A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production
Abstract
:1. Introduction
1.1. Homogeneous Catalysis
1.2. Heterogeneous Catalysis
1.3. Enzymatic Catalysis
1.4. Non-Catalyzed Process
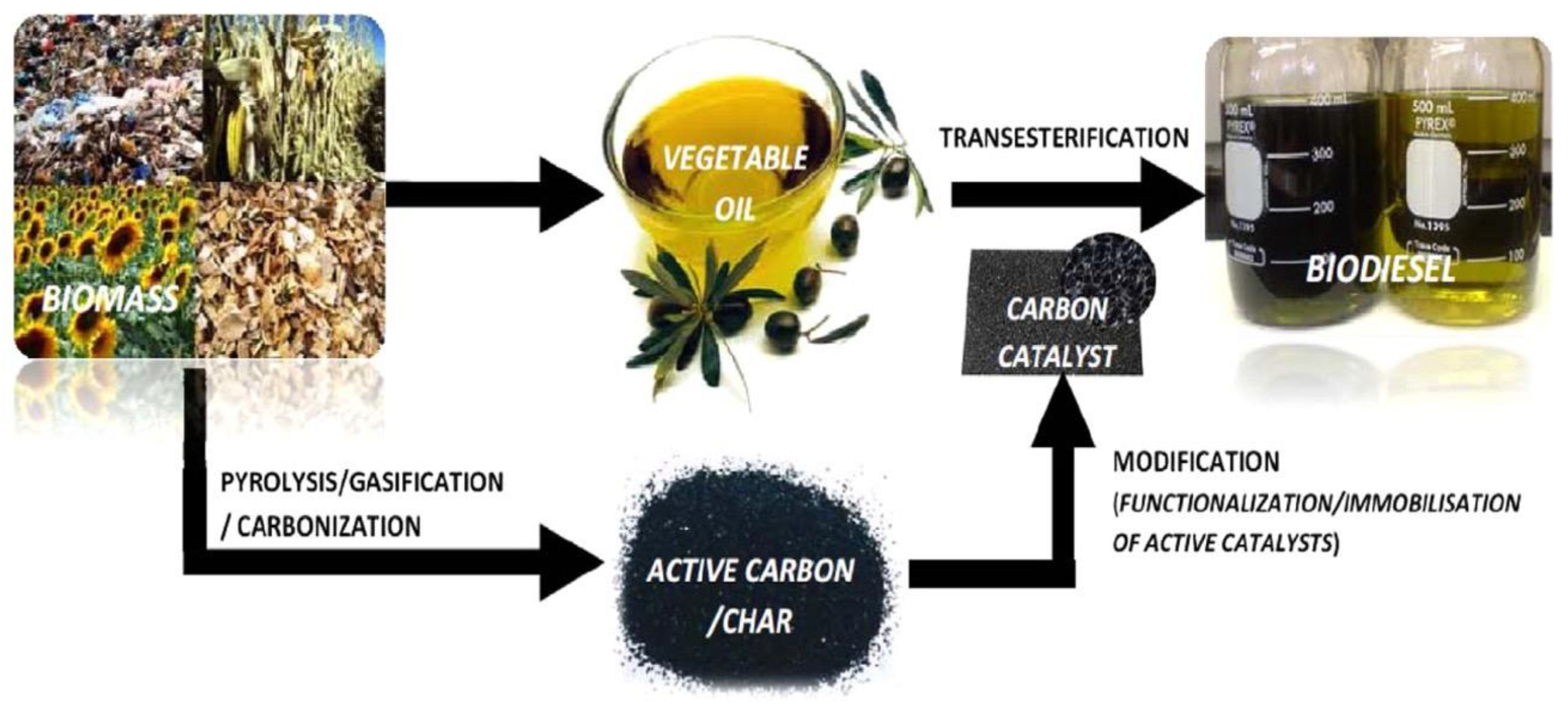
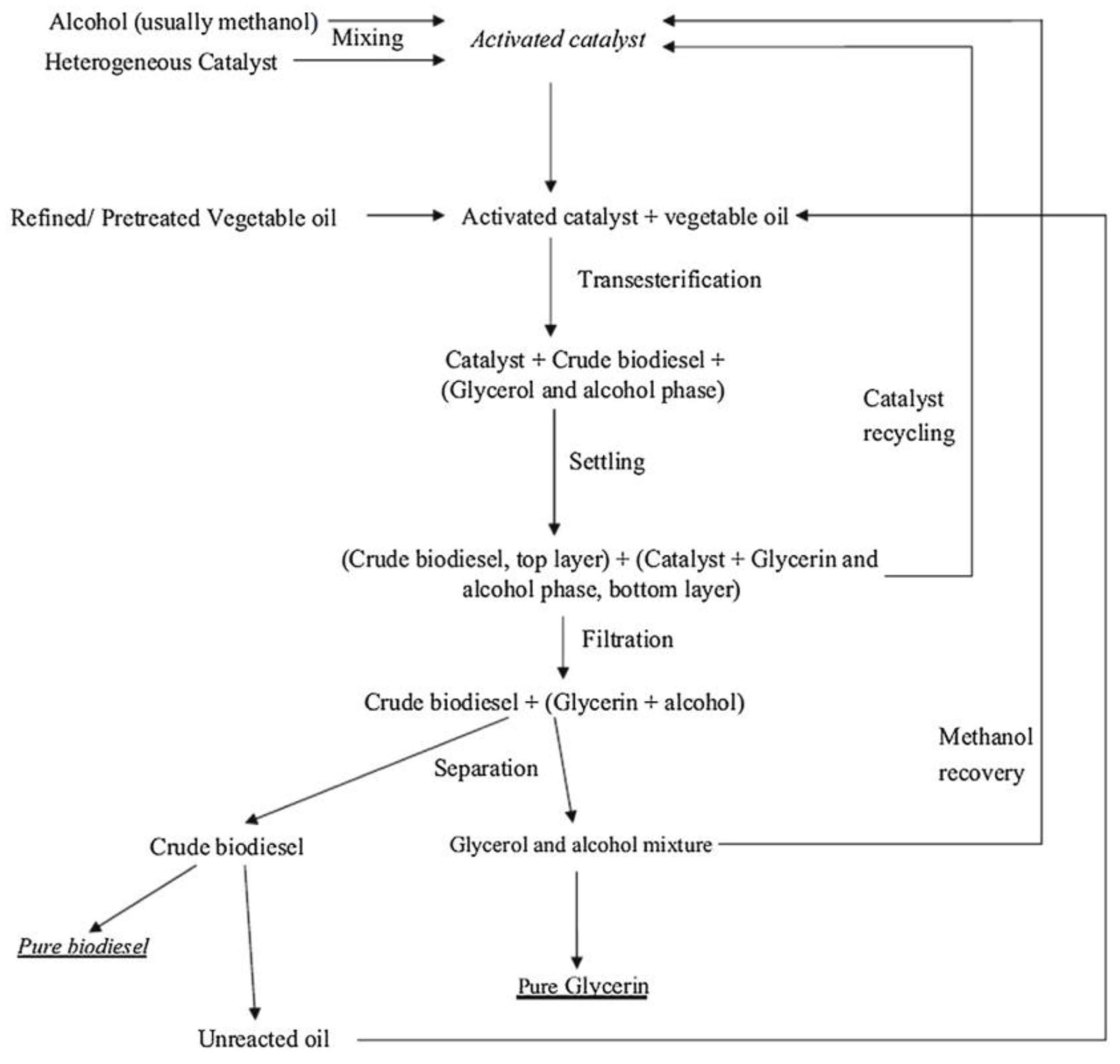
2. Common Methodology for the Synthesis of Heterogeneous Catalyst
2.1. Hydrothermal
2.2. Carbonization
2.3. Calcination
2.4. Pyrolysis
2.5. Impregnation
2.6. Sol-Gel
2.7. Co-Solvent Preparation
3. Biodiesel Production Using Heterogeneous Catalysts
3.1. Edible Plant Oils
3.2. Non-Edible Plant Oils
3.3. Waste Cooking Oil
3.4. Animal Fats
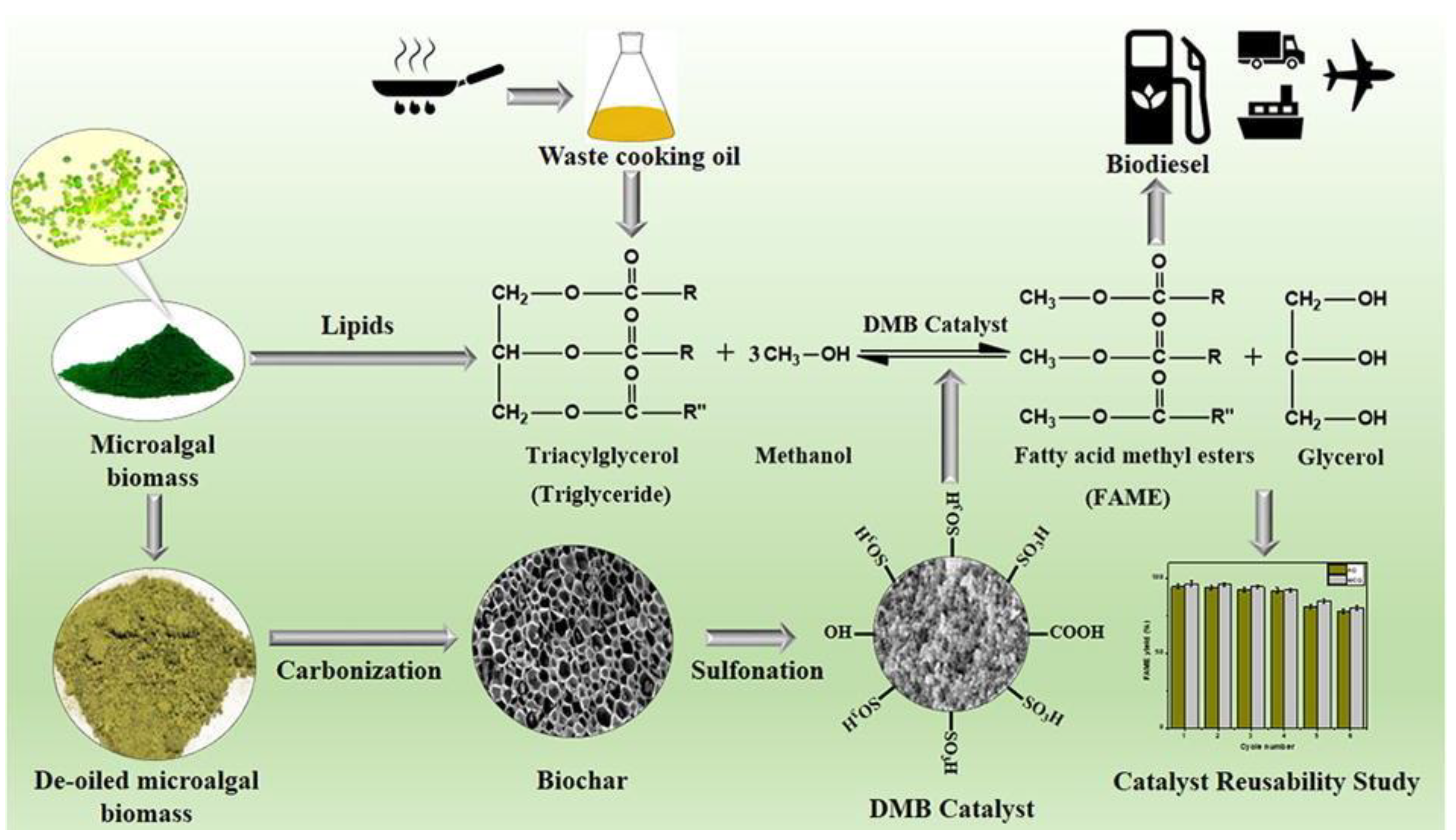
3.5. Algal Oil
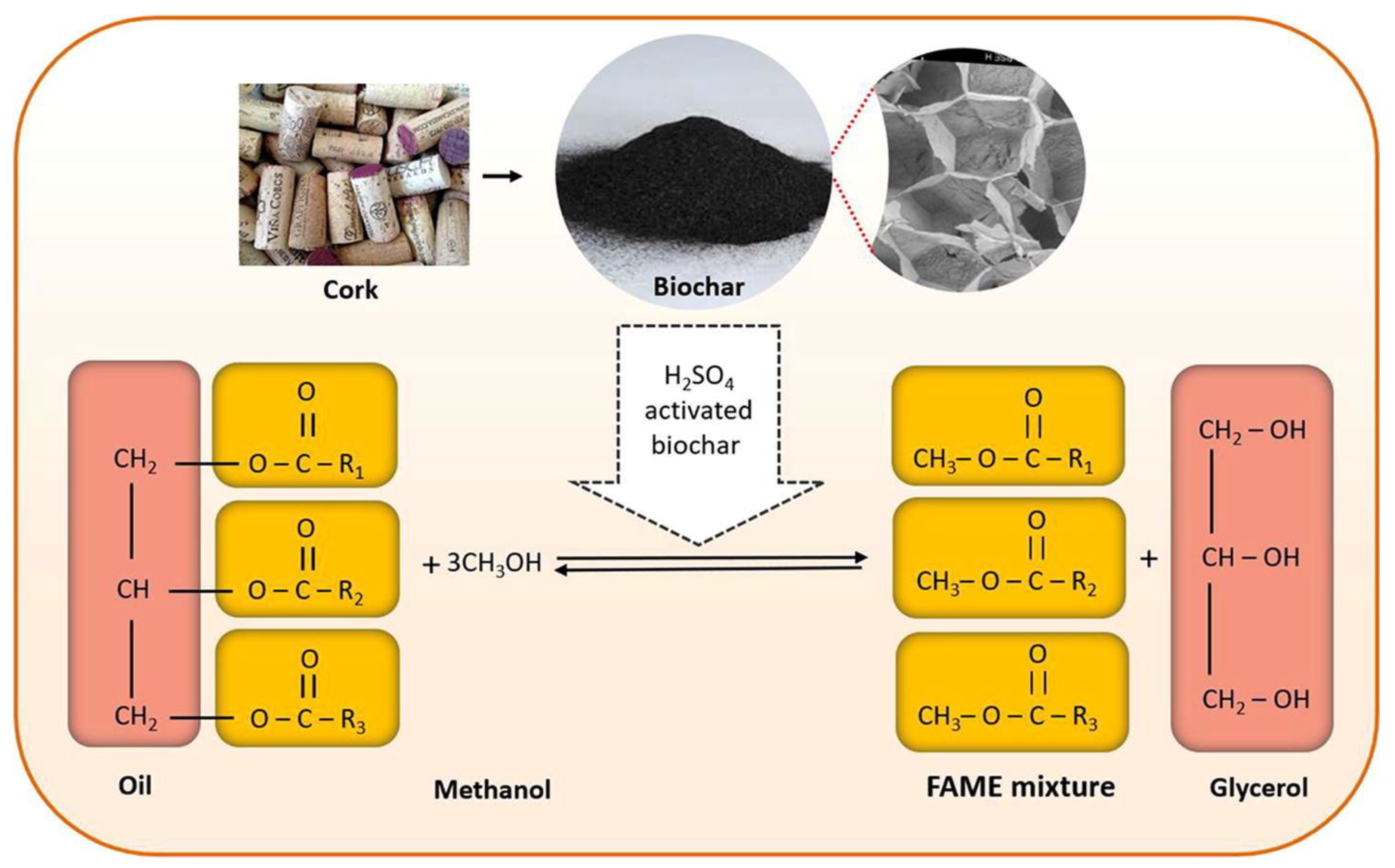
3.6. Biodiesel Production for Biomass-Derived Heterogeneous Catalysts
4. Optimization of Reaction Parameters for Biodiesel Production Using Heterogeneous Catalysts
5. Summary and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef] [PubMed]
- Amjith, L.; Bavanish, B. A review on biomass and wind as renewable energy for sustainable environment. Chemosphere 2022, 293, 133579. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts-A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Rathore, S.S.; Singh, R.; Kumar, S.; Singh, V.K.; Yadav, S.; Yadav, V.; Raj, R.; Yadav, D.; Shekhawat, K. Exploring agricultural waste biomass for energy, food and feed production and pollution mitigation: A review. Bioresour. Technol. 2022, 360, 127566. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Effect of sewage sludge-based fertilizers on biomass growth and heavy metal accumulation in plants. J. Environ. Manag. 2022, 305, 114417. [Google Scholar] [CrossRef]
- Agathokleous, E.; Guo, J.; Peñuelas, J. Low doses of toxicants can enhance algae potential as biodiesel and biomass feedstocks. Renew. Sustain. Energy Rev. 2022, 168, 112858. [Google Scholar] [CrossRef]
- Aggarwal, N.K.; Kumar, N.; Mittal, M. Potential of Weed Biomass for Bioethanol Production. In Bioethanol Production; Springer: Berlin/Heidelberg, Germany, 2022; pp. 65–71. [Google Scholar]
- Rashedi, A.; Gul, N.; Hussain, M.; Hadi, R.; Khan, N.; Nadeem, S.G.; Khanam, T.; Asyraf, M.; Kumar, V. Life cycle environmental sustainability and cumulative energy assessment of biomass pellets biofuel derived from agroforest residues. PLoS ONE 2022, 17, e0275005. [Google Scholar] [CrossRef]
- Beidaghy Dizaji, H.; Zeng, T.; Lenz, V.; Enke, D. Valorization of Residues from Energy Conversion of Biomass for Advanced and Sustainable Material Applications. Sustainability 2022, 14, 4939. [Google Scholar] [CrossRef]
- Pang, S.; Yip, A.; Zhao, P. Waste Wood Processing Technologies Review; Envirolink: Māpua, New Zealand, 2022. [Google Scholar]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Borges, M.; Díaz, L.; Gavín, J.; Brito, A. Estimation of the content of fatty acid methyl esters (FAME) in biodiesel samples from dynamic viscosity measurements. Fuel Process. Technol. 2011, 92, 597–599. [Google Scholar] [CrossRef]
- Lukić, I.; Kesić, Ž.; Skala, D. Kinetics of heterogeneous biodiesel synthesis using supported ZnO as catalyst. Chem. Eng. Technol. 2014, 37, 1879–1884. [Google Scholar] [CrossRef]
- Jokiniemi, T.; Ahokas, J. A review of production and use of first generation biodiesel in agriculture. Agron. Res. 2013, 11, 239–248. [Google Scholar]
- Bhuiya, M.; Rasul, M.; Khan, M.M.K.; Ashwath, N.; Azad, A.K.; Hazrat, M. Second generation biodiesel: Potential alternative to-edible oil-derived biodiesel. Energy Procedia 2014, 61, 1969–1972. [Google Scholar] [CrossRef] [Green Version]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic applications in the production of biodiesel from vegetable oils. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 278–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; McLaren, J.; Madl, R.; Wang, D. Biofuels from lignocellulosic biomass. In Sustainable Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–41. [Google Scholar]
- Kumar, S.; Singhal, M.K.; Sharma, M.P. Utilization of mixed oils for biodiesel preparation: A review. Energy Sources Part A Recovery Util. Environ. Eff. 2021. [Google Scholar] [CrossRef]
- Kumar, H.; Sarma, A.; Kumar, P. A comprehensive review on preparation, characterization, and combustion characteristics of microemulsion based hybrid biofuels. Renew. Sustain. Energy Rev. 2020, 117, 109498. [Google Scholar] [CrossRef]
- Prado, C.M.; Antoniosi Filho, N.R. Production and characterization of the biofuels obtained by thermal cracking and thermal catalytic cracking of vegetable oils. J. Anal. Appl. Pyrolysis 2009, 86, 338–347. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Mansir, N.; Shamsuddin, M.R.; Joseph, C.G.; Goto, M.; Taufiq-Yap, Y.H. Sustainable biofuel production approach: Critical methanol green transesterification by efficient and stable heterogeneous catalyst. Renew. Sustain. Energy Rev. 2022, 169, 112889. [Google Scholar] [CrossRef]
- Aslam, M. Transformation of 1-G and 2-G liquid biomass to green fuels using hydroprocessing technology: A promising technology for biorefinery development. Biomass Bioenergy 2022, 163, 106510. [Google Scholar] [CrossRef]
- Kalita, P.; Basumatary, B.; Saikia, P.; Das, B.; Basumatary, S. Biodiesel as renewable biofuel produced via enzyme-based catalyzed transesterification. Energy Nexus 2022, 6, 100087. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Razali, N.; Mootabadi, H.; Salamatinia, B. Critical technical areas for future improvement in biodiesel technologies. Environ. Res. Lett. 2007, 2, 034001. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Okechukwu, O.D.; Joseph, E.; Nonso, U.C.; Kenechi, N.-O. Improving heterogeneous catalysis for biodiesel production process. Clean. Chem. Eng. 2022, 3, 100038. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. BioEnergy Res. 2021, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M.B.; Akhtar, M.N.; Iqbal, H.M. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials–A review. Fuel 2022, 328, 125254. [Google Scholar] [CrossRef]
- Shende, V.S.; Saptal, V.B.; Bhanage, B.M. Recent advances utilized in the recycling of homogeneous catalysis. Chem. Rec. 2019, 19, 2022–2043. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of 12-tungestophosphoric acid supported on Zr/MCM-41 composite with excellent heterogeneous catalyst and promising adsorbent of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127753. [Google Scholar] [CrossRef]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Bakry, A.M.; Alamier, W.M.; Salama, R.S.; El-Shall, M.S.; Awad, F.S. Remediation of water containing phosphate using ceria nanoparticles decorated partially reduced graphene oxide (CeO2-PRGO) composite. Surf. Interfaces 2022, 31, 102006. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.A.; Soudagar, M.E.M.; Fattah, I.R. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Abbas, M.M.; Veza, I.; Soudagar, M.E.M.; Abdelrahman, A.; Kalam, M.A. Heterogeneous Catalyzed Biodiesel Production Using Cosolvent: A Mini Review. Sustainability 2022, 14, 5062. [Google Scholar] [CrossRef]
- Ferreira, R.S.B.; dos Passos, R.M.; Sampaio, K.A.; Batista, E. Heterogeneous catalysts for biodiesel production: A review. Food Public Health 2019, 9, 125–137. [Google Scholar]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel production through lipase catalyzed transesterification: An overview. J. Mol. Catal. B Enzym. 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Majidian, P.; Tabatabaei, M.; Zeinolabedini, M.; Naghshbandi, M.P.; Chisti, Y. Metabolic engineering of microorganisms for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 3863–3885. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Stojković, I.J.; Stamenković, O.S.; Povrenović, D.S.; Veljković, V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Teo, S.H.; Taufiq-Yap, Y.H.; Rashid, U.; Islam, A. Hydrothermal effect on synthesis, characterization and catalytic properties of calcium methoxide for biodiesel production from crude Jatropha curcas. RSC Adv. 2015, 5, 4266–4276. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G.; Onida, B.; Cocina, D.; Tagarelli, A.; Giuffrè, A.M. Biodiesel production process by homogeneous/heterogeneous catalytic system using an acid–base catalyst. Appl. Catal. A Gen. 2010, 378, 160–168. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Choong, T.S.Y.; Asim, N.; Zhao, Y. Microwave-assisted hydrothermal synthesis of sulfonated TiO2-GO core–shell solid spheres as heterogeneous esterification mesoporous catalyst for biodiesel production. Energy Convers. Manag. 2021, 238, 114165. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Hazmi, B.; Ibrahim, M.L.; Tsubota, T.; Alharthi, F.A. Potential heterogeneous nano-catalyst via integrating hydrothermal carbonization for biodiesel production using waste cooking oil. Chemosphere 2022, 286, 131913. [Google Scholar] [CrossRef]
- Cardoso, R.K.; Silva, G.V.; Alves, B.T.; Freire, V.A.; Alves, J.J.; Barbosa, B.V. Evaluation of the effect of Si/Mo and oil/alcohol ratios in the production of biodiesel from soybean oil. Arab. J. Chem. 2022, 15, 104074. [Google Scholar] [CrossRef]
- Atelge, M. Production of biodiesel and hydrogen by using a double-function heterogeneous catalyst derived from spent coffee grounds and its thermodynamic analysis. Renew. Energy 2022, 198, 1–15. [Google Scholar] [CrossRef]
- Fernández, J.V.; Faria, D.N.; Santoro, M.C.; Mantovaneli, R.; Cipriano, D.F.; Brito, G.M.; Carneiro, M.T.W.; Schettino, M.A.; Gonzalez, J.L.; Freitas, J.C. Use of Unmodified Coffee Husk Biochar and Ashes as Heterogeneous Catalysts in Biodiesel Synthesis. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, A.; Zheng, X. Shrimp shell catalyst for biodiesel production. Energy Fuels 2009, 23, 3859–3865. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gebeyehu, K.B.; Selvakumar, K.V.; Parvathy, S.; Kim, W.; Karmegam, N. Waste Ox bone based heterogeneous catalyst synthesis, characterization, utilization and reaction kinetics of biodiesel generation from Jatropha curcas oil. Chemosphere 2022, 288, 132534. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Bepari, S.; Banerjee, A. Application of calcined waste fish (Labeo rohita) scale as low-cost heterogeneous catalyst for biodiesel synthesis. Bioresour. Technol. 2011, 102, 3610–3618. [Google Scholar] [CrossRef]
- Nair, P.; Singh, B.; Upadhyay, S.; Sharma, Y. Synthesis of biodiesel from low FFA waste frying oil using calcium oxide derived from Mereterix mereterix as a heterogeneous catalyst. J. Clean. Prod. 2012, 29, 82–90. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Kawi, S.; Mokhtar, W.N.A.W.; Yaw, T.C.S.; Moser, B.R.; Alsalme, A. Palm fatty acid distillate esterification using synthesized heterogeneous sulfonated carbon catalyst from plastic waste: Characterization, catalytic efficacy and stability, and fuel properties. Process Saf. Environ. Prot. 2022, 162, 1139–1151. [Google Scholar] [CrossRef]
- Mahesh, S.E.; Ramanathan, A.; Begum, K.M.S.; Narayanan, A. Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energy Convers. Manag. 2015, 91, 442–450. [Google Scholar] [CrossRef]
- Alsharifi, M.; Znad, H.; Hena, S.; Ang, M. Biodiesel production from canola oil using novel Li/TiO2 as a heterogeneous catalyst prepared via impregnation method. Renew. Energy 2017, 114, 1077–1089. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Gao, D.; Yang, J.; Zeng, Y.-N.; Li, J.-G.; Wang, Y.-J.; Wang, X.-M.; Wang, F.-P.; Yu, Q.; Liu, T.-J. Highly stable heterogeneous catalysts from electric furnace dust for biodiesel production: Optimization, performance and reaction kinetics. Catal. Today 2022, 404, 78–92. [Google Scholar] [CrossRef]
- Shimada, G.B.; Cestari, A. Synthesis of heterogeneous catalysts by the hydrolytic Sol-Gel method for the biodiesel production. Renew. Energy 2020, 156, 389–394. [Google Scholar] [CrossRef]
- Salinas, D.; Sepúlveda, C.; Escalona, N.; Gfierro, J.; Pecchi, G. Sol-gel La2O3-ZrO2 mixed oxide catalysts for biodiesel production. J. Energy Chem. 2018, 27, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Maleki, B.; Talesh, S.S.A. Optimization of ZnO incorporation to αFe2O3 nanoparticles as an efficient catalyst for biodiesel production in a sonoreactor: Application on the CI engine. Renew. Energy 2022, 182, 43–59. [Google Scholar] [CrossRef]
- Pham, T.; Nguyen, U.; Imamura, K.; Furuta, M.; Maeda, Y. Transesterification using isopropanol as a co-solvent for the production of green biodiesel fuel. Int. J. Energy Res. 2022, 46, 4352–4361. [Google Scholar] [CrossRef]
- Okitsu, K.; Sadanaga, Y.; Takenaka, N.; Maeda, Y.; Bandow, H. A new co-solvent method for the green production of biodiesel fuel–Optimization and practical application. Fuel 2013, 103, 742–748. [Google Scholar]
- Akkarawatkhoosith, N.; Kaewchada, A.; Jaree, A. Simultaneous development of biodiesel synthesis and fuel quality via continuous supercritical process with reactive co-solvent. Fuel 2019, 237, 117–125. [Google Scholar] [CrossRef]
- Bambase, M.E., Jr.; Almazan, R.A.R.; Demafelis, R.B.; Sobremisana, M.J.; Dizon, L.S.H. Biodiesel production from refined coconut oil using hydroxide-impregnated calcium oxide by cosolvent method. Renew. Energy 2021, 163, 571–578. [Google Scholar] [CrossRef]
- Szkudlarek, Ł.; Chałupka, K.; Maniukiewicz, W.; Albińska, J.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. The Influence of Si/Al Ratio on the Physicochemical and Catalytic Properties of MgO/ZSM-5 Catalyst in Transesterification Reaction of Rapeseed Oil. Catalysts 2021, 11, 1260. [Google Scholar] [CrossRef]
- Islam, M.G.U.; Jan, M.T.; Farooq, M.; Naeem, A.; Khan, I.W.; Khattak, H.U. Biodiesel production from wild olive oil using TPA decorated Cr-Al acid heterogeneous catalyst. Chem. Eng. Res. Des. 2022, 178, 540–549. [Google Scholar] [CrossRef]
- Pratika, R.A.; Wijaya, K.; Trisunaryanti, W. Hydrothermal treatment of SO4/TiO2 and TiO2/CaO as heterogeneous catalysts for the conversion of Jatropha oil into biodiesel. J. Environ. Chem. Eng. 2021, 9, 106547. [Google Scholar] [CrossRef]
- Pandiangan, K.D.; Simanjuntak, W.; Jamarun, N.; Arief, S. The use of MgO/SiO2 as catalyst for transesterification of rubber seed oil with different alcohols. J. Phys. Conf. Ser. 2021, 1751, 012100. [Google Scholar] [CrossRef]
- Khan, S.G.; Hassan, M.; Anwar, M.; Khan, U.M.; Zhao, C. Mussel shell based CaO nano-catalyst doped with praseodymium to enhance biodiesel production from castor oil. Fuel 2022, 330, 125480. [Google Scholar] [CrossRef]
- Rezania, S.; Korrani, Z.S.; Gabris, M.A.; Cho, J.; Yadav, K.K.; Cabral-Pinto, M.M.; Alam, J.; Ahamed, M.; Nodeh, H.R. Lanthanum phosphate foam as novel heterogeneous nanocatalyst for biodiesel production from waste cooking oil. Renew. Energy 2021, 176, 228–236. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Gouran, A.; Razmehgir, M.H. Transesterification of waste cooking oil using Clay/CaO as a solid base catalyst. Energy 2022, 242, 122536. [Google Scholar] [CrossRef]
- Kataria, J.; Mohapatra, S.; Kundu, K. Biodiesel production from waste cooking oil using heterogeneous catalysts and its operational characteristics on variable compression ratio CI engine. J. Energy Inst. 2019, 92, 275–287. [Google Scholar] [CrossRef]
- Mutreja, V.; Singh, S.; Ali, A. Biodiesel from mutton fat using KOH impregnated MgO as heterogeneous catalysts. Renew. Energy 2011, 36, 2253–2258. [Google Scholar] [CrossRef]
- Cherian, E.; Yazhini, D.; Victor, M.; Baskar, G. Production of biodiesel from pork fat using alumina-doped calcium oxide nanocomposite as heterogeneous catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 1386–1395. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Narula, V.; Khan, M.F.; Negi, A.; Kalra, S.; Thakur, A.; Jain, S. Low temperature optimization of biodiesel production from algal oil using CaO and CaO/Al2O3 as catalyst by the application of response surface methodology. Energy 2017, 140, 879–884. [Google Scholar] [CrossRef]
- Abdala, E.; Nur, O.; Mustafa, M.A. Efficient biodiesel production from algae oil using Ca-doped ZnO nanocatalyst. Ind. Eng. Chem. Res. 2020, 59, 19235–19243. [Google Scholar] [CrossRef]
- Malpani, M.; Varma, A.; Mondal, P. Production of bio-oil from algal biomass and its upgradation to biodiesel using CaO-based heterogeneous catalysts. Int. J. Green Energy 2016, 13, 969–976. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. Valorization of de-oiled microalgal biomass as a carbon-based heterogeneous catalyst for a sustainable biodiesel production. Bioresour. Technol. 2021, 337, 125424. [Google Scholar] [CrossRef]
- Yani, F.; Ulhaqi, R.; Pratiwi, W.; Pontas, K.; Husin, H. Utilization of water hyacinth-based biomass as a potential heterogeneous catalyst for biodiesel production. J. Phys. Conf. Ser. 2019, 1402, 055005. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Singh, K.; Sinuraya, J.S.; Supeno, M.; Sembiring, H.; Tarigan, K.; Rambe, S.M.; Karo-Karo, J.A.; Sitepu, E.K. Waste Passion Fruit Peel as a Heterogeneous Catalyst for Room-Temperature Biodiesel Production. ACS Omega 2022, 7, 7885–7892. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Conversion of biomass to fuel: Transesterification of vegetable oil to biodiesel using KF loaded nano-γ-Al2O3 as catalyst. Appl. Catal. B Environ. 2009, 89, 590–596. [Google Scholar] [CrossRef]
- Takagaki, A.; Toda, M.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Esterification of higher fatty acids by a novel strong solid acid. Catal. Today 2006, 116, 157–161. [Google Scholar] [CrossRef]
- Deshmane, C.A.; Wright, M.W.; Lachgar, A.; Rohlfing, M.; Liu, Z.; Le, J.; Hanson, B.E. A comparative study of solid carbon acid catalysts for the esterification of free fatty acids for biodiesel production. Evidence for the leaching of colloidal carbon. Bioresour. Technol. 2013, 147, 597–604. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Li, H.; Niu, S.; Lu, C.; Liu, M.; Huo, M. Use of lime mud from paper mill as a heterogeneous catalyst for transesterification. Sci. China Technol. Sci. 2014, 57, 438–444. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Wiwatnimit, W.; Wangnoi, S. Modified dolomites as catalysts for palm kernel oil transesterification. J. Mol. Catal. A Chem. 2007, 276, 24–33. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Han, H. Utilization of waste freshwater mussel shell as an economic catalyst for biodiesel production. Biomass Bioenergy 2011, 35, 3627–3635. [Google Scholar] [CrossRef]
- Cho, Y.B.; Seo, G. High activity of acid-treated quail eggshell catalysts in the transesterification of palm oil with methanol. Bioresour. Technol. 2010, 101, 8515–8519. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Liu, C.; Yan, B. Biodiesel production from palm oil using active and stable K doped hydroxyapatite catalysts. Energy Convers. Manag. 2015, 98, 463–469. [Google Scholar] [CrossRef]
- Ghanei, R.; Khalili Dermani, R.; Salehi, Y.; Mohammadi, M. Waste animal bone as support for CaO impregnation in catalytic biodiesel production from vegetable oil. Waste Biomass Valorization 2016, 7, 527–532. [Google Scholar] [CrossRef]
- Chakraborty, R.; Das, S.; Bhattacharjee, S.K. Optimization of biodiesel production from Indian mustard oil by biological tri-calcium phosphate catalyst derived from turkey bone ash. Clean Technol. Environ. Policy 2015, 17, 455–463. [Google Scholar] [CrossRef]
- Mohamed, R.; Kadry, G.; Abdel-Samad, H.; Awad, M. High operative heterogeneous catalyst in biodiesel production from waste cooking oil. Egypt. J. Pet. 2020, 29, 59–65. [Google Scholar] [CrossRef]
- Mares, E.K.L.; Goncalves, M.A.; da Luz, P.T.S.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Acai seed ash as a novel basic heterogeneous catalyst for biodiesel synthesis: Optimization of the biodiesel production process. Fuel 2021, 299, 120887. [Google Scholar] [CrossRef]
- Attari, A.; Abbaszadeh-Mayvan, A.; Taghizadeh-Alisaraie, A. Process optimization of ultrasonic-assisted biodiesel production from waste cooking oil using waste chicken eggshell-derived CaO as a green heterogeneous catalyst. Biomass Bioenergy 2022, 158, 106357. [Google Scholar] [CrossRef]
- Oueda, N.; Bonzi-Coulibaly, Y.L.; Ouédraogo, I.W. Deactivation processes, regeneration conditions and reusability performance of CaO or MgO based catalysts used for biodiesel production—A review. Mater. Sci. Appl. 2016, 8, 94–122. [Google Scholar] [CrossRef] [Green Version]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Heterogeneous catalysis for biodiesel production from Jatropha curcas oil (JCO). Energy 2011, 36, 2693–2700. [Google Scholar] [CrossRef]




| Biomass | Feedstock | Yield | Reference |
|---|---|---|---|
| D-glucose | Oleic acid and stearic acid | 44 μmol/min | [91] |
| Starch | Waste cooking oil | 95% | [92] |
| Cellulose | Waste cooking oil | 88% | [92] |
| Sucrose | Waste cooking oil | 80% | [92] |
| Lime stone | Soybean oil | 93% | [93] |
| Lime mud | Peanut oil | 94% | [94] |
| Red mud | Soybean oil | 94% | [95] |
| Dolomite rock | Palm kernel oil | 99% | [96] |
| Chicken eggshell | Palm olein oil | 94% | [97] |
| Snail shell | Palm olein oil | 93.20% | [97] |
| Mussel shell | Chinese tallow oil | 90% | [98] |
| Quail eggshell | Palm oil | 98.00% | [99] |
| Pig bones | Palm oil | >90.00% | [100] |
| Sheep bones | Canola oil | 95.15% | [101] |
| Turkey bones | mustard oil | 91.22% | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Sangaraju, S.; Lee, Y.R. A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production. Catalysts 2022, 12, 1501. https://doi.org/10.3390/catal12121501
Chandra Kishore S, Perumal S, Atchudan R, Sundramoorthy AK, Alagan M, Sangaraju S, Lee YR. A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production. Catalysts. 2022; 12(12):1501. https://doi.org/10.3390/catal12121501
Chicago/Turabian StyleChandra Kishore, Somasundaram, Suguna Perumal, Raji Atchudan, Ashok K. Sundramoorthy, Muthulakshmi Alagan, Sambasivam Sangaraju, and Yong Rok Lee. 2022. "A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production" Catalysts 12, no. 12: 1501. https://doi.org/10.3390/catal12121501