Structural Distortion of g-C3N4 Induced by N-Defects for Enhanced Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Chemical Reagents
2.2. Synthesis of Photocatalysts
2.3. Characterization
2.4. Photoelectrochemical Measurements
2.5. Photocatalytic H2 Evolution
2.6. Computational Details
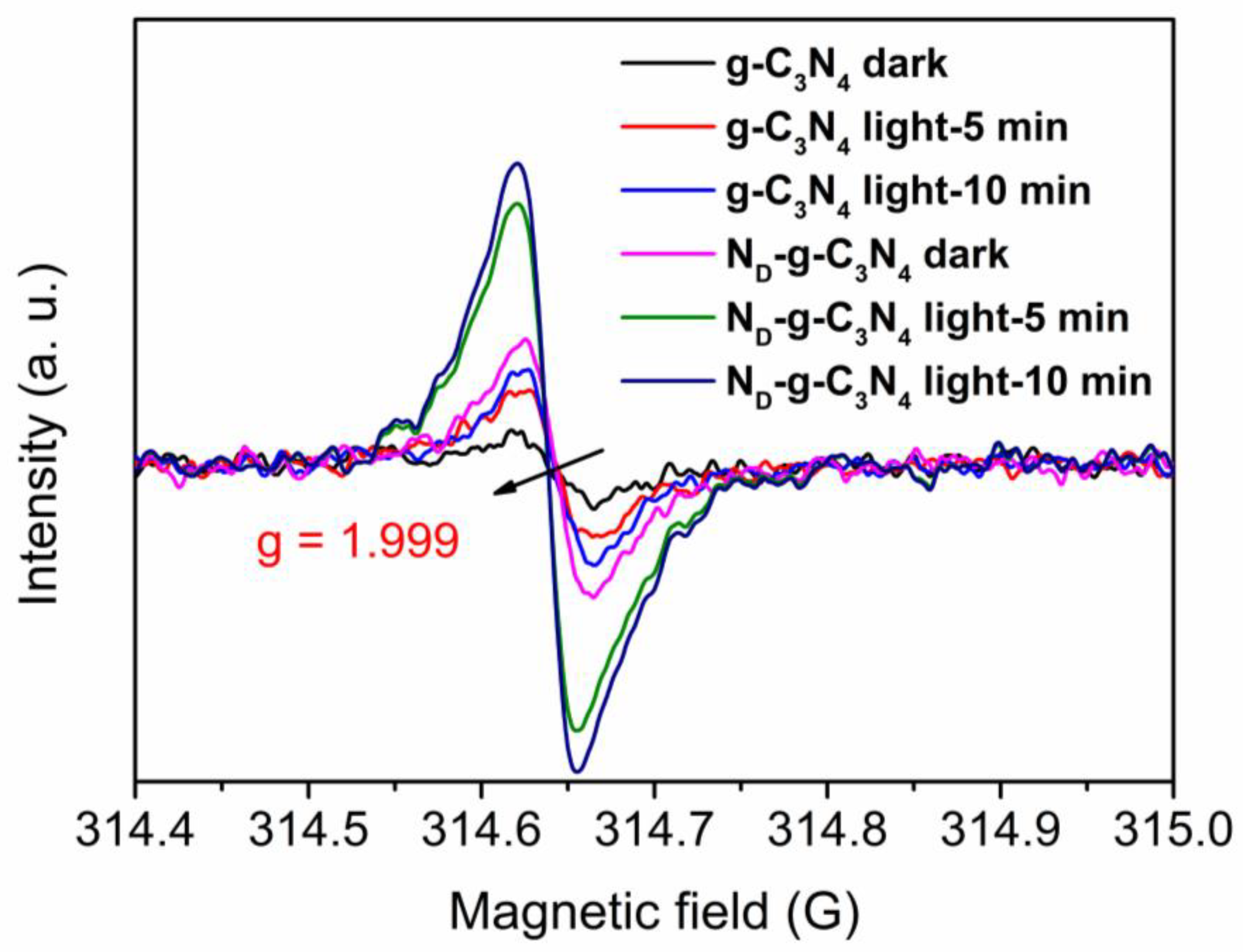
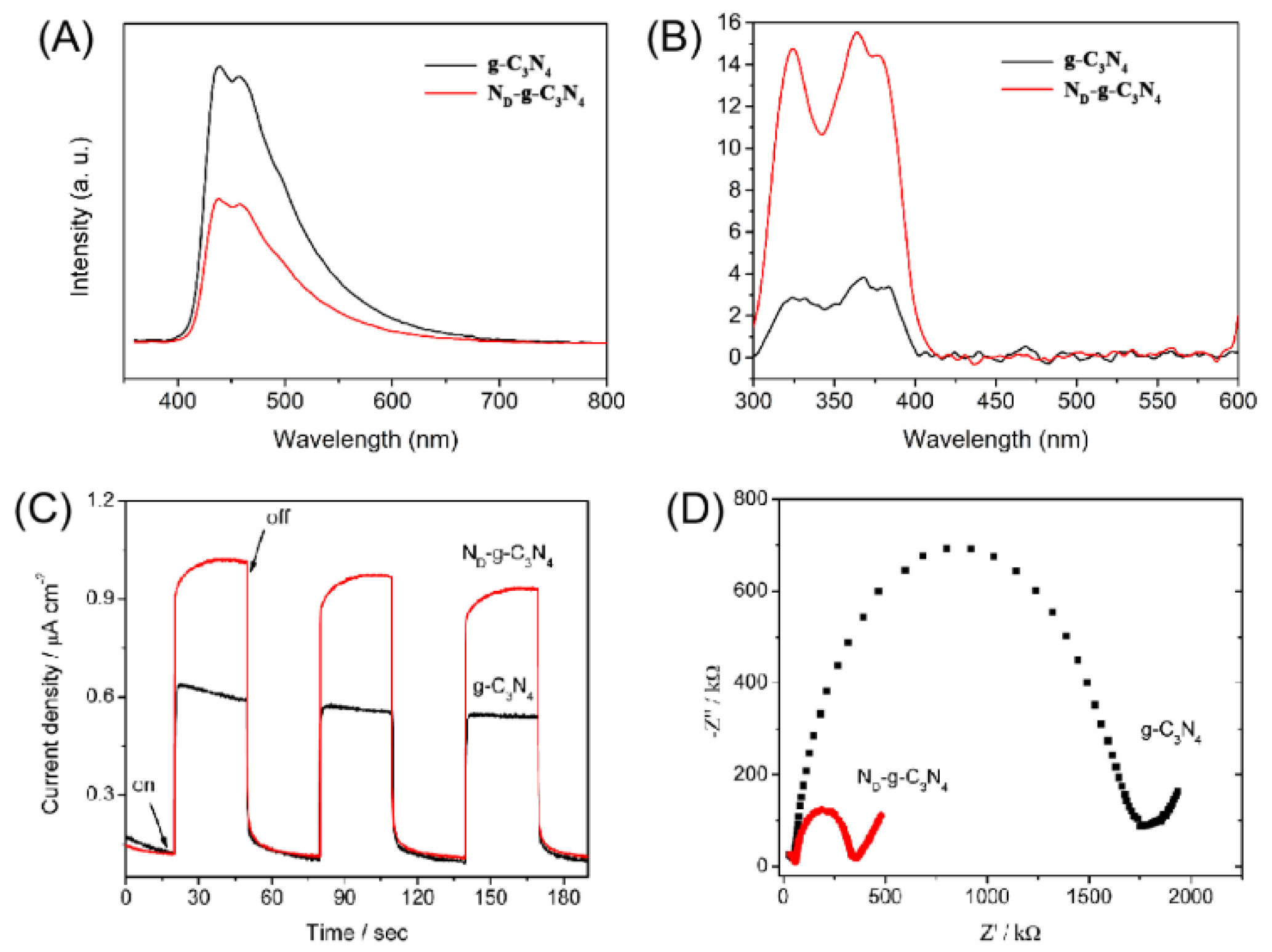
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Zhou, W.; Chi, L.; Zhang, X.; Hu, W.; Jiang, B.; Pan, K.; Tian, G.; Jiang, Z. Black N/H-TiO2 nanoplates with a flower-like hierarchical architecture for photocatalytic hydrogen evolution. ChemSusChem 2016, 9, 2841–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, F.Y.; Zhang, W.D. Fabrication and photoelectrochemical property of In2O3/ZnO composite nanotube arrays using ZnO nanorods as self-sacrificing templates. Mater. Lett. 2018, 211, 65–68. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability. Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Majdoub, M.; Anfar, Z.; Amedlou, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Zhao, L.; Wu, B.; Zhang, B.; Zhang, P.; Zhang, S.; Dong, C. Boosting exciton dissociation and charge transfer in P-doped 2D porous g-C3N4 for enhanced H2 production and molecular oxygen activation. Ceram. Int. 2022, 48, 4031–4046. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Tian, W.; Meng, A.; Li, Z.; Li, S.; Wang, L.; Li, G. High-energy ball-milling constructing P-doped g-C3N4/MoP heterojunction with MoN bond bridged interface and Schottky barrier for enhanced photocatalytic H2 evolution. Appl. Catal. B Environ. 2022, 303, 120933. [Google Scholar] [CrossRef]
- Andryushina, N.; Shvalagin, V.; Korzhak, G.; Grodzyuk, G.; Kuchmiy, S.; Skoryk, M. Photocatalytic evolution of H2 from aqueous solutions of two-component electron-donor substrates in the presence of g-C3N4 activated by heat treatment in the KCl+ LiCl melt. Appl. Surf. Sci. 2019, 475, 348–354. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, D.; Wang, G.; Gao, T.; Wang, J.; Lu, L.; Li, J. Carbon dots incorporated in hierarchical macro/mesoporous g-C3N4/TiO2 as an all-solid-state Z-scheme heterojunction for enhancement of photocatalytic H2 evolution under visible light. Appl. Surf. Sci. 2022, 601, 154167. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z.; Zhao, J.; Sun, X.; Wang, R.; Liao, G.; Jia, X. 1D/2D carbon-doped nanowire/ultra-thin nanosheet g-C3N4 isotype heterojunction for effective and durable photocatalytic H2 evolution. Int. J. Hydrog. Energy 2021, 46, 25436–25447. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Fu, H.; Ran, X.; Zhao, C.; An, X. Integrating optimal amount of carbon dots in g-C3N4 for enhanced visible light photocatalytic H2 evolution. Int. J. Hydrog. Energy 2022, 47, 18032–18043. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, Y.; Yang, L.; Zeng, Y.; Yuan, J.; Liu, T.; Zhang, S.; Liu, C.; Luo, S. Porous nitrogen-rich carbon materials from carbon self-repairing g-C3N4 assembled with graphene for high-performance supercapacitor. J. Mater. Chem. A 2016, 4, 14307–14315. [Google Scholar] [CrossRef]
- Wang, J.; Pan, R.; Hao, Q.; Gao, Y.; Ye, J.; Wu, Y.; van Ree, T. Constructing Defect-Mediated CdS/g-C3N4 by an In-situ Interlocking Strategy for Cocatalyst-free Photocatalytic H2 Production. Appl. Surf. Sci. 2022, 599, 153875. [Google Scholar] [CrossRef]
- Ye, L.; Deng, Y.; Wang, L.; Xie, H.; Su, F. Bismuth-based photocatalysts for solar photocatalytic carbon dioxide conversion. ChemSusChem 2019, 12, 3671–3701. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Shen, B.; Chen, Y.; Lin, B.; Gao, B. Enhancement of photocatalytic H2 evolution over nitrogen-deficient graphitic carbon nitride. J. Mater. Chem. A 2013, 1, 11754–11761. [Google Scholar] [CrossRef]
- Su, K.; Liu, H.; Gao, Z.; Fornasiero, P.; Wang, F. Nb2O5-Based Photocatalysts. Adv. Sci. 2021, 8, 2003156. [Google Scholar] [CrossRef]
- Jorge, A.B.; Martin, D.J.; Dhanoa, M.T.; Rahman, A.S.; Makwana, N.; Tang, J.; Sella, A.; Cora, F.; Darr, J.A.; Firth, S.; et al. H2 and O2 Evolution from Water half-splitting reactions by graphitic carbon nitride materials. J. Phys. Chem. C 2013, 117, 7178–7185. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Lin, S.; Zhang, Y.; Wang, X. Activation of n → π* transitions in two-dimensional conjugated polymers for visible light photocatalysis. J. Phys. Chem. C 2014, 118, 29981–29989. [Google Scholar] [CrossRef]
- Su, F.Y.; Xu, C.Q.; Yu, Y.X.; Zhang, W.D. Carbon self-doping induced activation of n–π* electronic transitions of g-C3N4 nanosheets for efficient photocatalytic H2 evolution. ChemCatChem 2016, 8, 3527–3535. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Kong, L.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, H.; Zhao, N. Light-assisted rapid preparation of a Ni/g-C3N4 magnetic composite for robust photocatalytic H2 evolution from water. J. Mater. Chem. A 2016, 4, 9998–10007. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.H.; Zhao, Y.; Zhang, Z.P.; Qu, L.T. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Peng, T.Y.; Zhang, X.H.; Li, K.; Ye, L.Q.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol. 2013, 3, 1253–1260. [Google Scholar] [CrossRef]
- Su, F.Y.; Zhang, W.D. Creating distortion in g-C3N4 framework by incorporation of ethylenediaminetetramethylene for enhancing photocatalytic generation of hydrogen. Mol. Catal. 2017, 432, 64–75. [Google Scholar] [CrossRef]
- Su, F.Y.; Zhang, W.D. Carbonyl-Grafted g-C3N4 Porous Nanosheets for Efficient Photocatalytic Hydrogen Evolution. Chem.–Asian J. 2017, 12, 515–523. [Google Scholar] [CrossRef]
- Li, K.; Xie, X.; Zhang, W. Photocatalysts based on g-C3N4-encapsulating carbon spheres with high visible light activity for photocatalytic hydrogen evolution. Carbon 2016, 110, 356–366. [Google Scholar] [CrossRef]
- Qin, Z.X.; Xue, F.; Chen, Y.B.; Shen, S.H.; Guo, L.J. Spatial charge separation of one-dimensional Ni2P-Cd0.9Zn0.1S/g-C3N4 heterostructure for high-quantum-yield photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 217, 551–559. [Google Scholar] [CrossRef]
- Araña, J.; Doña-Rodríguez, J.M.; Cabo, C.G.; González-Díaz, O.; Herrera-Melián, J.A.; Pérez-Peña, J. FTIR study of gas-phase alcohols photocatalytic degradation with TiO2 and AC-TiO2. Appl. Catal. B Environ. 2004, 53, 221–232. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Kong, L.; Mu, X.; Fan, X.; Li, R.; Zhang, Y.; Song, P.; Ma, F.; Sun, M. Site-selected N vacancy of g-C3N4 for photocatalysis and physical mechanism. Appl. Mater. Today 2018, 13, 329–338. [Google Scholar] [CrossRef]
- Ruan, D.; Kim, S.; Fujitsuka, M.; Majima, T. Defects rich g-C3N4 with mesoporous structure for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B Environ. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.; Tung, C.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, Z.; Tang, C.; Zhou, Y.; Zeng, L.; Huang, L. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B Environ. 2019, 240, 30–38. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Wang, Z.; Wu, H.; Zhou, X.; Ma, H.; Zhong, H.; Xing, Z.; Cai, G.; Jiang, C.; et al. C/N vacancy co-enhanced visible-light-driven hydrogen evolution of g-C3N4 nanosheets through controlled He+ ion irradiation. Sol. RRL 2019, 3, 1800298. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, H.; Luo, J.; Yuan, J.; Wang, L.; Liu, C.; Xia, Y.; Liu, M.; Luo, S.; Cai, T.; et al. Sea-urchin-structure g-C3N4 with narrow bandgap (~2.0 eV) for efficient overall water splitting under visible light irradiation. Appl. Catal. B Environ. 2019, 249, 275–281. [Google Scholar] [CrossRef]
- Yan, B.; Du, C.; Yang, G. Constructing Built-in Electric Field in Ultrathin Graphitic Carbon Nitride Nanosheets by N and O Codoping for Enhanced Photocatalytic Hydrogen Evolution Activity. Small 2020, 16, 1905700. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, J.; Wen, L.; Dong, C.L.; Huang, Y.C.; Zhang, Y.; Zong, S.; Diao, Z.; Shen, S.; Guo, L. Disordered nitrogen-defect-rich porous carbon nitride photocatalyst for highly efficient H2 evolution under visible-light irradiation. Carbon 2021, 181, 193–203. [Google Scholar] [CrossRef]
- Ge, L.; Zuo, F.; Liu, J.K.; Ma, Q.; Wang, C.; Sun, D.Z.; Bartels, L.; Feng, P.Y. Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 coupled with CdS quantum dots. J. Phys. Chem. C 2012, 116, 13708–13714. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Q.; Zeng, Y.; Liu, Z. Hollow spherical biomass derived-carbon dotted with SnS2/g-C3N4 Z-scheme heterojunction for efficient CO2 photoreduction into CO. Chem. Eng. J. 2022, 438, 135652. [Google Scholar] [CrossRef]







| Sample | N (wt%) | C (wt%) | H (wt%) | N/C (at.) |
|---|---|---|---|---|
| g-C3N4 | 60.02 | 34.26 | 2.242 | 1.502 |
| ND-g-C3N4 | 59.75 | 34.40 | 2.062 | 1.489 |
| Photocatalytic Materials | Cocatalyst | Light Source | Reaction Solutions | Hydrogen Evolution Rate | Ref. |
|---|---|---|---|---|---|
| ND-C3N4 | 3% wt% Pt | λ > 400 nm | 90 mL water and 10 mL triethanolamine | 865.2 μmol/g/h | This work |
| N-deficient g-C3N4 nanosheets | 3% wt% Pt | λ > 400 nm | 50 mL of aqueous solution containing 20 vol% triethanolamine | 3.1 mmol/g/h | [31] |
| N-deficient g-C3N4 nanosheets | 1 wt% Pt | λ > 420 nm | Lactic acid aqueous solution (20 mL, 25 vol%) | 6.9 mmol/g/h | [32] |
| Porous O-doped C3N4 with N vacancies | 3 wt% Pt | λ > 420 nm | 50 mL of aqueous solution (40 mL and 10 mL triethanolamine) | 258.18 μmol/g/h | [33] |
| N/C-deficient g-C3N4 nanosheets | 3 wt% Pt | λ > 420 nm | 72 mL water and 8 mL triethanolamine | 1271 μmol/g/h | [34] |
| Sea-urchin-structure N-deficient g-C3N4 | 3 wt% Pt | λ > 420 nm | Triethanolamine solution (15 vol%, 80 mL) | 41.5 μmol/g/h | [35] |
| N, O-codoped C3N4 | 3 wt% Pt | λ > 420 nm | 100 mL of aqueous solution containing triethanolamine (10 vol%) | 1284 μmol/g/h | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.; Wang, Z.; Xie, H.; Zhang, Y.; Ding, C.; Ye, L. Structural Distortion of g-C3N4 Induced by N-Defects for Enhanced Photocatalytic Hydrogen Evolution. Catalysts 2022, 12, 1496. https://doi.org/10.3390/catal12121496
Su F, Wang Z, Xie H, Zhang Y, Ding C, Ye L. Structural Distortion of g-C3N4 Induced by N-Defects for Enhanced Photocatalytic Hydrogen Evolution. Catalysts. 2022; 12(12):1496. https://doi.org/10.3390/catal12121496
Chicago/Turabian StyleSu, Fengyun, Zhishuai Wang, Haiquan Xie, Yezhen Zhang, Chenghua Ding, and Liqun Ye. 2022. "Structural Distortion of g-C3N4 Induced by N-Defects for Enhanced Photocatalytic Hydrogen Evolution" Catalysts 12, no. 12: 1496. https://doi.org/10.3390/catal12121496
APA StyleSu, F., Wang, Z., Xie, H., Zhang, Y., Ding, C., & Ye, L. (2022). Structural Distortion of g-C3N4 Induced by N-Defects for Enhanced Photocatalytic Hydrogen Evolution. Catalysts, 12(12), 1496. https://doi.org/10.3390/catal12121496




