Homogeneous Photo-Fenton Degradation and Mineralization of Model and Simulated Pesticide Wastewaters in Lab- and Pilot-Scale Reactors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Homogeneous Photocatalytic Oxidation of Model CLPR Pesticide in Laboratory Reactor
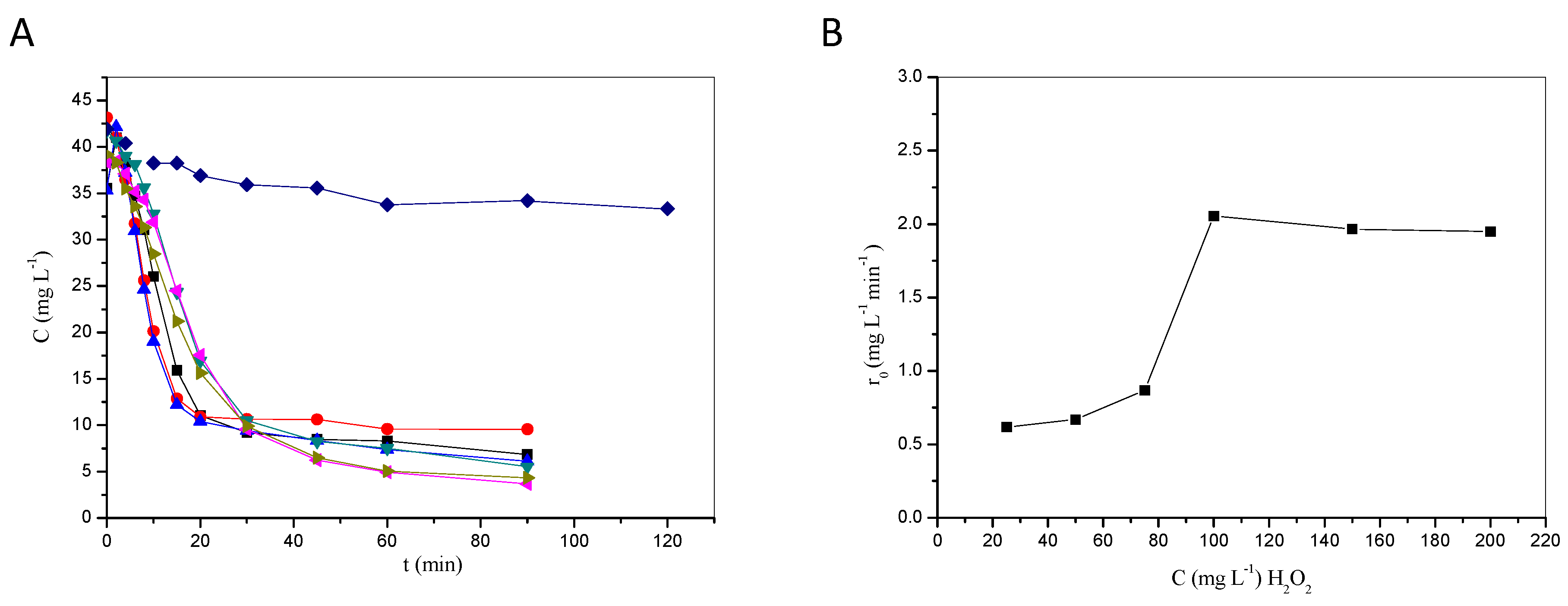
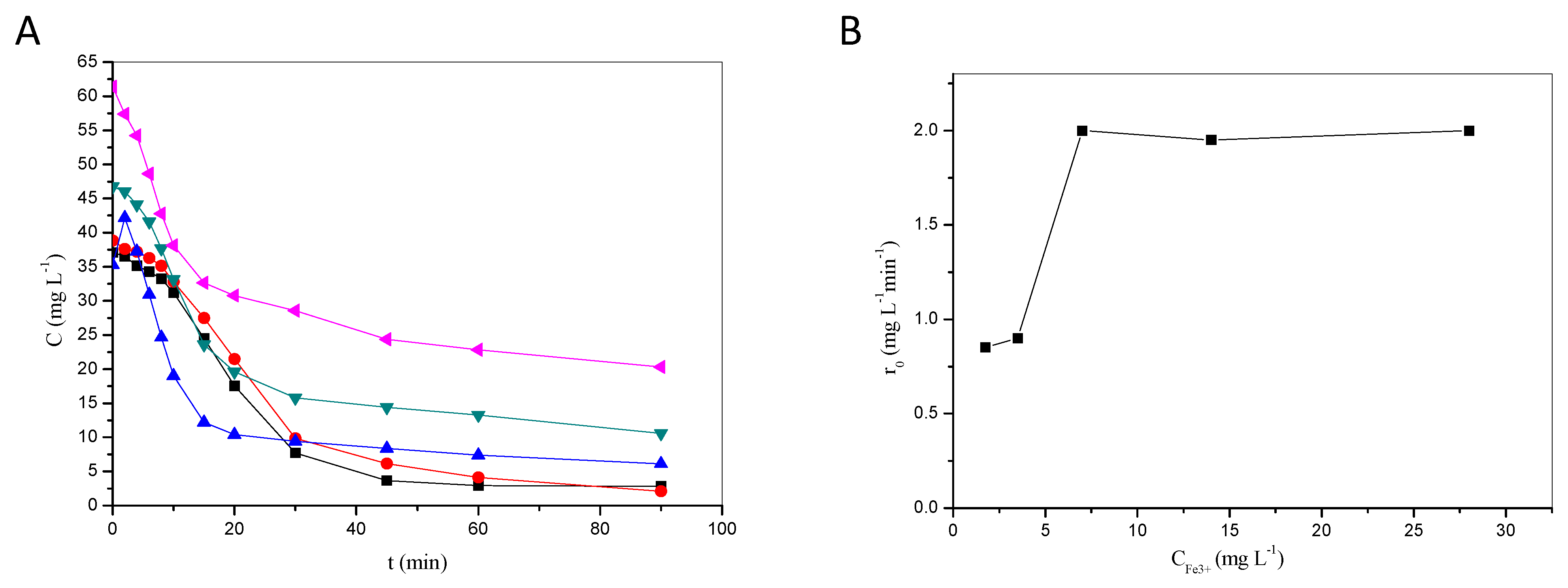
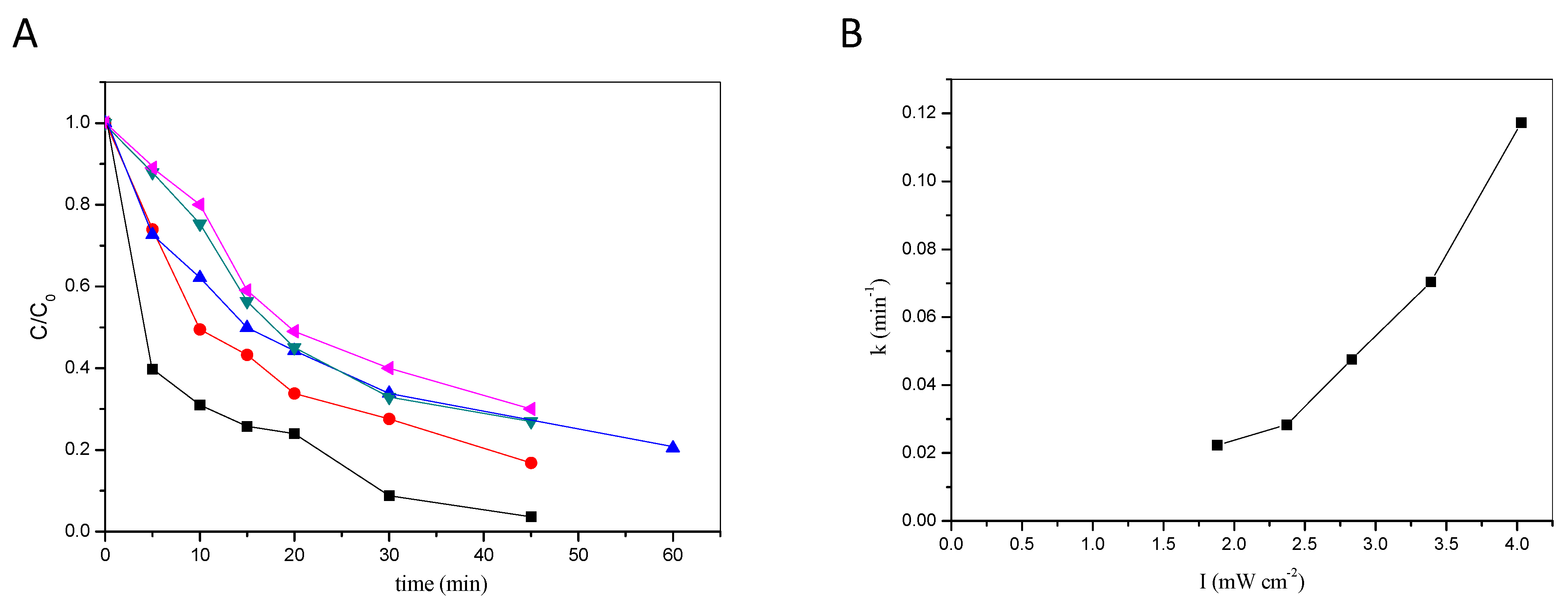
2.1.1. Effect of H2O2 and Fe3+ on Degradation Efficiency
2.1.2. Effect of Irradiation Type
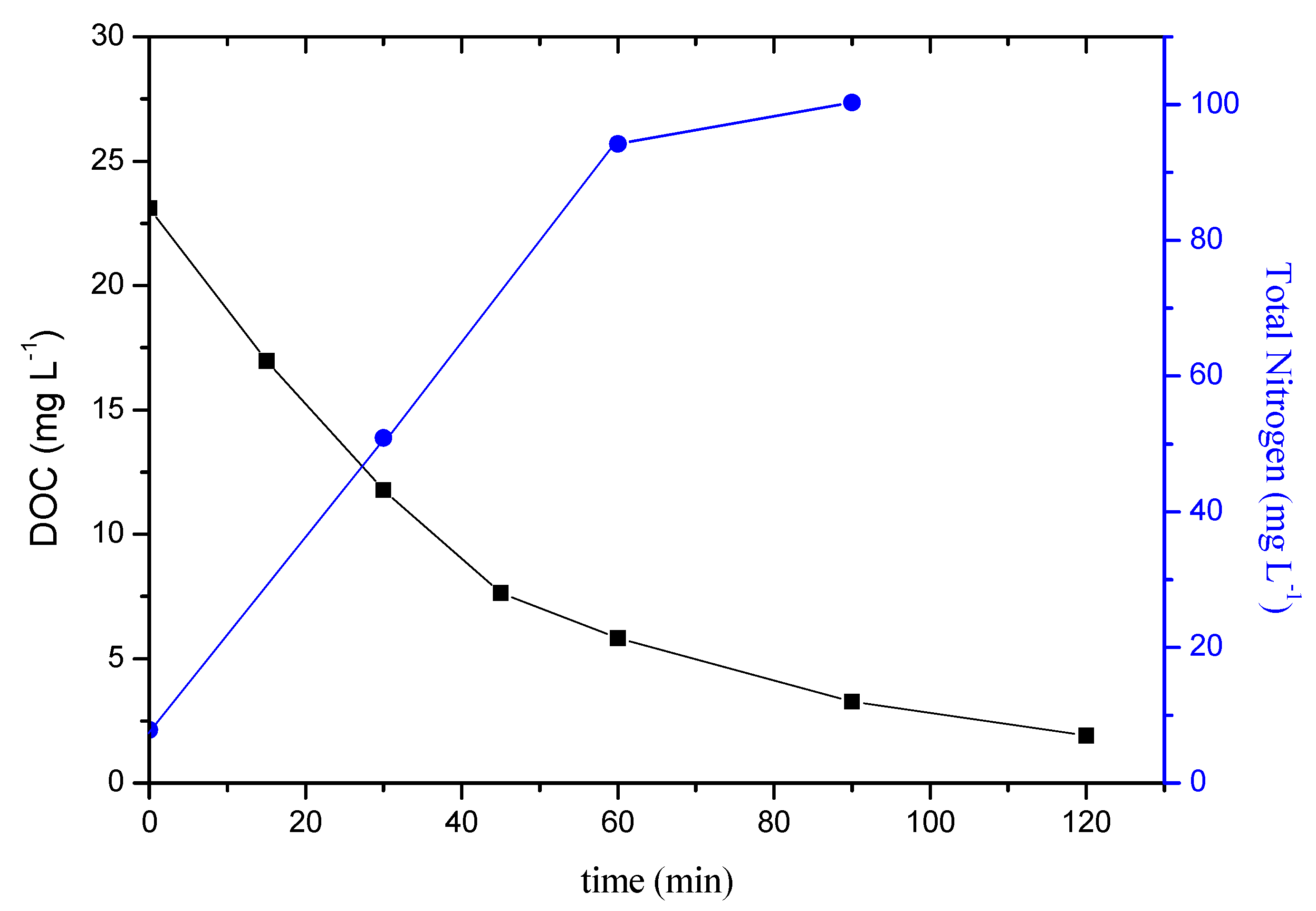
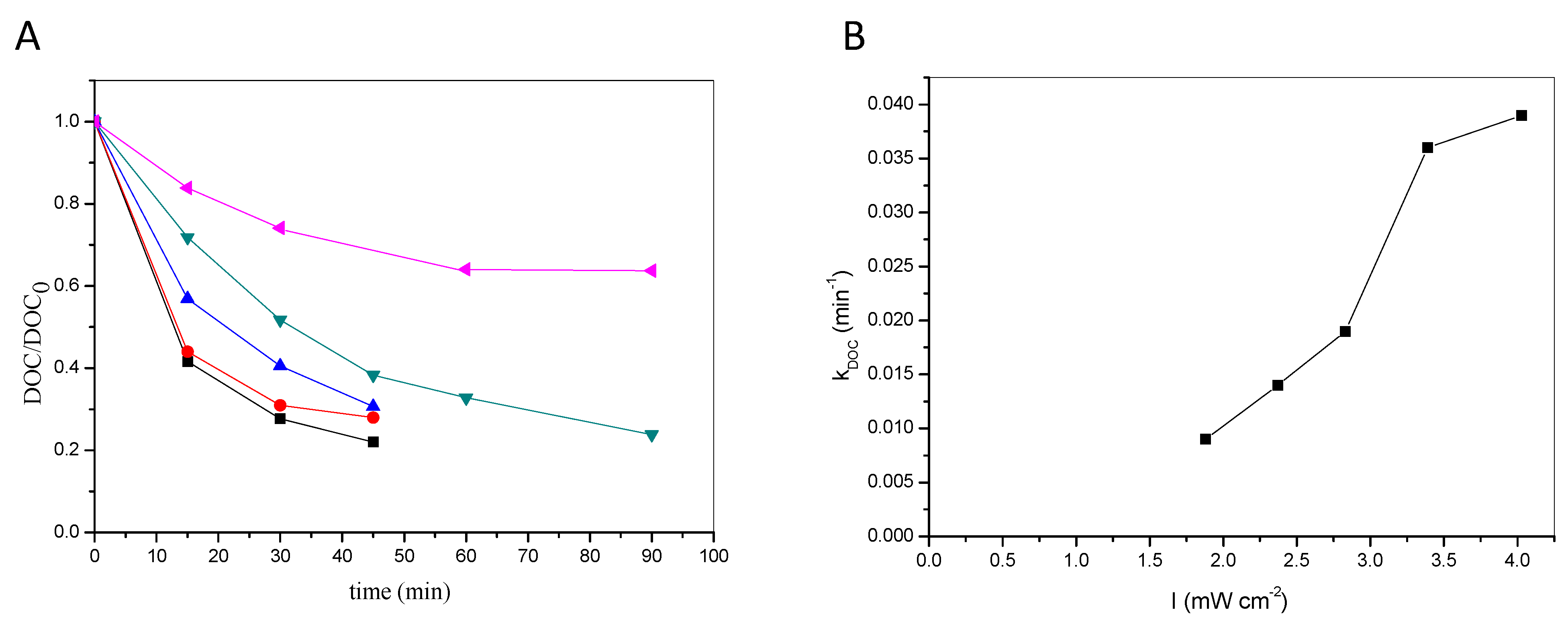
2.1.3. Mineralization
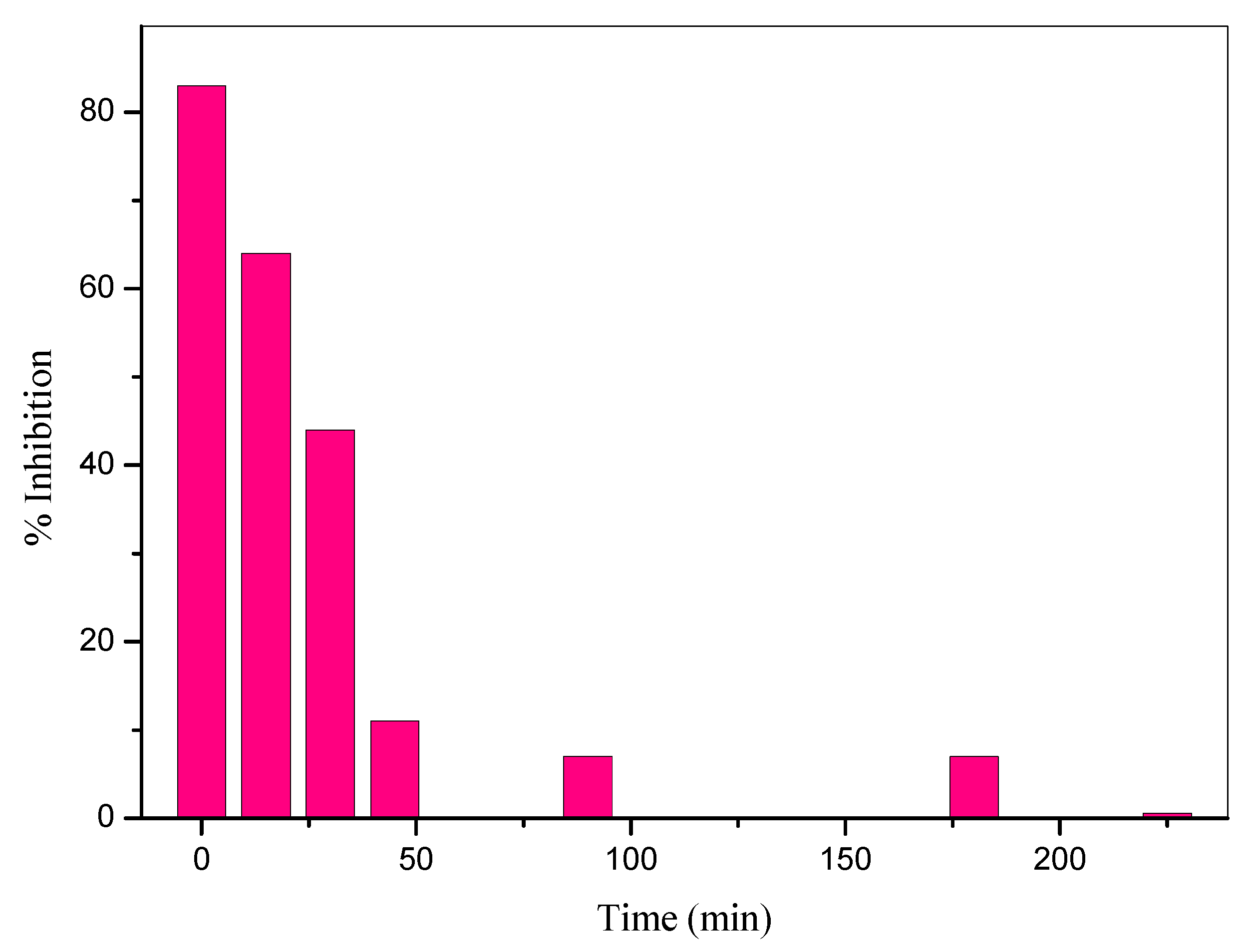
2.1.4. Determination of Acute Toxicity
2.2. Homogeneous Photocatalytic Oxidation of Simulated Wastewater Containing CLPR in the Pilot-Scale Photoreactor
2.2.1. Effect of Intensity of Incident UV-A Radiation
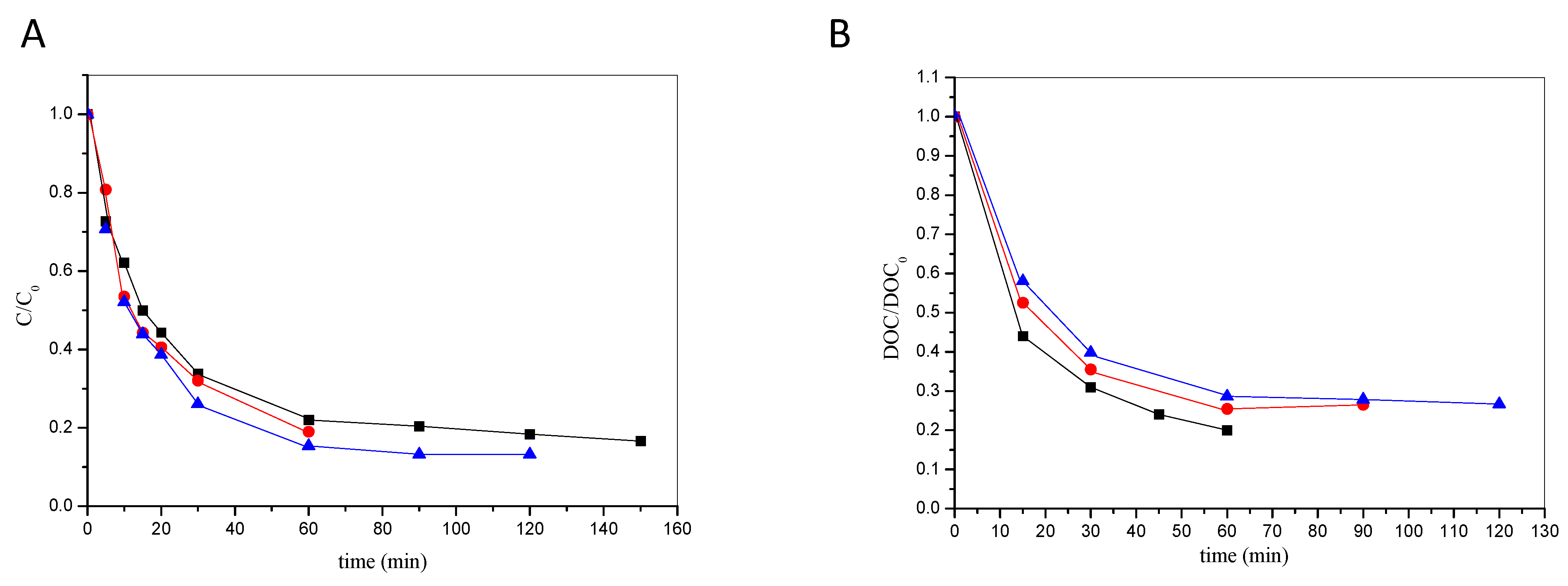
2.2.2. Effect of the Illuminated Volume
3. Materials and Methods
3.1. Materials
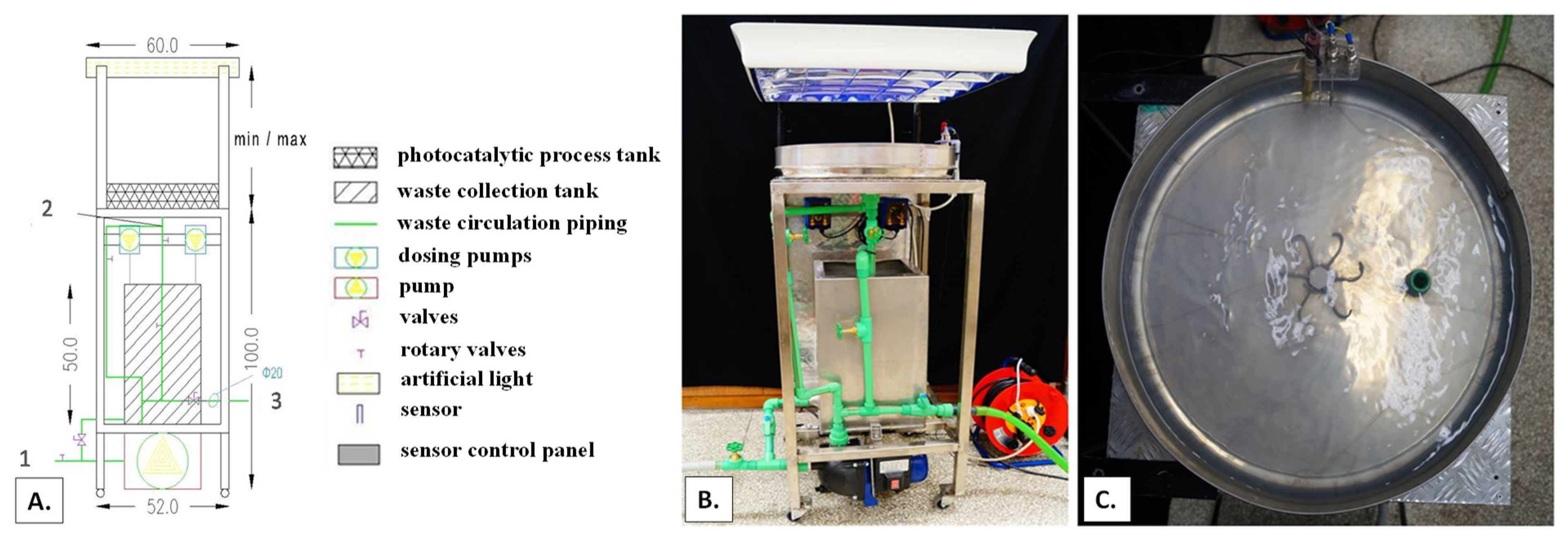
3.2. Photocatalytic Experiments
3.3. Analytical Procedures
3.4. Toxicity Analysis
4. Conclusions
- There usually is an optimum concentration of Fenton reagents to maximize treatment performance, beyond which degradation rates are not enhanced. This has been demonstrated in this work with lab-scale photocatalytic experiments. Excessive concentrations of peroxide and/or catalyst may (i) introduce unnecessary treatment costs, (ii) reduce performance due to scavenging effects, and (iii) raise environmental concerns associated with the disposal of, e.g., high concentrations of iron in the receiving water courses.
- Interestingly, photocatalytic oxidation under visible light resulted in comparable degradation rates to UV-A radiation; this pinpoints the potential of using renewable energy, i.e., natural sunlight, to drive the process.
- Moreover, the proposed process was capable of converting the organic carbon of CLPR to carbon dioxide, as well as the nitrogen content of the pyridine ring to NO3− and NH4+. Mineralization was accompanied by detoxification as has been demonstrated in experiments on marine bacteria.
- The process was tested in a pilot-scale, fountain-type reactor treating a commercial formulation of the pesticide under consideration. The results were promising, resulting in considerable degradation and mineralization, whose rates increased with increasing light intensity; an increase of the incident radiation from 1.88 mW cm−2 to 4.03 mW cm−2 led to a quadrupling of the reaction rate constant kDOC.
- The increase in the volume of the illuminated wastewater does not practically affect the initial rate of its degradation during the photocatalytic oxidation with the photo-Fenton reagent. Regarding mineralization, illumination of 5 L of simulated wastewater leads to slightly higher mineralization rates after 60 min of illumination (80%), compared to experiments where the illuminated volume was 3 or 8 L (~73%). Thus, wastewater volumes up to 8 L could be treated without efficiency loss, which shows the potential of the process for large-scale applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Appa, R.M.; Naidu, B.R.; Venkateswarlu, D.; Hanafiah, M.M.; Lakkaboyana, S.K.; Lakshmidevi, J.; Venkateswarlu, K. Water extract of pomegranate ash–I2 as sustainable system for external oxidant/metal/catalyst-free oxidative iodination of (hetero)arenes. Green Chem. Lett. Rev. 2021, 14, 700–712. [Google Scholar] [CrossRef]
- Venkateswarlu, K. Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett. 2021, 19, 3887–3950. [Google Scholar] [CrossRef]
- Semitsoglou-Tsiapou, S.; Templeton, M.R.; Graham, N.J.D.; Hernández Leal, L.; Martijn, B.J.; Royce, A.; Kruithof, J.C. Low pressure UV/H2O2 treatment for the degradation of the pesticides metaldehyde, clopyralid and mecoprop—Kinetics and reaction product formation. Water Res. 2016, 91, 285–294. [Google Scholar] [CrossRef]
- Carboneras Contreras, M.B.; Fourcade, F.; Assadi, A.; Amrane, A.; Jesus Fernandez-Morales, F. Electro Fenton removal of clopyralid in soil washing effluents. Chemosphere 2019, 237, 124447. [Google Scholar] [CrossRef] [PubMed]
- Rajah, Z.; Guiza, M.; Solís, R.R.; Rivas, F.J.; Ouederni, A. Catalytic and photocatalytic ozonation with activated carbon as technologies in the removal of aqueous micropollutants. J. Photochem. Photobiol. A 2019, 382, 111961. [Google Scholar] [CrossRef]
- Huang, X.; Fong, S.; Deanovic, L.; Young, T.M. Toxicity of herbicides in highway runoff. Environ. Toxicol. Chem. 2005, 24, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.O.S.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Saez, C.; Rodrigo, M.A. Biodegradability improvement of clopyralid wastes through electrolysis using different diamond anodes. Environ. Res. 2020, 188, 109747. [Google Scholar] [CrossRef]
- Millán, M.; García-Orozco, V.M.; Lobato, J.; Fernández-Marchante, C.M.; Roa-Morales, G.; Linares-Hernández, I.; Natividad, R.; Rodrigo, M.A. Toward more sustainable photovoltaic solar electrochemical oxidation treatments: Influence of hydraulic and electrical distribution. J. Environ. Manag. 2021, 285, 112064. [Google Scholar] [CrossRef] [PubMed]
- Solís, R.R.; Rivas, J.F.; Gimeno, O.; Pérez-Bote, J.L. Photocatalytic ozonation of pyridine-based herbicides by N-doped titania. J. Chem. Technol. Biotechnol. 2016, 91, 1998–2008. [Google Scholar] [CrossRef]
- Rajah, Z.; Guiza, M.; Solís, R.R.; Becheikh, N.; Rivas, F.J.; Ouederni, A. Clopyralid degradation using solar-photocatalytic/ozone process with olive stone activated carbon. J. Environ. Chem. Eng. 2019, 7, 102900. [Google Scholar] [CrossRef]
- Sojić, D.V.; Anderluh, V.B.; Orcić, D.Z.; Abramović, B.F. Photodegradation of clopyralid in TiO2 suspensions: Identification of intermediates and reaction pathways. J. Hazard. Mater. 2009, 168, 94–101. [Google Scholar] [CrossRef]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Improvement of electrochemical oxidation efficiency through combination with adsorption processes. J. Environ. Manag. 2020, 262, 110364. [Google Scholar] [CrossRef]
- Tizaoui, C.; Mezughi, K.; Bickley, R. Heterogeneous photocatalytic removal of the herbicide clopyralid and its comparison with UV/H2O2 and ozone oxidation techniques. Desalination 2011, 273, 197–204. [Google Scholar] [CrossRef]
- Gu, B.; Meldrum, B.; McCabe, T.; Phillips, S. Enhancing concentration and mass sensitivities for liquid chromatography trace analysis of clopyralid in drinking water. J. Sep. Sci. 2012, 35, 185–192. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Karahanidou, S.; Lambropoulou, D.A.; Kouras, A.; Kosma, C.I.; Albanis, T.A.; Poulios, I. Photocatalytic degradation of the herbicide clopyralid: Kinetics, degradation pathways and ecotoxicity evaluation. J. Chem. Technol. Biotechnol. 2016, 91, 2510–2518. [Google Scholar] [CrossRef]
- Pérez, J.F.; Llanos, J.; Sáez, C.; López, C.; Cañizares, P.; Rodrigo, M.A. On the design of a jet-aerated microfluidic flow-through reactor for wastewater treatment by electro-Fenton. Sep. Purif. Technol. 2019, 208, 123–129. [Google Scholar] [CrossRef]
- Sedlazeck, K.P.; Vollprecht, D.; Müller, P.; Mischitz, R.; Gill, J.; Trois, W.; Maunz, I.; Frate, R.; Mann, O.; Wruss, K. Decomposition of dissolved organic contaminants by combining a boron-doped diamond electrode, zero-valent iron and ultraviolet radiation. Chemosphere 2019, 217, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Carboneras, M.B.; Cañizares, P.; Rodrigo, M.A.; Villaseñor, J.; Fernandez-Morales, F.J. Improving biodegradability of soil washing effluents using anodic oxidation. Bioresour. Technol. 2018, 252, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lara-Ramos, J.A.; Saez, C.; Machuca-Martínez, F.; Rodrigo, M.A. Electro-ozonizers: A new approach for an old problem. Sep. Purif. Technol. 2020, 241, 116701. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced oxidation processes for water and wastewater viral disinfection. A systematic review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef]
- Pandit, V.U.; Arbuj, S.S.; Pandit, Y.B.; Naik, S.D.; Rane, S.B.; Mulik, U.P.; Gosavi, S.W.; Kale, B.B. Solar light driven dye degradation using novel organo-inorganic (6, 13-pentacenequinone-TiO2) nanocomposite. RSC Adv. 2015, 5, 10326–10331. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Lambropoulou, D.A.; Antoniadis, A.; Ntonou, E.; Zalidis, G.C.; Poulios, I. Evaluation of an alternative method for wastewater treatment containing pesticides using solar photocatalytic oxidation and constructed wetlands. J. Environ. Manag. 2017, 195 Pt 2, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Berberidou, C.; Kyzas, G.Z.; Paspaltsis, I.; Sklaviadis, T.; Poulios, I. Photocatalytic disinfection and purification of water employing reduced graphene oxide/TiO2 composites. J. Chem. Technol. Biotechnol. 2019, 94, 3905–3914. [Google Scholar] [CrossRef]

| Treatment | Photocatalytic Methods | Level of Implementation | Matrix | Main Outcome | Reference |
|---|---|---|---|---|---|
| heterogenous photocatalytic experiments with CLPR solutions containing TiO2 P25, TiO2 UV-100, ZnO, TiO2 Kronos 7000, Kronos 7001 and Kronos 7500 under UV-A irradiation | different operational conditions, e.g., type of photocatalyst, catalyst loading, initial pH and hydrogen peroxide (H2O2) concentration | lab | aqueous solutions of clopyralid | TiO2 mediated photocatalysis has the potential to provide a sustainable solution in the detoxification of wastewater containing recalcitrant pesticides, either alone or in combination with other methods | [16] |
| photocatalytic oxidation of CLPR in a bench-scale photocatalytic reactor, equipped with a central UV-A lamp | degradation and mineralization experiments in the presence of three reduced graphene oxide (rGO)/TiO2 composites containing a 1, 5 or 10 wt% nominal content in rGO and bare P25 for comparison purposes | lab | aqueous solutions of clopyralid | rGO, a low-cost, nontoxic material may serve as a reliable alternative in the enhancement of TiO2 photocatalytic efficiency in water processing applications | [17] |
| solar photocatalytic oxidation and constructed wetlands | photo-Fenton and ferrioxalate reagent, and combination of photo-Fenton with TiO2 P25 | pilot | simulated wastewater | the integrated system effectively detoxified wastewater containing clopyralid | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berberidou, C.; Kokkinos, P.; Poulios, I.; Mantzavinos, D. Homogeneous Photo-Fenton Degradation and Mineralization of Model and Simulated Pesticide Wastewaters in Lab- and Pilot-Scale Reactors. Catalysts 2022, 12, 1512. https://doi.org/10.3390/catal12121512
Berberidou C, Kokkinos P, Poulios I, Mantzavinos D. Homogeneous Photo-Fenton Degradation and Mineralization of Model and Simulated Pesticide Wastewaters in Lab- and Pilot-Scale Reactors. Catalysts. 2022; 12(12):1512. https://doi.org/10.3390/catal12121512
Chicago/Turabian StyleBerberidou, Chrysanthi, Petros Kokkinos, Ioannis Poulios, and Dionissios Mantzavinos. 2022. "Homogeneous Photo-Fenton Degradation and Mineralization of Model and Simulated Pesticide Wastewaters in Lab- and Pilot-Scale Reactors" Catalysts 12, no. 12: 1512. https://doi.org/10.3390/catal12121512
APA StyleBerberidou, C., Kokkinos, P., Poulios, I., & Mantzavinos, D. (2022). Homogeneous Photo-Fenton Degradation and Mineralization of Model and Simulated Pesticide Wastewaters in Lab- and Pilot-Scale Reactors. Catalysts, 12(12), 1512. https://doi.org/10.3390/catal12121512











