Synthesis of Oxygenated Hydrocarbons from Ethanol over Sulfided KCoMo-Based Catalysts: Influence of Novel Fiber- and Powder-Activated Carbon Supports
Abstract
1. Introduction
2. Results
2.1. Catalyst Characteristics
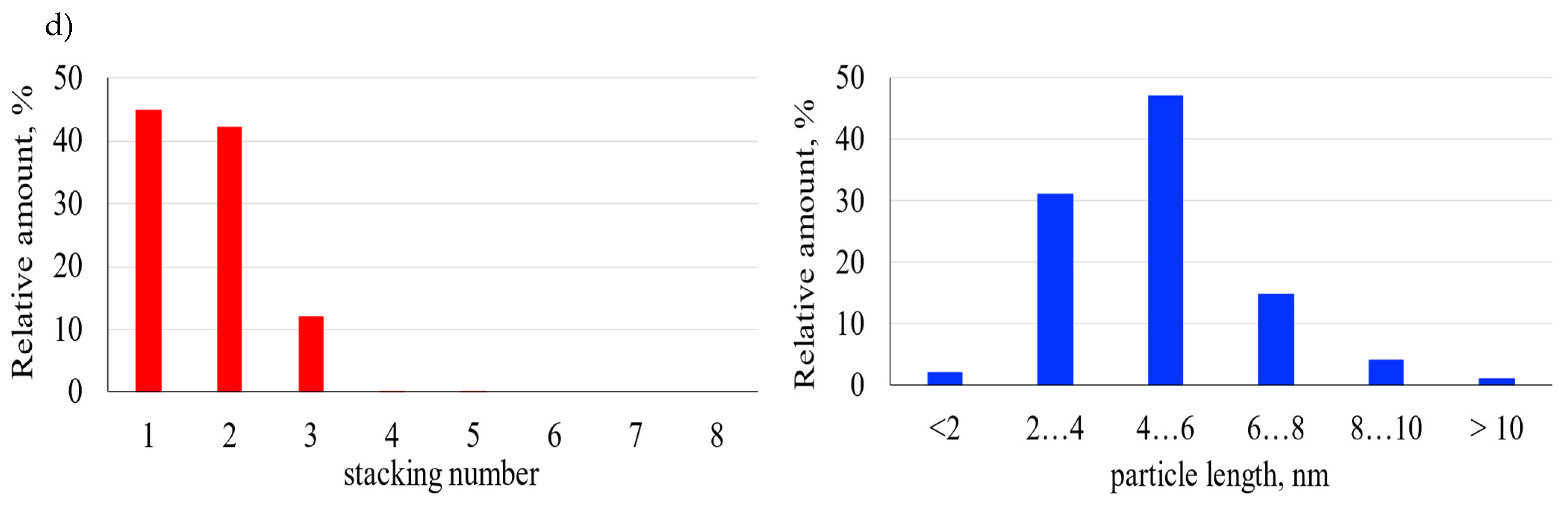
2.2. SEM and TEM
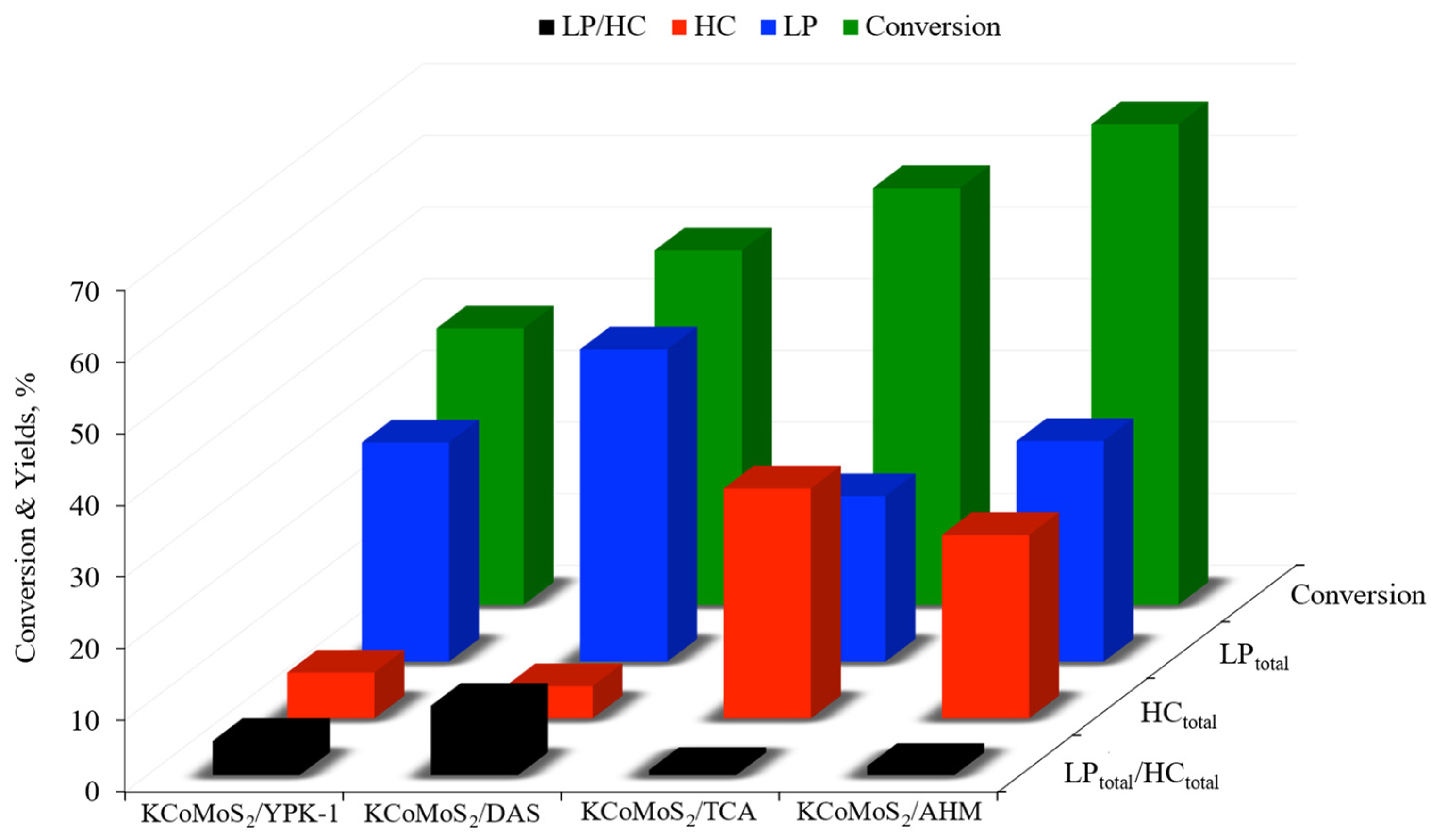
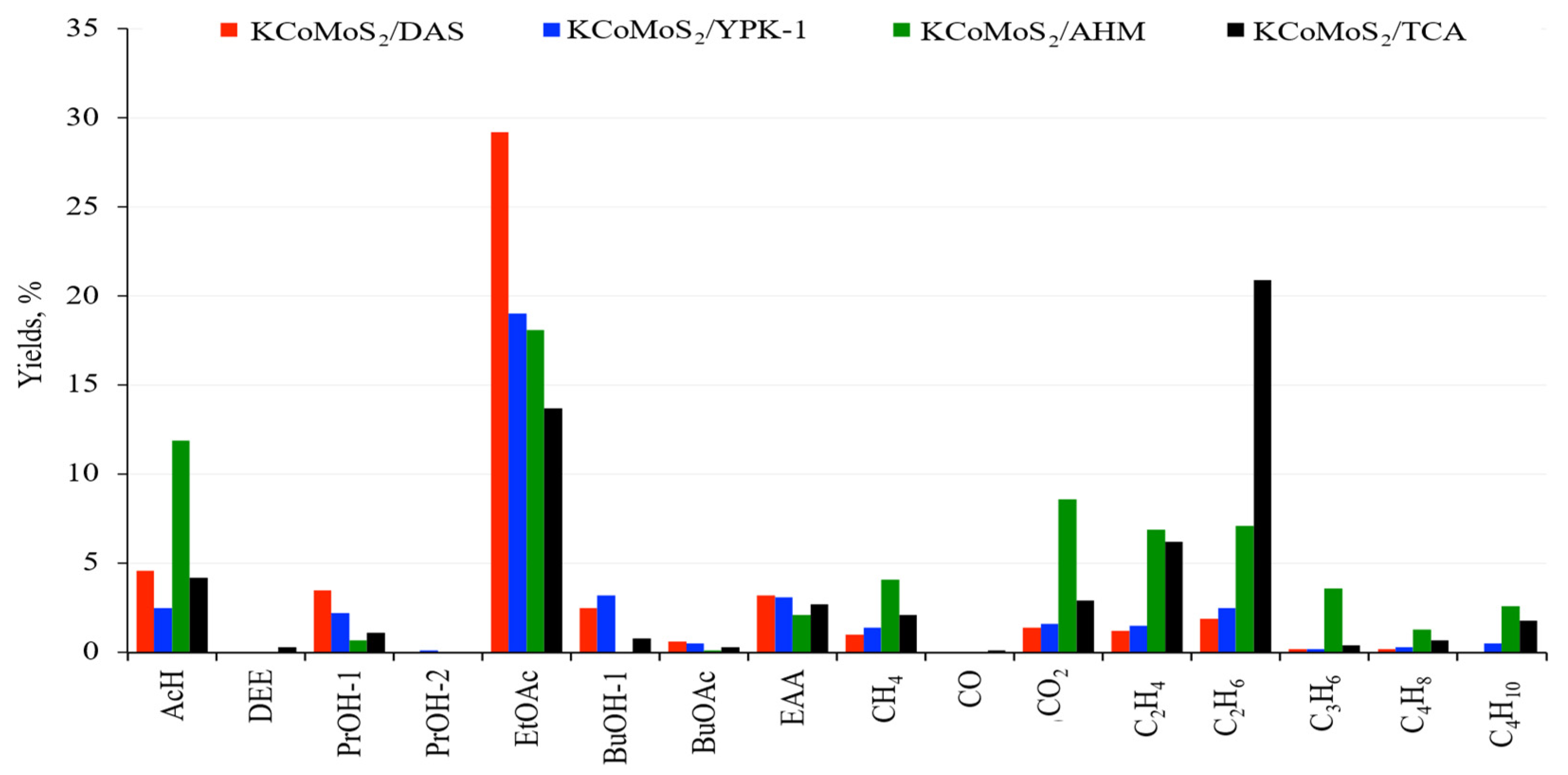
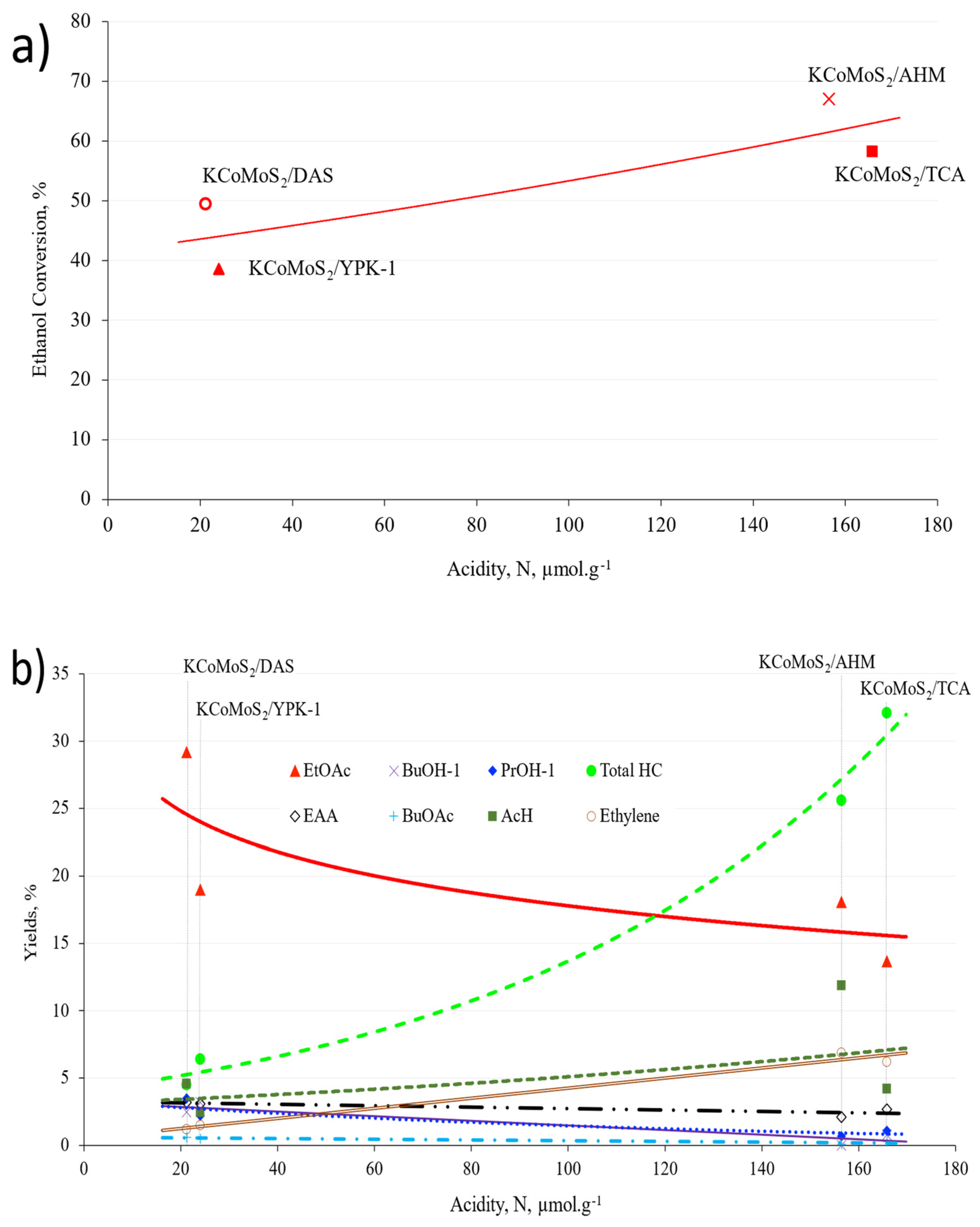
2.3. Catalytic Tests
3. Discussion
Industrial Relevance of Ethyl Acetate and Other Oxygenates
4. Materials and Methods
4.1. Carbonaceous Supports
4.2. Synthesis of K- and Co-Promoted MoS2 Catalysts
4.3. Physical Characteristics of Catalyst
4.4. Catalytic Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fadlalla, M.I.; Nyathi, T.M.; Claeys, M. Magnesium as a Methanation Suppressor for Iron-and Cobalt-Based Oxide Catalysts during the Preferential Oxidation of Carbon Monoxide. Catalysts 2022, 12, 118. [Google Scholar] [CrossRef]
- Morales, M.V.; Asedegbega-Nieto, E.; Bachiller-Baeza, B.; Guerrero-Ruiz, A. Bioethanol Dehydrogenation over Copper Supported on Functionalized Graphene Materials and a High Surface Area Graphite. Carbon 2016, 102, 426–436. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A Review on Commercial-Scale High-Value Products That Can Be Produced alongside Cellulosic Ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Bergthorson, J.M.; Thomson, M.J. A Review of the Combustion and Emissions Properties of Advanced Transportation Biofuels and Their Impact on Existing and Future Engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Dagle, R.A.; Winkelman, A.D.; Ramasamy, K.K.; Lebarbier Dagle, V.; Weber, R.S. Ethanol as a Renewable Building Block for Fuels and Chemicals. Ind. Eng. Chem. Res. 2020, 59, 4843–4853. [Google Scholar] [CrossRef]
- Ob-Eye, J.; Praserthdam, P.; Jongsomjit, B. Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. Catalysts 2019, 9, 66. [Google Scholar] [CrossRef]
- Wang, Q.N.; Shi, L.; Lu, A.H. Highly Selective Copper Catalyst Supported on Mesoporous Carbon for the Dehydrogenation of Ethanol to Acetaldehyde. ChemCatChem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent Advances in Catalytic Conversion of Ethanol to Chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Cheng, E.; McCullough, L.; Noh, H.; Farha, O.; Hupp, J.; Notestein, J. Isobutane Dehydrogenation over Bulk and Supported Molybdenum Sulfide Catalysts. Ind. Eng. Chem. Res. 2020, 59, 1113–1122. [Google Scholar] [CrossRef]
- McCullough, L.R.; Childers, D.J.; Watson, R.A.; Kilos, B.A.; Barton, D.G.; Weitz, E.; Kung, H.H.; Notestein, J.M. Acceptorless Dehydrogenative Coupling of Neat Alcohols Using Group VI Sulfide Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 4890–4896. [Google Scholar] [CrossRef]
- Zaki, T. Catalytic Dehydration of Ethanol Using Transition Metal Oxide Catalysts. J. Colloid Interface Sci. 2005, 284, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; El-Hakam, S.A.; Samra, S.E.; EL-Khouly, A.A.; Khder, A.S. Structural Characterization of Sulfated Zirconia and Their Catalytic Activity in Dehydration of Ethanol. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 317, 62–70. [Google Scholar] [CrossRef]
- Abd El-Raady, A.A.; Fouad, N.E.; Mohamed, M.A.; Halawy, S.A. Effect of the Preparation Method of Al-Mg-O Catalysts on the Selective Decomposition of Ethanol. Monatshefte Fur Chem. 2002, 133, 1351–1361. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Nickolov, R.; Mehandjiev, D. Effect of Chromium on Copper Containing Activated Carbon Catalysts for Methanol Decomposition. React. Kinet. Catal. Lett. 2001, 72, 383–390. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Vankova, S.; Bozhkov, O.; Mehandjiev, D. Effect of Rhenium on Copper Supported on Activated Carbon Catalysts for Methanol Decomposition. J. Mol. Catal. A Chem. 2005, 225, 245–251. [Google Scholar] [CrossRef]
- Takei, T.; Iguchi, N.; Haruta, M. Support Effect in the Gas Phase Oxidation of Ethanol over Nanoparticulate Gold Catalysts. New J. Chem. 2011, 35, 2227–2233. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. Reforming of Ethanol –Dehydrogenation to Ethyl Acetate and Steam Reforming to Acetic Acid over Copper-Based Catalysts. Bull. Chem. Soc. Jpn. 1991, 64, 2619–2623. [Google Scholar] [CrossRef]
- Dorokhov, V.S.; Ishutenko, D.I.; Nikul’shin, P.A.; Kotsareva, K.V.; Trusova, E.A.; Bondarenko, T.N.; Eliseev, O.L.; Lapidus, A.L.; Rozhdestvenskaya, N.N.; Kogan, V.M. Conversion of Synthesis Gas into Alcohols on Supported Cobalt-Molybdenum Sulfide Catalysts Promoted with Potassium. Kinet. Catal. 2013, 54, 243–252. [Google Scholar] [CrossRef]
- Kogan, V.M.; Rozhdestvenskaya, N.N.; Korshevets, I.K. Radioisotopic Study of CoMo-Al2O3 Sulfide Catalysts for HDS PartI. Active Site Monitoring. Appl. Catal. A Gen. 2002, 234, 207–219. [Google Scholar] [CrossRef]
- Kogan, V.M.; Rozhdestvenskaya, N.N. 35S Tracer Study of the Effect of Support on the Nature of the Active Sites of Co(Ni)Mo Sulphide Catalysts Supported on Al2O3 and Activated Carbon. Oil Gas Sci. Technol. 2006, 61, 547–559. [Google Scholar] [CrossRef]
- Kogan, V.M.; Isaguliants, G.V. The HDS Mechanism: Which “Auxiliary” Process Takes Place—Sulfur Isotopic Exchange or Replacement—And Why Is It Important to Know It? Catal. Today 2008, 130, 243–248. [Google Scholar] [CrossRef]
- Kogan, V.M.; Nikulshin, P.A. On the Dynamic Model of Promoted Molybdenum Sulfide Catalysts. Catal. Today 2010, 149, 224–231. [Google Scholar] [CrossRef]
- Kogan, V.M.; Nikulshin, P.A.; Rozhdestvenskaya, N.N. Evolution and Interlayer Dynamics of Active Sites of Promoted Transition Metal Sulfide Catalysts under Hydrodesulfurization Conditions. Fuel 2012, 100, 2–16. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Salnikov, V.A.; Mozhaev, A.V.; Minaev, P.P.; Kogan, V.M.; Pimerzin, A.A. Relationship between Active Phase Morphology and Catalytic Properties of the Carbon–Alumina-Supported Co(Ni)Mo Catalysts in HDS and HYD Reactions. J. Catal. 2014, 309, 386–396. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R.R.; Ruppert, A.F. Structure-Function Relations in Layered Transition Metal Sulfide Catalysts. Stud. Surf. Sci. Catal. 1993, 75, 571–584. [Google Scholar]
- Dorokhov, V.S.; Permyakov, E.A.; Nikulshin, P.A.; Maximov, V.V.; Kogan, V.M. Experimental and Computational Study of Syngas and Ethanol Conversion Mechanisms over K-Modified Transition Metal Sulfide Catalysts. J. Catal. 2016, 344, 841–853. [Google Scholar] [CrossRef]
- Dorokhov, V.S.; Kamorin, M.A.; Rozhdestvenskaya, N.N.; Kogan, V.M. Synthesis and Conversion of Alcohols over Modified Transition Metal Sulphides. Comptes Rendus Chim. 2016, 19, 1184–1193. [Google Scholar] [CrossRef]
- Taborga Claure, M.; Chai, S.H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of Higher Alcohol Selectivity and Productivity in CO Hydrogenation Reactions over K/MoS2 Domains Supported on Mesoporous Activated Carbon and Mixed MgAl Oxide. J. Catal. 2015, 324, 88–97. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Heracleous, E.; Triantafyllidis, K.S.; Lemonidou, A.A. K-Promoted NiMo Catalysts Supported on Activated Carbon for the Hydrogenation Reaction of CO to Higher Alcohols: Effect of Support and Active Metal. Appl. Catal. B Environ. 2015, 165, 296–305. [Google Scholar] [CrossRef]
- Liu, C.; Virginie, M.; Griboval-Constant, A.; Khodakov, A. Impact of Potassium Content on the Structure of Molybdenum Nanophases in Alumina Supported Catalysts and Their Performance in Carbon Monoxide Hydrogenation. Appl. Catal. A Gen. 2015, 504, 565–575. [Google Scholar] [CrossRef]
- Dugulan, A.I.; van Veen, J.A.R.; Hensen, E.J.M. On the Structure and Hydrotreating Performance of Carbon-Supported CoMo- and NiMo-Sulfides. Appl. Catal. B Environ. 2013, 142–143, 178–186. [Google Scholar] [CrossRef]
- Breysse, M.; Afanasiev, P.; Geantet, C.; Vrinat, M. Overview of Support Effects in Hydrotreating Catalysts. Catal. Today 2003, 86, 5–16. [Google Scholar] [CrossRef]
- Luk, H.T.; Forster, T.; Mondelli, C.; Siol, S.; Curulla-Ferré, D.; Stewart, J.A.; Pérez-Ramírez, J. Carbon Nanofibres-Supported KCoMo Catalysts for Syngas Conversion into Higher Alcohols. Catal. Sci. Technol. 2018, 8, 187–200. [Google Scholar] [CrossRef]
- Osman, M.E.; Maximov, V.V.; Dipheko, T.D.; Sheshko, T.F.; Cherednichenko, A.G.; Nikulshin, P.A.; Kogan, V.M. Synthesis of Higher Alcohols from Syngas over a K-Modified CoMoS Catalyst Supported on Novel Powder and Fiber Commercial Activated Carbons. ACS Omega 2022, 7, 21346–21356. [Google Scholar] [CrossRef]
- Castillo-Villalón, P.; Ramírez, J.; Cuevas, R.; Vázquez, P.; Castañeda, R. Influence of the Support on the Catalytic Performance of Mo, CoMo, and NiMo Catalysts Supported on Al2O3 and TiO2 during the HDS of Thiophene, Dibenzothiophene, or 4,6-Dimethyldibenzothiophene. Catal. Today 2016, 259, 140–149. [Google Scholar] [CrossRef]
- Han, W.; Nie, H.; Long, X.; Li, M.; Yang, Q.; Li, D. Effects of the Support BrØnsted Acidity on the Hydrodesulfurization and Hydrodenitrogention Activity of Sulfided NiMo/Al2O3 Catalysts. Catal. Today 2017, 292, 58–66. [Google Scholar] [CrossRef]
- van Veen, J.A.R.; Gerkema, E.; van der Kraan, A.M.; Hendriks, P.A.J.M.; Beens, H. A57 CO Mössbauer Emission Spectrometric Study of Some Supported CoMo Hydrodesulfurization Catalysts. J. Catal. 1992, 133, 112–123. [Google Scholar] [CrossRef]
- Lv, R.; Cui, T.; Jun, M.S.; Zhang, Q.; Cao, A.; Su, D.S.; Zhang, Z.; Yoon, S.H.; Miyawaki, J.; Mochida, I.; et al. Open-Ended, N-Doped Carbon Nanotube-Graphene Hybrid Nanostructures as High-Performance Catalyst Support. Adv. Funct. Mater. 2011, 21, 999–1006. [Google Scholar] [CrossRef]
- Sheng Su, D.; Perathoner, S.; Centi, G. Nanocarbons for the Development of Advanced Catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar]
- Dipheko, T.D.; Maximov, V.V.; Osman, M.E.; Permyakov, E.A.; Mozhaev, A.V.; Nikulshin, P.; Cherednichenko, A.G.; Kogan, V.M. Catalytic Conversion of Ethanol Over Supported Kcomos2 Catalysts for Synthesis of Oxygenated Hydrocarbons. Fuel 2022, 330, 125512. [Google Scholar] [CrossRef]
- Duchet, J.C.; van Oers, E.M.; de Beer, V.H.J.; Prins, R. Carbon-Supported Sulfide Catalysts. J. Catal. 1983, 80, 386–402. [Google Scholar] [CrossRef]
- Vissers, J.P.R.; Scheffer, B.; de Beer, V.H.J.; Moulijn, J.A.; Prins, R. Effect of the Support on the Structure of Mo-Based Hydrodesulfurization Catalysts: Activated Carbon versus Alumina. J. Catal. 1987, 105, 277–284. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Influence of Porous Characteristics of the Carbon Support on Alkali-Modified Trimetallic Co-Rh-Mo Sulfided Catalysts for Higher Alcohols Synthesis from Synthesis Gas. Appl. Catal. A Gen. 2011, 393, 50–58. [Google Scholar] [CrossRef]
- Vradman, L.; Landau, M.V.; Kantorovich, D.; Koltypin, Y.; Gedanken, A. Evaluation of Metal Oxide Phase Assembling Mode inside the Nanotubular Pores of Mesostructured Silica. Microporous Mesoporous Mater. 2005, 79, 307–318. [Google Scholar] [CrossRef]
- Das, D.; Mishra, H.K.; Parida, K.M.; Dalai, A.K. Preparation, Physico-Chemical Characterization and Catalytic Activity of Sulphated ZrO2-TiO2 Mixed Oxides. J. Mol. Catal. A Chem. 2002, 189, 271–282. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems, with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Bansal, P.B.; Goyal, M. Activated Carbon Adsorption, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; Volume 3, pp. 97–111. [Google Scholar]
- Boahene, P.E.; Surisetty, V.R.; Sammynaiken, R.; Dalai, A.K. Higher Alcohol Synthesis Using K-Doped CoRhMoS2/MWCNT Catalysts: Influence of Pelletization, Particle Size and Incorporation of Binders. Top. Catal. 2014, 57, 538–549. [Google Scholar] [CrossRef]
- Legras, A.; Kondor, A.; Heitzmann, M.T.; Truss, R.W. Inverse Gas Chromatography for Natural Fibre Characterisation: Identification of the Critical Parameters to Determine the Brunauer–Emmett–Teller Specific Surface Area. J. Chromatogr. A 2015, 1425, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luo, Y.; He, D.; Xu, Z.; He, S.; Xie, D.; Mei, Y. An Exploration into Potassium (K) Containing MoS2 Active Phases and Its Transformation Process over MoS2 Based Materials for Producing Methanethiol. Catal. Today 2020, 339, 93–104. [Google Scholar] [CrossRef]
- Andersen, A.; Kathmann, S.M.; Lilga, M.A.; Albrecht, K.O.; Hallen, R.T.; Mei, D. Adsorption of Potassium on MoS2(100) Surface: A First-Principles Investigation. J. Phys. Chem. C 2011, 115, 9025–9040. [Google Scholar] [CrossRef]
- Maximov, V.V.; Permyakov, E.A.; Dorokhov, V.S.; Wang, A.; Kooyman, P.J.; Kogan, V.M. Effect of Promoter Nature on Synthesis Gas Conversion to Alcohols over (K)MeMoS2/Al2O3 Catalysts. ChemCatChem 2020, 12, 1443–1452. [Google Scholar] [CrossRef]
- Dorokhov, V.S.; Ishutenko, D.I.; Nikul’Shin, P.A.; Eliseev, O.L.; Rozhdestvenskaya, N.N.; Kogan, V.M.; Lapidus, A.L. The Mechanism of Synthesis Gas Conversion to Alcohols Catalyzed by Transition Metal Sulfides. Dokl. Chem. 2013, 451, 191–195. [Google Scholar] [CrossRef]
- Osman, M.E.; Maximov, V.V.; Dorokhov, V.S.; Mukhin, V.M.; Sheshko, T.F.; Kooyman, P.J.; Kogan, V.M. Carbon-Supported KCoMoS2 for Alcohol Synthesis from Synthesis Gas. Catalysts 2021, 11, 1321. [Google Scholar] [CrossRef]
- Osman, M.E.; Maximov, V.V.; Dipheko, T.D.; Sheshko, T.F.; Cherednichenko, A.G.; Kogan, V.M. Effect of Textural Characteristics on the Catalytic Performance of Supported KCoMoS2 in the Synthesis of Higher Alcohols from Syngas. Mendeleev Commun. 2022, 32, 510–513. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Alcohols as Alternative Fuels: An Overview. Appl. Catal. A Gen. 2011, 404, 1–11. [Google Scholar] [CrossRef]
- Triantafyllidis, K.; Lappas, A.; Stocker, M. The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 157. [Google Scholar]
- Carrasco-Marín, F.; Mueden, A.; Moreno-Castilla, C. Surface-Treated Activated Carbons as Catalysts for the Dehydration and Dehydrogenation Reactions of Ethanol. J. Phys. Chem. B 1998, 102, 9239–9244. [Google Scholar] [CrossRef]
- Osman, M.E.; Dipheko, T.D.; Maximov, V.V.; Sheshko, T.F.; Markova, E.B.; Trusova, E.A.; Cherednichenko, A.G.; Kogan, V.M. Higher Alcohols Synthesis from Syngas and Ethanol over KCoMoS 2—Catalysts Supported on Graphene Nanosheets. Chem. Eng. Commun. 2022, 32, 510–513. [Google Scholar]
- Rychlicki, G.; Terzyk, A.P. Catalytic Conversion of Ethanol on Carbon Catalysts. Carbon N. Y. 1994, 32, 265–271. [Google Scholar]
- Santacesaria, E.; Carotenuto, G.; Tesser, R.; Di Serio, M. Ethanol Dehydrogenation to Ethyl Acetate by Using Copper and Copper Chromite Catalysts. Chem. Eng. J. 2012, 179, 209–220. [Google Scholar] [CrossRef]
- Carotenuto, G.; Tesser, R.; Di Serio, M.; Santacesaria, E. Kinetic Study of Ethanol Dehydrogenation to Ethyl Acetate Promoted by a Copper/Copper-Chromite Based Catalyst. Catal. Today 2013, 203, 202–210. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R.R. Structure-Function Relations in Molybdenum Sulfide Catalysts: The “Rim-Edge” Model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Jiang, F.; Chu, Y.; Nie, H. The Relation between Morphology of (Co)MoS2 Phases and Selective Hydrodesulfurization for CoMo Catalysts. Catal. Today 2010, 149, 35–39. [Google Scholar] [CrossRef]
- Freni, S.; Mondello, N.; Cavallaro, S.; Cacciola, G.; Parmon, V.N.; Sobyanin, V.A. Hydrogen Production by Steam Reforming of Ethanol: A Two Step Process. React. Kinet. Catal. Lett. 2000, 71, 143–152. [Google Scholar] [CrossRef]
- Shabaker, J.W.; Davda, R.R.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-Phase Reforming of Methanol and Ethylene Glycol over Alumina-Supported Platinum Catalysts. J. Catal. 2003, 215, 344–352. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Ordóñez, S. Ethanol Catalytic Condensation over Mg-Al Mixed Oxides Derived from Hydrotalcites. Catal. Today 2011, 164, 436–442. [Google Scholar] [CrossRef]
- Inui, K.; Kurabayashi, T.; Sato, S.; Ichikawa, N. Effective Formation of Ethyl Acetate from Ethanol over Cu-Zn-Zr-Al-O Catalyst. J. Mol. Catal. A Chem. 2004, 216, 147–156. [Google Scholar] [CrossRef]
- Anashkin, Y.V.; Ishutenko, D.I.; Maximov, V.V.; Pimerzin, A.A.; Kogan, V.M.; Nikulshin, P.A. Effect of Carrier Properties on the Activity of Supported KCoMoS Catalysts in the Synthesis of Alcohol from Syngas. React. Kinet. Mech. Catal. 2019, 127, 301–314. [Google Scholar] [CrossRef]
- Çakmak, A.; Kapusuz, M.; Özcan, H. Experimental Research on Ethyl Acetate as Novel Oxygenated Fuel in the Spark-Ignition (SI) Engine. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–16. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM Study of Nanosized Metal Films and Metal Nanoparticles Obtained by Magnetron Sputtering. Russ. Chem. Bull. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-Oriented Analysis of Gaseous, Liquid and Solid Chemical Systems by Mass Spectrometry, Nuclear Magnetic Resonance Spectroscopy and Electron Microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on Pore Systems in Catalysts: V. The t Method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
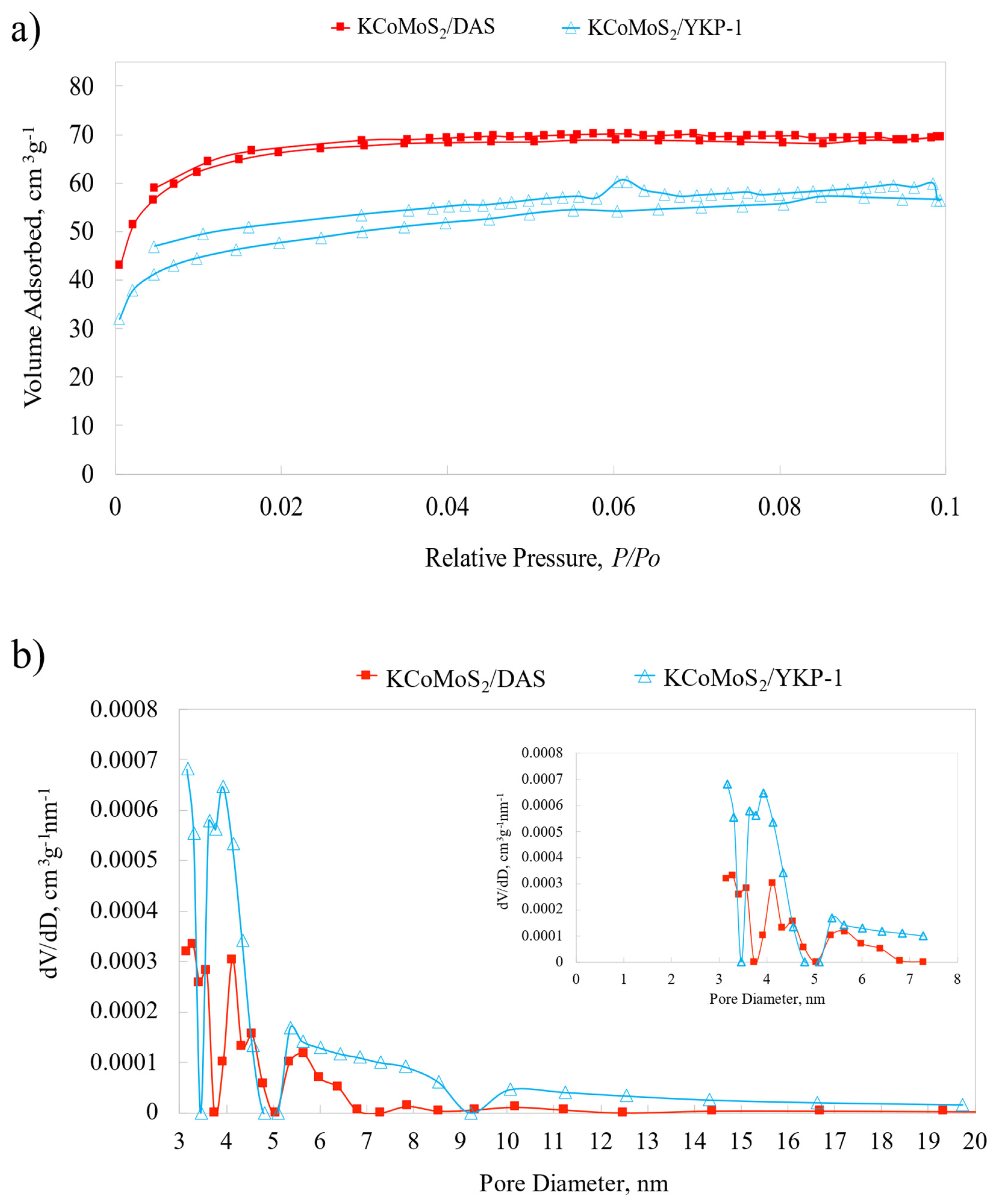
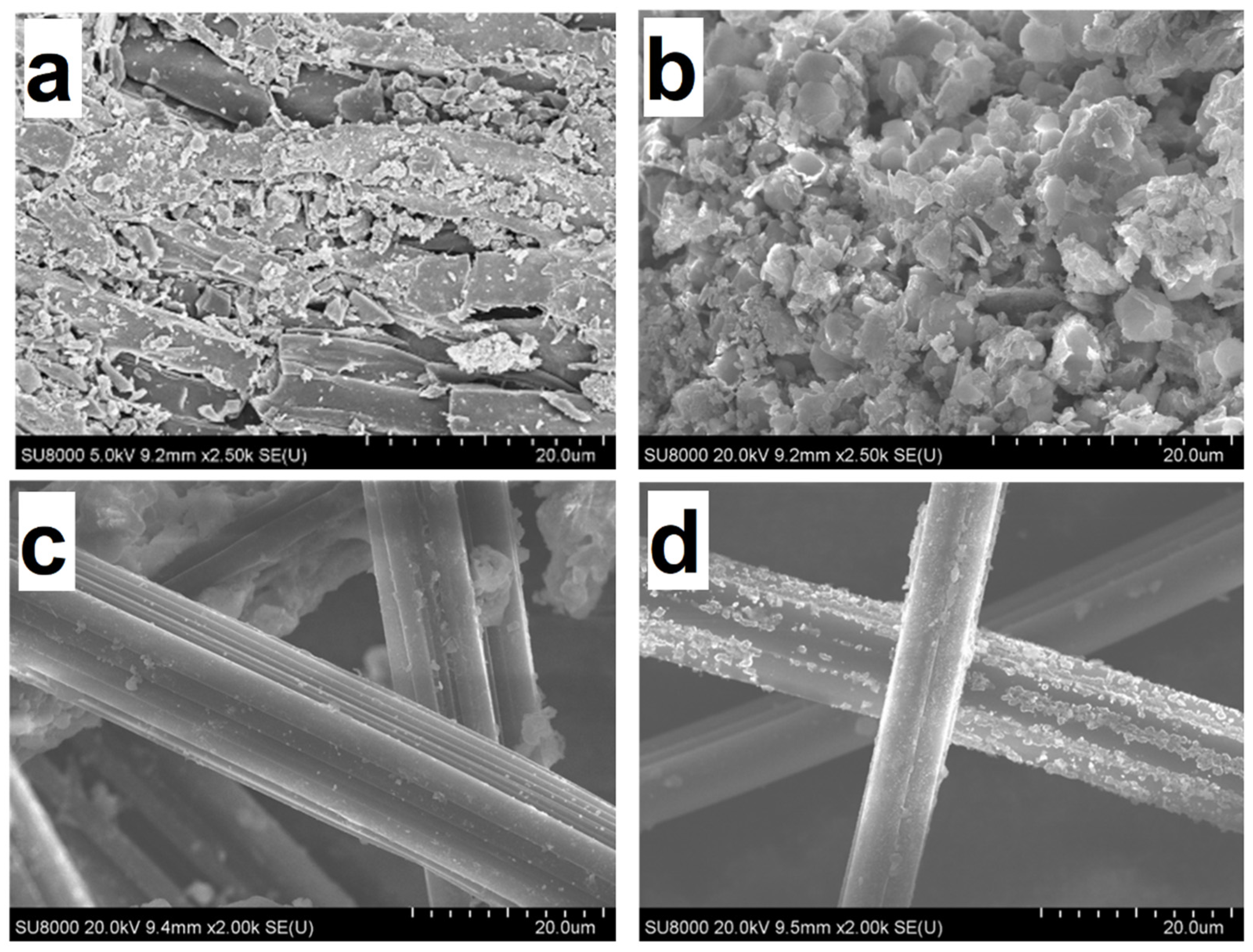
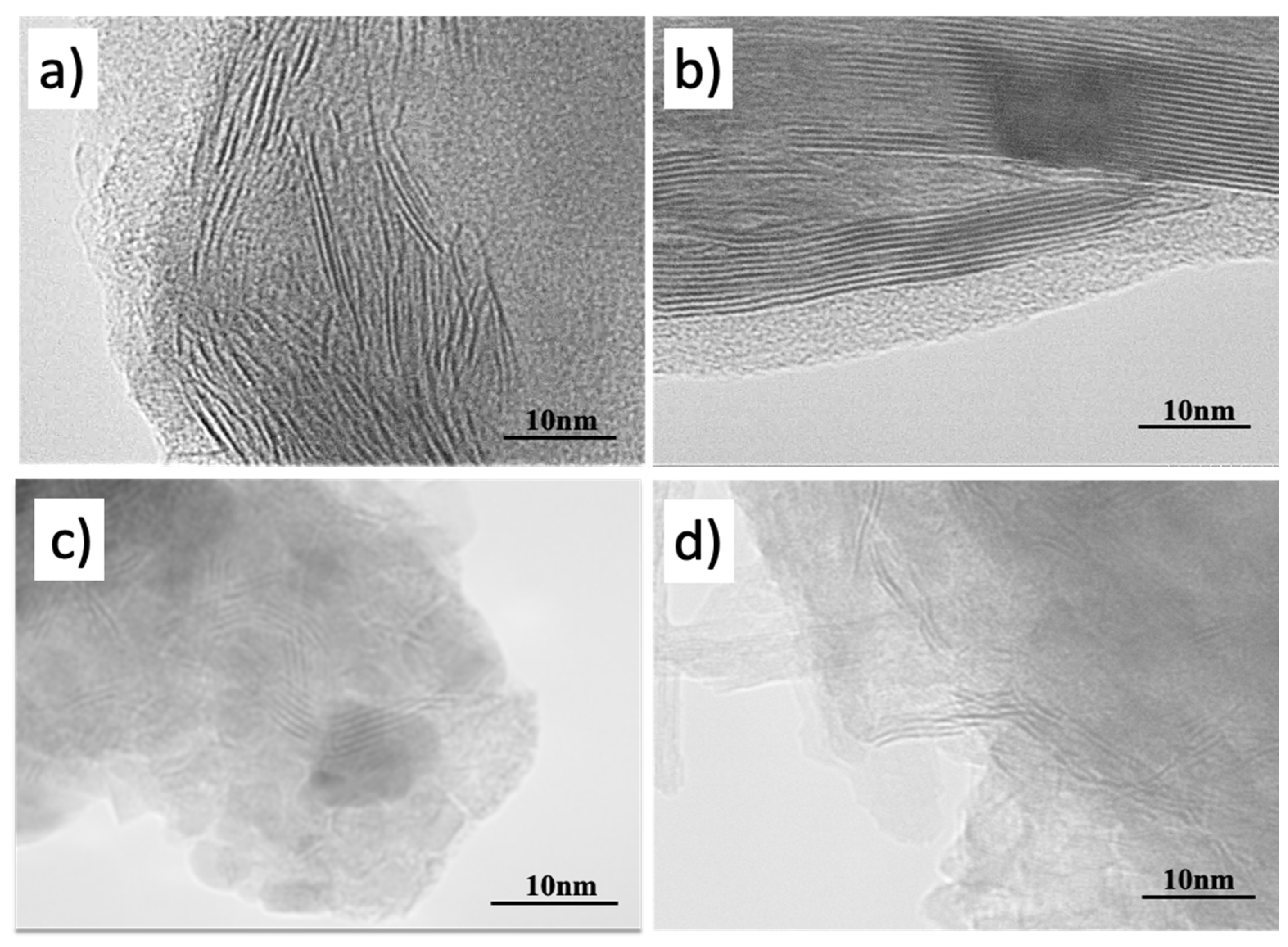

| Catalysts | DAS | YPK-1 | KCoMoS2/DAS | KCoMoS2/YPK-1 |
|---|---|---|---|---|
| SBET (m2/g) | 724 | 771 | 250 | 178 |
| Smicro (m2/g) | 662 | 660 | 243 | 142 |
| Smeso (m2/g) | 62 | 51 | 7 | 35 |
| Vtotal (cm3/g) | 0.34 | 0.32 | 0.11 | 0.09 |
| Vmicro (cm3/g) | 0.28 | 0.27 | 0.10 | 0.06 |
| Vmeso (cm3/g) | 0.06 | 0.05 | 0.01 | 0.03 |
| Dp (nm) | 3.2 | 3.9 | 3.3 | 3.2 |
| NSBET | – | – | 0.46 | 0.31 |
| BE | – | – | 0.54 | 0.69 |
| %ME | 0.18 | 0.16 | 0.11 | 0.33 |
| Catalysts | Targeted Compositions (wt.%) | Measured Compositions (wt.%) | ||||
|---|---|---|---|---|---|---|
| K | Co | Mo | K | Co | Mo | |
| KCoMoS2/DAS | 10 | 3.7 | 12 | 8.5 | 3.7 | 11.9 |
| KCoMoS2/YPK-1 | 10 | 3.7 | 12 | 8.4 | 3.9 | 15.8 |
| KCoMoS2/TCA | 10 | 3.7 | 12 | 11.3 | 4.2 | 13.9 |
| KCoMoS2/AHM | 10 | 3.7 | 12 | 11.1 | 4.6 | 15.1 |
| Catalysts | Acidity (µmol·g−1) |
|---|---|
| DAS | 65.81 |
| YPK-1 | 39.31 |
| AHM | 79.67 |
| TCA | 47.37 |
| KCoMoS2/DAS | 21.19 |
| KCoMoS2/YPK-1 | 24.05 |
| KCoMoS2/AHM | 156.43 |
| KCoMoS2/TCA | 165.81 |
| Catalysts | KCoMoS2/DAS | KCoMoS2/YPK-1 | KCoMoS2/AHM | KCoMoS2/TCA |
|---|---|---|---|---|
| Acetaldehyde | 4.6 | 2.5 | 11.9 | 4.2 |
| Ethyl acetate | 29.2 | 19 | 18.1 | 13.7 |
| Ethyl acetoacetate | 3.2 | 3.1 | 2.1 | 2.7 |
| BuOH | 2.5 | 3.2 | 0 | 0.8 |
| n-PrOH | 3.5 | 2.2 | 0.7 | 1.1 |
| i-PrOH | 0 | 0.1 | 0 | 0 |
| BuOAc | 0.6 | 0.5 | 0.1 | 0.3 |
| Dethyl ether | 0 | 0 | 0 | 0.3 |
| CO | 0 | 0 | 0 | 0.1 |
| CO2 | 1.4 | 1.6 | 8.6 | 2.9 |
| LPtotal | 43.6 | 30.6 | 29.8 | 23.1 |
| HCtotal | 4.5 | 6.4 | 24.6 | 32.1 |
| LPtotal/HCtotal | 9.7 | 4.8 | 1.3 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipheko, T.D.; Maximov, V.V.; Osman, M.E.; Eliseev, O.L.; Cherednichenko, A.G.; Sheshko, T.F.; Kogan, V.M. Synthesis of Oxygenated Hydrocarbons from Ethanol over Sulfided KCoMo-Based Catalysts: Influence of Novel Fiber- and Powder-Activated Carbon Supports. Catalysts 2022, 12, 1497. https://doi.org/10.3390/catal12121497
Dipheko TD, Maximov VV, Osman ME, Eliseev OL, Cherednichenko AG, Sheshko TF, Kogan VM. Synthesis of Oxygenated Hydrocarbons from Ethanol over Sulfided KCoMo-Based Catalysts: Influence of Novel Fiber- and Powder-Activated Carbon Supports. Catalysts. 2022; 12(12):1497. https://doi.org/10.3390/catal12121497
Chicago/Turabian StyleDipheko, Tshepo D., Vladimir V. Maximov, Mohamed E. Osman, Oleg L. Eliseev, Alexander G. Cherednichenko, Tatiana F. Sheshko, and Victor M. Kogan. 2022. "Synthesis of Oxygenated Hydrocarbons from Ethanol over Sulfided KCoMo-Based Catalysts: Influence of Novel Fiber- and Powder-Activated Carbon Supports" Catalysts 12, no. 12: 1497. https://doi.org/10.3390/catal12121497
APA StyleDipheko, T. D., Maximov, V. V., Osman, M. E., Eliseev, O. L., Cherednichenko, A. G., Sheshko, T. F., & Kogan, V. M. (2022). Synthesis of Oxygenated Hydrocarbons from Ethanol over Sulfided KCoMo-Based Catalysts: Influence of Novel Fiber- and Powder-Activated Carbon Supports. Catalysts, 12(12), 1497. https://doi.org/10.3390/catal12121497







