Raw natural gas contains some quantities of impurities. This issue causes problems in operations containing natural gas transfer or in other processes using it as a feed [
1]. Therefore, some purification processes such as dehydration, sweetening, and other operations are used after extraction of natural gas [
2]. Processes such as dehydration are used for minimizing the amount of water in the gas to about 0.1 ppm. Hydrocarbon dew point-tuning operations act with heavy hydrocarbon removal [
3]. These operations adjust the hydrocarbon dew point in a specified point based on the gas usage in downstream processes. In this work, an adsorption method for water and heavy hydrocarbons removal from natural gas is investigated. In this regard, an industrial unit with industrial data is modeled and analyzed. The simulation results are verified and compared with process data of a refinery currently in operation. Specifically, dehydration of natural gas decreases the possibility of corrosion and hydration and freezing of the water in the pipeline. This operation eliminates the slowing down of flow arising from vapor condensation. The presence of water in the gas stream causes the following fundamental problems: 1—corrosion; 2—liquid water formation; 3—ice formation; and 4—hydration [
4]. By decreasing the temperature and pressure, the heavy hydrocarbons content of natural gas causes liquid phase formation inside the gas pipeline and consequently pressure drop occurrence. Other problems originating from these liquid gases include undesirable combustion in furnaces, turbines, and heating equipment. In addition, using such a gas as petrochemical feed causes undesirable chemical compounds. Dehydration is suggested to avoid these problems. Different methods could be used such as water condensation with cooling, dehydration with liquid absorbents, and dehydration with solid adsorbents. The last one is used significantly because of its advantages.
Mehrpoya et al. simulated mercaptan removal from natural gas by the adsorption method using five beds. Operating conditions and the amount of mercaptans in the output product were examined, and optimum conditions were found [
5]. In another study, the effect of carbon dioxide in natural gas was investigated. In that work, a 4A molecular sieve adsorbent was used, and simulation of the process was carried out by Aspen Adsim software. It was observed that increasing the concentration of carbon dioxide increases the amount of water saturation in the natural gas and also increases the amount of absorbent and raises the operating pressure up to 35 bar [
6]. Gholami and et al. [
7] studied mathematical modeling of water removal from natural gas and compared it with experimental results. In this study, the Ergun equation and the Langmuir isotherm were implemented for the pressure drop and for the adsorber equilibrium curve, respectively. It was shown that the breakthrough time curve linearly decreased with the square of the particle diameter, and also the pressure drop increased with the inverse of the particle diameter [
7]. In another study, water and heavy hydrocarbon removal was investigated by two beds including three layers filled with adsorbents. The first two layers of these three layers were filled with silica gel and 13x adsorbents, which were used to remove water. Another layer was filled with activated carbon adsorbent to remove organic compounds. By comparing the modeling of this process with the experimental results, good validation was achieved [
8]. Michal Netusil et al. [
9] compared natural gas water removal in three different ways, namely, water removal by absorption, adsorption, and condensation. The allowable amount of water in the natural gas was equivalent to a dew point of −10 degrees Celsius at 4 MPa. As a result of this comparison, the amount of energy consumption was presented in terms of vapor pressure of water in natural gas. The dehydration process with 3A adsorbent was used to adjust the dew point. In addition, the impact of operating conditions in that study was investigated. Dehydration process efficiency decreased by increasing the amount of water in the natural gas and increased by inlet pressure [
10]. In a study [
11], the value of adsorption and regeneration of water on 13x adsorbent was investigated. In that work, in the regeneration temperature of 90 °C, the adsorption capacity decreased from 23% (
w/w) to 19% (
w/w) by increasing the adsorption temperature from 35 to 60 °C. The adsorption capacity increased by the temperature of regeneration. Shirani et al. [
12] studied the adsorption of water and mercaptans by a 13x adsorbent. In that process, three beds were considered, and two of them were used in the adsorption mode. The effect of the pressure and the bed length on the breakthrough curve was investigated. In [
13], CO
2 adsorption with a polyaniline adsorber by the TSA process was investigated. In that study, a Langmuir–Freundlich isotherm for the adsorption equilibrium curve was used. The amount of carbon dioxide, according to operating conditions such as adsorption temperature, feed pressure, and desorption temperature was investigated to obtain the optimal conditions. Gandhidasan et al. [
14] studied the process of dehydration by silica gel adsorbent in two beds. The effect of different operating conditions on the process performance and energy consumption of the regeneration were considered in their work. The process of mercaptan removal from natural gas by the PVSA method and achieving an allowable amount of 10 ppm was investigated. As a result of that study, the PVSA process had better performance than PTSA. Comparing these two methods with the same operating conditions and feed, the PVSA method had a shorter cycle time and better purity in the feed [
15]. The process of mercaptan and water removal from natural gas by the PTSA method was simulated with two beds filled with 13x adsorbents and active alumina. As a result of that simulation, good validation was obtained with industrial data. By reviewing the operating conditions, the value of adsorption pressure was reduced from 6.8 bar to 6.1 bar [
16]. In [
17], a comparison of PSA and TPSA methods for carbon dioxide recovery with 13x adsorbent was carried out. In another study [
18], a comparison of the amount of carbon dioxide adsorption by molecular adsorbents and 13x at different operating pressures was investigated.
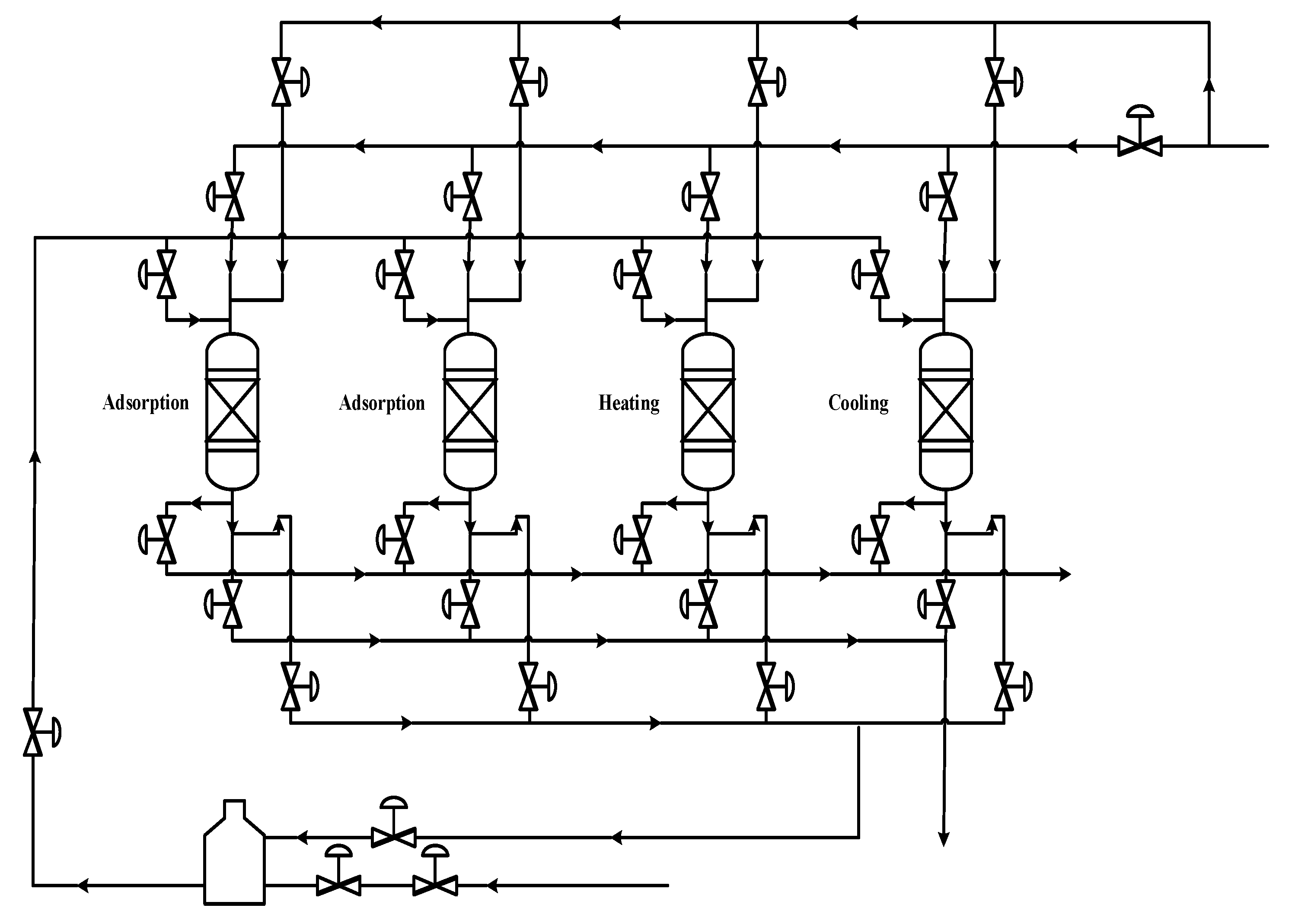
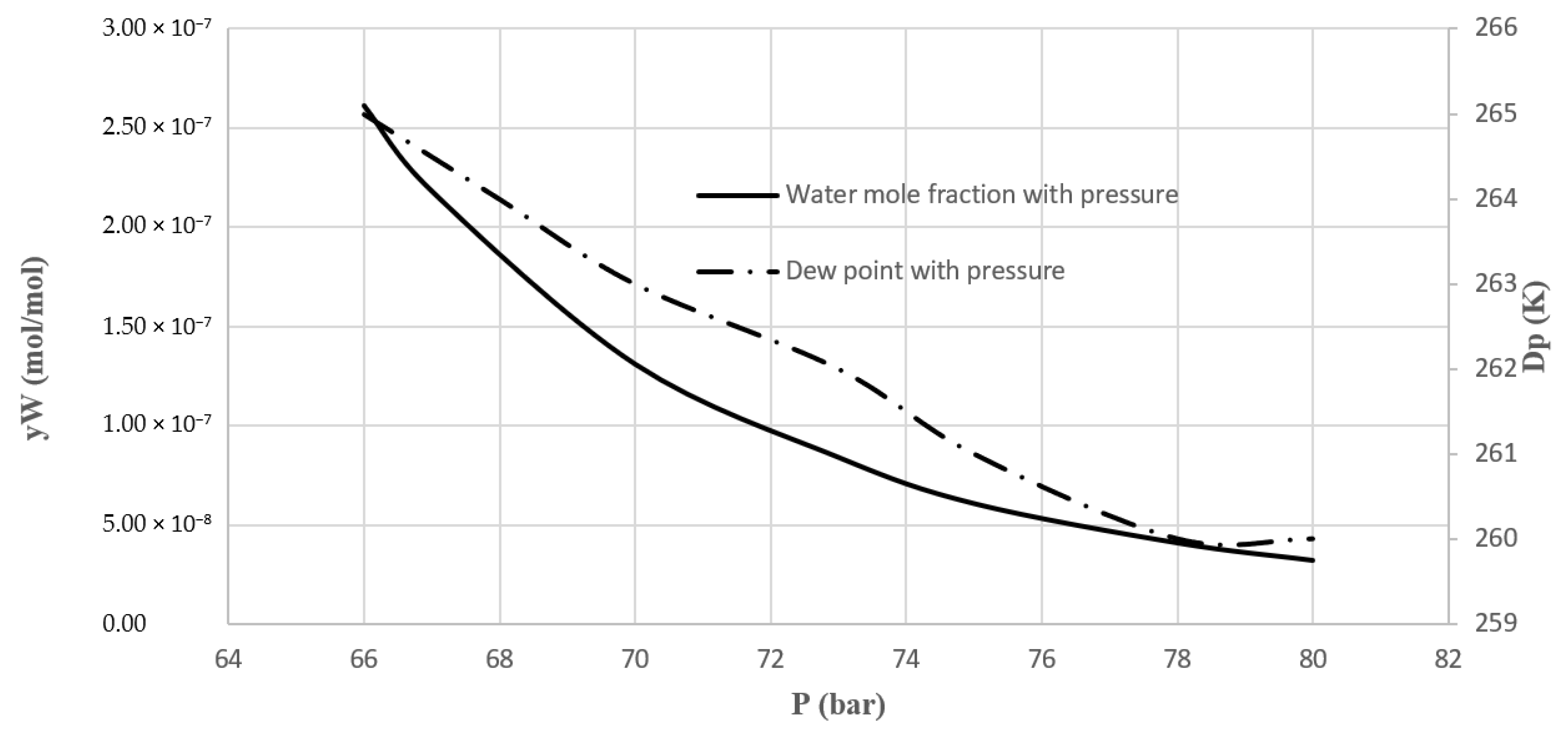
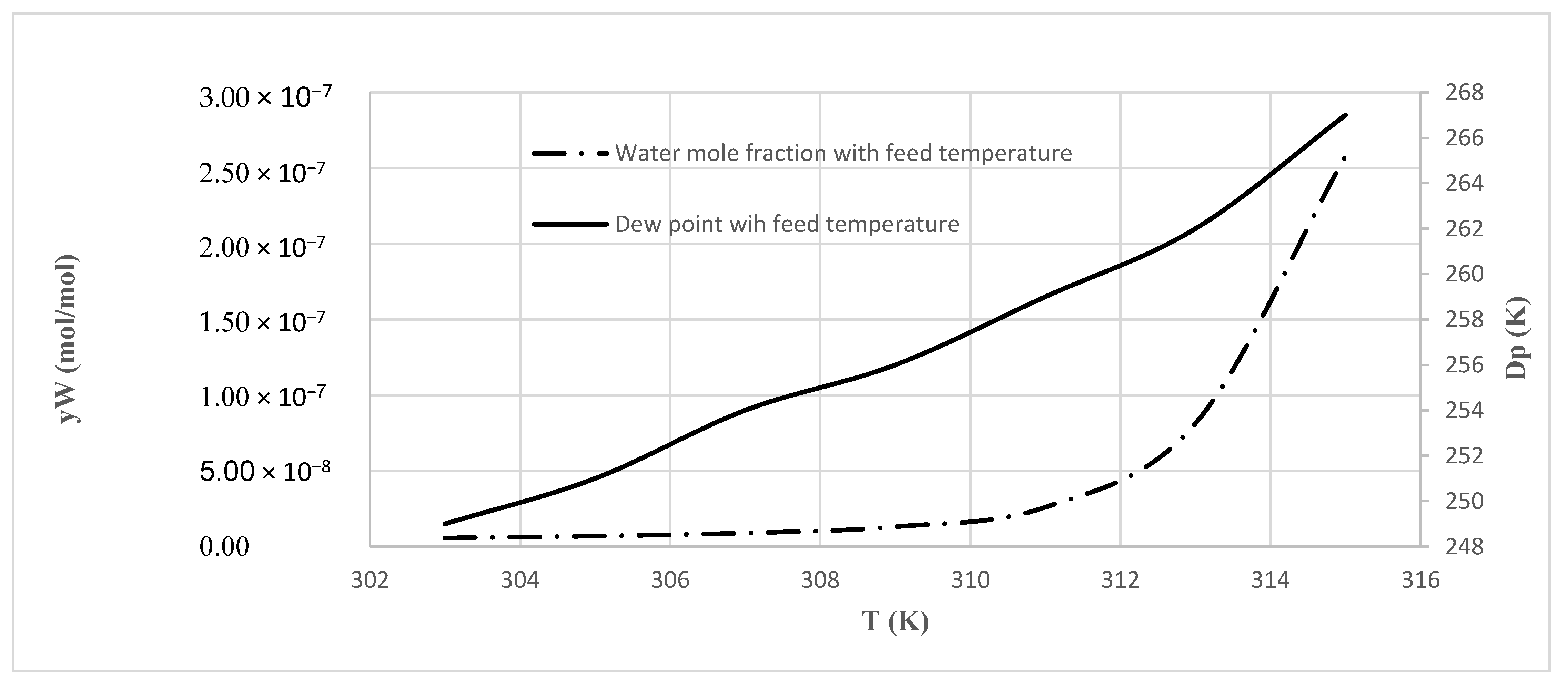
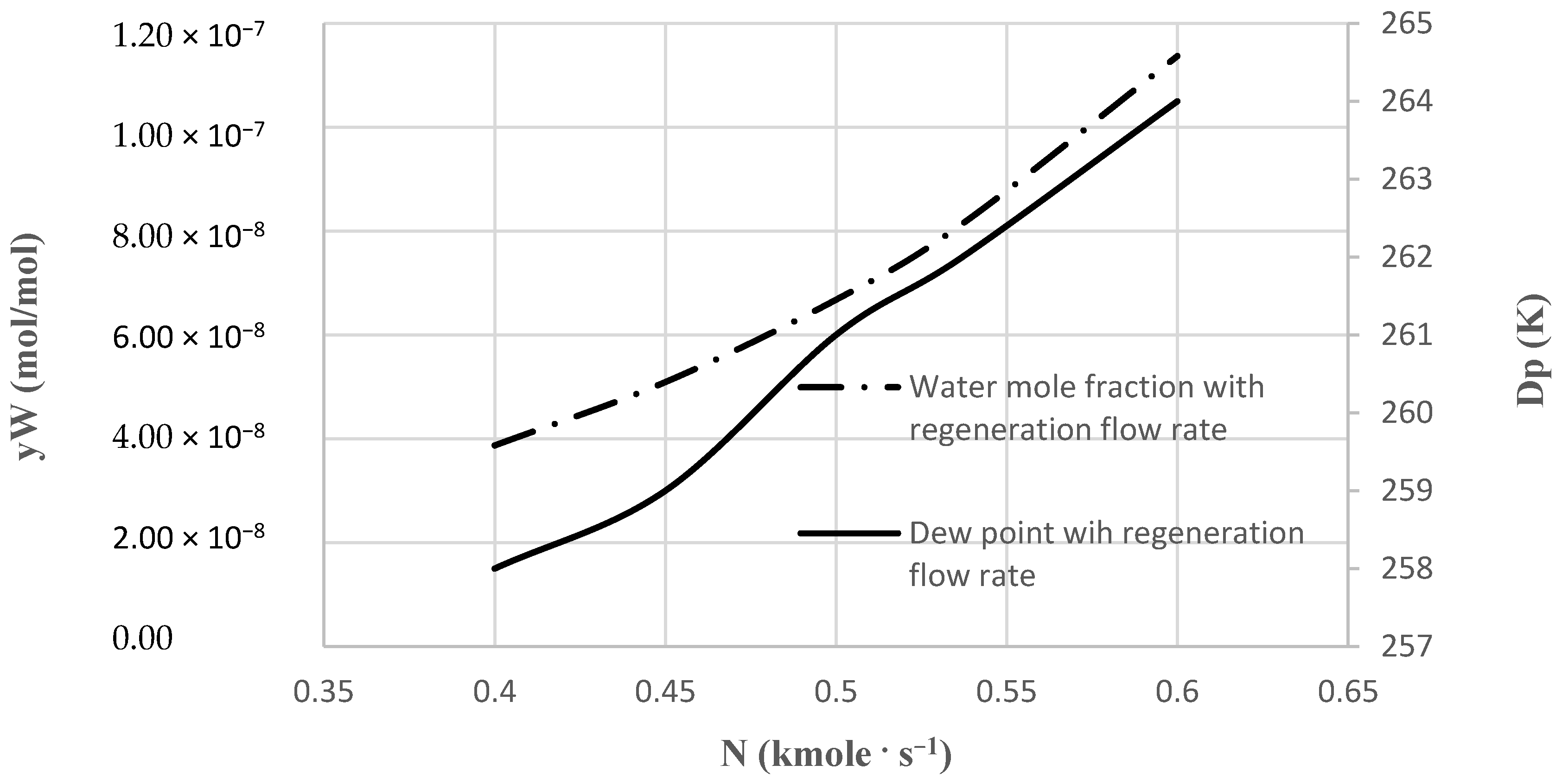
In this work, an industrial natural gas purification process, which is currently in operation, was considered and modeled. Simulation and determination of the optimal design of water and heavy hydrocarbons removal from natural gas by TSA method were conducted. A model was trained exactly based on the industrial data, including the inlet feed composition, the operating conditions, the adsorption kinetics of the adsorbers used, and the process configuration and equipment. The model outputs were compared with industrial data. The process performance through varying operating parameters was analyzed. Operating conditions such as temperature and pressure were investigated in different situations. Sensitivity analysis was conducted, and optimum conditions were investigated based on the developed model. In addition, this model can be used for optimization and retrofit design projects.