Pulse Electrolysis Technique for Preparation of Bimetal Tin-Containing Electrocatalytic Materials
Abstract
1. Introduction
- the adsorption of organic particles on platinum:
- the adsorption of oxygen-containing particles on the tin component, or tin in the oxide form can itself be a source of oxygen-containing particles:
- the chemical interaction of particles of organic nature and oxygen-containing particles:
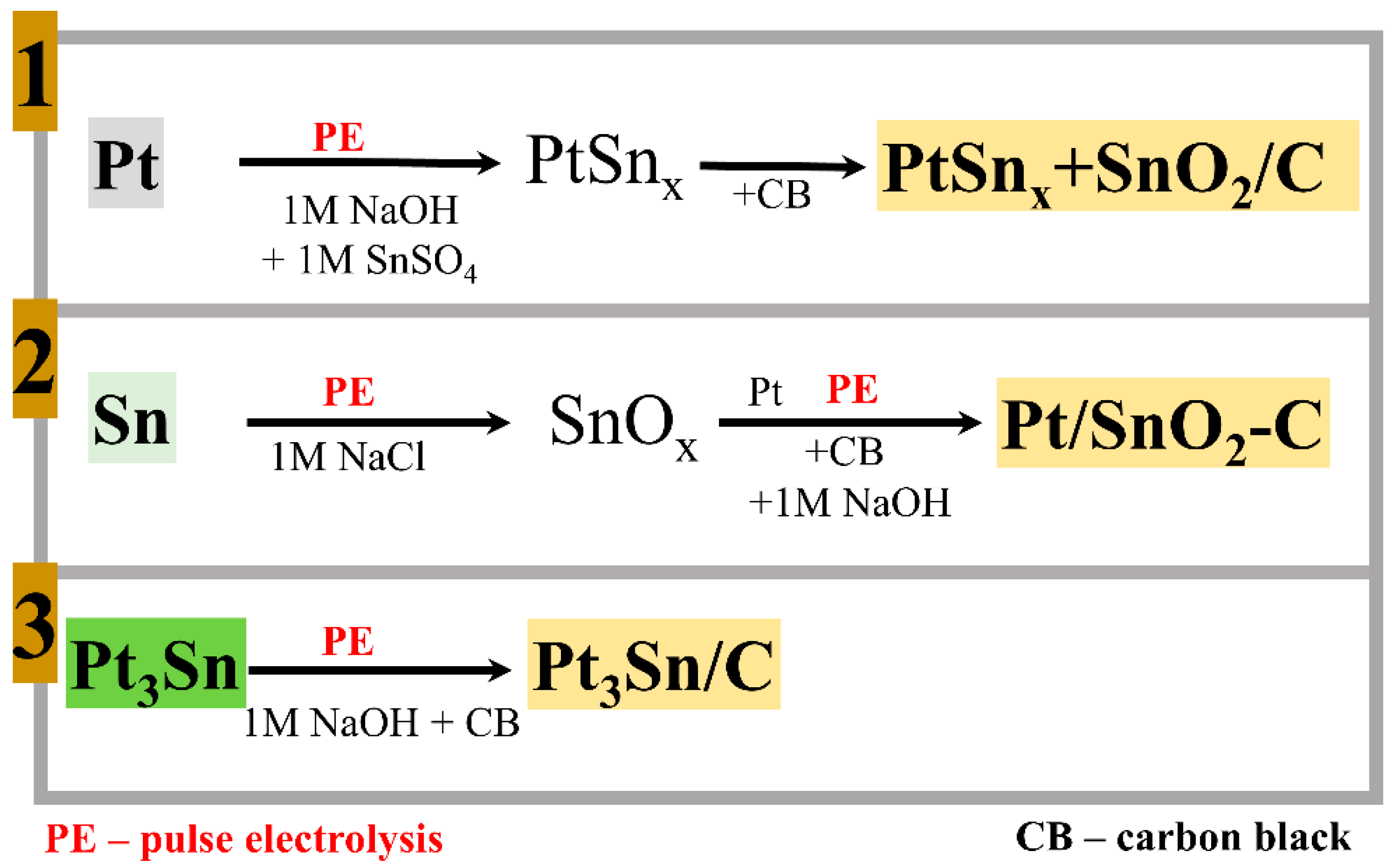
2. Results and Discussion
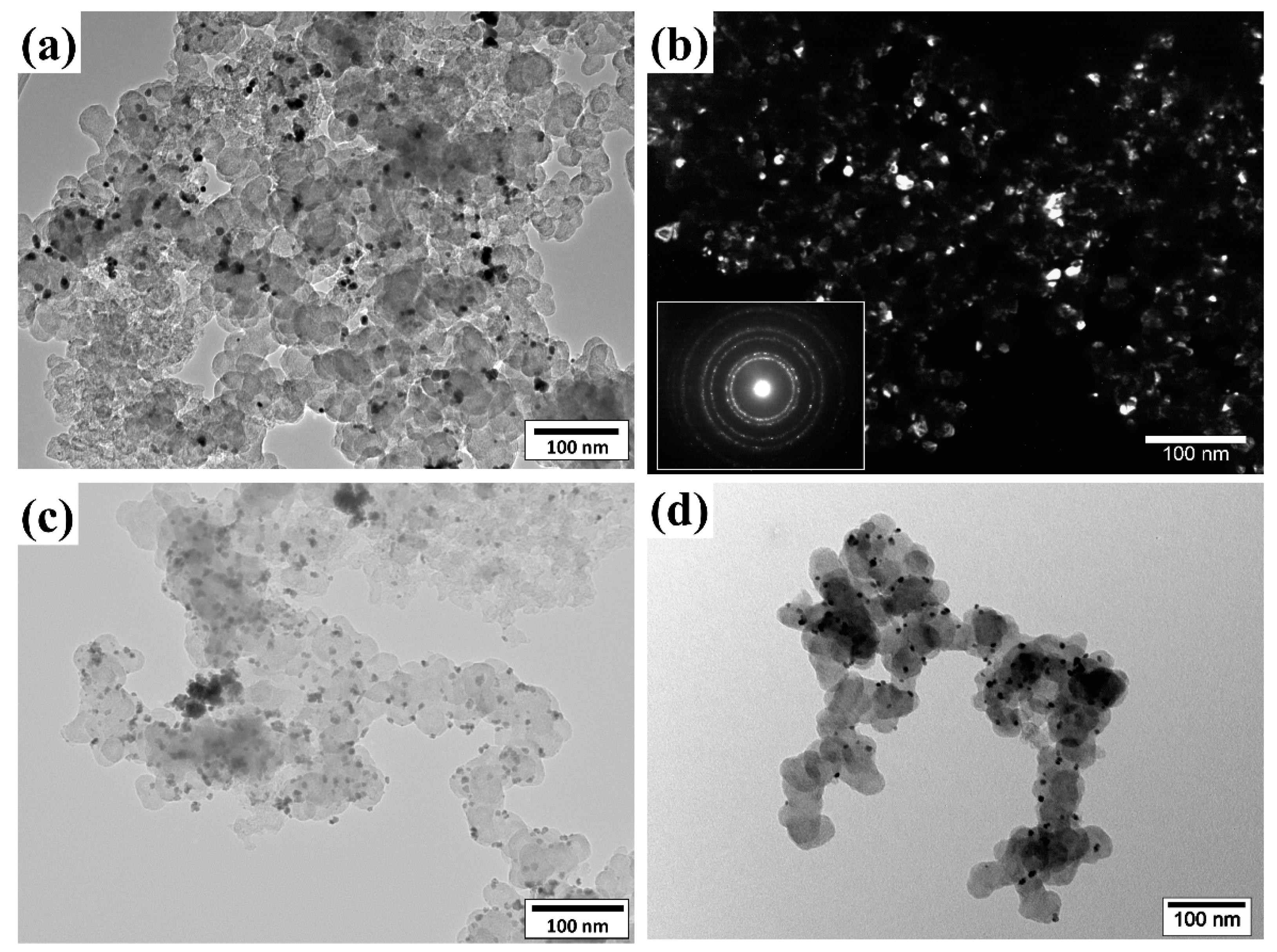
2.1. Composition and Microstructure Characterization
- in the form of a platinum–tin alloy Pt3Sn supported onto carbon black (strategy 3, Figure 1).
- the composition of the platinum–tin alloy nanoparticles obtained as a result of the dispersion of the Pt3Sn alloy electrodes (strategy 3) corresponds to the composition of the initial alloy electrode: using energy-dispersive X-ray spectroscopy (EDS) (Figure S1), it was found that the atomic ratio of the elements in a dispersed electrode is as follows: Pt:Sn = 3:1;
- the PtSnx-based material (strategy 1) was characterized by the presence of SnO2 in its composition, the concentration of which in relation to the PtSnx particles was 11 wt.%, while EDS mapping of PtSnx + SnO2/C showed a fairly uniform distribution of the Pt, Sn, O, and C elements (Figure S3b);
- 3D spectra of the Pt3Sn/C material (XPS analysis, Figure S4) demonstrate the presence of both an oxidized tin surface and tin in the metallic state.
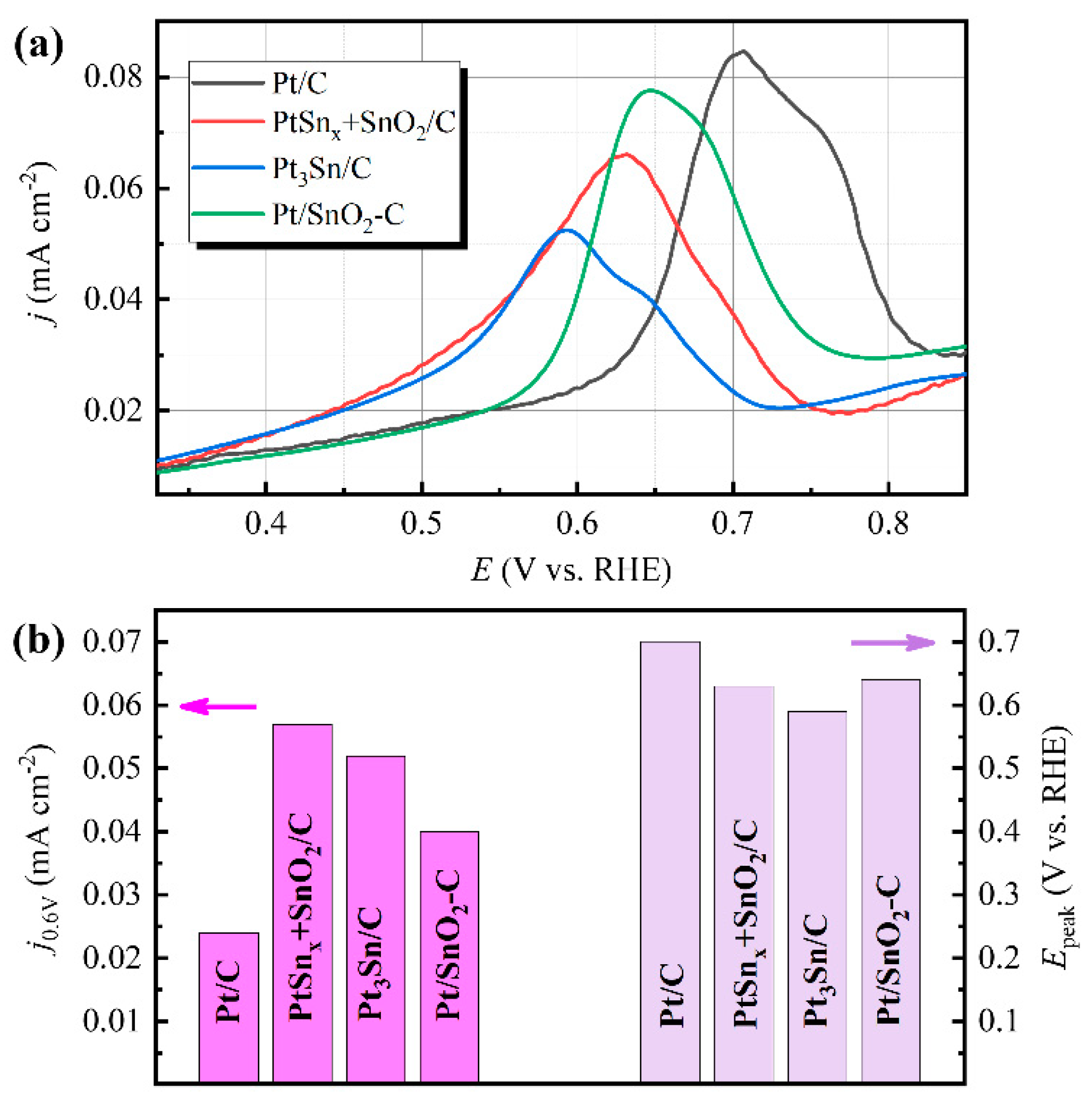
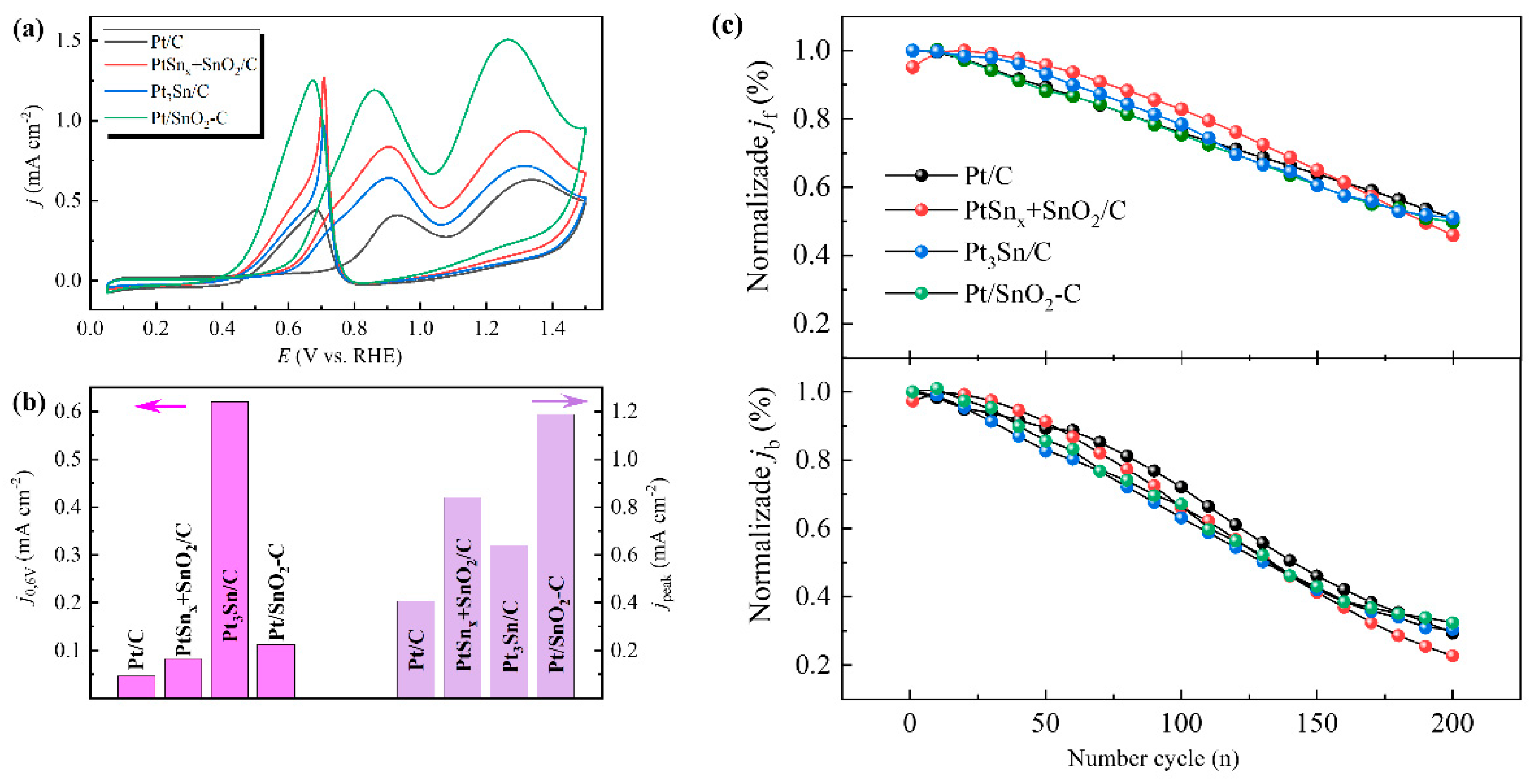
2.2. Electrocatalytic Properties
3. Materials and Methods
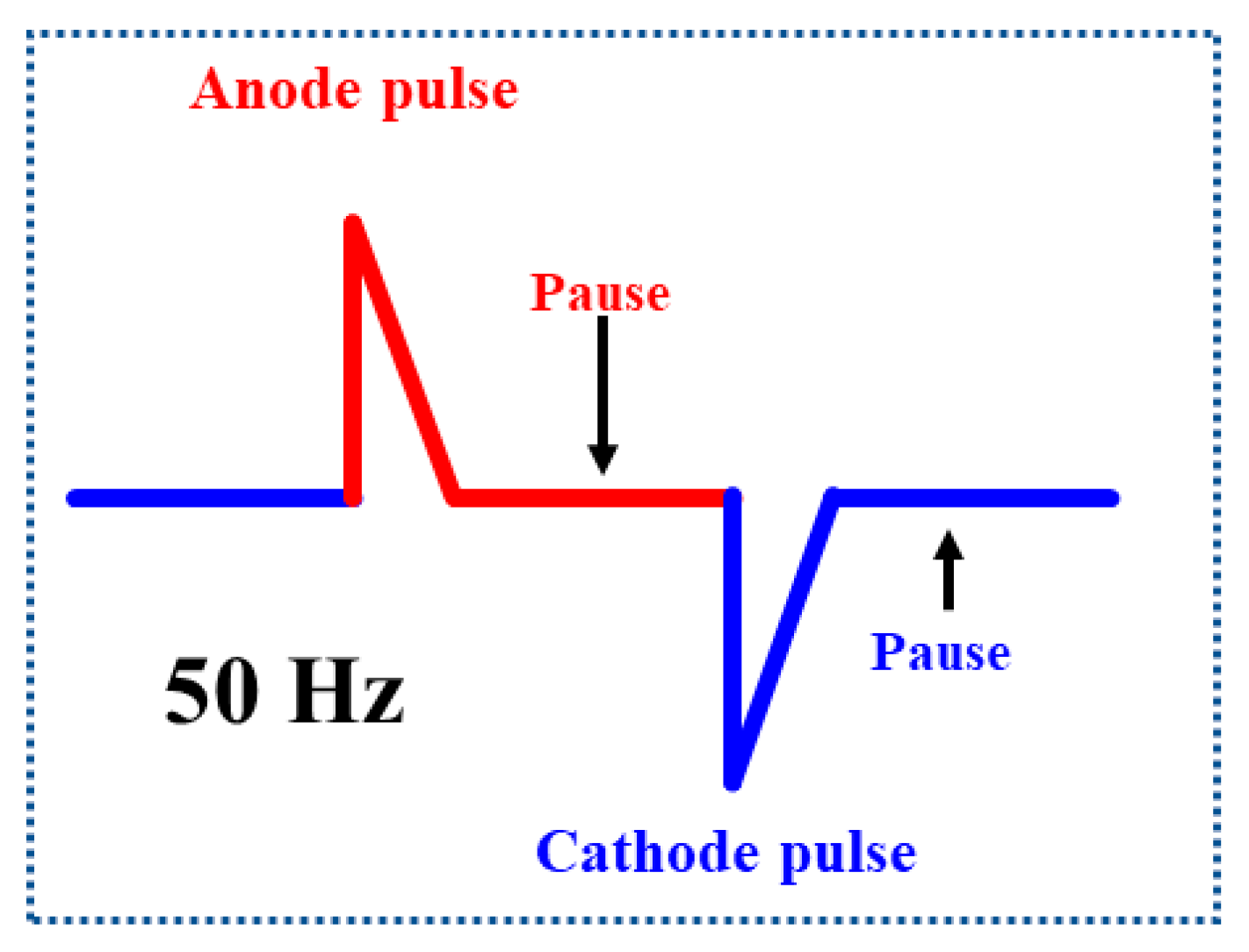
3.1. Electrocatalyst Preparation
3.2. Composition and Microstructure Studies
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baldizzone, C.; Mezzavilla, S.; Carvalho, H.W.; Meier, J.C.; Schuppert, A.K.; Heggen, M.; Galeano, C.; Grunwaldt, J.D.; Schüth, F.; Mayrhofer, K. Confined-Space Alloying of Nanoparticles for the Synthesis of Efficient PtNi Fuel-Cell Catalysts. Angew. Chem. Int. Ed. 2014, 53, 14250–14254. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2013, 42, 2880–2904. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Nitze, F.; Gracia-Espino, E.; Ma, J.; Barzegar, H.R.; Sharifi, T.; Jia, X.; Shchukarev, A.; Lu, L.; Ma, C. Small palladium islands embedded in palladium–tungsten bimetallic nanoparticles form catalytic hotspots for oxygen reduction. Nat. Commun. 2014, 5, 5253. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Yan, S.-Y.; Huang, Y.-R.; Yang, C.-Y.; Liu, C.-W.; Wang, J.-H.; Wang, K.-W. Enhanced activity of ethanol oxidation reaction on PtM (M = Au, Ag and Sn): The importance of oxophilicity and surface oxygen containing species. Electrochim. Acta 2018, 259, 733–741. [Google Scholar] [CrossRef]
- Wala, M.; Simka, W. Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review. Molecules 2021, 26, 2144. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef]
- Li, H.H.; Cui, C.H.; Zhao, S.; Yao, H.B.; Gao, M.R.; Fan, F.J.; Yu, S.H. Mixed-PtPd-Shell PtPdCu nanoparticle nanotubes templated from copper nanowires as efficient and highly durable electrocatalysts. Adv. Energy Mater. 2012, 2, 1182–1187. [Google Scholar] [CrossRef]
- Barbosa, A.F.; Del Colle, V.; Galiote, N.A.; Tremiliosi-Filho, G. Effect of tin deposition over electrogenerated random defects on Pt (111) surfaces onto ethanol electrooxidation: Electrochemical and FTIR studies. J. Electroanal. Chem. 2020, 857, 113734. [Google Scholar] [CrossRef]
- Chandran, P.; Puthusseri, D.; Ramaprabhu, S. 1D-2D integrated hybrid carbon nanostructure supported bimetallic alloy catalyst for ethanol oxidation and oxygen reduction reactions. Int. J. Hydrogen Energy 2019, 44, 4951–4961. [Google Scholar] [CrossRef]
- Kuriganova, A.B.; Smirnova, N.V. Pt/SnOx-C composite material for electrocatalysis. Mendeleev Commun. 2014, 6, 351–352. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Huang, J.; Zhang, W.; Ji, W.; Hui, Y.; Yao, X. Electrospinning synthesis of porous carbon fiber supported Pt-SnO2 anode catalyst for direct ethanol fuel cell. Solid State Sci. 2019, 94, 64–69. [Google Scholar] [CrossRef]
- Kuriganova, A.B.; Leontyev, I.N.; Ulyankina, A.A.; Smirnova, N.V. Electrochemical dispersion technique for the preparation of Sn-doped Pt particles and their use as electrocatalysts. Mendeleev Commun. 2020, 30, 663–665. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Xin, H.; Liu, J.; Xiong, G.; Wu, P.; Li, X. Sn-doped Pt catalyst supported on hierarchical porous ZSM-5 for the liquid-phase hydrogenation of cinnamaldehyde. Catal. Sci. Technol. 2019, 9, 3226–3237. [Google Scholar] [CrossRef]
- Novikova, K.; Kuriganova, A.; Leontyev, I.; Gerasimova, E.; Maslova, O.; Rakhmatullin, A.; Smirnova, N.; Dobrovolsky, Y. Influence of carbon support on catalytic layer performance of proton exchange membrane fuel cells. Electrocatalysis 2018, 9, 22–30. [Google Scholar] [CrossRef]
- Kuriganova, A.B.; Vlaic, C.A.; Ivanov, S.; Leontyeva, D.V.; Bund, A.; Smirnova, N.V. Electrochemical dispersion method for the synthesis of SnO2 as anode material for lithium ion batteries. J. Appl. Electrochem. 2016, 46, 527–538. [Google Scholar] [CrossRef]
- Ulyankina, A.A.; Kuriganova, A.B.; Smirnova, N.V. Photocatalytic properties of SnO2–SnO nanocomposite prepared via pulse alternating current synthesis. Mendeleev Commun. 2019, 29, 215–217. [Google Scholar] [CrossRef]
- Kuriganova, A.; Leontyeva, D.; Ivanov, S.; Bund, A.; Smirnova, N. Electrochemical dispersion technique for preparation of hybrid MOx–C supports and Pt/MOx–C electrocatalysts for low-temperature fuel cells. J. Appl. Electrochem. 2016, 46, 1245–1260. [Google Scholar] [CrossRef]
- Alias, M.; Kamarudin, S.; Zainoodin, A.; Masdar, M. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. Approaches to overcome the barrier issues of passive direct methanol fuel cell–Review. Renew. Sustain. Energy Rev. 2017, 67, 1087–1104. [Google Scholar] [CrossRef]
- Wasmus, S.; Küver, A. Methanol oxidation and direct methanol fuel cells: A selective review. J. Electroanal. Chem. 1999, 461, 14–31. [Google Scholar] [CrossRef]
- Badwal, S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Kamarudin, M.; Kamarudin, S.; Masdar, M.; Daud, W. Direct ethanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef]
- Bittins-Cattaneo, B.; Wilhelm, S.; Cattaneo, E.; Buschmann, H.; Vielstich, W. Intermediates and products of ethanol oxidation on platinum in acid solution. Ber. Bunsenges. Phys. Chem. 1988, 92, 1210–1218. [Google Scholar] [CrossRef]
- Gootzen, J.; Visscher, W.; Van Veen, J. Characterization of ethanol and 1, 2-ethanediol adsorbates on platinized platinum with Fourier transform infrared spectroscopy and differential electrochemical mass spectrometry. Langmuir 1996, 12, 5076–5082. [Google Scholar] [CrossRef]
- Hitmi, H.; Belgsir, E.; Léger, J.-M.; Lamy, C.; Lezna, R. A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium. Electrochim. Acta 1994, 39, 407–415. [Google Scholar] [CrossRef]
- Iwasita, T.; Pastor, E. A DEMS and FTIR spectroscopic investigation of adsorbed ethanol on polycrystalline platinum. Electrochim. Acta 1994, 39, 531–537. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Ianniello, R.; Pastor, E.; González, S. Electrochemical reactivity of ethanol on porous Pt and PtRu: Oxidation/reduction reactions in 1 M HClO4. J. Phys. Chem. 1996, 100, 17901–17908. [Google Scholar] [CrossRef]
- Willsau, J.; Heitbaum, J. Elementary steps of ethanol oxidation on Pt in sulfuric acid as evidenced by isotope labelling. J. Electroanal. Chem. Interfacial Electrochem. 1985, 194, 27–35. [Google Scholar] [CrossRef]
- Marinkovic, N.S.; Li, M.; Adzic, R.R. Pt-Based Catalysts for Electrochemical Oxidation of Ethanol. In Electrocatal; Shao, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–39. [Google Scholar]
- Huang, H.; Blackman, O.F.; Celorrio, V.; Russell, A.E. Isolating the contributions of surface Sn atoms in the bifunctional behaviour of PtSn CO oxidation electrocatalysts. Electrochim. Acta 2021, 390, 138811. [Google Scholar] [CrossRef]
- Baranova, E.A.; Padilla, M.A.; Halevi, B.; Amir, T.; Artyushkova, K.; Atanassov, P. Electrooxidation of ethanol on PtSn nanoparticles in alkaline solution: Correlation between structure and catalytic properties. Electrochim. Acta 2012, 80, 377–382. [Google Scholar] [CrossRef]
- Charlton, J.S.; Cordey-Hayes, M.; Harris, I.R. A study of the 119Sn mössbauer isomer shifts in some platinum-tin and gold-tin alloys. J. Less Common Met. 1970, 20, 105–112. [Google Scholar] [CrossRef]
- Leontyev, I.N.; Kulbakov, A.A.; Allix, M.; Rakhmatullin, A.; Kuriganova, A.B.; Maslova, O.A.; Smirnova, N.V. Thermal expansion coefficient of carbon-supported Pt nanoparticles: In-situ X-ray diffraction study. Phys. Status Solidi 2017, 254, 1600695. [Google Scholar] [CrossRef]
- Rudi, S.; Cui, C.; Gan, L.; Strasser, P. Comparative Study of the Electrocatalytically Active Surface Areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and CO-stripping voltammetry. Electrocatalysis 2014, 5, 408–418. [Google Scholar] [CrossRef]
- Rizo, R.; Sebastián, D.; Lázaro, M.J.; Pastor, E. On the design of Pt-Sn efficient catalyst for carbon monoxide and ethanol oxidation in acid and alkaline media. Appl. Catal. B Environ. 2017, 200, 246–254. [Google Scholar] [CrossRef]
- Rizo, R.; Arán-Ais, R.M.; Padgett, E.; Muller, D.A.; Lázaro, M.J.; Solla-Gullón, J.; Feliu, J.M.; Pastor, E.; Abruña, H.D. Pt-Richcore/Sn-Richsubsurface/Ptskin Nanocubes as Highly Active and Stable Electrocatalysts for the Ethanol Oxidation Reaction. J. Am. Chem. Soc. 2018, 140, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cano, L.A.; Rosado-Ortiz, G.; Valenzuela-Muñiz, A.M.; Ordóñez, L.C.; Gauvin, R.; Verde Gómez, Y. Solvent effect in the synthesis of nanostructured Pt–Sn/CNT as electrocatalysts for the electrooxidation of ethanol. Int. J. Hydrogen Energy 2019, 44, 12430–12438. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, J.S.; Guo, X.; Zhao, J.H.; Song, C.Y.; Wang, L.C. Improving the Stability and Ethanol Electro-Oxidation Activity of Pt Catalysts by Selectively Anchoring Pt Particles on Carbon-Nanotubes-Supported-SnO2. Fuel Cells 2012, 12, 898–903. [Google Scholar] [CrossRef]
- Higuchi, E.; Miyata, K.; Takase, T.; Inoue, H. Ethanol oxidation reaction activity of highly dispersed Pt/SnO2 double nanoparticles on carbon black. J. Power Sources 2011, 196, 1730–1737. [Google Scholar] [CrossRef]
| Sample | a, Å | D (XRD), nm | D (TEM), nm |
|---|---|---|---|
| Pt/C | 3.9131 | 7.3 ± 1.0 | 10.4 ± 1.0 |
| PtSnx + SnO2/C | 3.9174 | 5.6 ± 0.6 | - |
| Pt/SnO2/C | 3.9131 | 7.3 ± 1.0 | 8.0 ± 0.8 |
| Pt3Sn/C | 4.0047 | 5.7 ± 0.8 | 7.6 ± 0.7 |
| Sample | CO Stripping | Ethanol Electrooxidation | |||||
|---|---|---|---|---|---|---|---|
| ECSA, m2 g−1 | j0.6V, mA cm−2 | Epeak, V | j0.6V, mA cm−2 | j peak, mA cm−2 | Eonset, V | Epeak, V | |
| Pt/C | 13.1 ± 1.3 | 0.024 | 0.70 | 0.046 | 0.406 | 0.77 | 0.93 |
| PtSnx + SnO2/C | 12.3 ± 1.2 | 0.057 | 0.63 | 0.083 | 0.84 | 0.62 | 0.90 |
| Pt/SnO2/C | 13.1 ± 1.5 | 0.040 | 0.64 | 0.113 | 1.19 | 0.62 | 0.86 |
| Pt3Sn/C | 13.5 ± 1.5 | 0.052 | 0.59 | 0.62 | 0.64 | 0.61 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuriganova, A.; Kubanova, M.; Leontyev, I.; Molodtsova, T.; Smirnova, N. Pulse Electrolysis Technique for Preparation of Bimetal Tin-Containing Electrocatalytic Materials. Catalysts 2022, 12, 1444. https://doi.org/10.3390/catal12111444
Kuriganova A, Kubanova M, Leontyev I, Molodtsova T, Smirnova N. Pulse Electrolysis Technique for Preparation of Bimetal Tin-Containing Electrocatalytic Materials. Catalysts. 2022; 12(11):1444. https://doi.org/10.3390/catal12111444
Chicago/Turabian StyleKuriganova, Alexandra, Marina Kubanova, Igor Leontyev, Tatiana Molodtsova, and Nina Smirnova. 2022. "Pulse Electrolysis Technique for Preparation of Bimetal Tin-Containing Electrocatalytic Materials" Catalysts 12, no. 11: 1444. https://doi.org/10.3390/catal12111444
APA StyleKuriganova, A., Kubanova, M., Leontyev, I., Molodtsova, T., & Smirnova, N. (2022). Pulse Electrolysis Technique for Preparation of Bimetal Tin-Containing Electrocatalytic Materials. Catalysts, 12(11), 1444. https://doi.org/10.3390/catal12111444








