Abstract
This work reports on the synthesis of iron-platinum on Vulcan carbon (FePt/VC) as an effective catalyst for the electrooxidation of molecular hydrogen at the anode, and electroreduction of molecular oxygen at the cathode of a proton exchange membrane fuel cell. The catalyst was synthesized by using the simple polyol route and characterized by XRD and HRTEM along with EDS. The catalyst demonstrated superior electrocatalytic activity for the oxygen reduction reaction and the oxidation of hydrogen with a 2.4- and 1.2-fold increase compared to platinum on Vulcan carbon (Pt/VC), respectively. Successful application of FePt/VC catalyst in a self-breathing fuel cell also showed a 1.7-fold increase in maximum power density compared to Pt/VC. Further analysis by accelerated stress test demonstrated the superior stability of FePt on the VC substrate with a 4% performance degradation after 60,000 cycles. In comparison, a degradation of 6% after 10,000 cycles has been reported for Pt/Ketjenblack.
1. Introduction
The cost of Polymer Electrolyte Membrane Fuel Cells (PEMFCs) currently hinders their wide deployment and this is mainly due to the cost of platinum (Pt) which represent 40% of the total cost of the PEMFCs [1,2]. In current PEMFCs, the majority of the Pt (80%) is placed at the cathode side to overcome the sluggish nature of the oxygen reduction reaction (ORR) [3,4]. Indeed, the ORR is 5 order of magnitude slower than the hydrogen oxidation reaction (HOR) at the anode [2].
Over the last two decades research efforts have focused on developing better ORR catalysts to improve their catalytic activity and durability [5,6]. In particular, two main approaches have emerged: (i) minimise the amount of Pt needed by alloying Pt with low-cost transition metals such as nickel (Ni), cobalt (Co), chromium (Cr) and copper (Cu); [7] and (ii) develop non-noble metal based catalysts, e.g., based on a combination of carbon, nitrogen (N), and transition metals including Co and, iron (Fe), manganese (Mn) [8,9,10,11].
One of the promising Pt alloys recently reported, PtCe, showed an enhanced ORR activity (half wave potential, E1/2 = 0.73 V vs. standard hydrogen electrode (SHE), 0.1 M HClO4) over Pt/C (E1/2 = 0.68 V vs. SHE, 0.1 M HClO4) [12]. Similarly, PtNi showed superior ORR performance (E1/2 = 0.8 V vs. SHE, 0.1 M HClO4) compared to Pt/C (E1/2 = 0.75 V vs. SHE, 0.1 M HClO4) [13]. Alternatives of Pt in the form of iron/nitrogen/carbon have also shown reasonable electrocatalytic activities [14]. For example, an activity of E1/2 = 0.74 vs. SHE (0.1 M HClO4) was reported for CoNC [15]. Similarly, bimetallic catalysts, such as FeCoNC (0.1 M HClO4) and MnCoNC (0.5 M H2SO4) were reported to both have an activity of E1/2 = 0.80 V vs. SHE [9,15]. However, these catalysts still suffer from relatively low activity compared to Pt and poor stability. Accordingly, to date, Pt remains the catalyst of choice. The use of Pt alloys has also been reported to result in a better durability of the membrane electrode assembly (MEA) in comparison to Pt only [16], and this is because alloys of Pt have been shown to favour the four-electron path of the ORR which does not involve the formation of peroxides. The latter can lead to an accelerated degradation of the proton exchange membrane, e.g., Nafion [17]. Upon alloying Pt with transition metals, the Pt-Pt bond distance is expected to decrease [5], and this results in a downshift of the d-band centre of Pt and improved electrocatalytic activity as the intermediate species (OH•, OOH• and O•) bind less strongly to the catalytic active sites [5,8]. In addition, the formation of an oxide layer at the surface of Pt alloys upon ORR has been reported to be less pronounced due to the weaker adsorption of the intermediate species [8].
Although Pt plays a major role at the anode for HOR, less work has been done to substitute Pt on the HOR side of PEMFCs. Among the few studies, the use of palladium (Pd) alloyed with other elements such as Pt, iridium (Ir) and ruthenium (Rh) has been investigated. For example, the electrocatalytic activity of PdIr was found to exceed that of Pt by 1.8 fold (at 0.2 V vs. SHE, 0.5 M H2SO4) [18]. A recent work on PtCu reported a 2.4 fold improvement of HOR activity as compared to Pt only (at 0.2 V vs. SHE,0.1 M HClO4) [8].
Regardless of the promising results reported with these catalysts, it is difficult to estimate their practical viability because most of these have only been tested at the surface of a rotating disk electrode (RDE) and not within operating fuel cells [5]. One of the issues concerns the durability of these catalysts under real operating conditions because it has been reported that the stability of shape-controlled catalysts (both Pt and Pt-alloy), owing to their ‘metastable nature’, is lower than conventional Pt supported on carbon [19,20].
In this respect, Pt-Fe alloys have been extensively investigated because of their good electrocatalytic activity towards ORR. In particular, face-centred tetragonal (fct) FePt has been found to be highly stable under acidic environments [21]. It is assumed that upon alloying Pt with Fe, a charge transfer from Fe to Pt would occur. This would alter the electronic density around Pt, and thus lead to a weaker adsorption of OH• intermediates [22,23] and as such the ORR would occur along the preferred 4 electron path [24].
However, dispersion of such alloys at the surface of conventional carbon black, to increase the number of active sites, has been reported to lead to extensive particle instability, including detachment from the substrate [25]. Carbon black was also reported to be susceptible to corrosion [26]. Extensive carbon corrosion would occur at the cathode of PEMFC at voltages >1.44 V [26]. Carbon corrosion would also be accelerated at high fuel cell operating temperature, i.e., >80 °C [27,28].Accordingly, efforts have focused on the use of alternative carbon substrates such as porous carbon fibres and nitrogen doped carbons, [22,29] and/or the synthesis of substrate free FePt nanoparticles [30]. For example, a N-doped carbon shell was developed to individually cover the PtFe nanoparticles and prevent their agglomeration during synthesis and stabilise a Pt-rich surface [21]. Similarly, PtFe nanoparticles were encapsulated within a Pt shell to increase the electrocatalytic activity [31].
Although these approaches have demonstrated the possibility to stabilise highly dispersed FePt fct particles, and limit carbon corrosion, the synthesis methods used are relatively complicated and impractical to some extent. Herein, we report on a simple, surfactant free, and one step method to synthesize a highly durable FePt fct catalyst supported on Vulcan carbon (FePt/VC) that can be used as a catalyst at both the anode and cathode of a PEMFC. The choice of VC was mainly driven by the fact that it usually leads to a high dispersion of nanoparticles, and thus a higher electrochemical surface area (ECSA, 103 m2 g−1) [32] and smaller inter-particles distances (<5 nm) [32]. The latter is important to ensure optimum electrocatalysis [32,33]. Among the various carbon substrates, VC has also been shown to be the less susceptible to corrosion [34].
2. Results and Discussion
2.1. Synthesis of FePt/VC
Ethylene glycol is commonly used in the polyol process because it provides good control over the particles size distribution and spatial dispersion of catalysts at the surface of the support. During synthesis, it is expected that the dehydration of ethylene glycol in the presence of H2PtCl6 and FeCl3 will result in the formation of acetaldehyde which oxidizes during Pt4+ and Fe3+ reduction to result in diacetyl [8,35]. In this process, Pt and Fe nuclei are generated in solution and heterogenous nucleation and growth is expected to occur at the defective sites of the VC [36].
Upon TEM analysis of FePt/VC, particles with an average size of 2 ± 0.3 nm uniformly dispersed on VC were observed (Figure 1a). These particles had a d spacing of 0.219 nm and this would correspond to FePt (111) (Figure 1b,c). The average inter-particle distance between FePt particles was 2.1 ± 0.5 nm (Figure S1). This is expected to result in better electrocatalytic activity because the resulting overlapping of the double layer around the individual particles is expected to further alter the adsorption energy of oxygen containing species (O•, OH•) and increase the ORR [37,38]. Further, STEM and elemental mapping of FePt/VC revealed uniformly distributed Pt and Fe, and this would suggest that these elements are co-located on the VC support.

Figure 1.
(a,b) HRTEM images, (c) SAD, and (d) EDS elemental mapping of FePt/VC.
The XRD pattern of as-synthesised FePt/VC revealed diffraction peaks at 40.2 and 68.0° assigned to fct FePt (111) and FePt (220) (Figure 2) [29,39]. The peak positions are shifted slightly towards higher diffraction angles compared to Pt/VC (Figure S3). This shift towards higher diffraction angles could be due to a decrease in the lattice spacing as a result of alloying Pt with the smaller Fe atom [40].
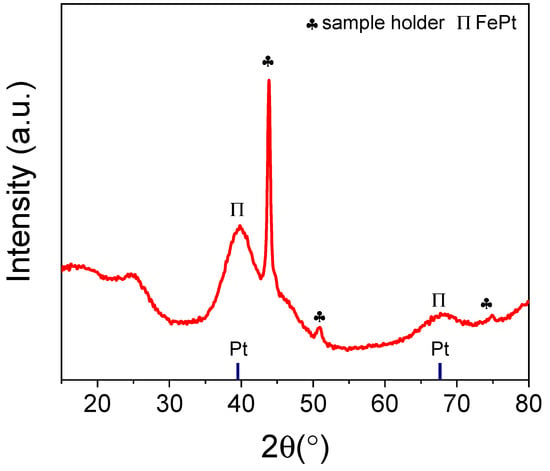
Figure 2.
XRD pattern of as-synthesised FePt/VC.
2.2. HOR Electrocatalytic Activity of FePt/VC
Figure 3 and Figure S4, respectively, show the CV of as-synthesised FePt/VC and Pt/VC in 0.1 M aqueous HClO4 at the scan rate of 50 mV s−1 under saturated Ar and H2. Under saturated Ar, the peaks associated with the adsorption and desorption of underpotential deposited H (HUPD) were clearly distinct. Similarly, the peaks associated with the formation and reduction of Pt oxides in the region 0.7–1 V and 0.6–1 V, respectively, were also visible and in agreement with previous reports on Pt/C HOR electroactivity [41]. This implies that the surface composition of FePt is dominated by Pt [40]. The electrochemical surface area (ECSA) of FePt/VC was calculated as 54 m2 g−1 and 64 m2 g−1 for Pt/VC (Figure S5a).
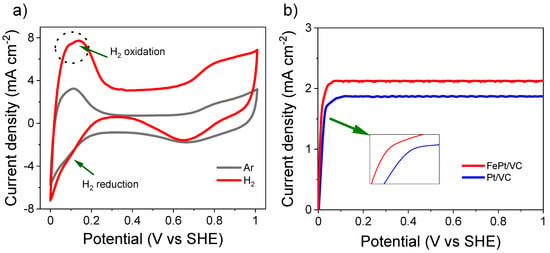
Figure 3.
HOR activity of FePt/VC, including (a) its CV at scan rate 50 mV s−1 @ 1600 rpm in 0.1 M aqueous HClO4 under saturated Ar and H2, and (b) LSV comparison between FePt/VC and Pt/VC at the scan rate of 10 mV s−1 @ 1600 rpm under saturated H2. Catalyst loading was 50 µg cm−2.
Under saturated H2, the HUPD was more prominent particularly in the region 0.02–0.32 V and the entire CV profile exhibited larger currents associated with the occurring HOR. This signifies that FePt/VC is a good HOR catalyst in comparison to Pt/VC (Figure S4a). The improved HOR performance of FePt/VC is assumed to be due to a weakened binding energy of adsorbed hydrogen (H• −0.38 eV) at the surface of FePt/VC as compared to Pt (−1.05 eV) [42,43]. As such alloying Pt with Fe enhances the HOR. Better performance of FePt/VC for HOR was further confirmed by LSV measurement, where the hydrogen oxidation at FePt/VC started at a lower potential (17 mV vs. SHE) compared to Pt/VC (Figure 3b).
2.3. ORR Electrocatalytic Activity of FePt/VC
FePt/VC was further examined for ORR under saturated O2 (Figure 4). Pt oxide formation and reduction at the surface of FePt/VC was highly pronounced with larger currents under saturated O2 suggesting that the ORR is proceeding (Figure 4a). This was further confirmed by LSV measurement. Indeed, the half-wave potential of FePt/VC shifted towards higher potentials by + 57 mV in comparison to Pt/VC only (Figure 4b and Figure S4b). The mass activity of FePt/VC (460 mA mgPt−1) for ORR was also found to be superior to that of Pt/VC (164 mA mgPt−1) (Figure S5b).
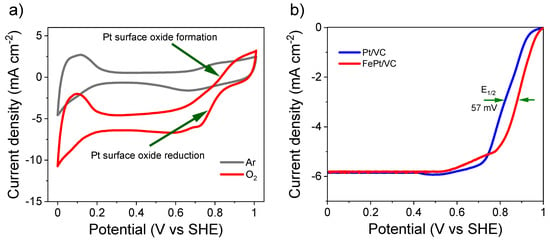
Figure 4.
ORR activity of FePt/VC, including (a) its CV at scan rate 50 mV s−1 @ 1600 rpm in 0.1 M aqueous HClO4 under saturated Ar and O2, and (b) the LSV comparison between FePt/VC and Pt/VC at the scan rate of 10 mV s−1 @ 1600 rpm under saturated O2. Catalyst loading was 50 µg cm−2.
This better catalytic performance could be explained by a 4-electron path. If the ORR follows the 4-electron path, molecular O2 forms two O• radicals (reaction 1) which reacts with H+ to lead to O• and OH• (reaction 2). Based on theoretical studies, it has been predicted that the final step of converting species OH• to H2O (rection 3) occurs at a lower energy barrier of 0.18 eV on FePt (111) instead of the 0.34 eV calculated for Pt (111) [44]. As such, a lower energy barrier is expected to facilitate ORR through the four electron-path (reaction 1–4).
This 4-electron path was further confirmed by calculating the number of electrons transferred during the ORR of FePt and this was found to be 3.8 instead of 3.6 for Pt/VC (Figure S6). Accordingly, the occurrence of a 2 electron path as per reaction (5–6) leading to the formation of H2O2 as the main product [44] cannot be excluded, however, it is most likely that at FePt the ORR proceeds accruing to a 4 electron path. Further comparison of parameters including the onset and Tafel slope against values reported in the literatures is shown Table S1 and Figure S7.
2.4. Performance of FePt/VC in Self-Breathing PEMFC
Owing to its excellent electrocatalytic activity for both HOR and ORR, FePt/VC was further evaluated under self-breathing PEMFC operation (Figure 5). For comparison, two MEAs were prepared, one with FePt/VC on both sides of the MEA and the other with Pt/VC at both the anode and cathode of the fuel cell. As expected, the performance of FePt/VC was superior (112 mW cm−2) to that of Pt/VC (66 mW cm−2). This is in line with the observations from RDE where both HOR and ORR activities of FePt/VC were superior to that of Pt/VC.
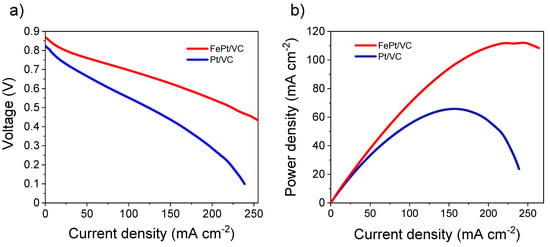
Figure 5.
Performance of FePt/VC and Pt/VC in a self-breathing PEMFC, (a) current density vs. voltage, (b) current density vs. power density, catalyst loading at the active area of both the anode and cathode was the same, i.e., 0.8 mg cm−2. The H2 flow rate was 10 mL min−1. Catalyst loading was the same at both MEAs (0.8 mg cm−2) and the self-breathing PEMFC operation was done at an ambient condition (25 °C, H2 at 1 bar and 20%RH at the anode).
The performance of FePt/VC was further evaluated through an accelerated stress test under self-breathing PEMFC operation. Figure 6 shows the evolution of the polarization curves up to 60,000 cycles, with a degradation in fuel cell performance of 4% only (at 200 mA cm−2) after 60,000 cycles. Under these conditions, degradation in maximum power density was 3% (Figure 6b).
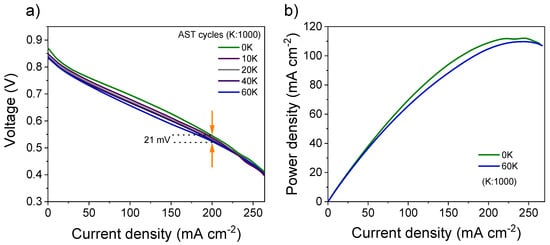
Figure 6.
(a) Fuel cell polarisation curves at various cycles of operation under AST, (b) comparison of power density between first cycle (0K) and final cycle (60 K). Performance was monitored in the voltage range of 0.5–0.6 V at the current density of 200 mA cm−2.
Due to the unique nature of self-breathing operation, this work cannot be directly compared with the other accelerated stress tests. However, as summarised in Table 1, the performance of the FePt/VC catalyst reported here is far superior to previous catalysts. The performance degradation of 21 mV in this work after 60,000 fuel cell cycles is remarkable, and this may be attributed to the stability of FePt fct phase under acidic environments [45].

Table 1.
Performance of various Pt and Pt-alloy cathode catalysts recently reported.
A degradation of 6% after 10,000 cycles was reported for Pt/Ketjenblack, and this was mainly due to the agglomeration of Pt particles during cycling [27]. Such an effect has not been observed with Pt-alloy nanoparticles, e.g., PtFe and PtY [39,46]. In this case, performance degradation was mainly attributed to a leaching of the transition metal [28].
This demonstrates the capability of FePt/VC as a catalyst to withstand abrupt load conditions which is important particularly in demanding applications. The maximum power density with FePt/VC as anode and cathode catalyst degraded by 3% after 60,000 cycles. As per our knowledge, FePt/VC catalyst have not been evaluated under self-breathing PEMFC in the past. Among the very few Pt-alloys operated under self-breathing PEMFC conditions, PtCu delivered a maximum power density of 45.16 mW cm−2 [8]. The performance of FePt/VC reported in this work is superior to this. The better performance of FePt in comparison to other Pt alloys could be due to the better dispersion of FePt nanoparticles with a short interparticle distance of 2.1 ± 0.5 nm.
3. Materials and Methods
3.1. Materials
Chloroplatinic acid hexahydrate (H2PtCl6∙6H2O, ≥ 99.9% trace metals basis), Iron (III) chloride (FeCl3, anhydrous >98%), ethylene glycol (anhydrous 99.8%), 2-propanol (99.7%), and perchloric acid (70%, 99.99%) were purchased from Sigma-Aldrich, Sydney, NSW, Australia. Vulcan XC 72R, NafionTM 212, Nafion TM dispersion (10 wt%), Gas Diffusion Layer (GDL): (Freudenberg H23C2), and 40 wt% Platinum on Vulcan Carbon (VC) (Fuel Cell grade) were purchased from Fuel Cell Store, College Station, TX, USA. For all the synthesis Milli Q water was used.
3.2. Synthesis of Iron-Platinum on Vulcan Carbon (FePt/VC)
Iron-platinum on VC was prepared by using the polyol process [8]. In brief, 50 mg of VC was mixed with 33 mL of ethylene glycol, 17 mL of Milli Q water, 35 mg of H2PtCl6∙6H2O and 16 mg of FeCl3 in a 100 mL single neck round bottom flask. After sonication for 5 min for homogenisation, the mixture was continuously stirred for 15 h at room temperature. The following day, the round flask, containing the total mixture, was heated to 120 °C for 2 h under reflux and continuously stirred for the reduction of Pt and Fe on the carbon substrate. After cooling, the obtained catalyst was washed three times with Milli Q water and separated by centrifugation at 10,000 rpm. The resulting material was dried under vacuum at 60 °C for 15 h. The synthesised catalyst is referred to as FePt/VC in the manuscript. The same procedure was repeated to synthesise Pt/VC catalyst with the same amount of Pt loading without FeCl3 addition. This material is referred to as Pt/VC in the manuscript. By ICP-OES, it was confirmed that FePt/VC contained 19.1 ± 0.2% of Pt and 8 ± 0.1% of Fe and this is in agreement with the amount used during synthesis.
3.3. Preparation of the Catalyst Ink
1.1 mg of as-synthesised catalyst, 480 mL of Milli Q water, 20 µL of Nafion 10% in water and 120 mL of 2-propanol were mixed and sonicated for 5 min to form a homogenous mixture. A glassy carbon electrode was polished with the help of an alumina suspension on a microcloth disk. 2 µL of the catalyst ink was pipetted and dropped onto the polished glassy carbon electrode surface (3 mm diameter) and left to dry at room temperature. The catalyst loading for all the experiments by Rotating Disk Electrode (RDE) was 50 µg cm−2.
For making the fuel cell, 24 mg of as-synthesised catalyst was added to a vial. 125 µL of Milli Q water, 100 µL Nafion 10% in water and 500 µL of 2-propanol were added. The final volume was adjusted by adding 1.5 mL of 2-propanol. The mixture was sonicated for 5 min and left to stir overnight at room temperature.
3.4. Preparation of the Membrane Electrode Assembly
The catalyst ink was dispersed on the microporous layer of the GDL by using the doctor blade technique. This was then dried in an oven at 60 °C for 1 h. Nafion 212 was used without pre-treatment. The MEA was formed by placing the catalyst coated GDL on either side of the Nafion membrane and hot pressing at 0.18 MPa and 90 °C for 2 min. The catalyst (loading in all the MEAs was 0.8 mg cm−2, the ionomer (Nafion) in the MEA was 30 wt% of the solid dispersed in the catalyst ink. The active area of the MEA was 2 cm2.
3.5. Characterization
High-Resolution Transmission Electron Microscopy (HRTEM), Selected Area Diffraction (SAD) and Energy Dispersive X-ray Spectroscopy (EDS) were performed with a JEOL JEM-F200 (Tokyo, Japan) cold field emission gun operated at 200 kV with an attached windowless 100 mm2 silicon drift X-ray detector. To achieve this aim, the as-synthesised catalysts were dispersed in ethanol and sonicated before being dropped casted onto a carbon coated copper grid.
X-ray Diffraction (XRD) was performed by using a Philips X’pert Multipurpose XRD system (Tokyo, Japan) operated at 40 mA and 45 kV and equipped with a monochromated Cu Kα radiation (λ = 1.541 Å). Diffraction patterns were recorded between 15 to 80°. X-ray Photoelectron Spectroscopy (XPS) was performed by using a Thermo ESCALAB250Xi XPS (Waltham MA, USA) system operated at 160 W and equipped with a mono-chromated Al K-α (1486.68 eV) X-ray source.
The amount of Pt and Fe in the as-synthesised catalysts was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) by using an Optima 7300 DV (PerkinElmer, Waltham, MA, USA). For this, the materials were digested in acid (3HCl + 1HNO3).
Cyclic Voltammetry (CV) and Linear Sweep Voltammetry (LSV) were performed by using a VMP3-Biologic potentiostat. The potentiostat was connected to a Rotating Disk Electrode (RDE), Basi RDE 2 having a 3 electrodes electrochemical cell. Ag/AgCl saturated with 3 M NaCl was used as the reference electrode and a Pt wire as the counter electrode. Freshly prepared 0.1 M HClO4 was used as the electrolyte. The catalyst activity was determined by subtracting the background measurement and iR correction.
Fuel cell testing was done by using a self-breathing single PEMFC as described Figure 7 [48]. The anode had a mixed serpentine flow field (Figure 7a) and the opening at the air cathode was 35% of the active area of the cell, i.e., 2 cm2. A silicon gasket was used for sealing (Figure 7d). Full detailed design of the self-breathing PEMFC can be found in reference [48].
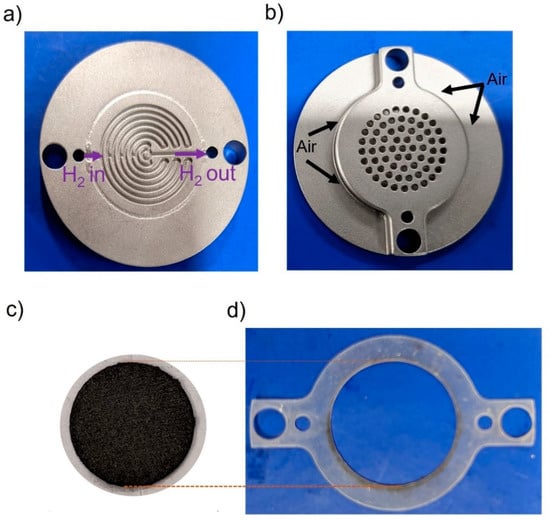
Figure 7.
(a) Photo of the self-breathing PEMFC showing the (a) anode, (b) open cathode, (c) MEA, (d) silicon sealing gasket.
Accelerated Stress Test (AST) was achieved by adjusting the protocol recommended by the US DOE and other research groups [11,27,49,50]. In brief, polarisation curves were performed between the Open Circuit Voltage (OCV) and 0.4 V, and between the successive cycles the potential was held for 3s at the OCV and 0.4 V. The total number of cycles performed were 60,000 at room temperature (25 °C) with H2 humidified at 20% RH at the anode. Although various AST protocols have been suggested to determine the durability of Pt under fuel cell operation, [11,49,50,51] most of these protocols rely on using N2 instead of air/O2 at the cathode for 30,000 cycles [49]. However, higher fuel cell degradation was reported when O2/air was used [11]. Assessment under O2/air mimics a more realistic fuel cell environment and should also be noted that the existing testing protocols have been designed for the scenario of vehicular applications, [49] and do not address the operating environments of stationary and portable applications. As per our knowledge, no research was conducted in the past where the performance of Pt or Pt alloy catalysts were determined under AST at low temperature, humidity or under self-breathing conditions.
4. Conclusions
The synthesis of a FePt/VC catalyst of average size 2 ± 0.3 nm and fct phase, suitable for electroreduction of molecular oxygen and electrooxidation of molecular hydrogen was reported. This FePt/VC catalyst exceeds the performance of Pt 2.8-fold in terms of mass activity. The enhanced performance was attributed to the weaker adsorption of intermediate species such as OH•, OOH• and O• at the catalyst surface and this was believed to facilitate a four-electron reaction path for the ORR reaction instead of the two-electron path which results in H2O2.
A maximum power density of 112 mW cm−2 (245 mA cm−2) was observed under ambient condition which is 1.7 times that of platinum/carbon under the same operating condition. An accelerated stress test of 60,000 cycles was performed on the MEA developed with FePt/VC. The catalyst showed exceptional performance stability with 4% only degradation in performance between the first and the 60,000 cycle. In comparison, Pt alloy catalysts are reported to degrade more than 4% after 30,000 cycles in conventional fuel cells [31,46]. This also shows the high stability of FePt particles on VC as a substrate. With the simple and scalable method of preparation reported here and their superior stability under PEMFC environment these catalysts have potential to replace existing platinum carbon catalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12111369/s1, Figure S1: Inter-particle distance between the FePt nanoparticles; Figure S2: TEM image of as-synthesized Pt/VC, Figure S3: XRD pattern of as-synthesized Pt/VC, Figure S4: CV of as-synthesized Pt/VC, Figure S5: Electrochemical surface area and mass activity of as-synthesized catalysts, Figure S6: Koutecky–Levich plots, Figure S7: Tafel plots derived from ORR polarization curve, Figure S8: LSV of FePt/VC at various rotational speed, Figure S9: LSV of Pt/VC at various rotational speed, Table S1: Comparison of ORR performance of various catalysts [8,52,53,54,55,56,57,58,59,60].
Author Contributions
P.S. carried out all the experimental work which was conceived and designed with K.-F.A.-Z., S.L. performed analysis on electron microscopy. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sapkota, P.; Kim, H. Zinc–air fuel cell, a potential candidate for alternative energy. J. Ind. Eng. Chem. 2009, 15, 445–450. [Google Scholar] [CrossRef]
- Sapkota, P.; Boyer, C.; Dutta, R.; Cazorla, C.; Aguey-Zinsou, K.-F. Planar polymer electrolyte membrane fuel cells: Powering portable devices from hydrogen. Sustain. Energy Fuels 2020, 4, 439–468. [Google Scholar] [CrossRef]
- Banham, D.; Zou, J.; Mukerjee, S.; Liu, Z.; Yang, D.; Zhang, Y.; Peng, Y.; Dong, A. Ultralow platinum loading proton exchange membrane fuel cells: Performance losses and solutions. J. Power Sources 2021, 490, 229515. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, G.-M.; Yao, R.; Yu, T.; Han, M.-F.; Huang, R.-S. High Oxygen Reduction Activity of Pt-Ni Alloy Catalyst for Proton Exchange Membrane Fuel Cells. Catalysts 2022, 12, 250. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.Y. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Alekseenko, A.; Pavlets, A.; Moguchikh, E.; Tolstunov, M.; Gribov, E.; Belenov, S.; Guterman, V. Platinum-Containing Nanoparticles on N-Doped Carbon Supports as an Advanced Electrocatalyst for the Oxygen Reduction Reaction. Catalysts 2022, 12, 414. [Google Scholar] [CrossRef]
- Garcia-Cardona, J.; Alcaide, F.; Brillas, E.; Sirés, I.; Cabot, P.L. Testing PtCu Nanoparticles Supported on Highly Ordered Mesoporous Carbons CMK3 and CMK8 as Catalysts for Low-Temperature Fuel Cells. Catalysts 2021, 11, 724. [Google Scholar] [CrossRef]
- Sapkota, P.; Boyer, C.; Lim, S.; Aguey-Zinsou, K.-F. High performing platinum—copper catalyst for self—breathing polymer electrolyte membrane fuel cell. Res. Chem. Intermed. 2022, 48, 3019–3037. [Google Scholar] [CrossRef]
- Chao, G.; Zhang, Y.; Zhang, L.; Zong, W.; Zhang, N.; Xue, T.; Fan, W.; Liu, T.; Xie, Y. Nitrogen-coordinated single-atom catalysts with manganese and cobalt sites for acidic oxygen reduction. J. Mater. Chem. A 2022, 10, 5930–5936. [Google Scholar] [CrossRef]
- Osmieri, L. Transition Metal–Nitrogen–Carbon (M–N–C) Catalysts for Oxygen Reduction Reaction. Insights on Synthesis and Performance in Polymer Electrolyte Fuel Cells. Chem. Eng. 2019, 3, 16. [Google Scholar] [CrossRef]
- Osmieri, L.; Cullen, D.A.; Chung, H.T.; Ahluwalia, R.K.; Neyerlin, K.C. Durability evaluation of a Fe–N–C catalyst in polymer electrolyte fuel cell environment via accelerated stress tests. Nano Energy 2020, 78, 105209. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Leng, D.; Yin, F. The enhanced activity of Pt-Ce nanoalloy for oxygen electroreduction. Sci. Rep. 2020, 10, 14837. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, S.; Fan, X.; Tang, M.; Zhao, X.; Chen, W.; Yang, Q.; Quan, Z. Controlled Synthesis of PtNi Hexapods for Enhanced Oxygen Reduction Reaction. Front. Chem. 2018, 6, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yin, S.H.; Chen, Y.H.; Chen, C.; Yan, W.; Cheng, X.Y.; Li, Y.R.; Zhang, T.N.; Yang, J.; Jiang, Y.X.; et al. Experimental and DFT studies of oxygen reduction reaction promoted by binary site Fe/Co-N-C catalyst in acid. J. Electroanal. Chem. 2022, 914, 116322. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Bonville, L.J.; Slattery, D.K. Evaluation of the Durability of Polymer Electrolyte Membranes in Fuel Cells Containing Pt/C and Pt-Co/C Catalysts under Accelerated Testing. ECS Trans. 2011, 41, 1461–1469. [Google Scholar] [CrossRef]
- Antolini, E. Iron-containing platinum-based catalysts as cathode and anode materials for low-temperature acidic fuel cells: A review. RSC Adv. 2016, 6, 3307–3325. [Google Scholar] [CrossRef]
- Tzorbatzoglou, F.; Brouzgou, A.; Jing, S.; Wang, Y.; Song, S.; Tsiakaras, P. Oxygen reduction and hydrogen oxidation reaction on novel carbon supported Pd x Ir y electrocatalysts. Int. J. Hydrogen Energy 2018, 43, 11766–11777. [Google Scholar] [CrossRef]
- Choi, S.I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X.; et al. Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mg(Pt) for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Strmcnik, D.S.; Tripkovic, D.V.; Sun, X.; Kang, Y.; Chi, M.; Snyder, J.D.; van der Vliet, D.; Tsai, Y.; et al. Functional links between Pt single crystal morphology and nanoparticles with different size and shape: The oxygen reduction reaction case. Energy Environ. Sci. 2014, 7, 4061–4069. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, K.; Jiang, H.; Zhu, J.; Ji, H.; Lu, C.; Zhang, L.; Li, J.; Chen, Z.; Ke, C.; et al. Pt3 Fe Nanoparticles Triggered High Catalytic Performance for Oxygen Reduction Reaction in Both Alkaline and Acidic Media. Chem. Electro. Chem. 2022, 9, e202101458. [Google Scholar] [CrossRef]
- Ye, X.; Xue, Y.; Li, K.; Tang, W.; Han, X.; Zhang, X.; Song, Z.; Zheng, Z.; Kuang, Q. Design of ternary Pt–CoZn alloy catalysts coated with N-doped carbon towards acidic oxygen reduction. Mater. Adv. 2021, 2, 5479–5486. [Google Scholar] [CrossRef]
- Jin, N.; Han, J.; Wang, H.; Zhu, X.; Ge, Q. A DFT study of oxygen reduction reaction mechanism over O-doped graphene-supported Pt4, Pt3Fe and Pt3V alloy catalysts. Int. J. Hydrogen Energy 2015, 40, 5126–5134. [Google Scholar] [CrossRef]
- Schonvogel, D.; Hülstede, J.; Wagner, P.; Kruusenberg, I.; Tammeveski, K.; Dyck, A.; Agert, C.; Wark, M. Stability of Pt Nanoparticles on Alternative Carbon Supports for Oxygen Reduction Reaction. J. Electrochem. Soc. 2017, 164, F995–F1004. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.M.; Ye, Y.; Mun, Y.; Lee, S.; Kim, O.-H.; Rhee, H.-W.; Lee, H.I.; Sung, Y.-E.; Lee, J. Development of Highly Stable and Mass Transfer-Enhanced Cathode Catalysts: Support-Free Electrospun Intermetallic FePt Nanotubes for Polymer Electrolyte Membrane Fuel Cells. Adv. Energy Mater. 2015, 5, 142093. [Google Scholar] [CrossRef]
- Kobayashi, A.; Fujii, T.; Harada, C.; Yasumoto, E.; Takeda, K.; Kakinuma, K.; Uchida, M. Effect of Pt and Ionomer Distribution on Polymer Electrolyte Fuel Cell Performance and Durability. ACS Appl. Energy Mater. 2021, 4, 2307–2317. [Google Scholar] [CrossRef]
- Stariha, S.; Macauley, N.; Sneed, B.T.; Langlois, D.; More, K.L.; Mukundan, R.; Borup, R.L. Recent Advances in Catalyst Accelerated Stress Tests for Polymer Electrolyte Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, F492–F501. [Google Scholar] [CrossRef]
- Mao, L.; Fu, K.; Jin, J.; Yang, S.; Li, G. PtFe alloy catalyst supported on porous carbon nanofiber with high activity and durability for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 18083–18092. [Google Scholar] [CrossRef]
- Kuroki, H.; Imura, Y.; Fujita, R.; Tamaki, T.; Yamaguchi, T. Carbon-Free Platinum–Iron Nanonetworks with Chemically Ordered Structures as Durable Oxygen Reduction Electrocatalysts for Polymer Electrolyte Fuel Cells. ACS Appl. Nano Mater. 2020, 3, 9912–9923. [Google Scholar] [CrossRef]
- Li, J.; Xi, Z.; Pan, Y.T.; Spendelow, J.S.; Duchesne, P.N.; Su, D.; Li, Q.; Yu, C.; Yin, Z.; Shen, B.; et al. Fe Stabilization by Intermetallic L10-FePt and Pt Catalysis Enhancement in L10-FePt/Pt Nanoparticles for Efficient Oxygen Reduction Reaction in Fuel Cells. J. Am. Chem. Soc. 2018, 140, 2926–2932. [Google Scholar] [CrossRef]
- Speder, J.; Altmann, L.; Bäumer, M.; Kirkensgaard, J.J.K.; Mortensen, K.; Arenz, M. The particle proximity effect: From model to high surface area fuel cell catalysts. RSC Adv. 2014, 4, 14971–14978. [Google Scholar] [CrossRef]
- Inaba, M.; Zana, A.; Quinson, J.; Bizzotto, F.; Dosche, C.; Dworzak, A.; Oezaslan, M.; Simonsen, S.B.; Kuhn, L.T.; Arenz, M. The Oxygen Reduction Reaction on Pt: Why Particle Size and Interparticle Distance Matter. ACS Catal. 2021, 11, 7144–7153. [Google Scholar] [CrossRef]
- Yu, P.T.; Gu, W.; Zhang, J.; Makharia, R.; Wagner, F.T.; Gasteiger, H.A. Carbon-Support Requirements for Highly Durable Fuel Cell Operation. In Polymer Electrolyte Fuel Cell Durability; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–53. [Google Scholar]
- Holade, Y.; Sahin, N.; Servat, K.; Napporn, T.; Kokoh, K. Recent Advances in Carbon Supported Metal Nanoparticles Preparation for Oxygen Reduction Reaction in Low Temperature Fuel Cells. Catalysts 2015, 5, 310–348. [Google Scholar] [CrossRef]
- Dennany, L.; Sherrell, P.; Chen, J.; Innis, P.C.; Wallace, G.G.; Minett, A.I. EPR characterisation of platinum nanoparticle functionalised carbon nanotube hybrid materials. Phys. Chem. Chem. Phys. 2010, 12, 4135–4141. [Google Scholar] [CrossRef]
- Nesselberger, M.; Roefzaad, M.; Hamou, R.F.; Biedermann, P.U.; Schweinberger, F.F.; Kunz, S.; Schloegl, K.; Wiberg, G.K.; Ashton, S.; Heiz, U.; et al. The effect of particle proximity on the oxygen reduction rate of size-selected platinum clusters. Nat. Mater. 2013, 12, 919–924. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Li, Q.; Wu, L.; Wu, G.; Su, D.; Lv, H.; Zhang, S.; Zhu, W.; Casimir, A.; Zhu, H.; Mendoza-Garcia, A.; et al. New approach to fully ordered fct-FePt nanoparticles for much enhanced electrocatalysis in acid. Nano Lett. 2015, 15, 2468–2473. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, M.; Li, K.; Liu, C.; Xing, W. Superior electrocatalytic activity from nanodendritic structure consisting of a PtFe bimetallic core and Pt shell. Chem. Commun. 2015, 51, 3215–3218. [Google Scholar] [CrossRef]
- Prass, S.; St-Pierre, J.; Klingele, M.; Friedrich, K.A.; Zamel, N. Hydrogen Oxidation Artifact During Platinum Oxide Reduction in Cyclic Voltammetry Analysis of Low-Loaded PEMFC Electrodes. Electrocatalysis 2020, 12, 45–55. [Google Scholar] [CrossRef]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Yano, H.; Tryk, D.A.; Nohara, S.; Uchida, H. High hydrogen evolution activity and suppressed H2O2 production on Pt-skin/PtFe alloy nanocatalysts for proton exchange membrane water electrolysis. Phys. Chem. Chem. Phys. 2019, 21, 2861–2865. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Q.; Xu, G.-L.; Qin, X.; Hwang, I.; Sun, C.-J.; Liu, M.; Hua, W.; Wu, H.-w.; Zhu, S.; et al. Atomically dispersed Pt and Fe sites and Pt–Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 2022, 5, 503–512. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Achievements in Pt nanoalloy oxygen reduction reaction catalysts: Strain engineering, stability and atom utilization efficiency. Chem. Commun. 2021, 57, 12898–12913. [Google Scholar] [CrossRef] [PubMed]
- Schwämmlein, J.N.; Harzer, G.S.; Pfändner, P.; Blankenship, A.; El-Sayed, H.A.; Gasteiger, H.A. Activity and Stability of Carbon Supported PtxY Alloys for the ORR Determined by RDE and Single-Cell PEMFC Measurements. J. Electrochem. Soc. 2018, 165, J3173–J3185. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Peng, J.K.; Kariuki, N.N.; Myers, D.J.; Rasouli, S.; Ferreira, P.J.; Yang, Z.; Martinez-Bonastre, A.; Fongalland, D.; et al. Durability of De-Alloyed Platinum-Nickel Cathode Catalyst in Low Platinum Loading Membrane-Electrode Assemblies Subjected to Accelerated Stress Tests. J. Electrochem. Soc. 2018, 165, F3316–F3327. [Google Scholar] [CrossRef]
- Sapkota, P.; Brockbank, P.; Aguey-Zinsou, K.-F. Development of self-breathing polymer electrolyte membrane fuel cell stack with cylindrical cells. Int. J. Hydrogen Energy 2022, 47, 23833–23844. [Google Scholar] [CrossRef]
- DOE, U. Multi-year Research, Development and Demonstration Plan: 3.4 Fuel Cells. Fuel Cell Technol. Off. 2017, 3, 1–58. [Google Scholar]
- Padgett, E.; Yarlagadda, V.; Holtz, M.E.; Ko, M.; Levin, B.D.A.; Kukreja, R.S.; Ziegelbauer, J.M.; Andrews, R.N.; Ilavsky, J.; Kongkanand, A.; et al. Mitigation of PEM Fuel Cell Catalyst Degradation with Porous Carbon Supports. J. Electrochem. Soc. 2019, 166, F198–F207. [Google Scholar] [CrossRef]
- Bloom, I.; Walker, L.K.; Basco, J.K.; Malkow, T.; Saturnio, A.; De Marco, G.; Tsotridis, G. A comparison of Fuel Cell Testing protocols–A case study: Protocols used by the U.S. Department of Energy, European Union, International Electrotechnical Commission/Fuel Cell Testing and Standardization Network, and Fuel Cell Technical Team. J. Power Sources 2013, 243, 451–457. [Google Scholar] [CrossRef]
- Garsany, Y.; Baturina, O.A.; Swider-Lyons, K.E.; Kocha, S.S. Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction. Anal. Chem. 2010, 82, 6321–6328. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.F.; Guo, P.; Li, J.Z.; Zhao, L.; Sui, X.L.; Wang, Y.; Wang, Z.B. How to appropriately assess the oxygen reduction reaction activity of platinum group metal catalysts with rotating disk electrode. iScience 2021, 24, 103024. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Synthesis and electrocatalytic oxygen reduction properties of truncated octahedral Pt3Ni nanoparticles. Nano Res. 2010, 4, 72–82. [Google Scholar] [CrossRef]
- Paliteiro, C.; Martins, N. Electroreduction of oxygen on a (100)-like polycrystalline gold surface in an alkaline solution containing Pb(II). Electrochim. Acta 1998, 44, 1359–1368. [Google Scholar] [CrossRef][Green Version]
- St. John, S.; Dutta, I.; P. Angelopoulos, A. Synthesis and Characterization of Electrocatalytically Active Platinum Atom Clusters and Monodisperse Single Crystals. J. Phys. Chem. C 2010, 114, 13515–13525. [Google Scholar] [CrossRef]
- Seo, A.; Lee, J.; Han, K.; Kim, H. Performance and stability of Pt-based ternary alloy catalysts for PEMFC. Electrochim. Acta 2006, 52, 1603–1611. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, W.; Wang, K. Oxygen Reduction Reaction Catalyzed by Noble Metal Clusters. Catalysts 2018, 8, 65. [Google Scholar] [CrossRef]
- Paulus, U.A.; Wokaun, A.; Scherer, G.G.; Schmidt, T.J.; Stamenkovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim. Acta 2002, 47, 3787–3798. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).