -Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.; Wu, Z.Y.; Xiong, Y.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef]
- Tang, C.; Chen, L.; Li, H.J.; Li, L.Q.; Jiao, Y.; Zheng, Y.; Xu, H.L.; Davey, K.; Qiao, S.Z. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827. [Google Scholar] [CrossRef]
- Zhao, C.X.; Li, B.Q.; Liu, J.N.; Zhang, Q. Intrinsic electrocatalytic activity regulation of M-N-C single-atom catalysts for the oxygen reduction reaction. Angew. Chem. -Int. Ed. 2021, 60, 4448–4463. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.Q.; Ye, C.; Chen, S.M.; Sun, T.L.; Jiao, Y.; Liu, L.M.; Zhu, C.Z.; Song, L.; Han, Y.; Jaroniec, M.; et al. Short-range ordered iridium single atoms integrated into cobalt oxide spinel structure for highly efficient electrocatalytic water oxidation. J. Am. Chem. Soc. 2021, 143, 5201–5211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Zhang, L.; Yang, P.P.; Hu, C.L.; Luo, Z.B.; Chang, X.X.; Zhao, Z.J.; Gong, J.L. Low-coordinated edge sites on ultrathin palladium nanosheets boost carbon dioxide electroreduction performance. Angew. Chem. -Int. Ed. 2018, 57, 11544–11548. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.W.; Lou, X.W. Hollow structures based on prussian blue and its analogs for electrochemical energy storage and conversion. Adv. Mater. 2019, 31, 1706825. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, Q.; Ding, J.; Li, T.T.; Yu, D.; Nie, H.; Qian, J.; Yang, Z. Heteroepitaxial metal-organic frameworks derived cobalt and nitrogen codoped carbon nanosheets to boost oxygen reduction. J. Colloid Interface Sci. 2022, 623, 1210–1219. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Z.; Liu, X.; Ren, J.; Xing, T.; Li, Z.; Li, X.; Chen, Y. Strategic Design of Cellulose nanofibers@zeolitic imidazolate frameworks derived mesoporous carbon-supported nanoscale CoFe2O4/CoFe hybrid composition as trifunctional electrocatalyst for zn-air battery and self-powered overall water-splitting. J. Power Sources 2022, 521, 230925. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Wang, P.; Long, X.; Zheng, L.; Liu, G.; He, X.; Qiu, J. Core-shell ZIF-67@ZIF-8-derived multi-dimensional cobalt-nitrogen doped hierarchical carbon nanomaterial for efficient oxygen reduction reaction. J. Alloys Compd. 2022, 903, 163701. [Google Scholar] [CrossRef]
- Yao, W.; Chen, J.; Wang, Y.; Fang, R.; Qin, Z.; Yang, X.; Chen, L.; Li, Y. Nitrogen-doped carbon composites with ordered macropores and hollow walls. Angew. Chem. -Int. Ed. 2021, 60, 23729–23734. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cao, P.; Chen, W.; Ezeh, C.I.; Chen, Z.; Luo, Y.; Liu, Q.; Zhao, H.; Rui, Z.; Gao, S.; et al. Electrocatalysis enabled transformation of earth-abundant water, nitrogen and carbon dioxide for a sustainable future. Mater. Adv. 2022, 3, 1359–1400. [Google Scholar] [CrossRef]
- Liang, P.; Wang, Q.; Kang, J.; Tian, W.; Sun, H.; Wang, S. Dual-metal zeolitic imidazolate frameworks and their derived nanoporous carbons for multiple environmental and electrochemical applications. Chem. Eng. J. 2018, 351, 641–649. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yan, Y.; Larissa, T.Y.P.; Zhang, X.; Wuu, D.; Zhang, H.; Yang, Y.; Wang, X. Core-shell carbon materials derived from metal-organic frameworks as an efficient oxygen bifunctional electrocatalyst. Nano Energy 2016, 30, 368–378. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zheng, Z.; Zhang, J.; Jiang, S.P.; Zhao, Y.; Wang, H. Graphene oxide/core-shell structured metal-organic framework nano-sandwiches and their derived cobalt/N-doped carbon nanosheets for oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 10182–10189. [Google Scholar] [CrossRef]
- Wang, R.; Yan, T.; Han, L.; Chen, G.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Tuning the dimensions and structures of nitrogen-doped carbon nanomaterials derived from sacrificial g-C3N4/metal-organic frameworks for enhanced electrocatalytic oxygen reduction. J. Mater. Chem. A 2018, 6, 5752–5761. [Google Scholar] [CrossRef]
- Jose, V.; Jayakumar, A.; Lee, J.M. Bimetal/metal oxide encapsulated in graphitic nitrogen doped mesoporous carbon networks for enhanced oxygen electrocatalysis. Chemelectrochem 2019, 6, 1485–1491. [Google Scholar] [CrossRef]
- Yang, B.; Shao, M.; Xu, Y.; Du, Y.; Yang, H.; Bin, D.; Liu, B.; Lu, H. Core-shell ZIF-8@ZIF-67-derived cobalt nanoparticles in situ grown on n-doped carbon nanotube polyhedra for ultrasensitive electrochemical detection of chloramphenicol. Chemelectrochem 2022, 9, e202200438. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [Green Version]
- Saadat, N.; Dhakal, H.N.; Tjong, J.; Jaffer, S.; Yang, W.; Sain, M. Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sustain. Energy Rev. 2021, 138, 110535. [Google Scholar] [CrossRef]
- Lu, B.; Qi, W.; Luo, M.; Liu, Q.; Guo, L. Fischer-Tropsch Synthesis: ZIF-8@ZIF-67-Derived cobalt nanoparticle-embedded nanocage catalysts. Ind. Eng. Chem. Res. 2020, 59, 12352–12359. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, C.; Yuan, H.; Zhang, W.; Zheng, H.; Cao, R. PVP-assisted transformation of a metal-organic framework into Co-embedded N-enriched meso/microporous carbon materials as bifunctional electrocatalysts. Chem. Commun. 2018, 54, 7519–7522. [Google Scholar] [CrossRef]
- Gan, L.; Dong, M.; Han, Y.; Xiao, Y.; Yang, L.; Huang, J. Connection-improved conductive network of carbon nanotubes in a rubber cross-link network. Acs Appl. Mater. Interfaces 2018, 10, 18213–18219. [Google Scholar] [CrossRef]
- Li, J.C.; Meng, Y.; Zhang, L.L.; Li, G.Z.; Shi, Z.C.; Hou, P.X.; Liu, C.; Cheng, H.M.; Shao, M.H. Dual-Phasic Carbon with Co Single Atoms and Nanoparticles as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. Adv. Funct. Mater. 2021, 31, 2103360. [Google Scholar] [CrossRef]
- Sun, J.; Guo, N.K.; Song, T.S.; Hao, Y.R.; Sun, J.W.; Xue, H.; Wang, Q. Revealing the interfacial electron modulation effect of CoFe alloys with CoCX encapsulated in N-doped CNTs for superior oxygen reduction. Adv. Powder Mater. 2022, 1, 100023. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, K.A.; Zhu, H.B. KOH-promoted In-situ construction of zeolitic imidazolate framework-derived CoO/Co-N-C hybrids jointly boosting oxygen reduction reaction. J. Alloys Compd. 2022, 912, 165198. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorl-Amorós, D.; Linares-Solano, A. The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon 2003, 41, 1925–1932. [Google Scholar] [CrossRef]
- Pelsa, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhub, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Wang, P.; Li, K.; Li, S.; Zhang, Z.; He, X.; Duan, Y. Co/N-codoped carbon nanotube hollow polyhedron hybrid derived from salt-encapsulated core-shell ZIF-8@ZIF-67 for efficient oxygen reduction reaction. J. Alloys Compd. 2022, 904, 164083. [Google Scholar] [CrossRef]
- He, W.H.; Jiang, C.H.; Wang, J.B.; Lu, L.H. High-rate oxygen electroreduction over graphitic-n species exposed on 3d hierarchically porous nitrogen-doped carbons. Angew. Chem. -Int. Ed. 2014, 53, 9503–9507. [Google Scholar] [CrossRef]
- Yu, Y.; You, S.J.; Du, J.N.; Xing, Z.P.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.Q.; Zou, J.L. ZIF-67-derived CoO (tetrahedral Co2+)@nitrogen-doped porous carbon protected by oxygen vacancies-enriched SnO2 as highly active catalyst for oxygen reduction and Pt co-catalyst for methanol oxidation. Appl. Catal. B-Environ. 2019, 259, 118043. [Google Scholar] [CrossRef]
- Zuo, Y.; Huang, M.; Sheng, W.; Xu, Q.; Tao, W.; Li, Z. Highly stable and methanol tolerant oxygen reduction reaction electrocatalyst Co/CoO/SnO@N-C nanocubes by one-step introduction of functional components. Int. J. Hydrog. Energy 2022, 47, 917–927. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Kou, X.; Dong, X.; Chi, X.; Ma, H.; Wang, G. High-performance oxygen reduction electrocatalysts derived from bimetal-organic framework and sulfur-doped precursors for use in microbial fuel cells. J. Power Sources 2022, 521, 230944. [Google Scholar] [CrossRef]
- Liu, Z.F.; Ye, D.D.; Zhu, X.; Wang, S.L.; Zou, Y.N.; Lan, L.H.; Chen, R.; Yang, Y.; Liao, Q. ZIF-67-derived Co nanoparticles embedded in n-doped porous carbon composite interconnected by mwcnts as highly efficient orr electrocatalysts for a flexible direct formate fuel cell. Chem. Eng. J. 2022, 432, 134192. [Google Scholar] [CrossRef]

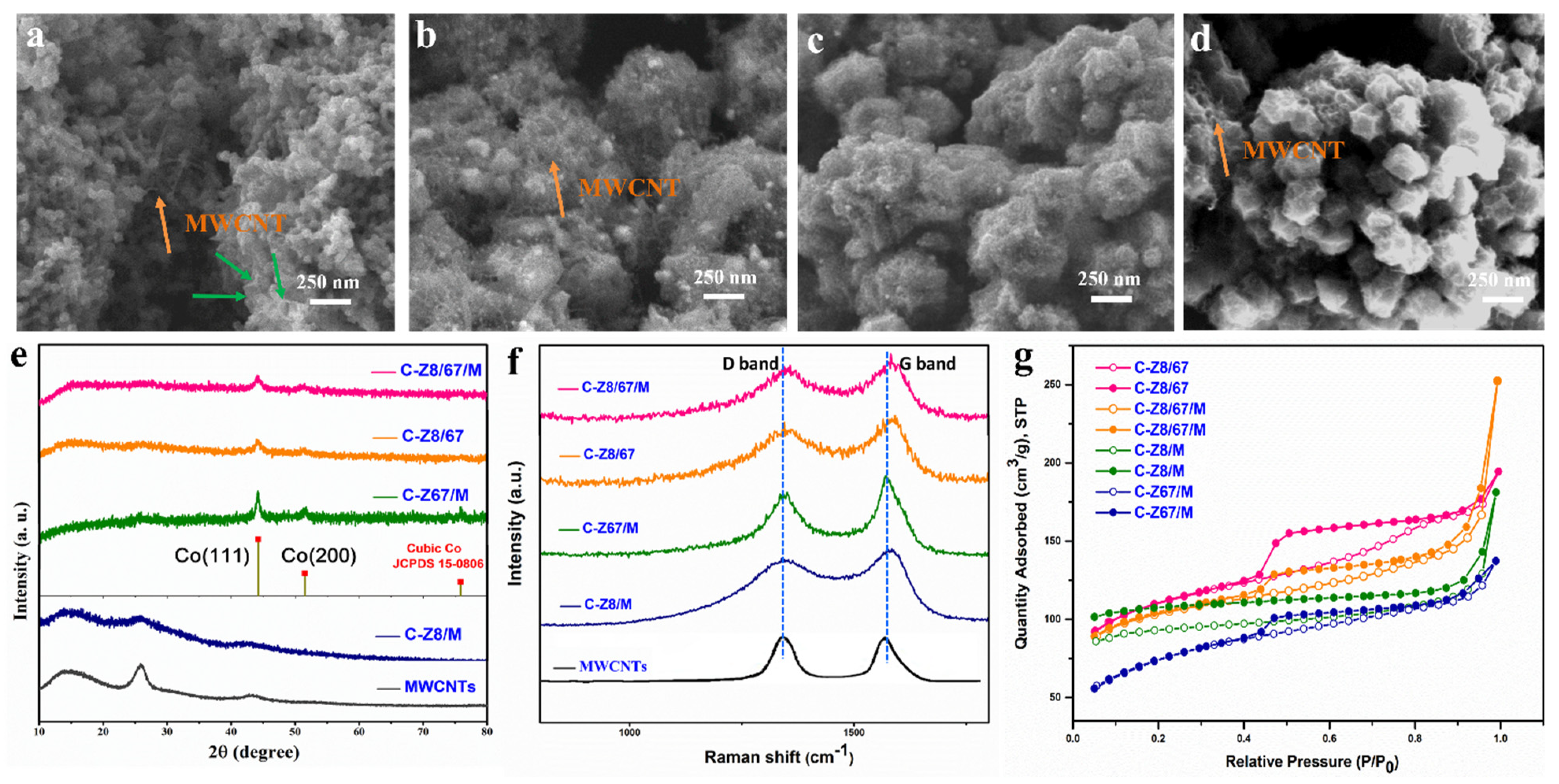
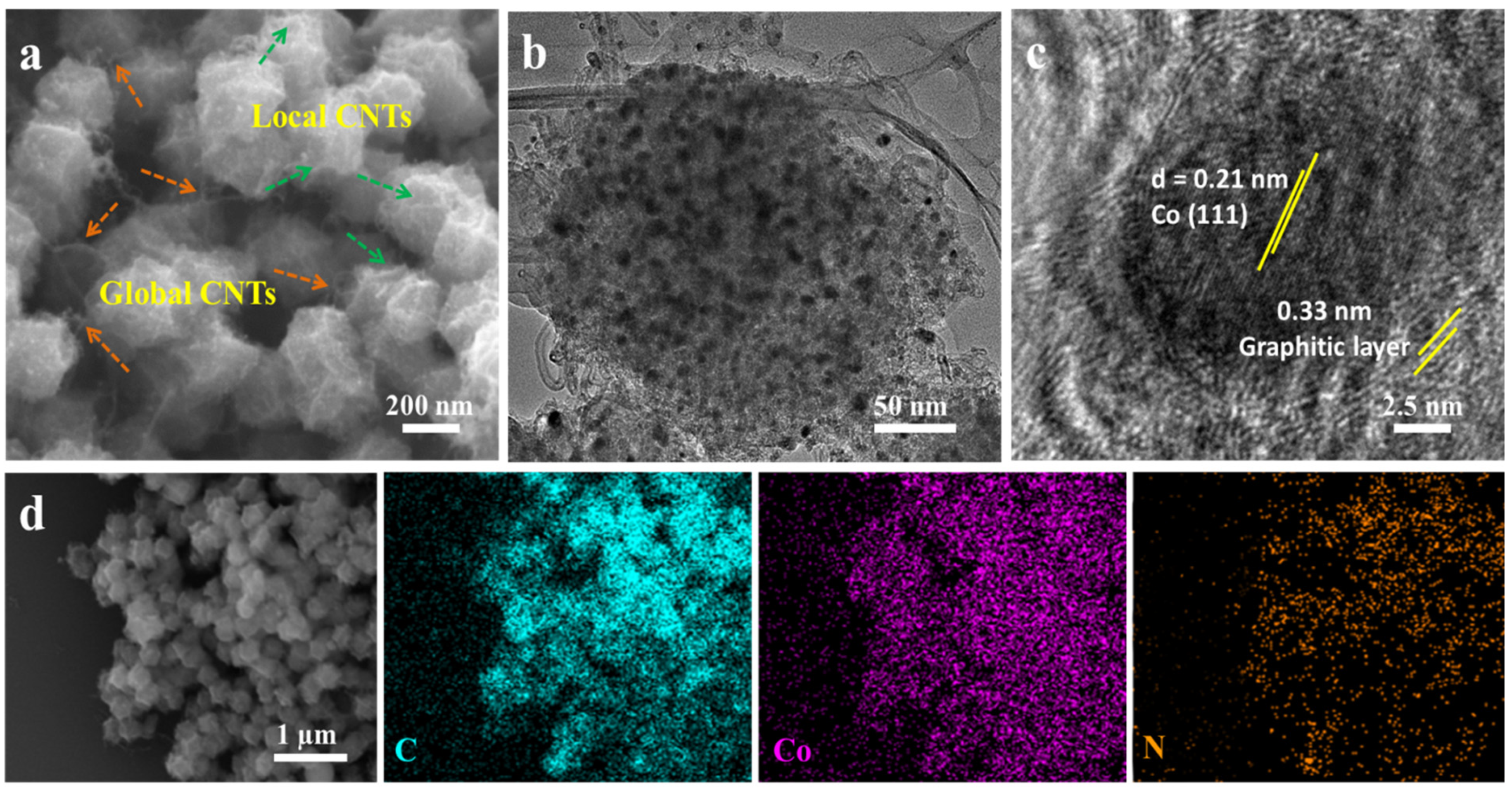
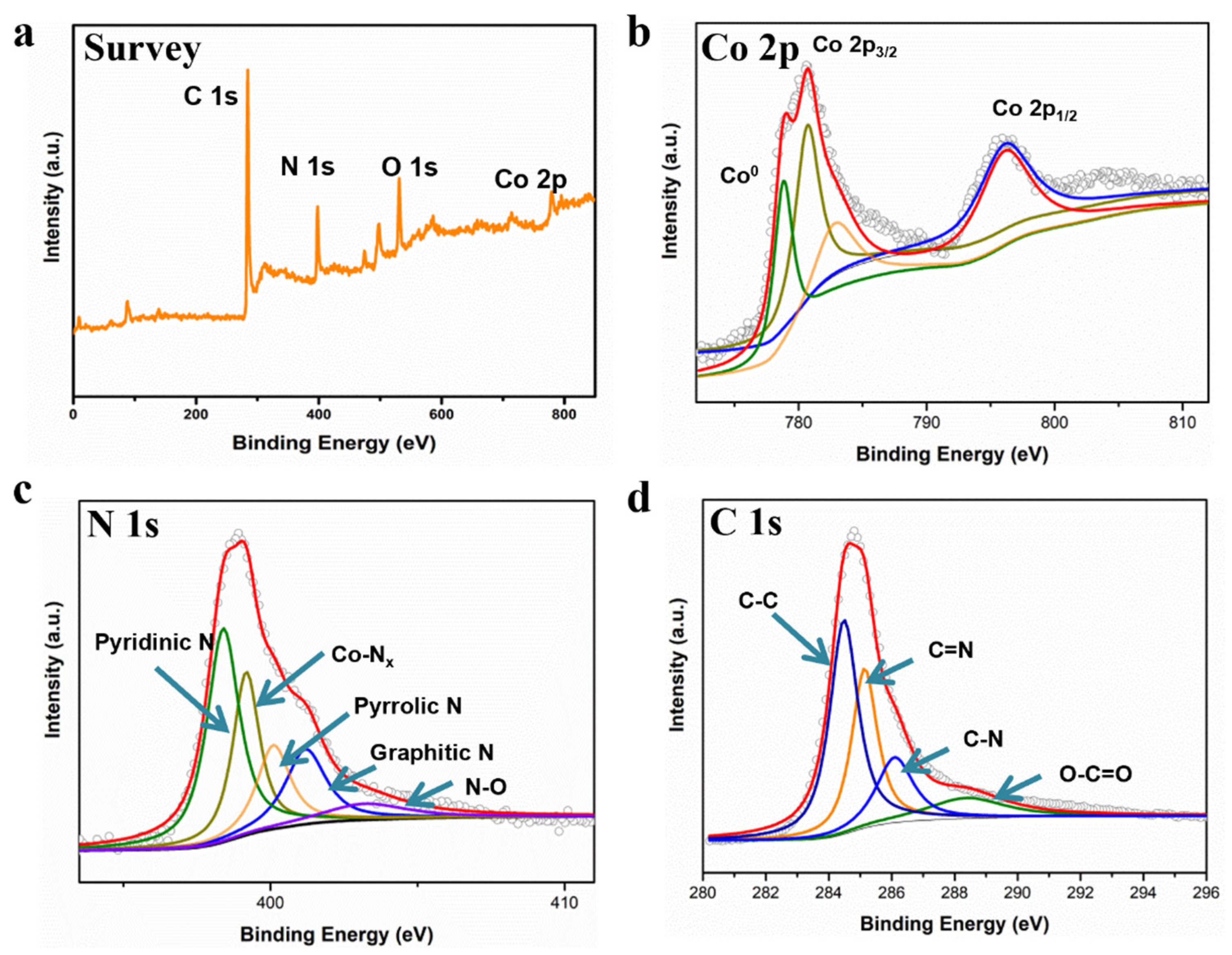
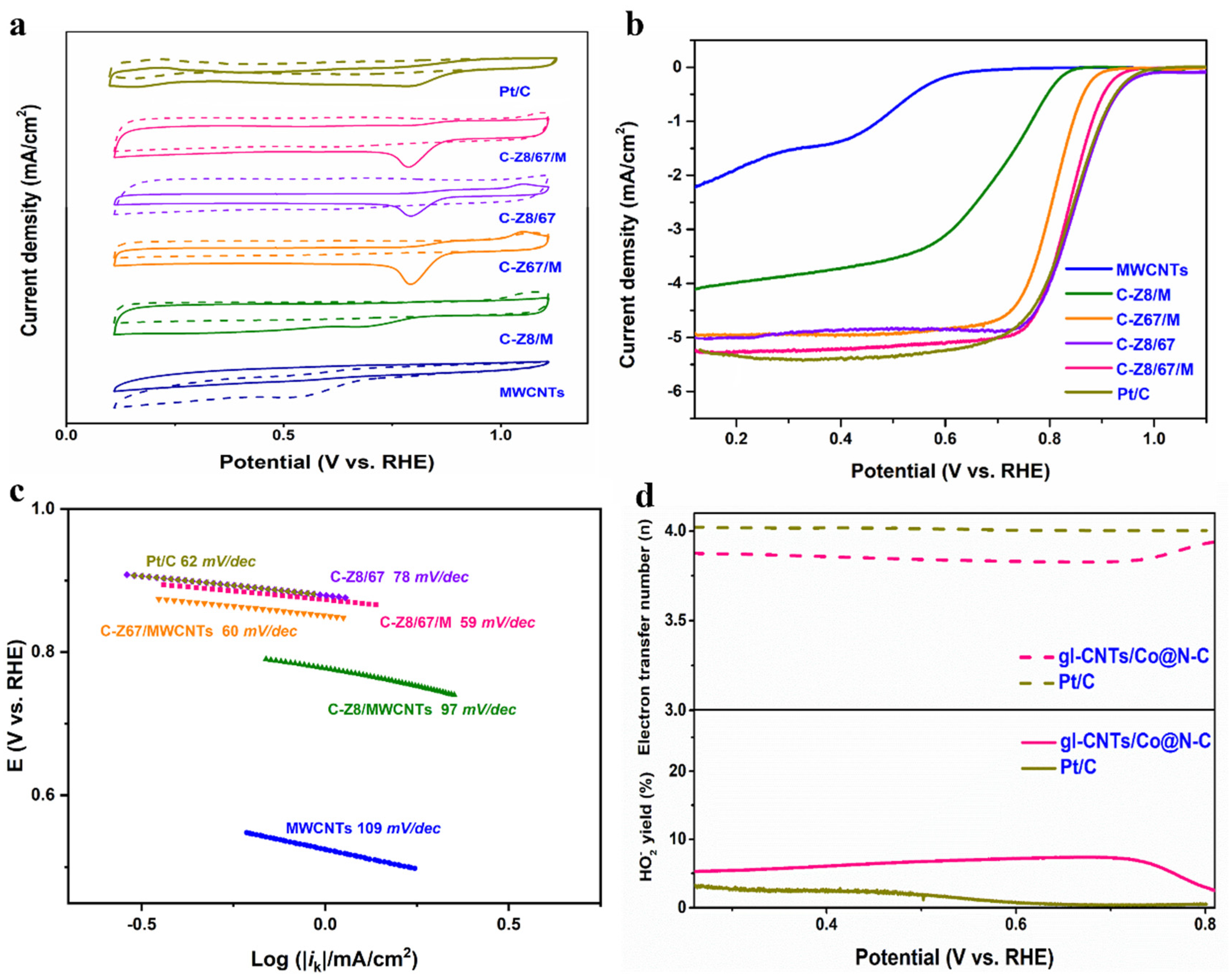

| Element | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| C-Z/8/M | 212.90 | 0.28 | 4.60 |
| C-Z67/M | 203.31 | 0.21 | 4.01 |
| C-Z8/67 | 280.72 | 0.30 | 4.08 |
| C-Z8/67/M | 275.09 | 0.39 | 5.22 |
| Catalyst | E1/2 (V) | Jd (mA cm−2) | Medium | Ref. |
|---|---|---|---|---|
| gl-CNTs/Co@N-C | 0.86 | 5.34 | 0.1 M KOH | this work |
| Co,N-C/TOCNF | 0.74 | 4.5 | 0.1 M KOH | [4] |
| CoNC-CNFs | 0.8 | 5.9 | 0.1 M KOH | [5] |
| Mn2O4/NCNTs | 0.76 | 6.06 | 0.1 M KOH | [7] |
| CNCo-20 | 0.81 | 6.0 | 0.1 M KOH | [8] |
| NC@GC | 0.89 | - | 0.1 M KOH | [20] |
| Co/N-CNTFs | 0.83 | 6 | 0.1 M KOH | [22] |
| CoNHPC | 0.87 | 5.41 | 0.1 M KOH | [31] |
| Co-N/S-C-3.5 | 0.75 | 3.7 | 0.1 M KOH | [39] |
| Co@NPC/C-MWCNTs | 0.79 | 4.32 | 0.1 M KOH | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zuo, Y.; Wang, H.; Shi, H.; Lu, S. Global-Local CNTs Conductive Network Couple with Co-Based Polyhedral Promotes the Electrocatalytic Reduction of Oxygen. Catalysts 2022, 12, 1508. https://doi.org/10.3390/catal12121508
Sun J, Zuo Y, Wang H, Shi H, Lu S. Global-Local CNTs Conductive Network Couple with Co-Based Polyhedral Promotes the Electrocatalytic Reduction of Oxygen. Catalysts. 2022; 12(12):1508. https://doi.org/10.3390/catal12121508
Chicago/Turabian StyleSun, Jinhua, Yuanhui Zuo, Hanyun Wang, Huancong Shi, and Shijian Lu. 2022. "Global-Local CNTs Conductive Network Couple with Co-Based Polyhedral Promotes the Electrocatalytic Reduction of Oxygen" Catalysts 12, no. 12: 1508. https://doi.org/10.3390/catal12121508






