Fabrication of a Plasmonic Heterojunction for Degradation of Oxytetracycline Hydrochloride and Removal of Cr(VI) from Water
Abstract
1. Introduction
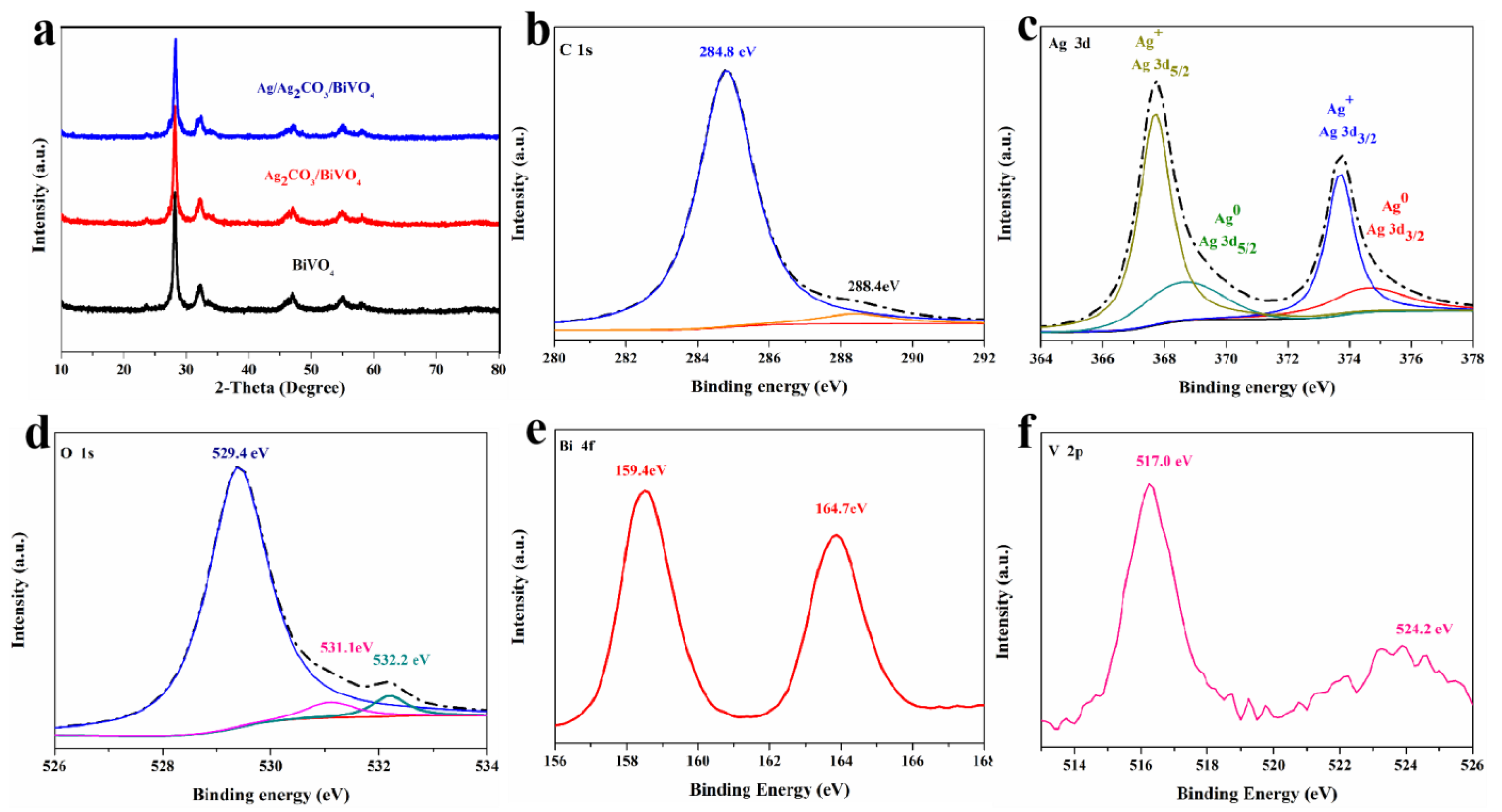
2. Results and Discussion
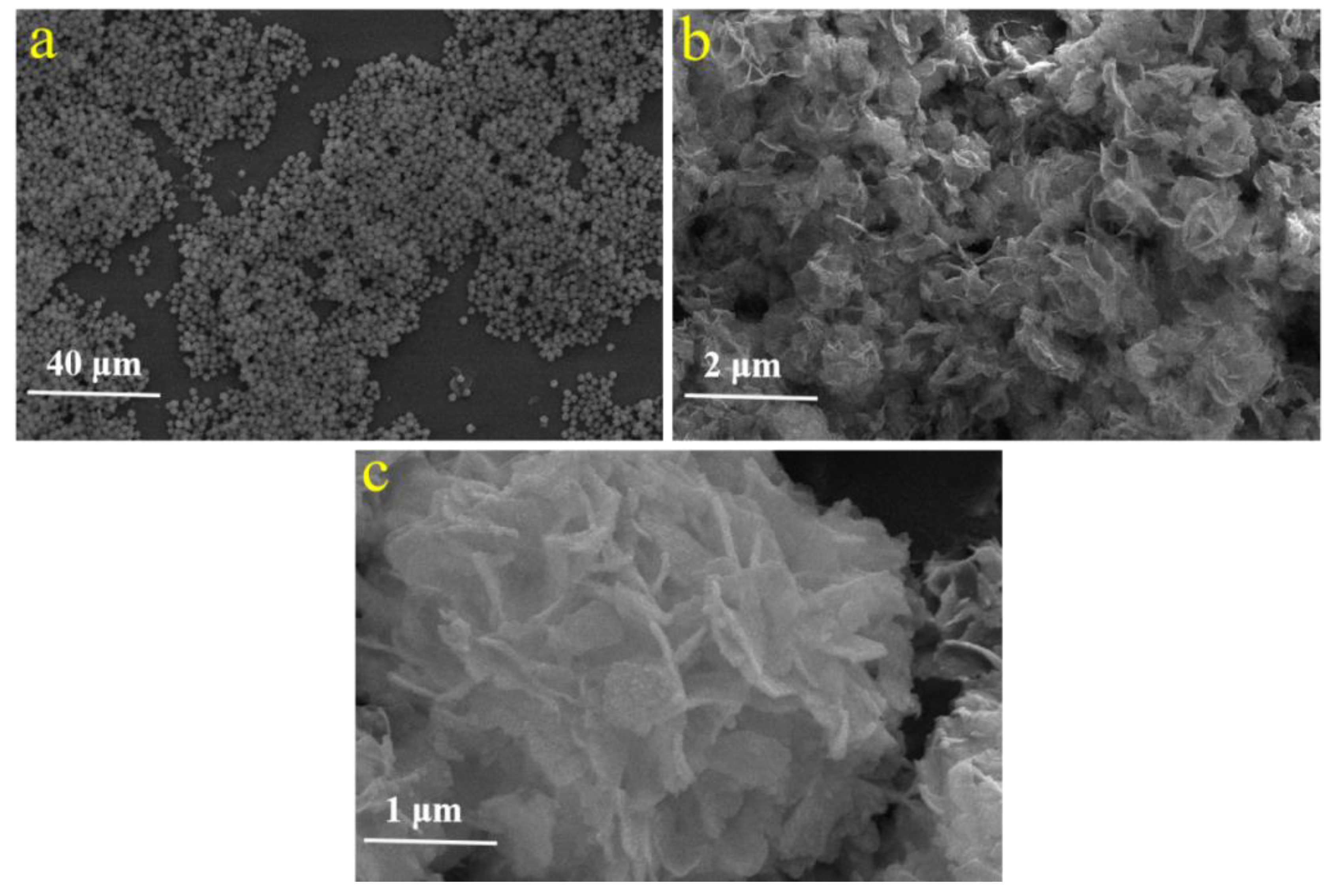
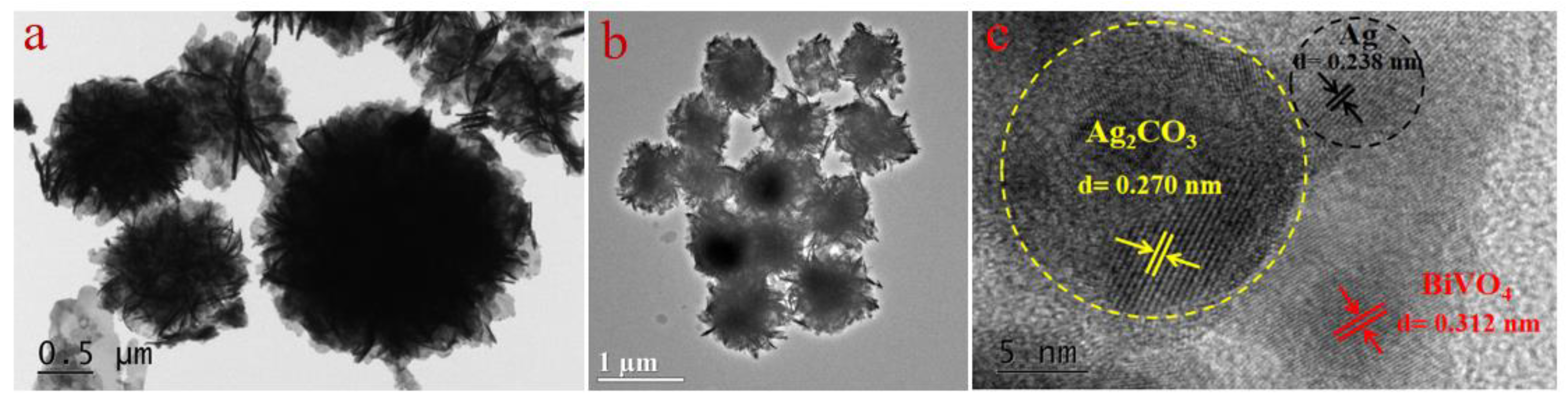
2.1. Morphology of Sample
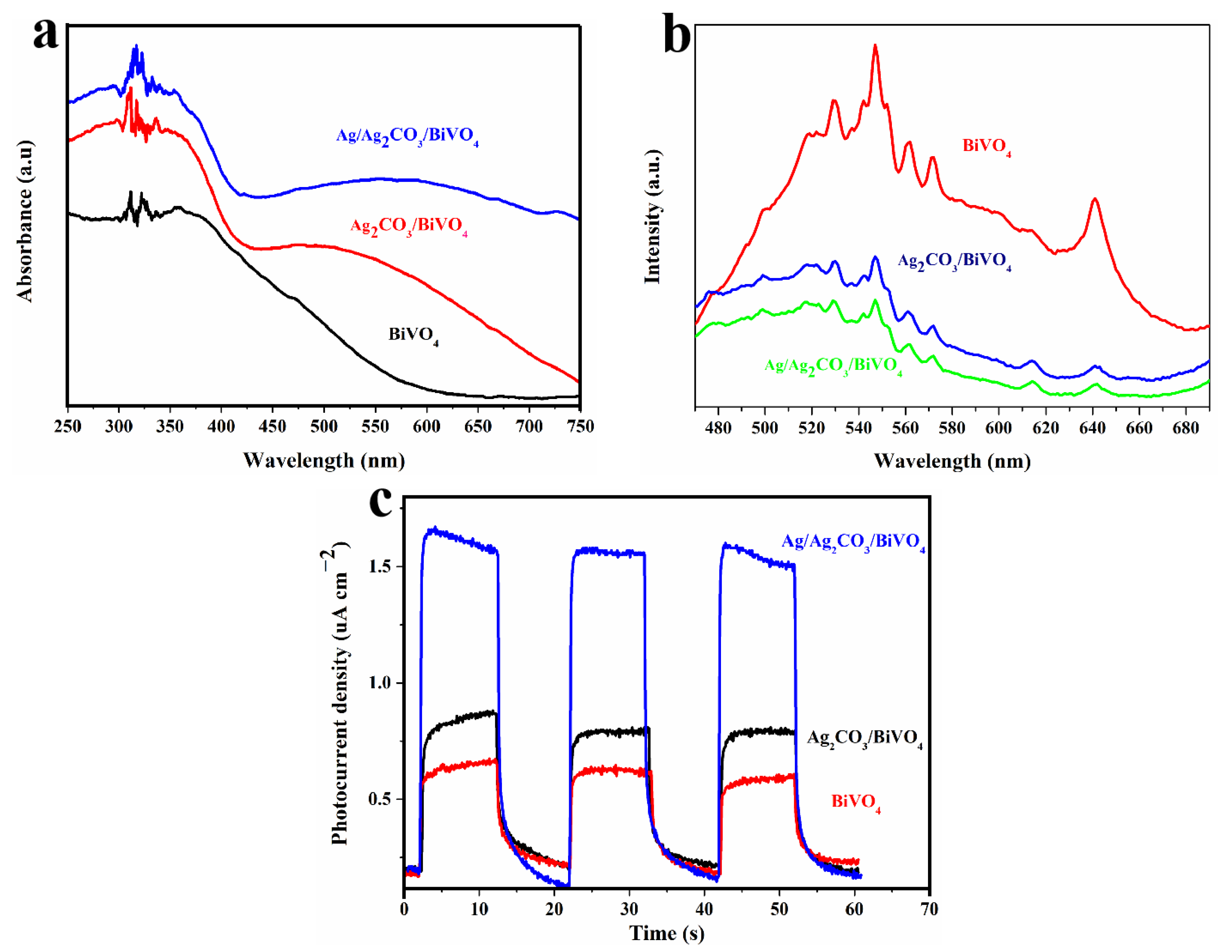
2.2. Optical Properties
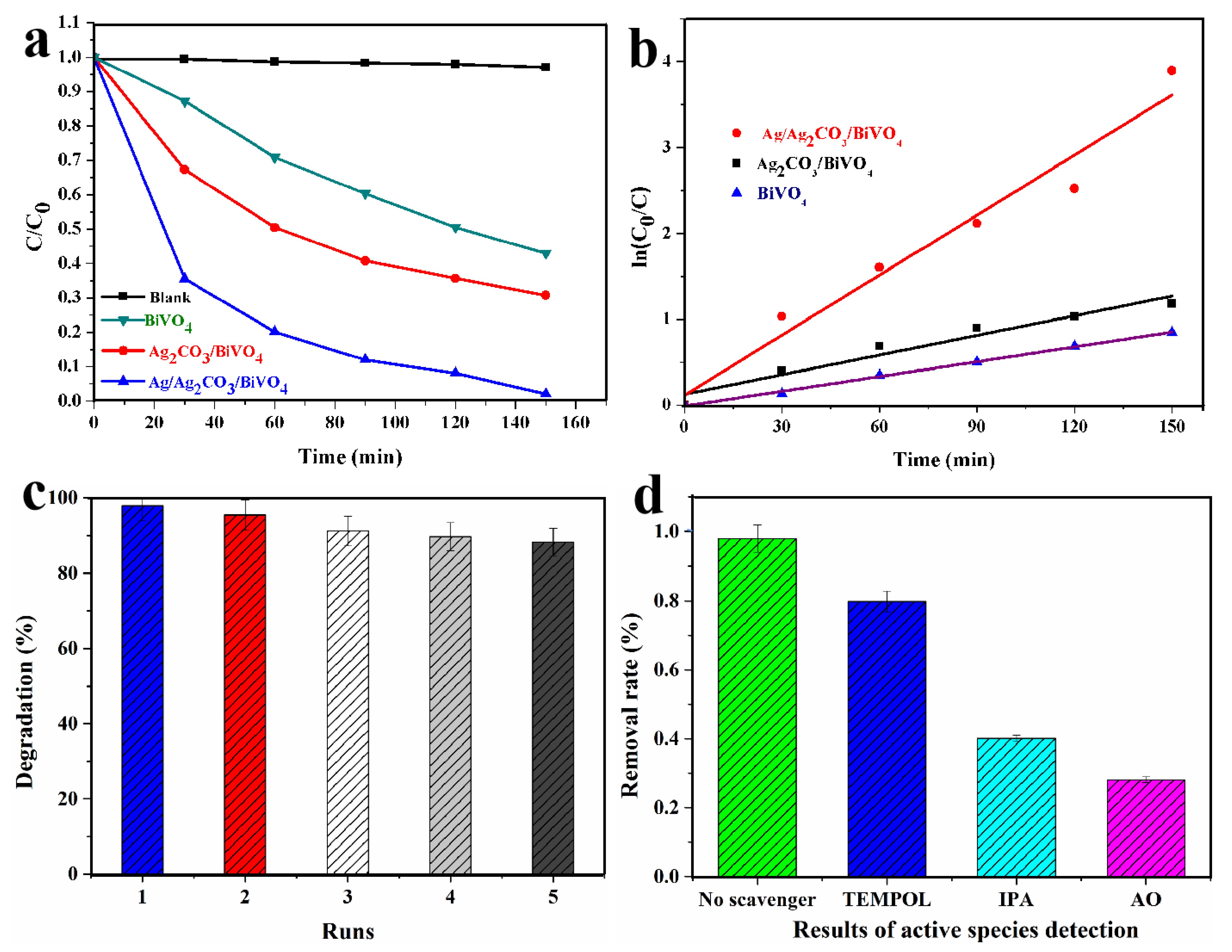
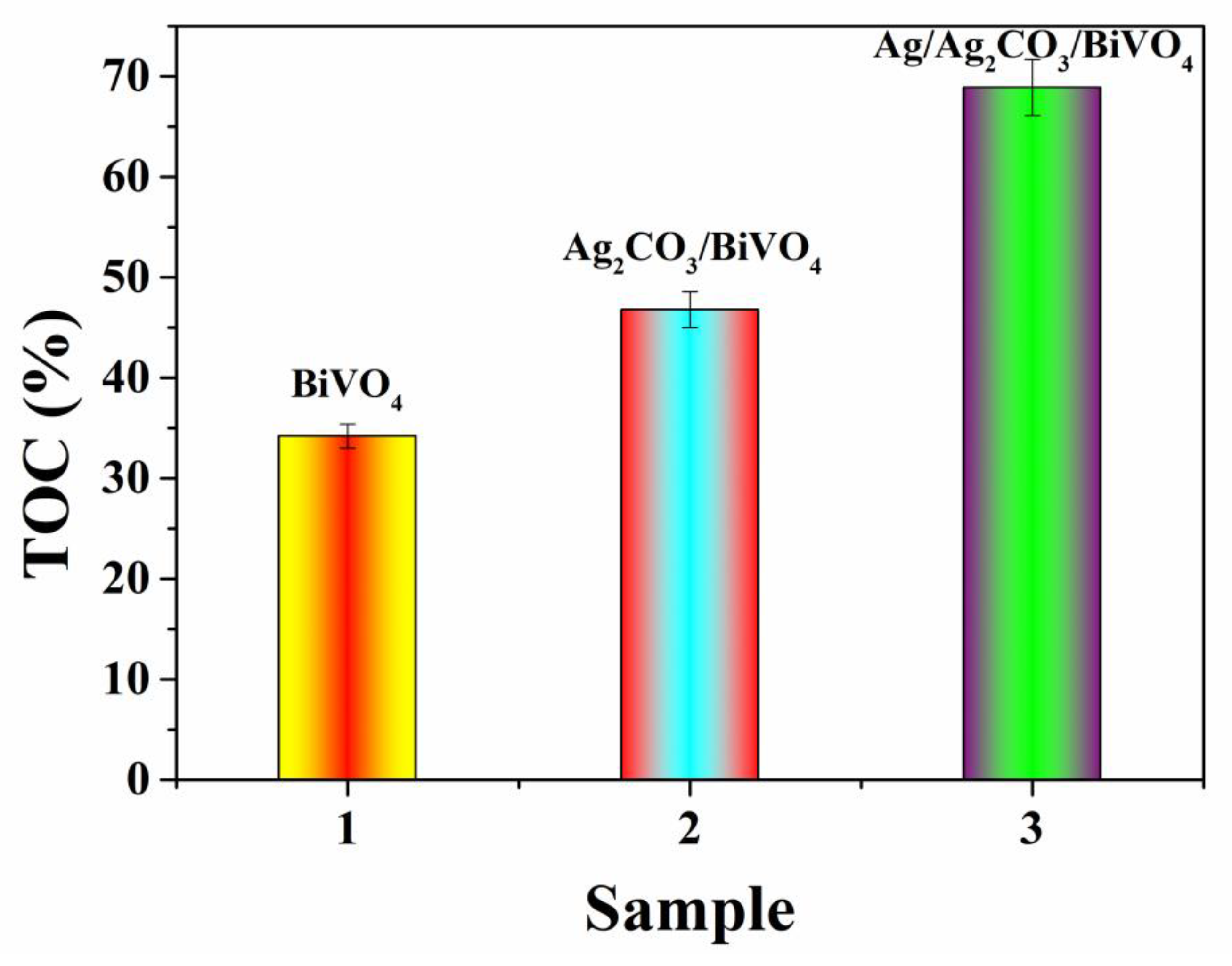
2.3. Photocatalytic Performance
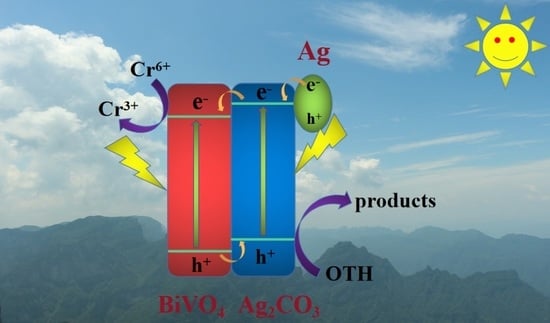
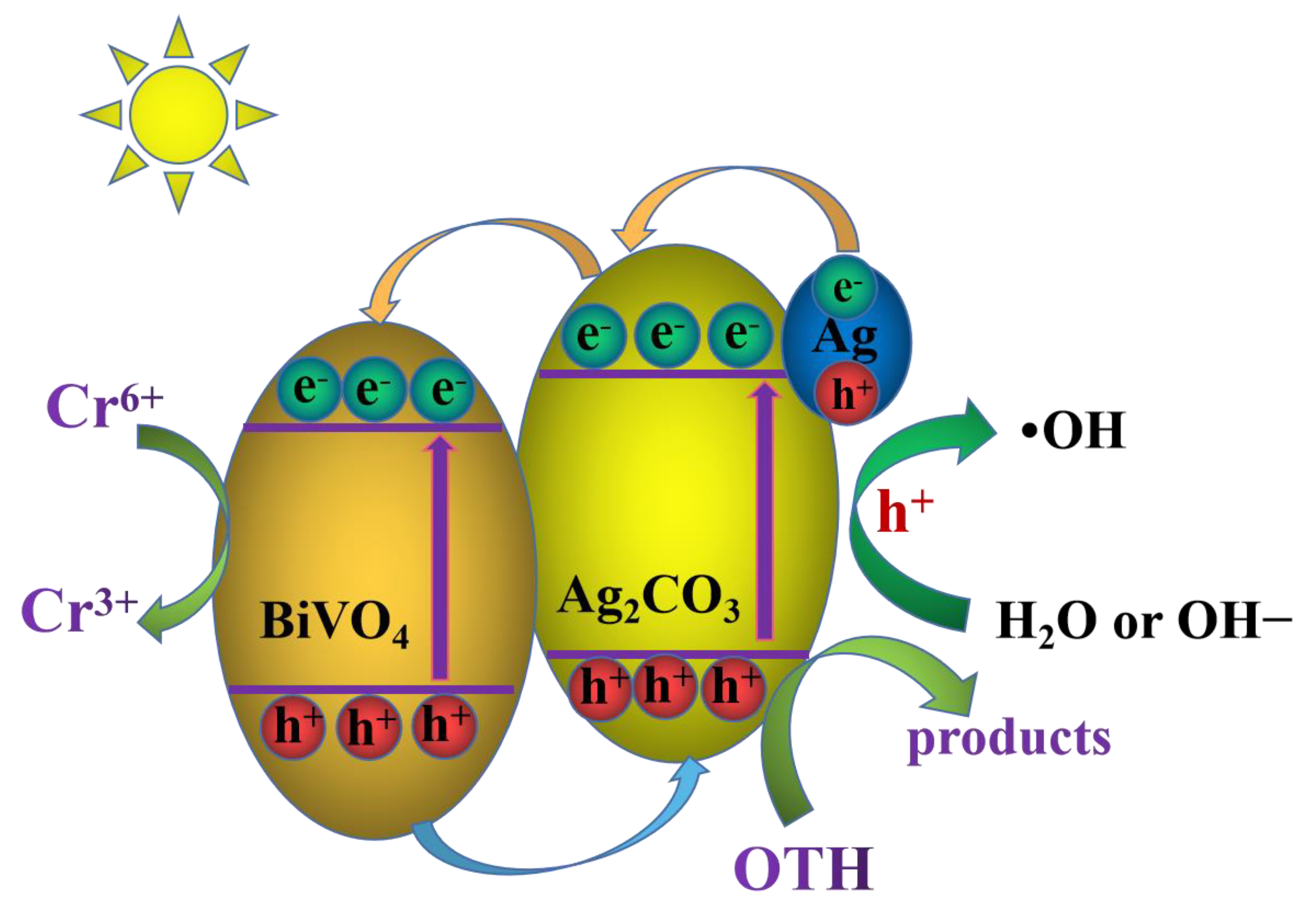
2.4. Photocatalytic Mechanism
3. Materials and Discussion
3.1. Materials
3.2. Preparation of Flower Globular BiVO4
3.3. Synthesized of Ag2CO3/BiVO4 Heterojunction Photocatalyst
3.4. Preparation of Ag/Ag2CO3/BiVO4 Photocatalyst
3.5. Photocatalytic Procedures
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, F.; Miao, X.; Cao, J.; Zhao, W.; Yang, G.; Zeng, H.; Li, S.; Sun, C. Integration of plasmonic effect and S-scheme heterojunction into gold decorated carbon nitride/cuprous oxide catalyst for photocatalysis. J. Clean. Prod. 2022, 360, 131948. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, S.; Yang, G.; Wang, G.; Zhang, L.; Xia, D.; Huang, H.; Cheng, Z.; Xu, J.; Sun, C.; et al. Z-scheme Au decorated carbon nitride/cobalt tetroxide plasmonic heterojunction photocatalyst for catalytic reduction of hexavalent chromium and oxidation of Bisphenol A. J. Hazard. Mater. 2021, 410, 124539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Dong, Q.; Sun, C.; Xia, D.; Huang, H.; Yang, G.; Wang, G.; Leung, D. A novel Au/g-C3N4 nanosheets/CeO2 hollow nanospheres plasmonic heterojunction photocatalysts for the photocatalytic reduction of hexavalent chromium and oxidation of oxytetracycline hydrochloride. Chem. Eng. J. 2021, 409, 128185. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, Y.; Wang, L.; Tang, H.; Meng, X.; She, X. Constructing 0D/1D Ag3PO4/TiO2 S-scheme heterojunction for efficient photodegradation and oxygen evolution. Chin. J. Catal. 2022, 43, 2558–2568. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Liu, Y.; Wang, H.; Yang, D. Ternary red phosphorus/CoP2/SiO2 microsphere boosts visible-light-driven photocatalytic hydrogen evolution from pure water splitting. J. Mater. Sci. Technol. 2022, 125, 59–66. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings 2019, 9, 465. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma. Chem. Eng. J. 2017, 313, 1033–1041. [Google Scholar] [CrossRef]
- Abdulla, S.M.; Jamil, D.M.; Aziz, K.H.H. Investigation in heavy metal contents of drinking water and fish from Darbandikhan and Dokan Lakes in Sulaimaniyah Province—Iraqi Kurdistan Region. IOP Conf. Ser. Earth Environ. Sci. 2020, 612, 012023. [Google Scholar] [CrossRef]
- Sun, F.; Xie, Y.; Qi, H.; He, W.; Xu, D.; Yu, W.; Yang, M.; Ma, Q.; Yang, Y.; Dong, X. Construction, structure and photocatalysis of janus nanofiber modified by g-C3N4 nanosheets heterostructure photocatalysts. Ceram. Int. 2021, 47, 28848–28858. [Google Scholar] [CrossRef]
- Haque, M.M.; Khan, A.; Umar, K.; Mir, N.A.; Muneer, M.; Harada, T.; Matsumura, M. Synthesis, Characterization and Photocatalytic Activity of Visible Light Induced Ni-Doped TiO2. Energy Environ. Focus 2013, 2, 73–78. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.Z.; Lu, Y.; Song, Z.; Wang, C.; Li, D.; Tang, X.; Zhou, X. Surface hydroxylation of TiO2/g-C3N4 photocatalyst for photo-Fenton degradation of tetracycline. Ceram. Int. 2022, 48, 1306–1313. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Saha, J.; De, G. Electrospun anatase TiO2nanofibers with ordered mesoporosity. J. Mater. Chem. A 2014, 2, 19029–19035. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, J.; Rao, F.; Liu, H.; Wang, Y.; Qu, D.; Zhong, P.; Zhu, G. Ti3C2@TiO2/g-C3N4 heterojunction photocatalyst with improved charge transfer for enhancing visible-light NO selective removal. Ceram. Int. 2021, 47, 31302–31310. [Google Scholar] [CrossRef]
- Wang, M.; Pang, X.; Zheng, D.; He, Y.; Sun, L.; Lin, C.; Lin, Z. Nonepitaxial growth of uniform and precisely size-tunable core/shell nanoparticles and their enhanced plasmon-driven photocatalysis. J. Mater. Chem. A 2016, 4, 7190–7199. [Google Scholar] [CrossRef]
- Umar, K.; Dar, A.A.; Haque, M.; Mir, N.; Muneer, M. Photocatalysed decolourization of two textile dye derivatives, Martius Yellow and Acid Blue 129, in UV-irradiated aqueous suspensions of Titania. Desalination Water Treat. 2012, 46, 205–214. [Google Scholar] [CrossRef]
- Huang, G.; Ye, W.; Lv, C.; Butenko, D.S.; Yang, C.; Zhang, G.; Lu, P.; Xu, Y.; Zhang, S.; Wang, H.; et al. Hierarchical red phosphorus incorporated TiO2 hollow sphere heterojunctions toward superior photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 108, 18–25. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Ye, W.; Butenko, D.S.; Lu, P.; Chen, Q.; Cai, R.; Sun, J.; Zhu, Y.; Yang, D. FeOx nanoclusters decorated TiO2 for boosting white LED driven photocatalytic Fenton-like norfloxacin degradation. Sep. Purif. Technol. 2022, 303, 122194. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Lv, K.; Chen, X. S-Scheme photocatalyst TaON/Bi2WO6 nanofibers with oxygen vacancies for efficient abatement of antibiotics and Cr(VI): Intermediate eco-toxicity analysis and mechanistic insights. Chin. J. Catal. 2022, 43, 2652–2664. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Tadé, M.O.; Liu, S. Quantum-sized BiVO4 modified TiO2 microflower composite heterostructures: Efficient production of hydroxyl radicals towards visible light-driven degradation of gaseous toluene. J. Mater. Chem. A 2015, 3, 21655–21663. [Google Scholar] [CrossRef]
- Lei, W.; Wang, H.; Zhang, X.; Yang, Z.; Kong, C. Cu2O-based binary and ternary photocatalysts for the degradation of organic dyes under visible light. Ceram. Int. 2022, 48, 1757–1764. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.; Yu, L.; Wang, Y.; Ning, J.; Xu, S.; Lou, X.W. Carbon-coated CdS petalous nanostructures with enhanced photostability and photocatalytic activity. Angew. Chem. 2013, 125, 5746–5749. [Google Scholar] [CrossRef]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Lei, L.; Wang, W.; Wang, C.; Zhang, M.; Zhong, Q.; Fan, H. In situ growth of boron doped g-C3N4 on carbon fiber cloth as a recycled flexible film-photocatalyst. Ceram. Int. 2021, 47, 1258–1267. [Google Scholar] [CrossRef]
- Boltersdorf, J.; Sullivan, I.; Shelton, T. Flux synthesis, optical and photocatalytic properties of n-type Sn2TiO4: Hydrogen and oxygen evolution under visible light. Chem. Mater. 2016, 28, 8876–8889. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Lv, R.; Lu, J.; Wang, T.; Wang, S.; Wang, H.; Gong, J. Photocatalysis: Selective deposition of Ag3PO4 on monoclinic BiVO4 (040) for highly efficient photocatalysis. Small 2013, 9, 3951–3956. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, G.; Wei, S.; Ren, H.; Xia, A.; Luo, Y. Microwave hydrothermal synthesis and photocatalytic properties of TiO2/BiVO4 composite photocatalysts. Ceram. Int. 2013, 39, 8597–8604. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhao, J.; Nail, B.A.; Yuan, X.; Guo, Y.; Osterloh, F.E. Photochemical charge separation at particle interfaces: The n-BiVO4–p-Silicon System. ACS Appl. Mater. Inter. 2015, 7, 5959–5964. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Liu, B. Synthesis of BiVO4 nanoparticles with tunable oxygen vacancy level: The phenomena and mechanism for their enhanced photocatalytic performance. Ceram. Int. 2021, 47, 9849–9855. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y.; Li, N.; Zhang, P.; Li, X. Rationally designed Ta3N5/BiOCl S-scheme heterojunction with oxygen vacancies for elimination of tetracycline antibiotic and Cr(VI): Performance, toxicity evaluation and mechanism insight. J. Mater. Sci. Technol. 2022, 123, 177–190. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Cai, M.; Yan, R.; Dong, K.; Zhang, J.; Liu, Y. Rationally designed tetra (4-carboxyphenyl) porphyrin/graphene quantum dots/bismuth molybdate Z-scheme heterojunction for tetracycline degradation and Cr(VI) reduction: Performance, mechanism, intermediate toxicity appraisement. J. Colloid Interface Sci. 2022, 619, 307–321. [Google Scholar] [CrossRef]
- Cai, M.; Wang, C.; Liu, Y.; Yan, R.; Li, S. Boosted photocatalytic antibiotic degradation performance of Cd0.5Zn0.5S/carbon dots/Bi2WO6 S-scheme heterojunction with carbon dots as the electron bridge. Sep. Purif. Technol. 2022, 300, 121892. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Wang, Y.; Zhu, B.; Yang, J. Fabrication of magnetically recoverable Fe3O4/CdS/g-C3N4 photocatalysts for effective degradation of ciprofloxacin under visible light. Ceram. Int. 2020, 46, 20974–20984. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, A.; Xiao, Y.; Yu, H.; Dong, X. Two-dimensional/two-dimensional Z-scheme photocatalyst of graphitic carbon nitride/bismuth vanadate for visible-light-driven photocatalytic synthesis of imines. Ceram. Int. 2020, 46, 16157–16165. [Google Scholar] [CrossRef]
- Yin, C.; Zhu, S.; Chen, Z.; Zhang, W.; Gu, J.; Zhang, D. One step fabrication of C-doped BiVO4 with hierarchical structures for a high-performance photocatalyst under visible light irradiation. J. Mater. Chem. A 2013, 1, 8367–8378. [Google Scholar] [CrossRef]
- Liu, N.; Ouyang, D.; Cai, Y.; Li, Y. Heterostructured Fe2O3/BiVO4 nano-photocatalyst for the reduction of nitroarenes into amines. Ceram. Int. 2020, 46, 24534–24543. [Google Scholar] [CrossRef]
- Zalfani, M.; Schueren, B.; Hu, Z.; Rooke, J.; Bourguiga, R.; Wu, M.; Li, Y.; Tendeloo, G.; Su, B. Novel 3DOM BiVO4/TiO2 nanocomposites for highly enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 21244–21256. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, S.; Liu, Y.; Shen, Y.; Li, F.; Zhu, S.; Zheng, X. Hydrothermal-photoreduction synthesis of novel Ag@AgBr/BiVO4 plasmonic heterojunction photocatalysts with enhanced activity under white light emitting diode (wLED) irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 17602–17611. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, T.; Wei, Y.; Zhang, P.; Gong, J. Monoclinic porous BiVO4 networks decorated by discrete g-C3N4 nano-islands with tunable coverage for highly efficient photocatalysis. Small 2014, 10, 2783–2790. [Google Scholar] [CrossRef]
- Wang, J.; Osterloh, F. Limiting factors for photochemical charge separation in BiVO4/Co3O4, a highly active photocatalyst for water oxidation in sunlight. J. Mater. Chem. A 2014, 2, 9405–9411. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.B.; Zheng, L.; Zhong, Y.; Hu, Y.; Lou, X.W.D. Formation of Mesoporous Heterostructured BiVO4/Bi2S3 Hollow Discoids with Enhanced Photoactivity. Angew. Chem. Int. Ed. 2014, 126, 6027–6031. [Google Scholar] [CrossRef]
- Gutkowski, R.; Peeters, D.; Schuhmann, W. Improved photoelectrochemical performance of electrodeposited metal-doped BiVO4 on Pt-nanoparticle modified FTO surfaces. J. Mater. Chem. A 2016, 4, 7875–7882. [Google Scholar] [CrossRef]
- Patil, S.; Dubal, D.; Tamboli, M.; Ambekar, J.; Kolekar, S.; Gomez-Romero, P.; Kale, B.; Patil, D. Ag:BiVO4 dendritic hybrid-architecture for high energy density symmetric supercapacitors. J. Mater. Chem. A 2016, 4, 7580–7584. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Wei, B. Fabrication of a ternary plasmonic photocatalyst of Ag/AgVO3/RGO and its excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 179, 9–20. [Google Scholar] [CrossRef]
- Hammond, J.S.; Gaarenstroom, S.W.; Winograd, N. X-ray photoelectron spectroscopic studies of cadmium- and silver-oxygen surfaces. Anal. Chem. 1975, 47, 2193–2199. [Google Scholar] [CrossRef]
- Akhter, S.; Allan, K.; Buchanan, D.; Cook, J.; Campion, A.; White, J. XPS and IR study of X-ray induced degradation of PVA polymer film. Appl. Surf. Sci. 1988, 35, 241–258. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, W.S.; Lin, L.; Gao, G.Q.; Guo, H.L.; Du, H.; Xu, A.W. Facile synthesis of the novel Ag3VO4/AgBr/Ag plasmonic photocatalyst with enhanced photocatalytic activity and stability. J. Phys. Chem. C 2013, 117, 5894–5900. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, J.; Yuan, J.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem. Eng. J. 2018, 331, 242–254. [Google Scholar] [CrossRef]
- Gauzzi, A.; Mathieu, H.J.; James, J.H.; Kellett, B. AES, XPS and SIMS characterization of YBa2Cu3O7 superconducting high Tc thin fifilms. Vacuum 1990, 41, 870–874. [Google Scholar] [CrossRef]
- Ayyoob, H.M.S. Electron spectroscopic studies of formic acid adsorption and oxidation on Cu and Ag dosed with barium. J. Chem. Soc. 1986, 82, 1651–1662. [Google Scholar] [CrossRef]
- Liang, S.Q.; Zhou, J.; Zhang, X.L.; Tang, Y.; Fang, G.Z.; Chen, T.; Tan, X.P. Hydrothermal synthesis of Ag/β-AgVO3 nanobelts with enhanced performance as a cathode material for lithium batteries. Cryst. Eng. Comm. 2013, 15, 9869–9873. [Google Scholar] [CrossRef]
- Zhou, X.F.; Hu, C.; Hu, X.X.; Peng, T.W.; Qu, J.H. Plasmon-Assisted Degradation of Toxic Pollutants with Ag-AgBr/Al2O3 under Visible-Light Irradiation. J. Phys. Chem. C. 2010, 114, 2746–2750. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Yu, S.; Shen, X. Efficient removal of organic contaminants by a visible light driven photocatalyst Sr6Bi2O9. Chem. Eng. J. 2010, 162, 171–177. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A Chem. 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Zhao, W.; Dai, B.; Zhu, F.; Tu, X.; Xu, J.; Zhang, L.; Li, S.; Dennis, Y.C.L.; Sun, C. A novel 3D plasmonic p-n heterojunction photocatalyst: Ag nanoparticles on flower-like p-Ag2S/n-BiVO4 and its excellent photocatalytic reduction and oxidation activities. Appl. Catal. Environ. 2018, 229, 171–180. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhang, M.; Yang, X.; Zeng, X.; Yang, Y.; Li, Y.; Zeng, H.; Zhao, W. Fabrication of a Plasmonic Heterojunction for Degradation of Oxytetracycline Hydrochloride and Removal of Cr(VI) from Water. Catalysts 2022, 12, 1498. https://doi.org/10.3390/catal12121498
Cao J, Zhang M, Yang X, Zeng X, Yang Y, Li Y, Zeng H, Zhao W. Fabrication of a Plasmonic Heterojunction for Degradation of Oxytetracycline Hydrochloride and Removal of Cr(VI) from Water. Catalysts. 2022; 12(12):1498. https://doi.org/10.3390/catal12121498
Chicago/Turabian StyleCao, Jihui, Meihua Zhang, Xinran Yang, Xiaojun Zeng, Yubo Yang, Yuanyi Li, Hehua Zeng, and Wei Zhao. 2022. "Fabrication of a Plasmonic Heterojunction for Degradation of Oxytetracycline Hydrochloride and Removal of Cr(VI) from Water" Catalysts 12, no. 12: 1498. https://doi.org/10.3390/catal12121498
APA StyleCao, J., Zhang, M., Yang, X., Zeng, X., Yang, Y., Li, Y., Zeng, H., & Zhao, W. (2022). Fabrication of a Plasmonic Heterojunction for Degradation of Oxytetracycline Hydrochloride and Removal of Cr(VI) from Water. Catalysts, 12(12), 1498. https://doi.org/10.3390/catal12121498