Tuning the Hydrophobicity and Lewis Acidity of UiO-66-NO2 with Decanoic Acid as Modulator to Optimise Conversion of Glucose to 5-Hydroxymethylfurfural
Abstract
:1. Introduction
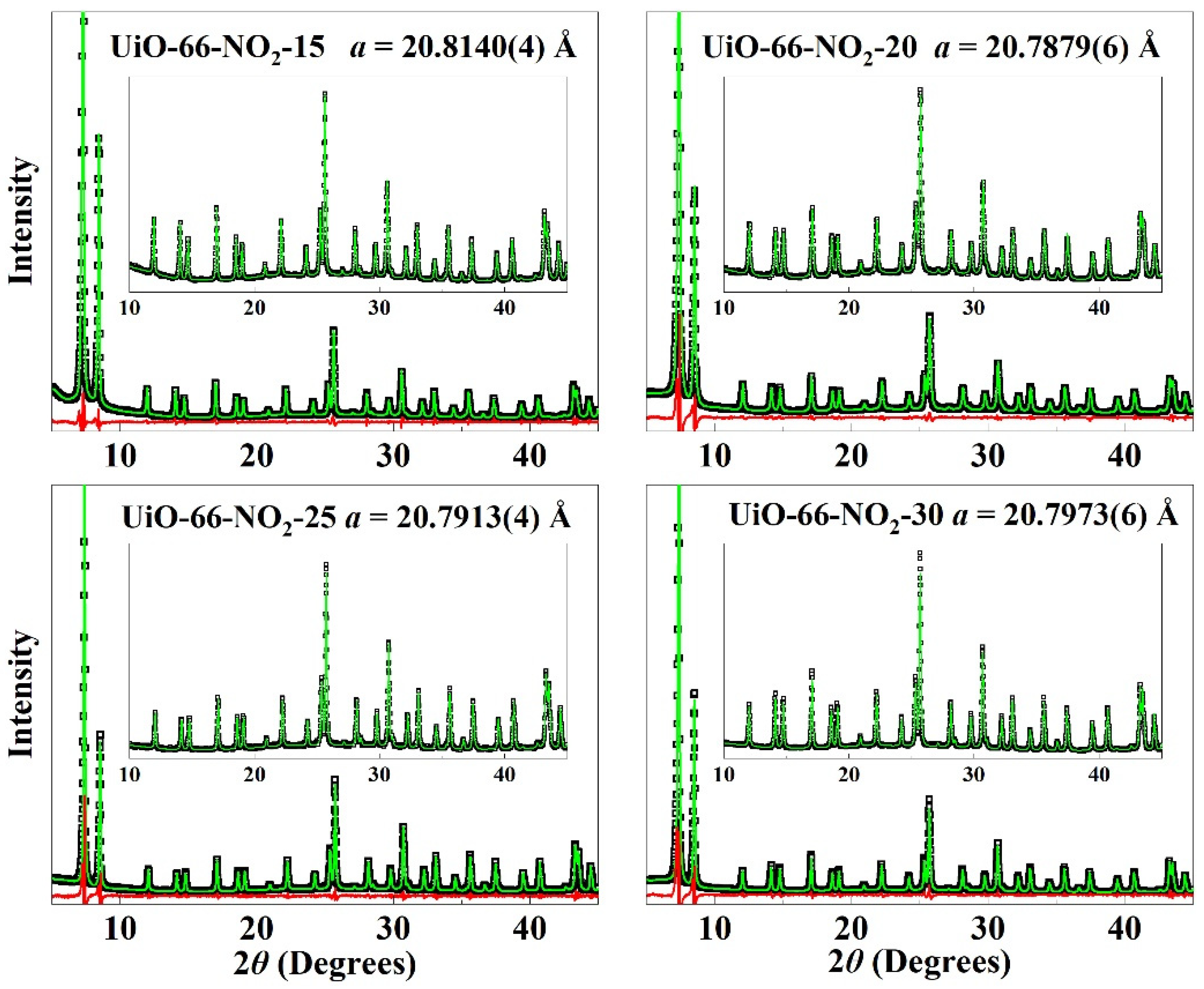
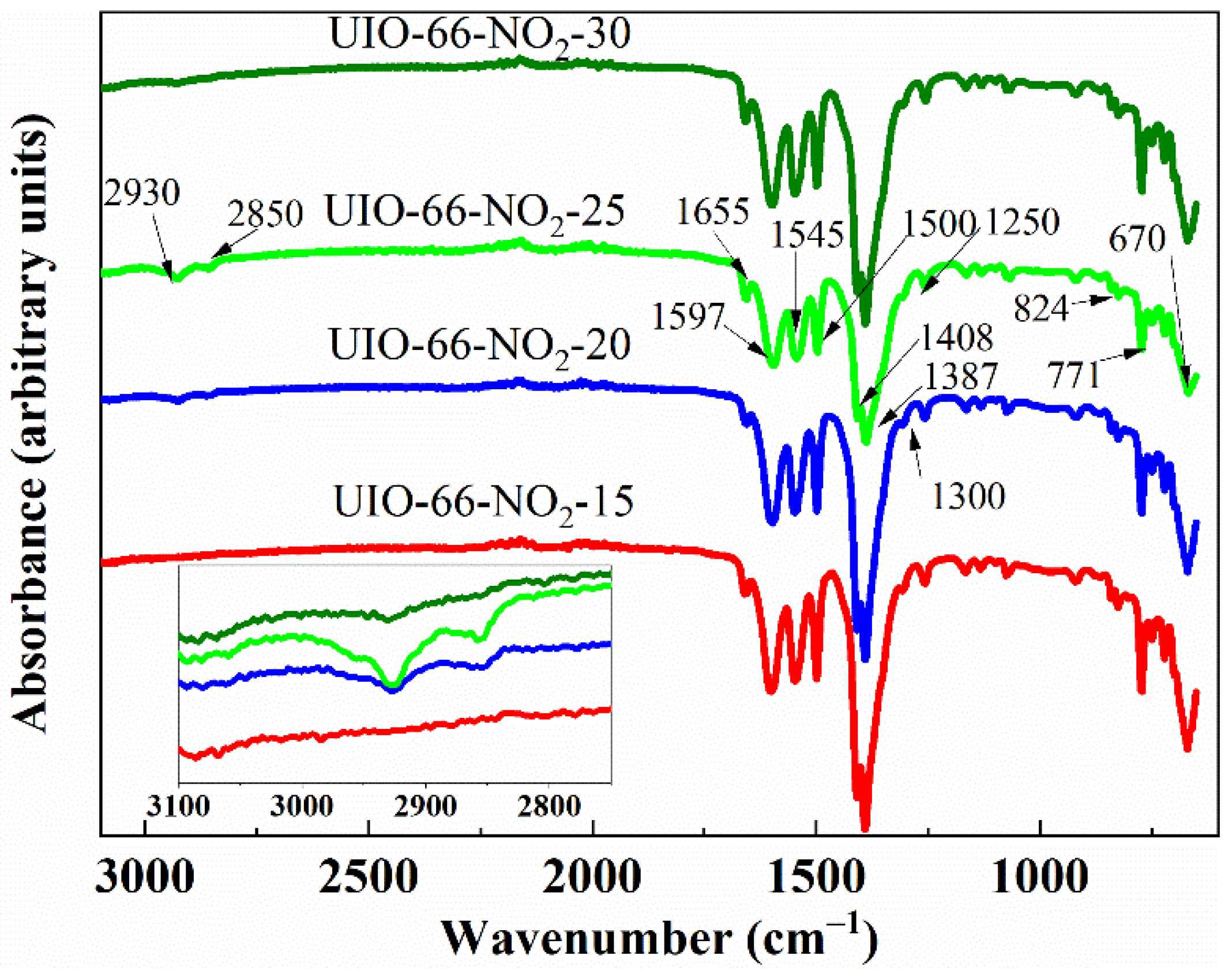
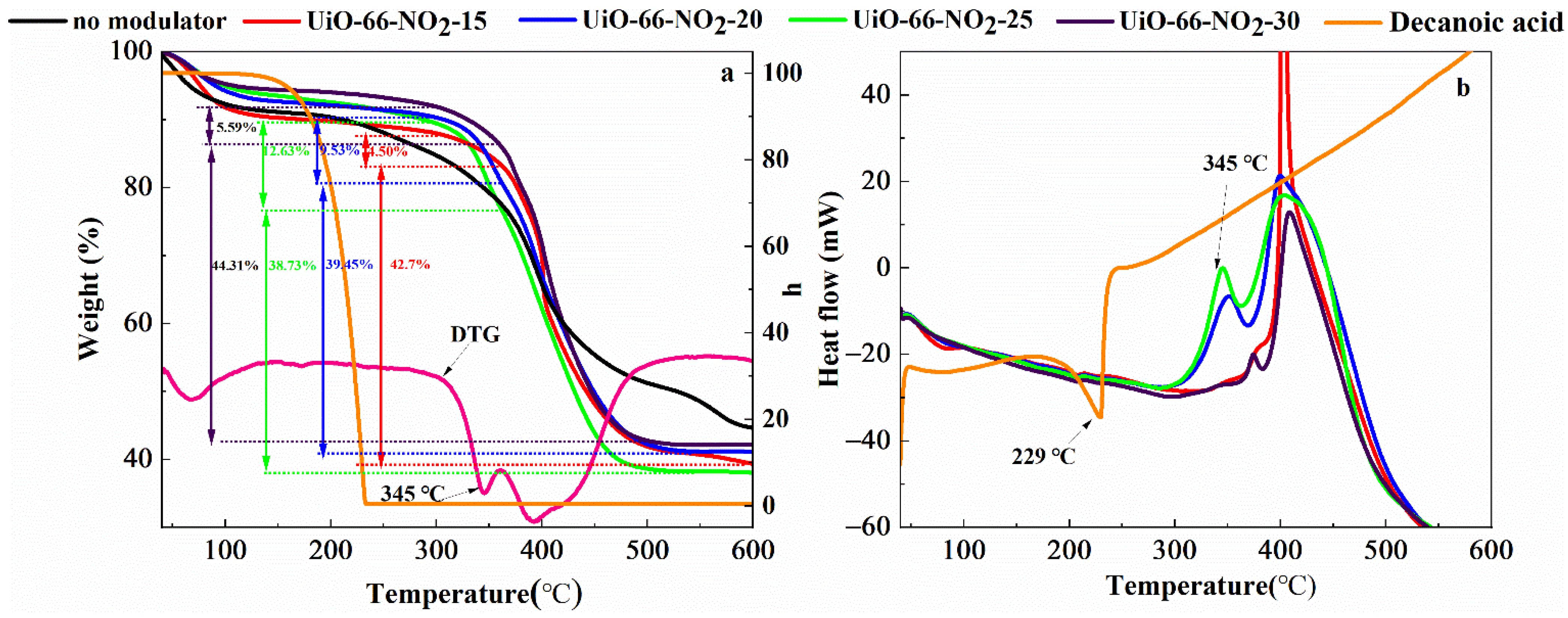
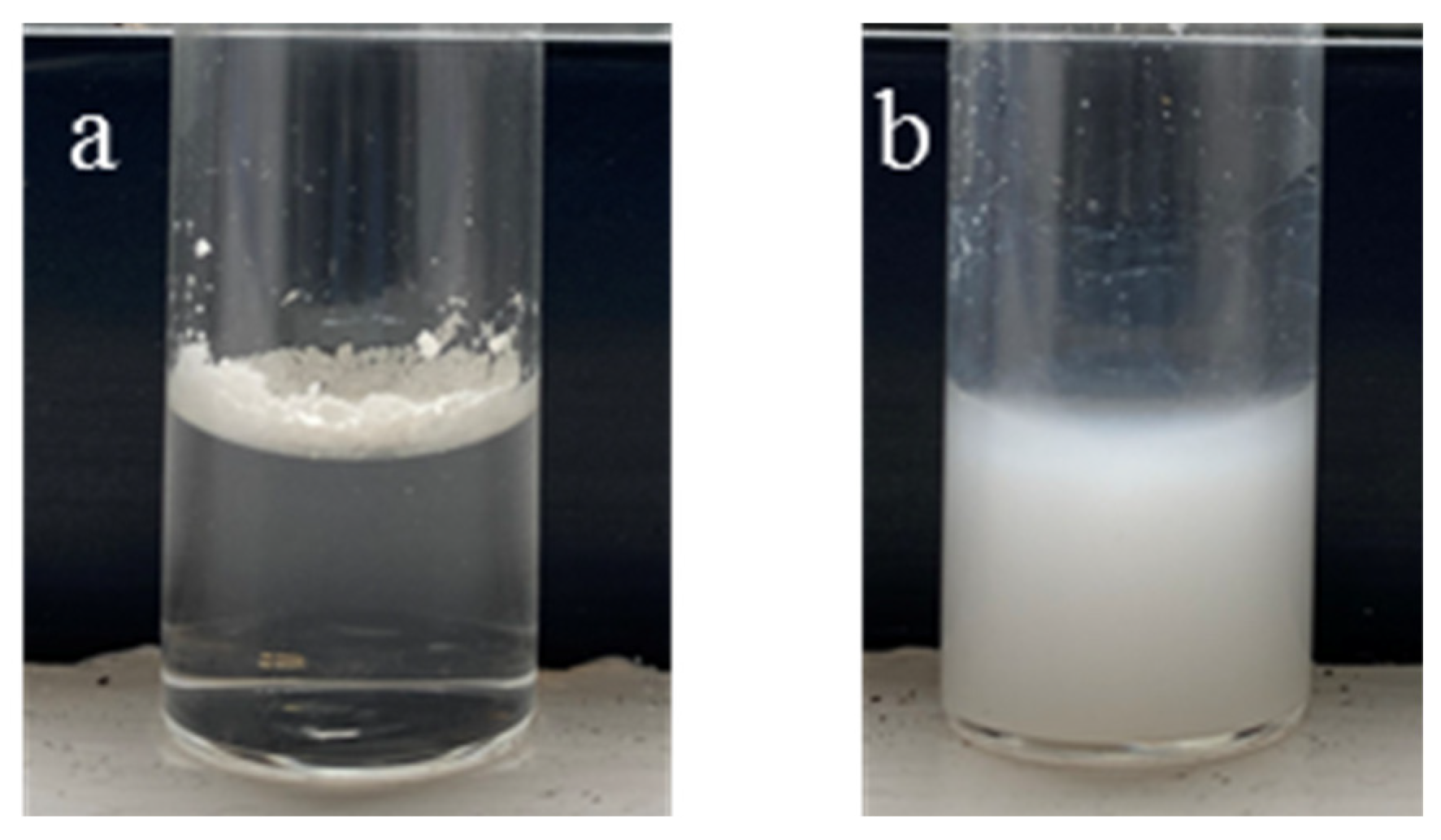
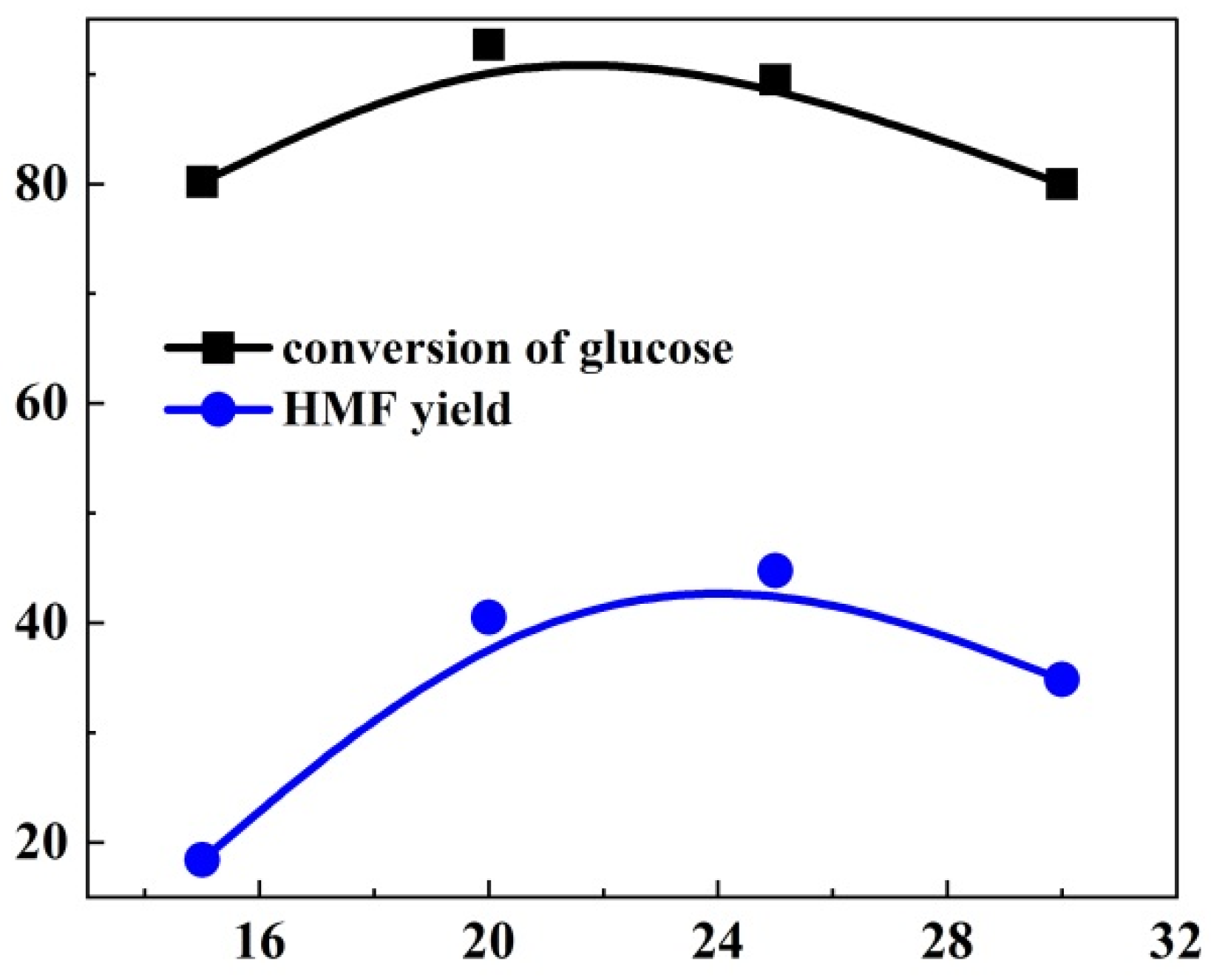
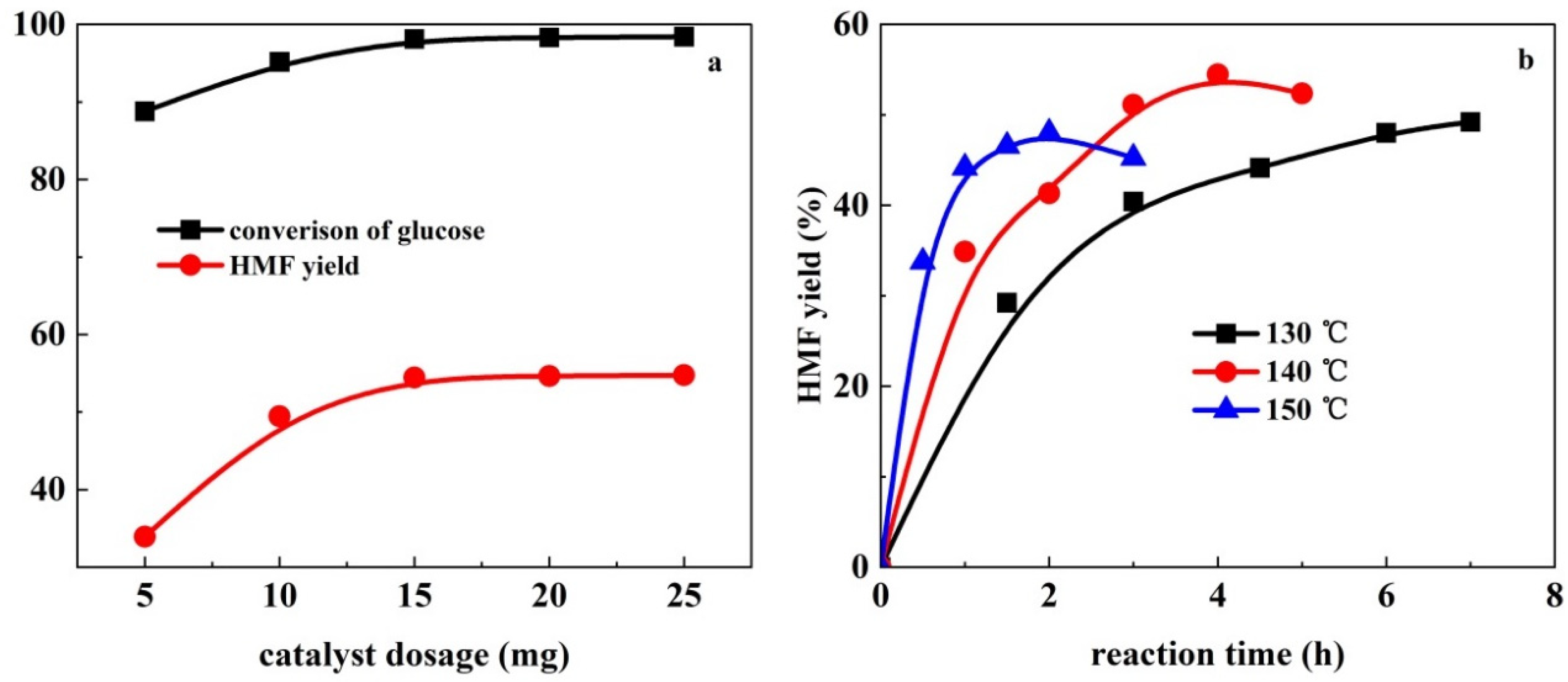
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of UiO-66-NO2
3.3. Materials Characterisation
3.4. Catalytic Reactions and Analysis Technique
3.5. Quantification of Accessible Lewis Acidic Sites
3.6. Recyclability of Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Putten, R.J.; van der Waal, J.C.; de Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Fan, W.; Verrier, C.; Queneau, Y.; Popowycz, F. 5-Hydroxymethylfurfural (HMF) in organic synthesis: A review of its recent applications towards fine chemicals. Curr. Org. Synth. 2019, 16, 583–614. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Queneau, Y. 5-Hydroxymethylfurfural and Furfural Chemistry Toward Biobased Surfactants. ChemSusChem 2022, 15, e202102660. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Kong, X.; Zhu, Y. Catalytic Conversion of 5-Hydroxymethylfurfural to High-Value Derivatives by Selective Activation of C−O, C=O, and C=C Bonds. ChemSusChem 2022, 15, e202200421. [Google Scholar] [CrossRef]
- Rosenfeld, C.; Konnerth, J.; Sailer-Kronlachner, W.; Solt, P.; Rosenau, T.; Van Herwijnen, H.W. Current situation of the challenging scale-up development of hydroxymethylfurfural production. ChemSusChem 2020, 13, 3544–3564. [Google Scholar] [CrossRef]
- Mason, J.B.; Sun, Y. Microwave-Assisted Production of 5-Hydroxymethylfurfural from Glucose. ChemistrySelect 2021, 6, 10582–10586. [Google Scholar] [CrossRef]
- Giovanelli, G.; Cappa, C. 5-hydroxymethylfurfural formation in bread as a function of heat treatment intensity: Correlations with browning indices. Foods 2021, 10, 417. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Huang, K.; Maravelias, C.T.; Dumesic, J.A. Solvent system for effective near-term production of hydroxymethylfurfural (HMF) with potential for long-term process improvement. Energy Environ. Sci. 2019, 12, 2212–2222. [Google Scholar] [CrossRef]
- Dashtban, M.; Gilbert, A.; Fatehi, P. Recent advancements in the production of hydroxymethylfurfural. RSC Adv. 2014, 4, 2037–2050. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Likozar, B. A review of bio-refining process intensification in catalytic conversion reactions, separations and purifications of hydroxymethylfurfural (HMF) and furfural. Chem. Eng. J. 2022, 429, 132325. [Google Scholar] [CrossRef]
- Tongtummachat, T.; Akkarawatkhoosith, N.; Jaree, A. Process intensification for 5-hydroxymethylfurfural production from sucrose in a continuous fixed-bed reactor. Chem. Eng. Res. Des. 2022, 182, 312–323. [Google Scholar] [CrossRef]
- Körner, P.; Jung, D.; Kruse, A. The effect of different Brønsted acids on the hydrothermal conversion of fructose to HMF. Green Chem. 2018, 20, 2231–2241. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Uozumi, R.; Satsuma, A. Enhanced production of hydroxymethylfurfural from fructose with solid acid catalysts by simple water removal methods. Catal. Commun. 2009, 10, 1849–1853. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, Y.; Gao, Y.; Qian, Y.; Ying, S.; Yuan, D.; Zhao, D. Direct synthesis of hierarchically porous metal–organic frameworks with high stability and strong Brønsted acidity: The decisive role of hafnium in efficient and selective fructose dehydration. Chem. Mater. 2016, 28, 2659–2667. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Navarro-Jaén, S.; Drault, F.; Ivanova, S. Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts 2021, 11, 1395. [Google Scholar] [CrossRef]
- He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Yu, H. Experimental and kinetic study on the production of furfural and HMF from glucose. Catalysts 2020, 11, 11. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.Y.; Willman, J.; Zhou, H.C. Hierarchy in metal–organic frameworks. ACS Cent. Sci. 2020, 6, 359–367. [Google Scholar] [CrossRef]
- Dissegna, S.; Vervoorts, P.; Hobday, C.L.; Duren, T.; Daisenberger, D.; Smith, A.J.; Kieslich, G. Tuning the mechanical response of metal-organic frameworks by defect engineering. J. Am. Chem. Soc. 2018, 140, 11581–11584. [Google Scholar] [CrossRef]
- Hao, J.; Mao, W.; Ye, G.; Xia, Y.; Wei, C.; Zeng, L.; Zhou, J. Tin–chromium bimetallic metal–organic framework MIL-101 (Cr, Sn) as a catalyst for glucose conversion into HMF. Biomass Bioenergy 2022, 159, 106395. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V. Exceptionally efficient and recyclable heterogeneous metal–organic framework catalyst for glucose isomerization in water. ChemCatChem 2018, 10, 706–709. [Google Scholar] [CrossRef]
- Burnett, D.L.; Oozeerally, R.; Pertiwi, R.; Chamberlain, T.W.; Cherkasov, N.; Clarkson, G.J.; Walton, R.I. A hydrothermally stable ytterbium metal-organic framework as a bifunctional solid-acid catalyst for glucose conversion. Chem. Comm. 2019, 55, 11446–11449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabushita, M.; Li, P.; Islamoglu, T.; Kobayashi, H.; Fukuoka, A.; Farha, O.K.; Katz, A. Selective metal-organic framework catalysis of glucose to 5-hydroxymethylfurfural using phosphate-modified NU-1000. Ind. Eng. Chem. Res. 2017, 56, 7141–7148. [Google Scholar] [CrossRef]
- Oozeerally, R.; Ramkhelawan, S.D.; Burnett, D.L.; Tempelman, C.H.; Degirmenci, V. ZIF-8 metal organic framework for the conversion of glucose to fructose and 5-hydroxymethyl furfural. Catalysts 2019, 9, 812. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, G.; Matsuda, R.; Sato, H.; Kitagawa, S. Catalytic glucose isomerization by porous coordination polymers with open metal sites. Asian J. Chem. 2014, 9, 2772–2777. [Google Scholar] [CrossRef]
- Zi, G.; Yan, Z.; Wang, Y.; Chen, Y.; Guo, Y.; Yuan, F.; Wang, J. Catalytic hydrothermal conversion of carboxymethyl cellulose to value-added chemicals over metal–organic framework MIL-53 (Al). Carbohydr. Polym. 2015, 115, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, R.; Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Cherkasov, N.; Walker, M.; Walton, R.I. Replacement of chromium by non-toxic metals in lewis-acid MOFs: Assessment of stability as glucose conversion catalysts. Catalysts 2019, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A decade of UiO-66 research: A historic review of dynamic structure, synthesis mechanisms, and characterization techniques of an archetypal metal–organic framework. Cryst. Growth Des. 2019, 20, 1347–1362. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Wei, Y.; Yan, C.; Meng, M.; Yan, Y. Direct synthesis of metal-organic frameworks catalysts with tunable acid–base strength for glucose dehydration to 5-hydroxymethylfurfural. J. Taiwan Inst. Chem. Eng. 2019, 96, 93–103. [Google Scholar] [CrossRef]
- Gong, J.; Katz, M.J.; Kerton, F.M. Catalytic conversion of glucose to 5-hydroxymethylfurfural using zirconium-containing metal–organic frameworks using microwave heating. RSC Adv. 2018, 8, 31618–31627. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.; Cirujano, F.G.; Krajnc, A.; Mali, G.; Henrion, M.; Smolders, S.; De Vos, D.E. Unexpected linker-dependent Brønsted acidity in the (Zr) UiO-66 metal organic framework and application to biomass valorization. Catal. Sci. Technol. 2020, 10, 4002–4009. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Kashtiban, R.J.; Huband, S.; Walton, R.I.; Degirmenci, V. Systematic modification of UiO-66 metal-organic frameworks for glucose conversion into 5-hydroxymethyl furfural in water. ChemCatChem 2021, 13, 2517–2529. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, W.; Song, H.; Wei, Y.; Jin, P.; Li, B.; Yan, Y. Coupled acid and base UiO-66-type MOFs supported on g-C3N4 as a bi-functional catalyst for one-pot production of 5-HMF from glucose. Microporous Mesoporous Mater. 2020, 305, 110328. [Google Scholar] [CrossRef]
- Li, Y.; Meng, X.; Luo, R.; Zhou, H.; Lu, S.; Yu, S.; Lyu, J. Aluminum/Tin-doped UiO-66 as Lewis acid catalysts for enhanced glucose isomerization to fructose. Appl. Catal. A-Gen. 2022, 632, 118501. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, K.; Gao, L.; Meng, M.; Yan, Y. Green Synthesis of Acid-Base Bifunctional UiO-66 Type Metal-Organic Frameworks Membranes Supported on Polyurethane Foam for Glucose Conversion. ChemistrySelect 2018, 3, 9378–9387. [Google Scholar] [CrossRef]
- Tangsermvit, V.; Pila, T.; Boekfa, B.; Somjit, V.; Klysubun, W.; Limtrakul, J.; Kongpatpanich, K. Incorporation of Al3+ Sites on Brønsted Acid Metal–Organic Frameworks for Glucose-to-Hydroxylmethylfurfural Transformation. Small 2021, 17, 2006541. [Google Scholar] [CrossRef] [PubMed]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Abdelkader-Fernández, V.K.; Cunha-Silva, L.; Balula, S.S. One-Pot Conversion of Glucose into 5-Hydroxymethylfurfural using MOFs and Brønsted-Acid Tandem Catalysts. Adv. Sustain. Syst. 2022, 6, 2100444. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Xamena, F.X.L.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lamberti, C. Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect engineering: Tuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Feng, X.; Song, Y.; Lin, W. Dimensional Reduction of Lewis Acidic Metal–Organic Frameworks for Multicomponent Reactions. J. Am. Chem. Soc. 2021, 143, 8184–8192. [Google Scholar] [CrossRef]
- Vo, T.K.; Nguyen, V.C.; Quang, D.T.; Park, B.J.; Kim, J. Formation of structural defects within UiO-66(Zr)-(OH)2 framework for enhanced CO2 adsorption using a microwave-assisted continuous-flow tubular reactor. Microporous Mesoporous Mater. 2021, 312, 110746. [Google Scholar] [CrossRef]
- Li, K.; Du, M.; Ji, P. Multifunctional tin-based heterogeneous catalyst for catalytic conversion of glucose to 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2018, 6, 5636–5644. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal-organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Guo, W.; Hensen, E.J.M.; Qi, W. Titanium Phosphate Grafted on Mesoporous SBA-15 Silica as a Solid Acid Catalyst for the Synthesis of 5-Hydroxymethylfurfural from Glucose. ACS Sustain. Chem. Eng. 2022, 10, 10157–10168. [Google Scholar] [CrossRef]
- Song, X.; Yue, J.; Zhu, Y.; Wen, C.; Chen, L.; Liu, Q.; Wang, C. Efficient Conversion of Glucose to 5-Hydroxymethylfurfural over a Sn-Modified SAPO-34 Zeolite Catalyst. Ind. Eng. Chem. Res. 2021, 60, 5838–5851. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-pot” synthesis of 5-(hydroxymethyl) furfural from carbohydrates using tin-beta zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]






| Entry | No Modulator | Formic Acid | UiO-66-NO2-X | |||
|---|---|---|---|---|---|---|
| X = 15 | X = 20 | X = 25 | X = 30 | |||
| Lewis acidic sites | - | 0.0004 | - | 0.0040 | 0.0061 | - |
| Wave Number (cm−1) | Assignment |
|---|---|
| 2930, 2850 | CH2 asymmetric and symmetric stretching |
| 1597, 1408 | C=O asymmetric and symmetric stretching |
| 1655 | C=O stretching in DMF |
| 1500 | C=C stretching in phenyl moieties |
| 1545, 1387 | N=O asymmetric and symmetric stretching |
| 1300 | C-N stretching distinctive of BDC-NO2 |
| 1250 | C-H stretching |
| 824, 771 | C-H anti-phase and in-phase bending |
| 670 | Zr-O stretching |
| Entry | UiO-66-NO2-X | |||||
|---|---|---|---|---|---|---|
| No Modulator | X = 15 | X = 20 | X = 25 | X = 30 | X = 25 * | |
| BET surface area(m2/g) | 367.52 | 871.96 | 772.42 | 842.13 | 853.88 | 935.62 |
| t-plot micropore volume (cm3/g) | 0.136 | 0.318 | 0.254 | 0.274 | 0.281 | 0.296 |
| Entry | Solvent | θ (°C) | Time (h) | Glucose Conversion (%) | HMF Yield (%) | Selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | Water | 140 | 3 | 55.2 | 5.6 | 10.1 |
| 2 | DMF | 3 | 88.3 | 4.8 | 5.4 | |
| 3 | Water/MIBK(v/v = 1/2) | 4 | 57.8 | 4.6 | 8.0 | |
| 4 | Water/DMSO(v/v = 1/9) | 4 | 72.6 | 24.3 | 33.5 | |
| 5 | Water/DMF(v/v = 1/9) | 4 | 100 | 0.1 | 0.1 | |
| 6 | Water/acetone(v/v = 1/9) | 4 | - | 1.4 | - | |
| 7 | Water/2-propanol(v/v = 1/9) | 4 | - | 3.9 | - |
| Catalyst | θ (°C) | Time (min) | Solvent | Glucose Conversion (%) | HMF Yield (%) | Ref |
|---|---|---|---|---|---|---|
| UiO-66-NO2-25 | 140 | 240 | DMSO | 98.1 | 54.5 | This work |
| MIL-88(Fe,Sc) | 140 | 180 | DMSO | 71.0 | 25.1 | [26] |
| MIL-101(Cr)-SO3H | 120 | 120 | DMSO | not given | 7 | [43] |
| MIL-101(Sn,Cr) | 140 | 60 | DMSO | 85.7 | 22.6 | [19] |
| UiO-66-NH2-SO3H | 140 | 600 | DMSO | not given | 45.2 | [28] |
| UiO-66-NH2-SO3H@g-C3N4 | 120 | 360 | Isopropanol/DMSO | 92 | 55 | [32] |
| UiO-66-SO3H | 140 | 180 | Water | 36 | 8 | [31] |
| MSDBC(50)-naphtha(50)-UiO-66 | 120 | 180 | Water | 33 | 7 | |
| SnPCP@MnO2-PDA | 150 | 180 | DMSO | 90 | 55.8 | [42] |
| P-3Ti/SBA-15 | 160 | 180 | Water/mTHF | 98 | 71 | [44] |
| Sn modified SAPO-34 | 150 | 90 | NaCl/Water/THF | 98.5 | 64.4 | [45] |
| Phosphate-modified NU-1000 | 140 | 420 | Water/ 2-propanol | >99 | 64 | [22] |
| Sn-Beta, HCl | 180 | 70 | NaCl/Water/THF | 79 | 56.9 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, B.; Das, S.; Degirmenci, V.; Walton, R.I. Tuning the Hydrophobicity and Lewis Acidity of UiO-66-NO2 with Decanoic Acid as Modulator to Optimise Conversion of Glucose to 5-Hydroxymethylfurfural. Catalysts 2022, 12, 1502. https://doi.org/10.3390/catal12121502
Zhang Y, Zhao B, Das S, Degirmenci V, Walton RI. Tuning the Hydrophobicity and Lewis Acidity of UiO-66-NO2 with Decanoic Acid as Modulator to Optimise Conversion of Glucose to 5-Hydroxymethylfurfural. Catalysts. 2022; 12(12):1502. https://doi.org/10.3390/catal12121502
Chicago/Turabian StyleZhang, Yongzhao, Baiwen Zhao, Satarupa Das, Volkan Degirmenci, and Richard I. Walton. 2022. "Tuning the Hydrophobicity and Lewis Acidity of UiO-66-NO2 with Decanoic Acid as Modulator to Optimise Conversion of Glucose to 5-Hydroxymethylfurfural" Catalysts 12, no. 12: 1502. https://doi.org/10.3390/catal12121502
APA StyleZhang, Y., Zhao, B., Das, S., Degirmenci, V., & Walton, R. I. (2022). Tuning the Hydrophobicity and Lewis Acidity of UiO-66-NO2 with Decanoic Acid as Modulator to Optimise Conversion of Glucose to 5-Hydroxymethylfurfural. Catalysts, 12(12), 1502. https://doi.org/10.3390/catal12121502








