Abstract
The widespread use of fossil fuels has caused high CO2 concentrations in the atmosphere, which have had a great impact on climate and the environment. Methods for efficiently utilizing CO2 to produce high value-added chemicals have received increasing attention. Among the products of CO2 hydrogenation, olefins, an important petrochemical feedstock, are one of the essential target products. Therefore, CO2 hydrogenation to olefins has been extensively studied, especially for the development of high-performance catalysts. Iron-based catalysts, which are widely used in Fischer–Tropsch synthesis reactions, have also been considered attractive for use in the CO2 hydrogenation to olefins due to their excellent performance in catalytic activity and reaction stability. Most studies have focused on the modulation of morphology; reduction and adsorption properties by tuning the methods of catalyst syntheses; pretreatment conditions and the composition of catalysts, in order to improve hydrogenation activity and olefin yield. In this review, we briefly discuss a thermodynamic overview of the CO2 hydrogenation to olefins reaction, the optimization of catalyst modifications, and current insights into the reaction mechanism; moreover, we summarize current challenges and future trends in the CO2 hydrogenation to olefins.
1. Introduction
Global CO2 emissions from the combustion of fossil fuels and industrial processes reached their highest level of 36.3 gigatons (Gt) in 2021, as shown in Figure 1; this was almost an increase of 6% compared to 2020 [1]. The widespread use of fossil fuels has led to a continuous increase in the atmospheric CO2 concentration, which reached 417.19 ppm in 2022, 1.51 times higher than that of 277.0 ppm at the beginning of the industrial era in 1750 [2]. It is expected to reach 560 ppm by the end of this century. As a major greenhouse gas, CO2 causes various environmental problems, such as climate warming [3]. The global surface temperature in 2021 was +1.12 °C higher relative to the 1880–1920 average in the GISS (Goddard Institute for Space Studies) analysis, and the eight warmest years on the record occurred in the past eight years; 2023 may set a new record [2]. However, since fossil fuels will remain the dominant source of energy for decades. It will be challenging to meet the goal of reducing CO2 emissions, even if we commit to improving energy efficiency and developing uses of renewable energy [4]. Therefore, approaches that utilize CO2 to create economic value have received a lot of attention [5,6,7,8]. Carbon capture and storage (CCS) has been considered the most effective technology [5,9,10] among the different climate change mitigation policies. However, it still faces several technical and economic barriers that must be overcome before it can be deployed on a global scale [11]. Hence, carbon utilization has drawn attention, because it involves the conversion of waste CO2 emissions into value-added products. CO2 has been used in various industries, such as food, agrochemicals, welding, foaming, fire extinguishers, propellants, etc. However, there is still a substantial market demand for developing technologies for the large-scale utilization and conversion of CO2. Therefore, the conversion of CO2 into chemicals and energy products offers a promising pathway that has been extensively investigated.
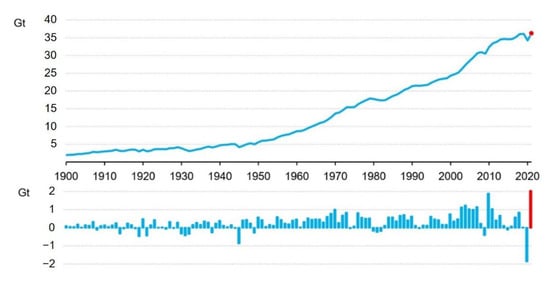
Figure 1.
Total CO2 emissions from energy combustion and industrial processes and their annual change, 1900–2021. Reprinted with permission from ref. [1]. Copyright 2022 IEA.
Thermodynamically, CO2 is stable, and energy is the basis for its conversion and utilization. As a high-energy material, catalytic CO2 hydrogenation that uses hydrogen generated from renewable energy sources, such as solar and wind, is an attractive way to achieve this conversion [7,12,13]. A wide variety of CO2 hydrogenation products can be produced with catalysts containing different active metals, including methane, formic acid and formate, hydrocarbons (especially olefins), alcohols and fuel oils [14,15]. Among the CO2 conversion pathways, CO2 hydrogenation to olefins is considered to be an important strategy for the synthesis of carbon-based fuels and feedstocks.
Olefins play a vital role in the petrochemical industry. As the most demanded chemical feedstock, olefins are not only abundant in downstream derivatives, including plastics, synthetic textiles, lubricants, detergents, solvents and cosmetics [16,17], they are also an important component of liquid fossil fuels, such as gasoline, diesel and jet fuel [18,19,20]. With continuing research on the reaction of CO2 hydrogenation to olefins, there are two generally accepted reaction pathways: (1) the methanol-to-olefins (MTO) pathway: hydrogenation of CO2 to methanol followed by the dehydration of methanol to form olefins [21,22,23,24]; (2) the direct conversion of CO2 pathway: hydrogenation of CO2 to CO via a reverse water-gas-shift (RWGS) reaction, followed by hydrogenation of CO via the classical Fischer–Tropsch synthesis (FTS) reaction to produce olefins. The MTO route usually leads to higher olefin selectivity, because it is not limited by the ASF distribution [25]. However, the temperature regimes for converting CO2 to methanol and methanol to olefins are different [26]. Methanol to olefins is thermodynamically favorable at high temperatures (400–450 °C), but CO2 hydrogenation at high temperatures favors CO formation via the RWGS reaction and not methanol [25,27]. Thus, for the MTO route, the low temperature kinetic limitations and high temperature thermodynamic limitations reduce the productivity of methanol and olefins [28]. For the direct conversion of CO2 pathway, CO formed via the RWGS reaction plays an important role in the downstream FTS process for the target olefin products. The RWGS reaction is endothermic, while the FTS reaction is exothermic, which makes CO2-FTS thermodynamically more favorable than the RWGS reaction alone [29]. In this review, we focus on the research progress of CO2 direct hydrogenation to the olefin pathway.
Since the direct CO2 hydrogenation route shares a common reaction step with the FTS reaction, a similar strategy has been adopted for catalyst design [30,31,32]. The Fe-based and Co-based catalysts that are commonly used for FTS reaction were also investigated for the CO2 hydrogenation reaction. Co-based catalysts limit the growth of carbon chains due to their low RWGS activity and strong adsorption of CO [33,34], resulting in the CO2 hydrogenation products being mainly methane. Even with the addition of promoters that can promote the RWGS reaction, the lower CO concentration on the catalyst surface makes it difficult to build up the FTS system [35]. Fe-based catalysts with high RWGS and FTS activity and reaction stability have been widely investigated. Usually, the product distribution of CO2 hydrogenation over Fe-based catalysts follows the Anderson–Schulz–Flory (ASF) law [36]. In order to synthesize the desired products, the ASF distribution limitation must be broken. Currently, most studies have focused on the modulation of morphology, crystal structure, reduction and adsorption properties of Fe-based catalysts, by tuning the methods of catalyst synthesis [37], roasting temperatures [38], pretreatment conditions [39,40,41] and support materials [42,43,44,45,46], to improve hydrogenation reaction activity and olefin selectivity. In addition, it was found that the doping of auxiliary substances could modulate the surface interface structure of Fe-based catalysts to obtain better catalytic activity and selectivity toward olefins.
2. Thermodynamic Analysis of CO2 Hydrogenation
The direct hydrogenation of CO2 is usually considered to be composed of two steps: RWGS reaction to form CO and H2O, followed by the FTS reaction to generate hydrocarbons [47,48,49]. Yao et al. [50] conducted a thermodynamic study of a CO2 hydrogenation system with high olefin selectivity, and assumed that the main products were light olefins and the formation of alkanes was negligible. The enthalpy and Gibbs free energy changes of related reactions at different temperatures are shown in Figure 2. The ΔrH of the RWGS reaction is greater than 0 at the temperature range from 373.15 K to 1273.15 K, and the ΔrG decreases with an increase in the temperature, which means the RWGS is an endothermic reaction and prefers a higher temperature. On the contrary, the ΔrH of the FTS reaction is less than 0, and is the same as the ΔrG below 650 K. As the temperature increases, ΔrG of the FTS reaction gradually increases and accelerates with an increase in the carbon number in the products, which means that the lower temperature is in favor of the exothermic FTS reaction, and the formation of higher olefins.
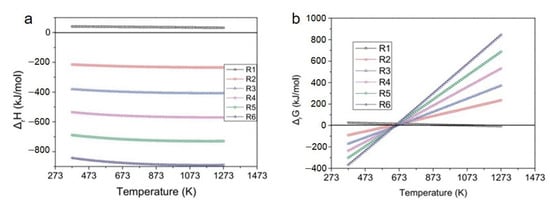
Figure 2.
The (a) ΔrH and (b) ΔrG of related reactions (R1 represents the RWGS reaction: CO2 + H2 ↔ CO + H2O; R2–R6 represents the reaction of nCO + 2nH2 → CnH2n + nH2O, n = 2, 3, 4, 5, 6). Reprinted with permission from ref. [50]. Copyright 2017 Wiley.
In order to simplify the calculation, Liu et al. [51] assumed that the concentration of CO in the system was approximately zero; the reaction mixture was treated as an ideal gas solution, and the hydrocarbon reaction products were low-carbon hydrocarbons (C1–C4). The thermodynamic calculations show that the theoretical maximum conversion of CO2 is 69–71% at a reaction temperature of 580–630 K, a pressure of 2.5–3.0 MPa, and with an H2/CO2 ratio of 3. However, the concentration of CO is not zero in the actual reaction system, and the simplified assumption brings a non-negligible effect on the calculation results. Therefore, Liang et al. [17] investigated the thermodynamic equilibrium of CO2 hydrogenation by considering the concentration of intermediate product CO. The intersection points of ΔG curves for olefins generation are near 320 °C, which is about 40 °C higher than that for alkanes, and the ΔG for olefins generation is already greater than zero at 320 °C. That is, the yield of alkanes will be greater than the yield of olefins in a reaction system that reaches thermodynamic equilibrium. Therefore, to improve the yield of olefins in the product, the operating conditions and catalysts design should be optimized from the kinetic direction [52].
3. Modification of Catalysts
As mentioned above, the CO2 hydrogenation reaction is composed of the RWGS reaction followed by the FTS reaction. The conventional Fe-based and Co-based FTS catalysts are generally considered to be suitable for CO2 hydrogenation as well. Compared with Co, Fe possesses stronger RWGS activity, and thus has higher catalytic activity in the reaction of CO2 hydrogenation to hydrocarbons [53]. To promote CO2 conversion and olefin selectivity, various strategies have been used to control the morphology, crystal and electronic structures, and redox properties of Fe-based catalysts [18,35,47,54,55,56,57,58,59,60]. The catalytic activity of various Fe-based catalysts reported recently are summarized in Table 1.

Table 1.
Summation of Fe-based catalysts used for CO2 hydrogenation to olefins.
3.1. Promoters
It was found that the incorporation of promoters could modulate the surface and interface structure of Fe-based catalysts. Various promoters, such as alkali metals [44,62,63,64,66,68,79,80,81,82,83,84,85] and non-noble metals [62,76,77,86,87,88] have been used to increase the specific surface area of Fe-based catalysts to modulate the CO2 adsorption characteristics or increase the oxygen vacancies. The addition of promoters further improves the carbonation rate of Fe-based species, as well as the surface basicity or surface electron migration processes, and regulates the interaction of Fe with the supports to obtain higher CO2 conversion and olefin selectivity.
3.1.1. Alkali Metals
Alkali metals always act as electronic promoters by donating electrons to metal atoms on the catalyst surface, and dramatically changing the catalytic activity. It was reported that Li+-promoted Fe-based catalysts have an inhibiting effect on the RWGS and FTS reactions [89,90]. Wang et al. [44] investigated the effects of five alkali metals on the catalytic activities of Fe/ZrO catalysts for CO2 hydrogenation. Similar to the FTS reaction, the addition of Li+ suppressed both the RWGS reaction and the following CO hydrogenation reaction in CO2 hydrogenation. The rest of the alkali metals, Na+, K+, Rb+ and Cs+, significantly decreased the selectivity to CH4 and light paraffins, and increased it to olefins. Simultaneously, the modification by Na+, K+, or Cs+ also increased the conversion of CO2. They found that the best performance for light olefin synthesis was obtained over the K+-modified Fe/ZrO2 catalyst, as a result of the accelerating effect of K+ on the formation of the Fe5C2 phase. Yang et al. [91] also compared the unpromoted Fe-based catalyst with the alkali-promoted ones, as seen in Figure 3. They found that Li or Na promoters did not practically affect the activity of iron carbides to produce C2+ hydrocarbons, while K, Rb, or Cs promoters inhibited the activity of the catalyst. Moreover, the overall concentration of iron carbides did not depend on the kind of promoter, but the K, Rb or Cs promoters regulated the spatial distribution of active species along the catalyst bed to improve the efficiency of C2+ hydrocarbons production.
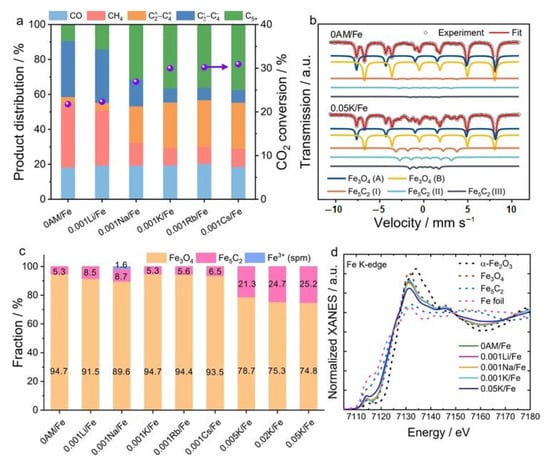
Figure 3.
(a) CO2 conversion and product distribution over 0AM/Fe and 0.001AM/Fe catalysts tested in CO2 -FTS at 15 bar and 300 °C, using a feed 3 H2/CO2/0.3 N2 with a GHSV of 1160 mL gcat−1 h−1 for 90 h. (b) Mössbauer spectra of spent 0AM/Fe and 0.05 K/Fe catalysts. (c) Composition of iron phases in spent catalysts as determined from Mössbauer spectra. (d) XANES spectra at Fe K-edge of spent 0AM/Fe, 0.001AM/Fe and 0.05 K/Fe catalysts. Reprinted with permission from ref. [91]. Copyright 2022 Wiley.
As a promising effect of K for tailoring the types of olefins in CO2 hydrogenation, many studies have focused on K-promoted Fe-based catalysts. Wang et al. [92] studied the promotion effect of K on the carbon chain length of olefin products with single-walled carbon nanotubes (SWNTs) that support Fe catalysts in the CO2 hydrogenation reaction. It was found that the content of K had an effect on the distribution of olefins in the products. The selectivity and productivity of light olefins (C2=–C4=) were highest at 7 wt.% of K, reaching 27% and 16 μmolCO2 gFe−1 s−1, respectively; when the mass fraction of K decreased to 3%, the selectivity and productivity of heavy olefins improved to 40% and 27 μmolCO2 gFe−1 s−1, respectively. Numpilai et al. [72] promoted Fe-Co/Al2O3 catalyst through the addition of K to increase the selectivity of light olefins from CO2 hydrogenation. The positive effect of K addition was attributed to the electron donor from the K atom to the Fe species, which enhanced the bonding strength of adsorbed CO2 and H2 species, and retarded the hydrogenation of olefins to paraffins. Increasing the K/Fe atomic ratio from 0 to 0.5 increased the olefins/paraffins (O/P) ratio by 25.4 times. Jiang et al. [83] demonstrated that the introduction of K effectively suppressed the production of CO and CH4, owing to its electron-donating property, promoting the adsorption and disassociation of CO, and thereby enhancing the formation of iron carbides and chain growth probability. Jiang et al. [93] investigated the surface adsorption properties of Fe–Mn samples with potassium loadings via in situ diffuse reflectance FTIR using NO, CO and CO + H2 as probe molecules. They found that the interaction between potassium and surface iron species promoted the formation of fine metallic iron clusters, while an excess of the potassium covered the formed Fe0 species.
Na is also one of the most commonly used alkali metal promoters, because Na+ in the catalyst can enhance the selectivity of light olefins by regulating the hydrogenation ability and facilitating the desorption of olefins [68]. Moreover, Na can facilitate the reduction by electron donation from Na to Fe as the oxygen vacancy formation energy is reduced by Na [20,62]. Yang et al. [84] compared the effect of sodium and potassium promoters to the zinc ferrite catalyst system on the concurrent conversion of CO and CO2 to olefins. The Na/Fe–Zn catalyst activity for apparent CO conversion was more than twice that of the K/Fe–Zn catalyst, and it also exhibited better reactivity in terms of chain growth probability and secondary reactions. They found that the spent Na/Fe–Zn catalyst mostly consisted of Fe5C2 and iron oxides, whereas the amount of Fe7C3 in the spent K/Fe–Zn was 2.5 times higher than that in the spent Na/Fe–Zn. The density functional theory (DFT) calculations revealed that the χ-Fe5C2 (001) surface showed weaker binding with C* than the Fe7C3 (001) surface, which led to the more favorable production of the linear α-olefins on the χ-Fe5C2 (001)-dominated surface of the Na/Fe–Zn catalyst.
Tu et al. [94] investigated in depth the role of Na in the dynamic transition of the working state of Fe5C2–ZnO catalysts, and found that Na additive did not alter the structures of Fe5C2–ZnO, but largely improved the stability of Fe5C2 by preventing phase oxidation during the reaction. Furthermore, Na also modified the surface properties of the catalyst, thus facilitating the reaction of CO* and H* as well as C–C bond formation, as illustrated in Figure 4. Malhi et al. [69] reported that the addition of Na into Fe catalysts altered the rate dependencies on reactants, and the reaction pathways for olefins formation, which may be a result of the modification of the reducibility and surface identities of Fe catalysts by Na atoms; this changed the rate ratios of paraffins and olefins. Liang et al. [79] and Wei et al. [63] also found that the addition of Na enhanced the adsorption of CO2, facilitated the formation and stability of active species Fe5C2, and inhibited the secondary hydrogenation of alkenes. However, Gnanamani et al. [82] revealed that the addition of alkali metals to iron oxalate decreased the bulk iron carbides formation of both the Hägg and theta carbides. The stability of iron carbides and the chain-growth probability factor for hydrocarbons and oxygenates increased with the increasing basicity of alkali added to iron for CO2 hydrogenation.
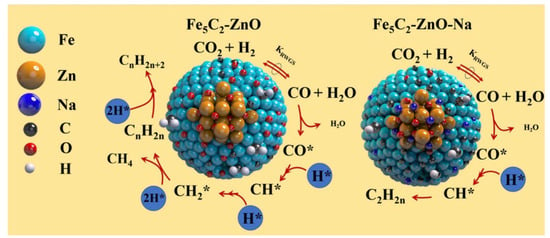
Figure 4.
Plausible mechanism of Na effects on Fe5C2 for CO2 hydrogenation. Reprinted with permission from ref. [94]. Copyright 2021 Elsevier.
3.1.2. Non-Noble Metals
Since the CO2 hydrogenation reaction is a combination of the RWGS and FTS reactions, the introduction of additional FTS active sites without WGS activity is expected to accelerate CO consumption to facilitate CO2 conversion through the FTS pathway, rather than through the WGS pathway. Jiang et al. [83] investigated the effect of introducing Co and Ru on Fe-based catalysts during CO2 hydrogenation. The addition of Co or Ru could enhance the CO2 conversion, only by promoting the FTS reaction rate of the CO intermediate without catalyzing the conversion of CO into CO2. It was found that the intimate contact between Fe and Co sites favored a higher selectivity to C2+ hydrocarbons, due to the easy spillover of the CO intermediate from Fe3O4 to Co sites; meanwhile, the increasing distance led to a higher selectivity to CH4 because of the lower CO concentration over Co sites. Xu et al. [75] prepared ternary ZnCoxFe2-xO4 spinel nanoparticles and used them in CO2 hydrogenation, which exhibited outstanding performance toward CO2 hydrogenation to produce light olefins; there was a 36.1% selectivity toward C2=–C4= products at a 49.6% conversion, and an unprecedentedly high iron time yield (FTY) for CO2 conversion to light olefins (~29.1 μmolCO2·gFe−1·s−1) at the gaseous hourly space velocity of 24,000 mL·gcat−1·h−1. Witoon et al. [95] studied the CO2 hydrogenation to light olefins over Fe–Co–Zn/K–Al2O3 catalysts with different Zn-loading contents (0–1.74 wt%). The 0.58 wt% Zn-promoted Fe–Co/K–Al2O3 catalyst showed superior activity for light olefins yield (19.9%) under the optimum operating conditions of 340 °C, 25 bar, 9000 mL gcat−1 h−1 and H2 to CO2 ratio = 4; this was due to the improvement in dispersion and the reducibility of iron oxides, and also due to an increase in active sites.
Mn is also an effective promoter to decrease methane selectivity and to increase C2–C4 olefins selectivity of Fe-based catalysts during CO2 hydrogenation [16]. It was reported that Mn acted as a structural promoter to disperse and stabilize the Fe particles [66], and promoted reduction by the presence of oxygen vacancy in MnO, as the oxygen in Fe oxide can spill over to the vacancy in MnO, spontaneously [62]. Dossary et al. [65] found that the loading amount of Mn to iron catalysts exerted a strong influence on CO2 conversion and selectivity to different products. Liu et al. [62] suggested that to improve the selectivity of olefins, the chain growth of the alkyl-metal intermediates should be suppressed, and the short alkyl-metal intermediates should be terminated via β-H abstraction to form light olefins rather than paraffins. They found that the spatial hindrance of Mn suppressed chain growth to increase the amount of surface short alkyl-metal intermediates; furthermore, the electron transfer from Na to Fe atoms and subsequent backdonation to C* in C-metal bonds favored the β-H abstraction of these short alkyl-metal intermediates to form light olefins. However, Xu et al. [66] demonstrated that close Fe–Mn contact could result in negative effects for both the intrinsic RWGS reaction rates and the olefin selectivity in CO2 hydrogenation. The reason for this result was because the active phase of the catalyst transformed from the RWGS-active FeO phase to the FTS-active Fe5C2 phase with the assistant of Mn, whereas the steric hindrance of the Mn moiety to the FTS steps on the Fe5C2 sites suppressed the formation of olefins.
ZnO and Cu metals are usually used as structural promoters to adjust the Fe particle size and the exposure of active Fe metal surface. Choi et al. [20] studied a ZnFe2O4-derived catalyst that contained well-dispersed iron particles with Zn, which served as a structural promoter to improve the selectivity of liquid-fuel to 58%, and obtained a high olefin-to-paraffin ratio of 11 in CO2 hydrogenation. Hwang et al. [96] found that Cu could incorporate into the Fe bulk lattice in the presence of K, and that the Fe–Cu–K catalyst exhibited a C5+ yield of 18.1%, which was 1.4 and 7.8 times that of the FeK (12.8%) and Fe–Cu (2.3%) catalysts. Chaipraditgul et al. [97] investigated the effect of adding transition metals to Fe/K–Al2O3 on its catalytic activity in the reaction of CO2 hydrogenation to olefins. It was found that Cu and Zn could significantly increase the amount of weakly adsorbed H atoms, thus improving the conversion of CO2 as well as the selectivity of long-chain alkanes. The addition of Co also improved the conversion of CO2, but provided higher yields of light olefins. On the other hand, Mn and V inhibited CO hydrogenation, and consequently made the conversion of CO2 low to some extent, but Mn reduced the weak adsorption of H atoms, and thus inhibited secondary hydrogenation of olefins to obtain a higher O/P (olefins/paraffins) value of 7.4.
3.2. Bimetallic Catalysts
Another strategy to increase CO2 conversion and olefin yields is to introduce a secondary metal, such as Mn, Co, Cu, or Zn, to increase the reducibility and number of active sites through the high dispersion of active metals.
Similar to Fe-based catalysts, Co-based catalysts also have been developed for industrial uses in FTS. Nevertheless, in CO2 hydrogenation at a relatively high temperature, the dominant product with Co catalysts is methane rather than C2+ olefins [98,99]. When the two metals are associated, the properties of the catalyst and the performance in CO2 hydrogenation are more likely due to the formation of an iron–cobalt alloy than the simple sum of their respective properties [100]. Satthawong et al. [57,58] discovered a strong bimetallic promotion of C2+ hydrocarbons formation from CO2 hydrogenation on Fe–Co catalysts by combining Fe and a small amount of Co on an alumina support. Inspired by previous research, Gnanamani et al. [35] investigated unsupported Co–Fe bimetallic catalysts in CO2 hydrogenation, which displayed high performance at a temperature range of 200–270 °C, and at a pressure of 0.92 MPa. An Increase in d spacing for the (111) plane of the metal oxalates precursor suggested that there was an incorporation of Fe into the cobalt lattice to form a Co–Fe bimetallic oxalate. After pretreatment in CO, the Co–Fe samples were composed of CoO, CoFe alloy, FeCx, Co2C, metallic Co and Fe, and the characterization results also showed the possibility of a presence of cobalt in the iron carbides (Figure 5a). In a subsequent study, the bimetallic alloy carbide was confirmed by Kim et al. [74], who reported that monodisperse nanoparticles of Na-promoted the CoFe2O4 catalysts being supported on carbon nanotubes for the preparation of C2–C4 olefin products by CO2 hydrogenation (Figure 5b). In comparison with the conventional Fe5C2 active site of pure iron catalysts and the Co2C active site of pure cobalt catalysts, the unique bimetallic alloy carbide (Fe1-xCox)5C2 led to higher CO2 conversion (~34%) and better selectivity toward light olefins (~39%) at 10 bar and 613 K, with an H2/CO2 ratio of 3:1.
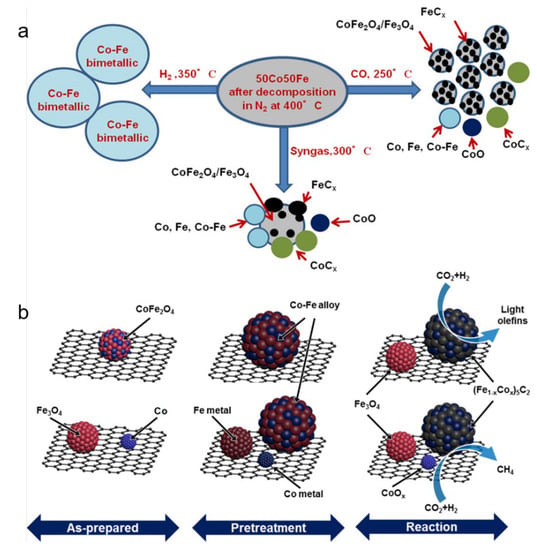
Figure 5.
(a) Structures of Co–Fe bimetallic catalysts pretreated in different conditions. Reprinted with permission from ref. [35]. Copyright 2016 American Chemical Society. (b) The phase transformations of as-prepared spinel CoFe2O4 NPs (top row) or (Fe3O4 + Co) NPs (bottom row) on CNT supports during pre-reduction and reaction steps. Reprinted with permission from ref. [74]. Copyright 2020 American Chemical Society.
According to previous studies, FeOx–MnOx bimetallic catalysts have been proven to have excellent performance in the Fischer–Tropsch-to-olefin (FTO) process. Even a small amount of MnOx can improve the dispersion of FeOx and the carburization of Fe [88,101]. Kinetic studies further demonstrated the effect of MnOx on promoting CO dissociation and monomer formation, reducing the effective sites of H2 dissociation, olefin reabsorption and secondary hydrogenation [102], which promoted the generation of low-carbon olefins. Unlike CO hydrogenation, Kuei et al. [103] concluded that manganese showed little promotion effect on the selectivity of olefinic hydrocarbons in CO2 hydrogenation. Generally, manganese decreased CO dissociation ability and the carbon content on catalysts, creating the proper oxide-carbide surface structure to produce olefins in CO hydrogenation. However, in CO2 hydrogenation, this oxide/carbide surface structure may be destroyed by severe oxidation, and active carbon was unstable in an atmosphere with high water content.
Cu has high activity in both the WGS and RWGS reactions, and did not lead to the methanation of CO2 [19]. Therefore, Fe–Cu bimetallic catalyst has attracted extensive attention in the field of CO2 hydrogenation to olefin. Compared with monometallic Fe, the addition of Cu to Fe can successfully suppress undesired CH4 formation, while enhancing CO and C2+ hydrocarbon formation [77]. Structural characterization revealed the generation of Cu–Fe alloy species, and the incorporation of Cu into the Fe bulk lattice in the presence of alkali metals [96]. Based on structural and electronic characterizations, Li et al. [78] demonstrated the reconstruction of CuFeO2 after the activation procedure. The Cu+ in the CuFeO2 lattice collapsed and aggregated to form a pure Cu phase with a partially oxidized surface. Meanwhile, Fe3+ in the CuFeO2 lattice underwent partial reduction and carbonization to form Fe3O4, χ-Fe5C2 and Fe3C. These species were in close contact with each other, forming multiple interfacial sites, such as an interface between Cu and χ-Fe5C2. Generally, interfacial sites between copper and iron carbides were regarded as the active center for CO insertion. As shown in Figure 6a and 6b, the DFT calculation proved that the Cu-χ-Fe5C2 interface significantly promoted CO insertion, relative to iron alone. Nie et al. [104,105] performed density functional theory calculations to investigate the adsorption, activation, initial hydrogenation and mechanism for CO2 hydrogenation to C1 and C2 hydrocarbons over Cu–Fe bimetallic catalysts. It was found that CO2 adsorption strength decreased monotonically as surface Cu coverage increased. On the monometallic Fe(100) surface, the favorable CH* species for production of CH4 and C2H4 was formed through a path of CO2* → CO* → HCO* → HCOH* → CH*. On the bimetallic Cu–Fe(100) surface, the preferred path for CH* formation went through CO2* → HCOO* → HCOOH* → HCO* → HCOH* → CH* (Figure 6c). Once CH* was produced, it was more selective to CH4 than C2H4 on the Fe(100) surface, since the energy barrier for C–C coupling of two CH* species was 0.3 eV higher than that for CH* to CH4. However, on the Cu–Fe (100) surface, the barrier of CH2 species hydrogenation was higher than that of the CH* +CH* coupling; thus, it became more favorable to produce C2H4 rather than CH4.
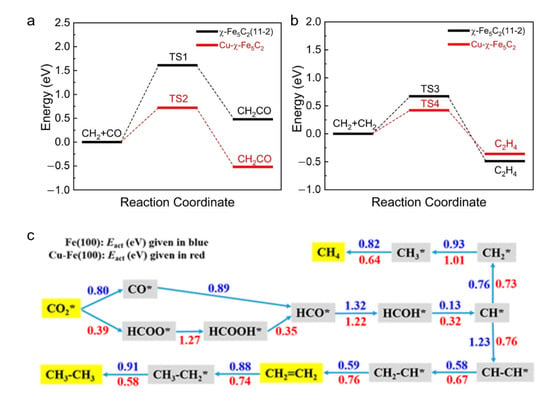
Figure 6.
(a) Comparison of energy barriers of CH2 + CO over Cu-χ-Fe5C2 and χ-Fe5C2(11-2) facets; (b) comparison of energy barriers of CH2 + CH2 over Cu-χ-Fe5C2 and χ-Fe5C2(11-2) facets. Reprinted with permission from ref. [78]. Copyright 2022 Springer. (c) Reaction pathways for production of CH4, C2H4 and C2H6 from CO2 on Fe(100) and the Cu–Fe(100) surface at 4/9 ML coverage. Kinetic barrier for each elementary step is given in eV. Reprinted with permission from ref. [105]. Copyright 2017 American Chemical Society.
Zn has been proven to be an effective structure promoter to disperse the active iron phases generated during the CO2 hydrogenation reaction. Therefore, Fe–Zn bimetallic catalysts have also been investigated for enhancing olefin yields in CO2 hydrogenation. Tu et al. [94] prepared ZnFe2O4 precursors, and obtained Fe5C2–ZnO bimetallic catalysts by CO reduction. In situ XRD detected the phase transformation of ZnFe2O4 to a mixture of FeO and ZnO, and then to a mixture of Fe5C2 and ZnO. Zhang et al. [67,106] used in situ XPS characterization to investigate the interactions of Fe–Zn bimetallic catalysts during CO2 hydrogenation to linear α-olefins, and found that the chemical state of ZnOx was between 0 and +2 (Znδ+), which demonstrated the electron donating interaction of Zn to Fe. They also found that adding Zn changed the balanced ratios of FeCx/FeOx during the reaction by preventing the oxidation of FeCx from H2O and CO2, thus promoting C–C coupling and olefins synthesis over FeCx species. An Fe2Zn1 catalyst showed a C2–C7 olefins selectivity of 57.8% in the gas phase, and a C4–C20 olefins selectivity of 60.7% with an 89.3% LAOs/olefins ratio, which was 2.4 times that of Fe2O3. Zhang et al. [68] proposed the reaction mechanism of CO2 hydrogenation to olefins over Na–Zn–Fe catalysts, as shown in Figure 7. They suggested that the CO2 and H2 molecules were first activated on the ZnO surface to form CO2* and H2*, followed by the hydrogenation of CO2* to produce CO* and OH*; then, OH* produced H2O by combining with H*. CO* species near the interface diffused to the adjacent v-Fe5C2 surface, where hydrogenation and C–C coupling reactions occurred, followed by the formation of olefins by dehydrogenation.
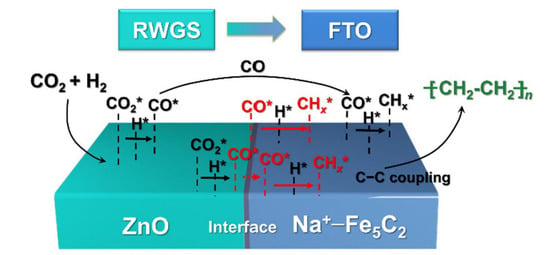
Figure 7.
Reaction mechanism for CO2 hydrogenation to olefins over the Na–Zn–Fe catalyst. Reprinted with permission from ref. [68]. Copyright 2021 Elsevier.
3.3. Supports
Using metal-support interactions to modify heterogeneous metal catalysts is an efficient way to improve the catalytic activity, and to control product selectivity during the heterogeneous catalytic reaction process. Electron transfer between metal and support [107], support-induced recombination of metal particles, and the formation of specific interfaces between metals and supports, all contributed to the occurrence of strong metal-support interactions (SMSI) [108], which had a positive effect on the performance of catalysts [109].
3.3.1. Metal Oxides
Metal oxide-supported catalysts generally have strong metal-support interactions, as the oxide covers the metal particles during the reduction process [110]. Furthermore, when the bond between the oxide and the metal particles is strong, the metal particles become well dispersed on the oxide support [111]. Wang et al. [44] investigated the effect of supports on the catalytic performances of K+-modified Fe catalysts. The results showed that SiO2 was unsuitable for CO2 hydrogenation to hydrocarbons because both the conversion of CO2 and the selectivity to hydrocarbons decreased over SiO2-supported Fe-based catalysts. K+-modified Fe catalysts loaded onto other typical metal oxide supports, such as Al2O3, TiO2, and ZrO2, provided much better performances for the formation of light olefins. The promotion of catalytic activity by SiO2 and Al2O3 was further investigated by Pandey et al. [112], who found that the SiO2-supported Ni–Fe catalysts showed lower enhancements of conversion and yield than those observed for the Al2O3-supported catalysts. For the SiO2-supported Ni–Fe catalysts, the enhancements appeared to be due to the additional metal sites formed. Moreover, from the DRIFT results, for the Al2O3-supported Fe–Ni catalyst, the adsorption of carbonate by the unreduced Fe3O4 species was a reason for the enhancements in catalytic activity. Ding et al. [113] found that the surface -OH of the ZrO2 support had better CO2 affinity compared to the Al2O3 support, and also possessed a higher surface oxygen vacancy density, all of which resulted in better catalytic activity of the catalysts. Huang et al. [71] studied the dynamic evolution of active Fe species during CO2 hydrogenation over two kinds of zirconia (m-ZrO2 and t-ZrO2)-supported iron catalysts. Catalysts of 15%Fe-K/m-ZrO2 presented a remarkable CO2 conversion (38.8%), and a high selectivity to light olefins (42.8%). More oxygen vacancies on the surface of m-ZrO2 and the electron-donating ability of iron elements boosted the charge transfer between Fe and the ZrO2 support, forming a more active Fe species.
3.3.2. Carbon Materials
Chernyak et al. [114] studied CO2 hydrogenation to hydrocarbons over spark plasma sintered Fe/CNT catalysts, which showed high activities of 5.4–12.2 molCO2 gFe−1 s−1 and promising C2+ selectivity of ~40–50 mol. % at low H2:CO2 ratios of 1 and 2, without pre-reduction. In contrast to oxide, the carbon support prevented the formation of inactive irreducible complex oxides, and promoted catalyst activation. Meanwhile, the stability of the sintered catalysts against particle migration was attributed to the framework structure of densely packed CNTs that hindered the mobility of iron-containing particles. Chew et al. [42] investigated the effects of CNT and SiO2 as supports on the catalytic activity of Fe-based catalysts during the CO2 hydrogenation reaction. In comparison, the iron oxide nanoparticles on SiO2 were more difficult to reduce than on the CNT support, indicating that the interaction between iron oxide and silica was substantially stronger than that with CNTs. The strong iron–silica interaction prevented the complete reduction and carbonization of the Fe/SiO2 catalyst, which primarily caused lower activity and high CO selectivity in CO2 hydrogenation. The CNT-supported Fe catalysts, on the contrary, were more active and selective to light olefins in the range from C2 to C5. Hwang et al. [45] synthesized well-defined mesoporous carbon (MPC) as a support for Fe catalyst, which exhibited excellent catalytic activity and selectivity towards long-chain hydrocarbons (C5+). The mesoporous structure of MPC provided the benefits of fast mass transfer of hydrocarbon molecules, and weak metal-support interaction of MPC and Fe metal promoted the formation of Fe7C3 during the reaction, which generally enhanced the CO2 conversion and C5+ hydrocarbon selectivity.
Metal–organic framework materials (MOFs), which consist of inorganic metal ions and organic ligands, have also been used as sacrificial templates or precursors to form different porosities and compositions of porous carbon supports by altering the pyrolysis temperature and the components of the initial MOFs [115,116]. Dong et al. [73] prepared a carbon-encapsulated Fe–Co bimetallic catalyst using ZIF-67 as precursor support, in order to convert CO2 to light olefins. The NC layer formed during the pyrolyzation of ZIF-67 could well embed and disperse the metal nanoparticles, which effectively promoted the reduction and carburization of the metal during the reaction. With an increase in pyrolysis temperature, the metal carburization in the reaction process became more accessible, leading to more active components of Fe5C2 and Co2C. The highest selectivity of light olefins (C2=–C4=) of 27% was achieved using an FeCo/NC-600 catalyst, coupled with a CO2 conversion of 37%. Similarly, Liu et al. [117,118] used ZIF-8 as a template to synthesize carbon encapsulated Fe-based catalysts, which exhibited enhanced catalytic activity toward olefins. In addition to using an MOF as a template, the corresponding catalyst could also be obtained through direct pyrolysis or carbonization of Fe-containing MOF materials, which also achieved high performance towards olefin formation [31,119]. The carbonates generated from the decarboxylation of the MOFs fully covered the particle surfaces, which could decompose to CO2 and lead to an active surface that was rich with dangling bonds for catalytic turnover [120].
3.3.3. Zeolites
For the conventional Fe-based catalysts, hydrocarbon products generally follow the Anderson–Schulz–Flory (ASF) distribution, which is mainly composed of low-carbon liner-olefins and liner-paraffins. Considering the unique framework topology of zeolite components, they are favorable candidates for precise control of product selectivity in the oligomerization/ aromatization/ isomerization of hydrocarbons. Different types of zeolites, such as HZSM-5, HMCM-22, HBeta and HY, demonstrated unusual characteristics in the skeletal isomerization reactions of hydrocarbons [70,121,122,123]. Therefore, the combination of Fe-based catalysts with zeolite into a multifunctional catalyst can shift product distribution towards gasoline-range hydrocarbons and aromatics.
Wei et al. [47,70] prepared highly efficient multifunctional Na-Fe3O4/HMCM-22 and Na–Fe3O4/HZSM-5 nanocomposite catalysts to directly convert CO2 into isoparaffins and higher hydrocarbons, respectively. Due to its unique pore structure and appropriate Brønsted acid properties, HMCM-22 effectively suppressed aromatization, while promoting isomerization to achieve a selectivity of 82% for C4+ hydrocarbons, of which isoparaffins accounted for 74%. The multiple active sites (Fe3O4, Fe5C2 and acid sites) in the Na–Fe3O4/HZSM-5 catalyst enabled a tandem reaction pathway, including the initial reduction of CO2 to CO over the Fe3O4 sites, the subsequent hydrogenation of CO to olefins over the Fe5C2 sites, and hydrocarbon isomerization over the Brønsted acid sites in H-ZSM-5; this lead to extremely high performance in CO2 hydrogenation with a C5–C11 hydrocarbons products selectivity of 78%, and CH4 of only 4% at a CO2 conversion of 22% under industrially relevant conditions. More importantly, it exhibited remarkable stability during more than 1000 h on stream, with the C5+ selectivity steadily maintained at 67 ± 2%.
3.3.4. Perovskite-Type Oxides
Perovskite structures are adopted by many oxides that have the chemical formula ABO3 (perovskite-type oxides, PTOs), where A and B are two positively charged ions (i.e., cations) with very different sizes [124]. Moreover, the A and B sites can be possibly substituted by other cations, resulting in a general formula of A1-yA′yBO3, AB1−xB′xO3 or A1-yA′yB1-xB′xO3 [125,126]. The unique crystal structure of PTOs can restrict the metal ions to A and B sites in specific crystals, thus providing a better dispersion of the related metal ions [127]. In addition, the metal valence state as well as the redox properties and interface structures of PTOs can be adjusted by elemental doping, or by partial substitution of the A and/or B sites, to provide controlled selectivity for the target products [128]. According to previous studies, perovskite-type catalysts have shown high catalytic activity in FTS [129,130,131] and RWGS reactions [132,133]. Therefore, as a potential material, Fe-doped perovskite catalysts have also been investigated for CO2 hydrogenation to hydrocarbons. Utsis et al. [134] investigated the effects of oxide precursors with different compositions and structures on the reactive sites of hydrogenating CO2 on Fe-based catalysts. The results showed that solid crystalline matrix containing Fe ions with different valence states and environments strongly affected the reaction rates of the RWGS and FTS steps during CO2 hydrogenation. The obtained ratio of rFTS/rRWGS for the LaFe–perovskite precursor was 0.15, which indicated that there was an imbalance between the RWGS and FTS steps, and the C5+ hydrocarbons productivity was directly limited by the rate of the FTS step. Hou et al. [135] reported on an Sr1-xKxFeO3 perovskite-derived catalyst with highly dispersed active sites and enhanced RWGS reaction activity, which obtained high CO2 conversion (30.82%) and light carbon olefin selectivity (29.61%) during CO2 hydrogenation. The K-substituted SKFx chalcogenide catalysts were found to promote the generation of active phases (Fe3O4 for RWGS, Fe5C2 for FTS), and the catalytic performance of Fe3O4, the redox effect of Sr2Fe2O5 on CO2 cracking, and the formation of SrCO3 carbonate, are responsible for the enhanced activity of the RWGS reaction. In addition, the reversible reaction between Sr2Fe2O5 and CO2 (Sr2Fe2O5 + 2 CO2 = 2 SrCO3 + Fe2O3) ensured the structural stability and high dispersion of the active phase. Although the unique properties of perovskite provide many innovative ideas for catalysts construction, there are relatively few reports on the use of perovskite-derived iron catalysts for CO2 hydrogenation to hydrocarbons. There are still many topics worthy of research in this field.
3.4. Particle Size
The size of metal particles has significant effects on their electronic and geometric effects; these changes can sometimes have unexpected effects on the catalytic activity of the catalyst. Kwak et al. [136] reported that the activity of atomically dispersed supported metals differed greatly from those of 3D metal particles in CO2 reduction. Xie et al. [137] investigated the CO2 hydrogenation catalytic activity of Al2O3-supported Fe catalysts with different pore sizes, and found that the particle size of Fe2O3 increased as the Al2O3 pore size increased, and the larger particle size facilitated the growth of C–C bonds. They found that only the appropriate Fe2O3 particle size (5–8 nm) was most favorable for CO2 conversion and C5+ product generation. Wu et al. [138]. reported that the large difference in Ni particle size strongly affected the kinetic parameters of CO2 hydrogenation, the formation pathways of CO and CH4, and the reaction selectivity. Iablokov et al. [139] found that for all Co nanoparticle sizes, the TOF of CO2 hydrogenation was found to be significantly higher on the larger nanoparticles, while the product distribution was similar. As for the CO2 hydrogenation reaction over Fe-based catalysts, it was more difficult to study the size effect due to the complexity of the reaction pathway and products formation. Hong et al. [140] investigated the deactivation of a co-precipitated Fe–Cu–K–Al catalyst in CO2 hydrogenation to hydrocarbons. It could be concluded that the deactivation of the Fe–Cu–K–Al catalyst was mainly caused by the growth in crystallite size and the component separation, which led to a decrease in the promoting effects of Cu and K. Zhu et al. [141] studied Fe-based catalysts with a particle size range of 2.5–12.9 nm in CO2 hydrogenation to hydrocarbons. The overall selectivity of C2+ hydrocarbons increased with increasing particle size, while that of CO decreased. They found that large particles were more favorable for both the primary (RWGS and methanation) and the secondary (FTS) processes, over which the HCOO* species could be formed more easily to promote further hydrogenation to CH4 as a primary product; moreover, large particles performed with a higher chain-growth probability. Numpilai et al. [38] found that the calcination temperature had significant impacts on metal oxides crystallite size, which affected CO2 conversion, product selectivity, as well as the yield of olefins. They synthesized a series of Fe–Co/K–Al2O3 catalysts calcined at a temperatures range of 400–800 °C. With an increase in calcination temperature, a growth of metal oxides particles and a lower degree of reduction of iron oxide species were obtained. The highest CO2 conversion (49%), hydrocarbons selectivity (90%) and olefins yield (18.1%) were achieved with Fe–Co/K–Al2O3 calcined at 400 °C, due to the combination of small metal oxides particles and easier reducibility of iron oxide species.
4. Active Site and Reaction Mechanism
The reaction mechanism of the CO2 hydrogenation reaction has been controversial, due to the complexity of its intermediate species. It is generally believed that there are two pathways for the hydrogenation of CO2 to hydrocarbons. One path is the direct hydrogenation of CO2 to hydrocarbons (Equation (1)), and the other is the RWGS–FTS pathway, in which CO2 is first converted to CO via the RWGS reaction (Equation (2)), and then converted to hydrocarbons via the FTS pathway of CO hydrogenation (Equation (3)). Zhu et al. [141] investigated the relationship between product selectivity and reactant conversion in the CO2 hydrogenation reaction. It was found that CO and CH4 selectivity were non-zero, and that C2+ selectivity was close to zero at a CO2 conversion of zero. Therefore, CO and CH4 were the primary products that were directly generated by the RWGS reaction and CO2 methanation, and the primary CO could be subsequently converted to CH4 and C2+ hydrocarbons. On this basis, the researchers proposed a reaction pathway for CO2 hydrogenation, and considered both CO2 methanation and the RWGS reaction as the primary reactions, and then the primary CO underwent the FTS process to form CH4 and C2+ hydrocarbons. As for the mechanism of the FTS process, the common views mainly involved the CO insertion mechanism [142] and the surface carbide mechanism [143,144]. With the advancements in catalyst characterization techniques, especially the operando and in situ characterizations of catalyst surfaces, the surface carbide mechanism is becoming increasingly accepted, due to the observations of an abundance of carbon rather than oxygen on the catalyst surface. Therefore, many scholars have studied the carbon species on the catalyst surface, in order to determine the nature of the active sites during the CO/CO2 hydrogenation reaction.
For Fe-based catalysts, the starting oxide phase is usually converted to an intermediate oxide, and eventually to a metal phase when pretreated under hydrogen-rich conditions, or to a final carbide phase under a CO-rich atmosphere [145,146], as seen in Figure 8. Since the binding strength and corresponding reactivity of the reactants vary with the Fe phases, it is necessary to understand the effect of each phase component on the catalytic reaction.
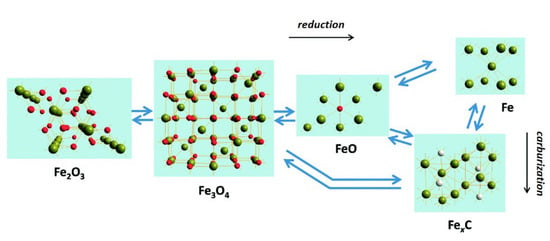
Figure 8.
Schematic of the common transformations observed between different iron phases during the Fischer–Tropsch and CO2 hydrogenation processes. Reprinted with permission from ref. [146]. Copyright 2018 Royal Society of Chemistry.
Han et al. [147] investigated the mechanism of the RWGS reaction on typical Fe-based catalyst surfaces using DFT calculations, and found that Fe3O4 was the active phase. The direct dissociation mechanism of CO2 dominated the RWGS reaction, and the OH* removal step was the rate-determining step for most Fe-based surfaces; thus, the adsorption energy of CO2* and OH* could be used as descriptors of the RWGS activity of Fe-based catalysts. Wang et al. [148] used DFT calculations to determine that HCOO* and CH* were key intermediates in the formation of CH4 and C2H4 during CO2 hydrogenation for hydrocarbon synthesis on the surface of χ-Fe5C2(510). The high surface coverage of O* could oxidize the carbide surface, and the resulting Fe5C2–Fe2O3 interfacial sites facilitated the conversion of adsorbed O* to H2O*, thus releasing more active sites to adsorb CHx species, and promoting C–C coupling to produce hydrocarbons. Thus far, the common experimental observations suggest that Fe3O4 and iron carbides are the active phases for the RWGS and FTS reactions, respectively. Cui et al. [149] demonstrated that bare Fe5C2 had no activity for CO2 conversion, and that CO2 was first reduced to CO by H2 via the RWGS reaction over Fe3O4, followed by the subsequent CO hydrogenation to olefins via the FTS reaction on Fe5C2 sites. Zhang et al. [61] explored the dynamic structure–performance relationship of the iron catalyst throughout its full lifecycle in CO2 hydrogenation to olefins. When pretreated in a CO atmosphere, the phase change process of iron in the catalyst followed Fe2O3 → Fe3O4 → Fe → Fe5C2. After the reaction gas was introduced, the phase change process had two main paths, which were Fe5C2 → Fe3O4 and Fe5C2 → Fe3C → Fe3O4. The results showed that the Fe5C2 phase was the active phase for the generation of C2–C4 olefins. The irreversible oxidation of the iron carbides to Fe3O4 under CO2 hydrogenation conditions was the main factor leading to catalyst deactivation (Figure 9).
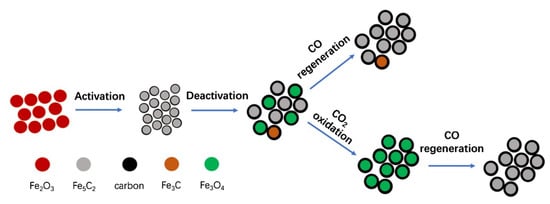
Figure 9.
Structure evolution of Fe catalyst in CO2 hydrogenation during its life cycle. Reprinted with permission from ref. [61]. Copyright 2019 Elsevier.
In fact, Fe-based catalysts have been observed to form a variety of iron carbides in the FTS reaction, such as ε-Fe2C, ε’-Fe2.2C, Fe7C3, χ-Fe5C2 and θ-Fe3C [150,151]. Figure 10 shows the structures of iron carbides and their transformation pathway obtained by the Ab initio atomistic thermodynamics method, which showed that iron carbides can be transformed into each other under certain conditions [152].
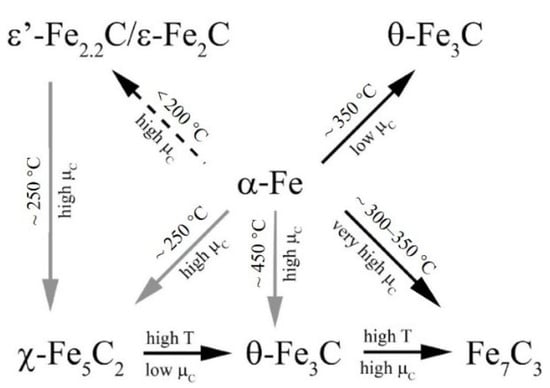
Figure 10.
Qualitative interpretation of the ab initio atomistic thermodynamics study of iron carbides structures (gray arrows indicate transformations where entropic contributions to the total free energy may be significant. The dashed arrow indicates that the transformation may be kinetically inhibited). Reprinted with permission from ref. [152]. Copyright 2010 American Chemical Society.
It is necessary to figure out the catalytic activities of different iron carbide phases. Smit et al. [152] investigated the stability and reactivity of each iron carbide phase at different carbon chemical potentials, and found that χ-Fe5C2 was susceptible to oxidation under FTS reaction conditions, while Fe3C and amorphous iron carbides exhibited lower activity and selectivity. However, Mazzucco et al. [153] presented direct evidence that nanoparticles with the cementite (Fe3C) structure were active for nanotube growth (C–C bond formation), while carbon-rich particles with the Hägg (Fe5C2) structure were inactive. Density functional theory calculations suggested that reduced activity may be due to lower carbon mobility and higher C–C bond formation energies on the surface of nanoparticles with a Fe5C2 structure. Zhang et al. [41] also found a significant difference in the catalytic activities of χ-Fe5C2 and θ-Fe3C generated from α-Fe2O3 and γ-Fe2O3 in the CO2 hydrogenation reaction. The higher selectivity of χ-Fe5C2 over light olefins was attributed to its higher effective barrier and weaker hydrogenation ability, while the high selectivity of C5+ hydrocarbons on θ-Fe3C was mainly attributed to its strong adsorption of CO2, enhancing the chain growth of the adsorbed carbonaceous species (Figure 11). Yang et al. [154] synthesized different iron carbides (Fe5C2, Fe5C2 and Fe3C mixture, Fe3C) by varying the carburization temperature of iron oxalate precursors between 320 and 450 °C, and tested their catalytic activity in a CO hydrogenation reaction. The results showed that both CO conversion and product selectivity were influenced by the iron carbides. The CO conversion and the selectivity of light olefins were higher when Fe5C2 was the dominant iron carbide. In addition, the selectivity of C5+ hydrocarbons in the products was higher when Fe3C was present alone.
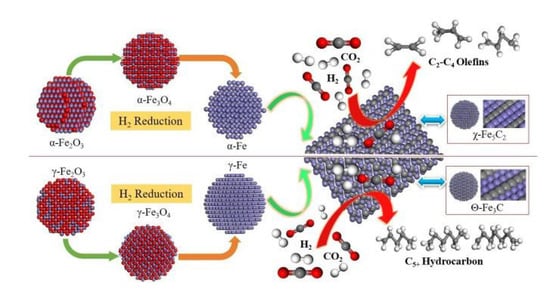
Figure 11.
Scheme of the structure–performance relationship of α-Fe2O3 and γ-Fe2O3 catalysts for CO2 hydrogenation during activation and reaction processes. Reprinted with permission from ref. [41]. Copyright 2018 John Wiley and Sons.
5. Conclusions
In the face of severe pressure for CO2 reduction, developing approaches that utilize CO2 resources efficiently is a challenging topic in C1 chemistry research. The direct synthesis of olefins via catalytic CO2 hydrogenation is one of the promising ways to realize the conversion and utilization of CO2. Fe-based catalysts are widely used for CO2 hydrogenation to hydrocarbons, due to their cost-effective performance and catalytic activity. However, under reaction conditions, complex and diverse phases such as iron oxide, metallic iron and iron carbide may co-exist on Fe-based catalysts as the active phases for the reactions of CO2 activation, H2 activation, CO hydrogenation, carbon chain growth and hydrogenation, respectively, which make the characterization of the dynamic structural evolution of Fe-based catalysts even more challenging. In recent years, many studies have tried to tune the morphology structures, reducibility, surface basicity, and adsorption properties of Fe-based catalysts, by altering the methods and temperatures of catalyst syntheses, supports, and promoters, or introducing other elements to form Fe-based bimetallic clusters. Although these studies are devoted to improving CO2 conversion and olefin selectivity, the catalytic consequences of the changes in structure at the surface and interface of Fe-based catalysts on the rate of CO2 hydrogenation activation, and the distribution pattern of carbon-containing products, are still insufficient and controversial. Meanwhile, the reaction pathways and kinetics of CO2 hydrogenation to olefins over Fe-based catalysts are still not well-established, which restrict the precise regulation of the catalysts’ active center, the design of industrial catalytic reactors, and limit the scale-up of CO2 hydrogenation to olefins.
Therefore, in addition to constructing highly active iron-based catalysts to further inhibit the generation of by-products such as CH4 and improve the selectivity of target products, it is also necessary to combine in situ or operando characterizations and DFT calculations to deeply analyze the active sites for CO2 activation and C=C bond formation on iron-based catalysts under realistic reaction conditions, and the resulting reaction pathways for CO2 hydrogenation. Clarification of the structure–performance relationships of iron-based catalysts in the CO2 hydrogenation process will facilitate the precise regulation of the active center of catalysts, and the design of industrial reactors that are suitable for scale-up of CO2 utilization.
Author Contributions
Conceptualization: W.L. and W.T.; validation: W.L. and W.T.; formal analysis: W.L., S.C., H.S.M. and W.T.; writing—original draft preparation: W.L. and W.T.; writing—review and editing: W.L., S.C., H.S.M., X.G., Z.Z. and W.T.; supervision: W.T. and Z.Z.; funding acquisition: W.T. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Project No. 22078307, 22238003, 22208314, 22278379), Natural Science Foundation of Henan Province (202300410432), the State Key Laboratory of Coal Conversion (J21-22-902), and the State Key Laboratory of High efficiency Utilization of Coal and Green Chemical Engineering (2022-K05 and 2022-K21).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Global Energy Review: CO2 Emissions in 2021; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 3 October 2022).
- Observatory, M.L. Atmospheric CO2. Available online: https://www.co2.earth/ (accessed on 3 October 2022).
- Broecker, W.S. CO2 Arithmetic. Science 2007, 315, 1371. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tan, C.S. A Review: CO2 Utilization. Aerosol. Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- Lim, X. How to make the most of carbon dioxide. Nature 2015, 526, 628–630. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Skrypnik, A.S.; Yang, Q.X.; Matvienko, A.A.; Bychkov, V.Y.; Tulenin, Y.P.; Lund, H.; Petrov, S.A.; Kraehnert, R.; Arinchtein, A.; Weiss, J.; et al. Understanding reaction-induced restructuring of well-defined FexOyCz compositions and its effect on CO2 hydrogenation. Appl. Catal. B-Environ. 2021, 291, 11. [Google Scholar] [CrossRef]
- Dokania, A.; Ramirez, A.; Bavykina, A.; Gascon, J. Heterogeneous Catalysis for the Valorization of CO2: Role of Bifunctional Processes in the Production of Chemicals. ACS Energy Lett. 2019, 4, 167–176. [Google Scholar] [CrossRef]
- Prieto, G. Carbon Dioxide Hydrogenation into Higher Hydrocarbons and Oxygenates: Thermodynamic and Kinetic Bounds and Progress with Heterogeneous and Homogeneous Catalysis. ChemSusChem 2017, 10, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cheng, K.; Kang, J.C.; Zhou, C.; Subramanian, V.; Zhang, Q.H.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Perez-Ramirez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef] [PubMed]
- Galvis, H.M.T.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Hou, B.; Su, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Progress in the catalytic hydrogenation of carbon dioxide to light olefins. Chem. Ind. Eng. Prog. 2015, 34, 3746–3754. [Google Scholar]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B-Environ. 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.N.; Choi, S.H.; Jang, J.H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef]
- Van Speybroeck, V.; De Wispelaere, K.; Van der Mynsbrugge, J.; Vandichel, M.; Hemelsoet, K.; Waroquier, M. First principle chemical kinetics in zeolites: The methanol-to-olefin process as a case study. Chem. Soc. Rev. 2014, 43, 7326–7357. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Spangsberg Holm, M.; Olsbye, U.; Lillerud, K.-P.; Svelle, S. Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A-Gen. 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of Methanol to Hydrocarbons: How Zeolite Cavity and Pore Size Controls Product Selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Qu, Y.; Liu, H.; Tang, C.; Miao, S.; Feng, Z.; An, H.; Li, C. Highly Selective Conversion of Carbon Dioxide to Lower Olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Numpilai, T.; Cheng, C.K.; Limtrakul, J.; Witoon, T. Recent advances in light olefins production from catalytic hydrogenation of carbon dioxide. Process Saf. Environ. Prot. 2021, 151, 401–427. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F. A review on CO2 hydrogenation to lower olefins: Understanding the structure-property relationships in heterogeneous catalytic systems. J. CO2 Util. 2021, 47, 101506. [Google Scholar] [CrossRef]
- Sedighi, M.; Mohammadi, M. CO2 hydrogenation to light olefins over Cu-CeO2/SAPO-34 catalysts: Product distribution and optimization. J. CO2 Util. 2020, 35, 236–244. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef]
- Ramirez, A.; Gevers, L.; Bavykina, A.; Ould-Chikh, S.; Gascon, J. Metal Organic Framework-Derived Iron Catalysts for the Direct Hydrogenation of CO2 to Short Chain Olefins. ACS Catal. 2018, 8, 9174–9182. [Google Scholar] [CrossRef]
- Hu, B.; Frueh, S.; Garces, H.F.; Zhang, L.; Aindow, M.; Brooks, C.; Kreidler, E.; Suib, S.L. Selective hydrogenation of CO2 and CO to useful light olefins over octahedral molecular sieve manganese oxide supported iron catalysts. Appl. Catal. B-Environ. 2013, 132–133, 54–61. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Li, Z.H. Research Progress of Catalysis for Low-Carbon Olefins Synthesis through Hydrogenation of CO2. J. Nanosci. Nanotechnol. 2019, 19, 3162–3172. [Google Scholar] [CrossRef] [PubMed]
- Visconti, C.G.; Lietti, L.; Tronconi, E.; Forzatti, P.; Zennaro, R.; Finocchio, E. Fischer–Tropsch synthesis on a Co/Al2O3 catalyst with CO2 containing syngas. Appl. Catal. A-Gen. 2009, 355, 61–68. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co–Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Abelló, S.; Montané, D. Exploring iron-based multifunctional catalysts for Fischer-Tropsch synthesis: A review. ChemSusChem 2011, 4, 1538–1556. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Chen, J.; Ma, Q.; Fan, S.; Zhao, T.-s. Effect of preparation methods on the structure and catalytic performance of Fe–Zn/K catalysts for CO2 hydrogenation to light olefins. Chin. J. Chem. Eng. 2018, 26, 761–767. [Google Scholar] [CrossRef]
- Numpilai, T.; Witoon, T.; Chanlek, N.; Limphirat, W.; Bonura, G.; Chareonpanich, M.; Limtrakul, J. Structure–activity relationships of Fe-Co/K-Al2O3 catalysts calcined at different temperatures for CO2 hydrogenation to light olefins. Appl. Catal. A-Gen. 2017, 547, 219–229. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Perez-Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J.L.G. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 2006, 243, 199–211. [Google Scholar] [CrossRef]
- Sirikulbodee, P.; Ratana, T.; Sornchamni, T.; Phongaksorn, M.; Tungkamani, S. Catalytic performance of Iron-based catalyst in Fischer-Tropsch synthesis using CO2 containing syngas. In Proceedings of the 2017 International Conference on Alternative Energy in Developing Countries and Emerging Economies, Bangkok, Thailand, 25–26 May 2017; pp. 998–1003. [Google Scholar]
- Zhang, Y.L.; Fu, D.L.; Liu, X.L.; Zhang, Z.P.; Zhang, C.; Shi, B.F.; Xu, J.; Han, Y.F. Operando Spectroscopic Study of Dynamic Structure of Iron Oxide Catalysts during CO2 Hydrogenation. Chemcatchem 2018, 10, 1272–1276. [Google Scholar] [CrossRef]
- Chew, L.M.; Kangvansura, P.; Ruland, H.; Schulte, H.J.; Somsen, C.; Xia, W.; Eggeler, G.; Worayingyong, A.; Muhler, M. Effect of nitrogen doping on the reducibility, activity and selectivity of carbon nanotube-supported iron catalysts applied in CO2 hydrogenation. Appl. Catal. A-Gen. 2014, 482, 163–170. [Google Scholar] [CrossRef]
- Yang, Q.; Skrypnik, A.; Matvienko, A.; Lund, H.; Holena, M.; Kondratenko, E.V. Revealing property-performance relationships for efficient CO2 hydrogenation to higher hydrocarbons over Fe-based catalysts: Statistical analysis of literature data and its experimental validation. Appl. Catal. B-Environ. 2021, 282, 119554. [Google Scholar] [CrossRef]
- Wang, J.J.; You, Z.Y.; Zhang, Q.H.; Deng, W.P.; Wang, Y. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts. Catal. Today 2013, 215, 186–193. [Google Scholar] [CrossRef]
- Hwang, S.M.; Zhang, C.D.; Han, S.J.; Park, H.G.; Kim, Y.T.; Yang, S.; Jun, K.W.; Kim, S.K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. [Google Scholar] [CrossRef]
- Suppiah, D.D.; Daud, W.M.A.W.; Johan, M.R. Supported Metal Oxide Catalysts for CO2 Fischer–Tropsch Conversion to Liquid Fuels─A Review. Energy Fuels 2021, 35, 17261–17278. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Wen, Z.; Ji, X.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Catalytic Hydrogenation of CO2 to Isoparaffins over Fe-Based Multifunctional Catalysts. ACS Catal. 2018, 8, 9958–9967. [Google Scholar] [CrossRef]
- Albrecht, M.; Rodemerck, U.; Schneider, M.; Broering, M.; Baabe, D.; Kondratenko, E.V. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl. Catal. B-Environ. 2017, 204, 119–126. [Google Scholar] [CrossRef]
- Riedel, T.; Schaub, G.; Jun, K.W.; Lee, K.W. Kinetics of CO2 hydrogenation on a K-promoted Fe catalyst. Ind. Eng. Chem. Res. 2001, 40, 1355–1363. [Google Scholar] [CrossRef]
- Yao, B.Z.; Ma, W.J.; Gonzalez-Cortes, S.; Xiao, T.C.; Edwards, P.P. Thermodynamic study of hydrocarbon synthesis from carbon dioxide and hydrogen. Greenh. Gases Sci. Technol. 2017, 7, 942–957. [Google Scholar] [CrossRef]
- Liu, Y.K.; Wang, L.; Hou, D.; Wan, H.; Feng, X. Study on thermodynamics of balanceable reaction system for hydrogenation of carbon dioxide to light alkenes. Chin. J. Catal. 2004, 25, 210–218. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Mattia, D.; Jones, M.D.; Plucinski, P.K. Formation of hydrocarbons via CO2 hydrogenation - A thermodynamic study. J. CO2 Util. 2014, 6, 34–39. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.S.; Jun, K.W.; Choi, M.J.; Kishan, G.; Lee, K.W. Comparative study of Fischer-Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe- and Co-based catalysts. Appl. Catal. Gen. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Torres Galvis, H.M.; Koeken, A.C.J.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; Jong, K.P.d. Supported Iron Nanoparticles as Catalysts for Sustainable Production of Lower Olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Bimetallic Fe–Co catalysts for CO2 hydrogenation to higher hydrocarbons. J. CO2 Util. 2013, 3-4, 102–106. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Light olefin synthesis from CO2 hydrogenation over K-promoted Fe–Co bimetallic catalysts. Catal. Today 2015, 251, 34–40. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Taguchi, A.; Abe, T.; Niu, W.; Lu, P.; Yoneyama, Y.; Tsubaki, N. Highly-Dispersed Metallic Ru Nanoparticles Sputtered on H-Beta Zeolite for Directly Converting Syngas to Middle Isoparaffins. ACS Catal. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cao, C.X.; Zhang, C.; Zhang, Z.P.; Liu, X.L.; Yang, Z.X.; Zhu, M.H.; Meng, B.; Xu, J.; Han, Y.F. The study of structure-performance relationship of iron catalyst during a full life cycle for CO2 hydrogenation. J. Catal. 2019, 378, 51–62. [Google Scholar] [CrossRef]
- Liu, B.; Geng, S.; Zheng, J.; Jia, X.; Jiang, F.; Liu, X. Unravelling the New Roles of Na and Mn Promoter in CO2 Hydrogenation over Fe3O4-Based Catalysts for Enhanced Selectivity to Light -Olefins. Chemcatchem 2018, 10, 4718–4732. [Google Scholar] [CrossRef]
- Wei, C.Y.; Tu, W.F.; Jia, L.Y.; Liu, Y.Y.; Lian, H.L.; Wang, P.; Zhang, Z.Z. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins. Appl. Surf. Sci. 2020, 525, 13. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Wen, Z.; Fang, C.; Ge, Q.; Xu, H. New insights into the effect of sodium on Fe3O4-based nanocatalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2016, 6, 4786–4793. [Google Scholar] [CrossRef]
- Al-Dossary, M.; Ismail, A.A.; Fierro, J.L.G.; Bouzid, H.; Al-Sayari, S.A. Effect of Mn loading onto MnFeO nanocomposites for the CO2 hydrogenation reaction. Appl. Catal. B-Environ. 2015, 165, 651–660. [Google Scholar] [CrossRef]
- Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S.; Ma, D. Highly Selective Olefin Production from CO2 Hydrogenation on Iron Catalysts: A Subtle Synergy between Manganese and Sodium Additives. Angew. Chem. Int. Ed. 2020, 59, 21736–21744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, M.J.; Yang, Z.X.; Zhu, M.H.; Gao, J.; Han, Y.F. Uncovering the electronic effects of zinc on the structure of Fe5C2-ZnO catalysts for CO2 hydrogenation to linear alpha-olefins. Appl. Catal. B-Environ. 2021, 295, 11. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yin, H.R.; Yu, G.D.; He, S.; Kang, J.C.; Liu, Z.M.; Cheng, K.; Zhang, Q.H.; Wang, Y. Selective hydrogenation of CO2 and CO into olefins over Sodium- and Zinc-Promoted iron carbide catalysts. J. Catal. 2021, 395, 350–361. [Google Scholar] [CrossRef]
- Malhi, H.S.; Sun, C.; Zhang, Z.; Liu, Y.; Liu, W.; Ren, P.; Tu, W.; Han, Y.-F. Catalytic consequences of the decoration of sodium and zinc atoms during CO2 hydrogenation to olefins over iron-based catalyst. Catal. Today 2022, 387, 28–37. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, S.; Wang, M.; Wang, X.; Gao, J.; Song, C. Dynamic Evolution of Fe and Carbon Species over Different ZrO2 Supports during CO Prereduction and Their Effects on CO2 Hydrogenation to Light Olefins. ACS Sustain. Chem. Eng. 2021, 9, 7891–7903. [Google Scholar] [CrossRef]
- Numpilai, T.; Chanlek, N.; Poo-Arporn, Y.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Chareonpanich, M.; Kongkachuichay, P.; Yigit, N.; Rupprechter, G.; et al. Tuning Interactions of Surface-adsorbed Species over Fe-Co/K-Al2O3 Catalyst by Different K Contents: Selective CO2 Hydrogenation to Light Olefins. Chemcatchem 2020, 12, 3306–3320. [Google Scholar] [CrossRef]
- Dong, Z.C.; Zhao, J.; Tian, Y.J.; Zhang, B.F.; Wu, Y. Preparation and Performances of ZIF-67-Derived FeCo Bimetallic Catalysts for CO2 Hydrogenation to Light Olefins. Catalysts 2020, 10, 455. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.; Noh, W.Y.; Shin, J.; Han, S.J.; Kim, S.K.; An, K.; Lee, J.S. Cobalt Ferrite Nanoparticles to Form a Catalytic Co-Fe Alloy Carbide Phase for Selective CO2 Hydrogenation to Light Olefins. ACS Catal. 2020, 10, 8660–8671. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Fan, G.; Yang, L.; Li, F. Unveiling the roles of Fe-Co interactions over ternary spinel-type ZnCoxFe2-xO4 catalysts for highly efficient CO2 hydrogenation to produce light olefins. J. Catal. 2021, 400, 355–366. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Wang, X.; Ma, Q.; Fan, S.; Zhao, T.S. Promotion effects of Ce added Fe–Zr–K on CO2 hydrogenation to light olefins. React. Kinet. Mech. Catal. 2018, 124, 575–585. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, X.; Wang, X.; Song, C. Fe–Cu bimetallic catalysts for selective CO2 hydrogenation to olefin-rich C2+ hydrocarbons. Ind. Eng. Chem. Res. 2018, 57, 4535–4542. [Google Scholar] [CrossRef]
- Li, Z.; Wu, W.; Wang, M.; Wang, Y.; Ma, X.; Luo, L.; Chen, Y.; Fan, K.; Pan, Y.; Li, H.; et al. Ambient-pressure hydrogenation of CO2 into long-chain olefins. Nat. Commun. 2022, 13, 2396. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Sun, T.; Ma, J.; Liu, X.; Xu, J.; Su, X.; Huang, Y.; Zhang, T. Effect of Na promoter on Fe-based catalyst for CO2 hydrogenation to alkenes. ACS Sustain. Chem. Eng. 2018, 7, 925–932. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Infantes-Molina, A.; Lietti, L.; Forzatti, P.; Iaquaniello, G.; Palo, E.; Picutti, B.; Brignoli, F. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst. Appl. Catal. B-Environ. 2017, 200, 530–542. [Google Scholar] [CrossRef]
- You, Z.Y.; Deng, W.P.; Zhang, Q.H.; Wang, Y. Hydrogenation of carbon dioxide to light olefins over non-supported iron catalyst. Chin. J. Catal. 2013, 34, 956–963. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Hamdeh, H.H.; Shafer, W.D.; Hopps, S.D.; Davis, B.H. Hydrogenation of carbon dioxide over iron carbide prepared from alkali metal promoted iron oxalate. Appl. Catal. A-Gen. 2018, 564, 243–249. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, B.; Geng, S.; Xu, Y.; Liu, X. Hydrogenation of CO2 into hydrocarbons: Enhanced catalytic activity over Fe-based Fischer–Tropsch catalysts. Catal. Sci. Technol. 2018, 8, 4097–4107. [Google Scholar] [CrossRef]
- Yang, S.; Chun, H.-J.; Lee, S.; Han, S.J.; Lee, K.-Y.; Kim, Y.T. Comparative Study of Olefin Production from CO and CO2 Using Na- and K-Promoted Zinc Ferrite. ACS Catal. 2020, 10, 10742–10759. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Highly selective conversion of CO2 to light olefins via Fischer-Tropsch synthesis over stable layered K-Fe-Ti catalysts. Appl. Catal. A-Gen. 2019, 573, 32–40. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective formation of light olefins from CO2 hydrogenation over Fe–Zn–K catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Dang, S.; Gao, P.; Liu, Z.; Chen, X.; Yang, C.; Wang, H.; Zhong, L.; Li, S.; Sun, Y. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]
- Gong, W.; Ye, R.-P.; Ding, J.; Wang, T.; Shi, X.; Russell, C.K.; Tang, J.; Eddings, E.G.; Zhang, Y.; Fan, M. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission. Appl. Catal. B-Environ. 2020, 278, 119302. [Google Scholar] [CrossRef]
- Ngantsoue-Hoc, W.; Zhang, Y.Q.; O’Brien, R.J.; Luo, M.S.; Davis, B.H. Fischer-Tropsch synthesis: Activity and selectivity for Group I alkali promoted iron-based catalysts. Appl. Catal. A-Gen. 2002, 236, 77–89. [Google Scholar] [CrossRef]
- Wang, P.; Kang, J.; Zhang, Q.; Wang, Y. Lithium ion-exchanged zeolite faujasite as support of iron catalyst for Fischer-Tropsch synthesis. Catal. Lett. 2007, 114, 178–184. [Google Scholar] [CrossRef]
- Yang, Q.; Kondratenko, V.A.; Petrov, S.A.; Doronkin, D.E.; Saraci, E.; Lund, H.; Arinchtein, A.; Kraehnert, R.; Skrypnik, A.S.; Matvienko, A.A.; et al. Identifying Performance Descriptors in CO2 Hydrogenation over Iron-Based Catalysts Promoted with Alkali Metals. Angew. Chem. Int. Ed. 2022, 61, e202116517. [Google Scholar] [CrossRef]
- Wang, S.; Ji, Y.; Liu, X.; Yan, S.; Xie, S.; Pei, Y.; Li, H.; Qiao, M.; Zong, B. Potassium as a Versatile Promoter to Tailor the Distribution of the Olefins in CO2 Hydrogenation over Iron-Based Catalyst. Chemcatchem 2022, 14, e202101535. [Google Scholar] [CrossRef]
- Jiang, M.; Koizumi, N.; Yamada, M. Characterization of potassium-promoted iron-manganese catalysts by insitu diffuse reflectance FTIR using NO, CO and CO+H2 as probes. Appl. Catal. A-Gen. 2000, 204, 49–58. [Google Scholar] [CrossRef]
- Tu, W.; Sun, C.; Zhang, Z.; Liu, W.; Malhi, H.S.; Ma, W.; Zhu, M.; Han, Y.-F. Chemical and structural properties of Na decorated Fe5C2-ZnO catalysts during hydrogenation of CO2 to linear α-olefins. Appl. Catal. B-Environ. 2021, 298, 120567. [Google Scholar] [CrossRef]
- Witoon, T.; Chaipraditgul, N.; Numpilai, T.; Lapkeatseree, V.; Ayodele, B.V.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Limtrakul, J. Highly active Fe-Co-Zn/K-Al2O3 catalysts for CO2 hydrogenation to light olefins. Chem. Eng. Sci. 2021, 233, 116428. [Google Scholar] [CrossRef]
- Hwang, S.M.; Han, S.J.; Min, J.E.; Park, H.G.; Jun, K.W.; Kim, S.K. Mechanistic insights into Cu and K promoted Fe-catalyzed production of liquid hydrocarbons via CO2 hydrogenation. J. CO2 Util. 2019, 34, 522–532. [Google Scholar] [CrossRef]
- Chaipraditgul, N.; Numpilai, T.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Wattanakit, C.; Limtrakul, J.; Witoon, T. Tuning interaction of surface-adsorbed species over Fe/K-Al2O3 modified with transition metals (Cu, Mn, V, Zn or Co) on light olefins production from CO2 hydrogenation. Fuel 2021, 283, 119248. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, J.; Zhao, N.; Dai, L.; Jiang, X.; Liu, C.; Lyu, S.; Li, Z. Preparation of SiO2 immobilized Co-based catalysts from ZIF-67 and the enhancement effect for Fischer-Tropsch synthesis. Appl. Catal. B-Environ. 2021, 289, 120027. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Nisa, M.U.; Li, Z. Combating poison with poison—Irreducible Co2SiO4 as a promoter to modify Co-based catalysts in Fischer-Tropsch synthesis. Appl. Catal. B-Environ. 2020, 267, 118377. [Google Scholar] [CrossRef]
- Duvenhage, D.J.; Coville, N.J. Fe:Co/TiO2 bimetallic catalysts for the Fischer-Tropsch reaction I. Characterization and reactor studies. Appl. Catal. A-Gen. 1997, 153, 43–67. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Zhang, C.; An, X.; Wan, H.; Tao, Z.; Xiang, H.; Li, Y.; Yi, F.; Xu, B. Effect of manganese on an iron-based Fischer-Tropsch synthesis catalyst prepared from ferrous sulfate. Fuel 2007, 86, 921–928. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Dai, W.W.; Xu, X.C.; Zhang, J.; Shi, B.F.; Xu, J.; Tu, W.F.; Han, Y.F. MnOx promotional effects on olefins synthesis directly from syngas over bimetallic Fe-MnOx/SiO2 catalysts. AIChE J. 2017, 63, 4451–4464. [Google Scholar] [CrossRef]
- Kuci, C.-K.; Chen, W.-S.; Lee, M.-D. Hydrogenation of Carbon Dioxide on Iron Manganese Catalysts. J. Chin. Chem. Soc. 1991, 38, 127–139. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Janik, M.J.; Chen, Y.; Guo, X.; Song, C. Mechanistic Insight into C-C Coupling over Fe-Cu Bimetallic Catalysts in CO2 Hydrogenation. J. Phys. Chem. C 2017, 121, 13164–13174. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Janik, M.J.; Guo, X.; Song, C. Computational Investigation of Fe-Cu Bimetallic Catalysts for CO2 Hydrogenation. J. Phys. Chem. C 2016, 120, 9364–9373. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, C.; Zhang, Y.; Liu, X.; Xu, J.; Zhu, M.; Tu, W.; Han, Y.-F. Unraveling the Role of Zinc on Bimetallic Fe5C2-ZnO Catalysts for Highly Selective Carbon Dioxide Hydrogenation to High Carbon alpha-Olefins. ACS Catal. 2021, 11, 2121–2133. [Google Scholar] [CrossRef]
- Daelman, N.; Capdevila-Cortada, M.; López, N. Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts. Nat. Mater. 2019, 18, 1215–1221. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.; Huang, J.; Haruta, M.; et al. Classical strong metal-support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Huertas Osta, E.; van Hoof, A.J.F.; Kimpel, T.F.; Kosinov, N.; Hensen, E.J.M. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. [Google Scholar] [CrossRef]
- Divins, N.J.; Angurell, I.; Escudero, C.; Perez-Dieste, V.; Llorca, J. Influence of the support on surface rearrangements of bimetallic nanoparticles in real catalysts. Science 2014, 346, 620–623. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Promotional effects in alumina and silica supported bimetallic Ni-Fe catalysts during CO2 hydrogenation. J. Mol. Catal. A Chem. 2014, 382, 23–30. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, W.X.; Zi, L.T.; Xu, X.; Liu, Q.; Zhong, Q.; Xu, Y. Promotional Effect of ZrO2 on supported FeCoK Catalysts for Ethylene Synthesis from catalytic CO2 hydrogenation. Int. J. Hydrogen Energy 2020, 45, 15254–15262. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Stolbov, D.N.; Maksimov, S.V.; Maslakov, K.I.; Chernavskii, P.A.; Pokusaeva, Y.A.; Koklin, A.E.; Bogdan, V.I.; Savilov, S.V. Sintered Fe/CNT framework catalysts for CO2 hydrogenation into hydrocarbons. Carbon 2020, 168, 475–484. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, W.; Lim, E.; Oh, M. One-pot synthesis of magnetic particle-embedded porous carbon composites from metal-organic frameworks and their sorption properties. Chem. Commun. 2014, 50, 5476–5479. [Google Scholar] [CrossRef] [PubMed]
- Wezendonk, T.A.; Santos, V.P.; Nasalevich, M.A.; Warringa, Q.S.E.; Dugulan, A.I.; Chojecki, A.; Koeken, A.C.J.; Ruitenbeek, M.; Meima, G.; Islam, H.-U.; et al. Elucidating the Nature of Fe Species during Pyrolysis of the Fe-BTC MOF into Highly Active and Stable Fischer-Tropsch Catalysts. ACS Catal. 2016, 6, 3236–3247. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Jiang, X.; Zhang, A.; Song, C.; Guo, X. Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Zhang, G.; Sun, Y.; Liu, M.; Ding, F.; Song, C.; Guo, X. Overcoating the Surface of Fe-Based Catalyst with ZnO and Nitrogen-Doped Carbon toward High Selectivity of Light Olefins in CO2 Hydrogenation. Ind. Eng. Chem. Res. 2019, 58, 4017–4023. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Liu, M.; Hu, S.; Ding, F.; Song, C.; Guo, X. Fe-MOF-derived highly active catalysts for carbon dioxide hydrogenation to valuable hydrocarbons. J. CO2 Util. 2017, 21, 100–107. [Google Scholar] [CrossRef]
- An, B.; Cheng, K.; Wang, C.; Wang, Y.; Lin, W. Pyrolysis of Metal-Organic Frameworks to Fe3O4@Fe5C2 Core-Shell Nanoparticles for Fischer-Tropsch Synthesis. ACS Catal. 2016, 6, 3610–3618. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Zhang, J.; Chen, S.; Wu, Y.; Zhang, Q.; Wang, J.; Han, Y.; Tan, Y. Synthesis of isoalkanes over a core (Fe–Zn–Zr)–shell (zeolite) catalyst by CO2 hydrogenation. Chem. Commun. 2016, 52, 7352–7355. [Google Scholar] [CrossRef]
- Roque-Malherbe, R.M.A.; Wendelbo, R.; Mifsud, A.; Corma, A.J.T.J.o.P.C. Diffusion of aromatic hydrocarbons in H-ZSM-5, H-Beta, and H-MCM-22 zeolites. J. Phys. Chem. 1995, 99, 14064–14071. [Google Scholar] [CrossRef]
- Chen, J.; Liang, T.; Li, J.; Wang, S.; Qin, Z.; Wang, P.; Huang, L.; Fan, W.; Wang, J. Regulation of Framework Aluminum Siting and Acid Distribution in H-MCM-22 by Boron Incorporation and Its Effect on the Catalytic Performance in Methanol to Hydrocarbons. ACS Catal. 2016, 6, 2299–2313. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B-Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, G.; Liu, Y. Perovskite-Type Oxides as the Catalyst Precursors for Preparing Supported Metallic Nanocatalysts: A Review. Ind. Eng. Chem. Res. 2018, 57, 1–17. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M.D. Structure and Reactivity of Perovskite-Type Oxides. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 36. [Google Scholar]
- Ren, J.; Mebrahtu, C.; van Koppen, L.; Martinovic, F.; Hofmann, J.P.; Hensen, E.J.M.; Palkovits, R. Enhanced CO2 methanation activity over La2-xCexNiO4 perovskite-derived catalysts: Understanding the structure-performance relationships. Chem. Eng. J. 2021, 426, 131760. [Google Scholar] [CrossRef]
- Bedel, L.; Roger, A.-C.; Rehspringer, J.-L.; Zimmermann, Y.; Kiennemann, A. La(1−y)Co0.4Fe0.6O3−δ perovskite oxides as catalysts for Fischer–Tropsch synthesis. J. Catal. 2005, 235, 279–294. [Google Scholar] [CrossRef]
- Ma, L.-H.; Gao, X.-H.; Ma, J.-J.; Hu, X.-D.; Zhang, J.-L.; Guo, Q.-J. K/LaFeMnO3 Perovskite-Type Oxide Catalyst for the Production of C2–C4 Olefins via CO Hydrogenation. Catal. Lett. 2022, 152, 1451–1460. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, G.; Zhao, C.; Xing, Y. Selective Iron Catalysts for Direct Fischer–Tropsch Synthesis to Light Olefins. Ind. Eng. Chem. Res. 2021, 60, 6137–6146. [Google Scholar] [CrossRef]
- Ao, M.; Pham, G.H.; Sage, V.; Pareek, V.; Liu, S. Perovskite-derived trimetallic Co-Ni-Cu catalyst for higher alcohol synthesis from syngas. Fuel Process. Technol. 2019, 193, 141–148. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon Dioxide Conversion by Reverse Water–Gas Shift Chemical Looping on Perovskite-Type Oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Lindenthal, L.; Popovic, J.; Rameshan, R.; Huber, J.; Schrenk, F.; Ruh, T.; Nenning, A.; Löffler, S.; Opitz, A.K.; Rameshan, C. Novel perovskite catalysts for CO2 utilization - Exsolution enhanced reverse water-gas shift activity. Appl. Catal. B-Environ. 2021, 292, 120183. [Google Scholar] [CrossRef]
- Utsis, N.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Novel bifunctional catalysts based on crystalline multi-oxide matrices containing iron ions for CO2 hydrogenation to liquid fuels and chemicals. Faraday Discuss. 2016, 188, 545–563. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Chen, M.; Gao, X.; Liu, Y.; Guo, Q. Sr1-xKxFeO3 Perovskite Catalysts with Enhanced RWGS Reactivity for CO2 Hydrogenation to Light Olefins. Atmosphere 2022, 13, 760. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 Reduction on Supported Ru/Al2O3 Catalysts: Cluster Size Dependence of Product Selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Xie, T.; Wang, J.; Ding, F.; Zhang, A.; Li, W.; Guo, X.; Song, C. CO2 hydrogenation to hydrocarbons over alumina-supported iron catalyst: Effect of support pore size. J. CO2 Util 2017, 19, 202–208. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Iablokov, V.; Beaumont, S.K.; Alayoglu, S.; Pushkarev, V.V.; Specht, C.; Gao, J.; Alivisatos, A.P.; Kruse, N.; Somorjai, G.A. Size-Controlled Model Co Nanoparticle Catalysts for CO2 Hydrogenation: Synthesis, Characterization, and Catalytic Reactions. Nano Lett. 2012, 12, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Hwang, J.S.; Jun, K.W.; Sur, J.C.; Lee, K.W. Deactivation study on a coprecipitated Fe-Cu-K-Al catalyst in CO2 hydrogenation. Appl. Catal. A-Gen. 2001, 218, 53–59. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Li, W.; Zhang, X.; Ding, F.; Song, C.; Guo, X. Deconvolution of the Particle Size Effect on CO2 Hydrogenation over Iron-Based Catalysts. ACS Catal. 2020, 10, 7424–7433. [Google Scholar] [CrossRef]
- Podgurski, H.; Kummer, J.; DeWitt, T.; Emmett, P. Preparation, Stability and Adsorptive Properties of the Carbides of Iron. J. Am. Chem. Soc. 1950, 72, 5382–5388. [Google Scholar] [CrossRef]
- Brady, R.C.; Pettit, R. On the Mechanism of the Fischer-Tropsch reaction. The chain propagation step. J. Am. Chem. Soc. 1981, 103, 1287–1289. [Google Scholar] [CrossRef]
- Chuang, S.; Tian, Y.; Goodwin Jr, J.; Wender, I. The use of probe molecules in the study of CO hydrogenation over SiO2-supported Ni, Ru, Rh, and Pd. J. Catal. 1985, 96, 396–407. [Google Scholar] [CrossRef]
- Riedel, T.; Schulz, H.; Schaub, G.; Jun, K.W.; Hwang, J.S.; Lee, K.W. Fischer-Tropsch on iron with H2/CO and H2/CO2 as synthesis gases: The episodes of formation of the Fischer-Tropsch regime and construction of the catalyst. Top. Catal. 2003, 26, 41–54. [Google Scholar] [CrossRef]
- Puga, A.V. On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catal. Sci. Technol. 2018, 8, 5681–5707. [Google Scholar] [CrossRef]
- Han, S.J.; Hwang, S.-M.; Park, H.-G.; Zhang, C.; Jun, K.-W.; Kim, S.K. Identification of active sites for CO2 hydrogenation in Fe catalysts by first-principles microkinetic modelling. J. Mater. Chem. A 2020, 8, 13014–13023. [Google Scholar] [CrossRef]
- Wang, H.; Nie, X.; Liu, Y.; Janik, M.J.; Han, X.; Deng, Y.; Hu, W.; Song, C.; Guo, X. Mechanistic Insight into Hydrocarbon Synthesis via CO2 Hydrogenation on χ-Fe5C2 Catalysts. ACS Appl. Mater. Interfaces 2022, 14, 37637–37651. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gao, P.; Li, S.; Yang, C.; Liu, Z.; Wang, H.; Zhong, L.; Sun, Y. Selective Production of Aromatics Directly from Carbon Dioxide Hydrogenation. ACS Catal. 2019, 9, 3866–3876. [Google Scholar] [CrossRef]
- Bukur, D.B.; Okabe, K.; Rosynek, M.P.; Li, C.P.; Wang, D.J.; Rao, K.R.P.M.; Huffman, G.P. Activation Studies with a Precipitated Iron Catalyst for Fischer-Tropsch Synthesis: I. Characterization Studies. J. Catal. 1995, 155, 353–365. [Google Scholar] [CrossRef]
- Raupp, G.B.; Delgass, W.N. Mössbauer investigation of supported Fe and FeNi catalysts: II. Carbides formed Fischer-Tropsch synthesis. J. Catal. 1979, 58, 348–360. [Google Scholar] [CrossRef]
- de Smit, E.; Cinquini, F.; Beale, A.M.; Safonova, O.V.; van Beek, W.; Sautet, P.; Weckhuysen, B.M. Stability and Reactivity of epsilon-chi-theta Iron Carbide Catalyst Phases in Fischer-Tropsch Synthesis: Controlling μ(c). J. Am. Chem. Soc. 2010, 132, 14928–14941. [Google Scholar] [CrossRef]
- Mazzucco, S.; Wang, Y.; Tanase, M.; Picher, M.; Li, K.; Wu, Z.; Irle, S.; Sharma, R. Direct evidence of active and inactive phases of Fe catalyst nanoparticles for carbon nanotube formation. J. Catal. 2014, 319, 54–60. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Liu, Y.; Ning, W.; Han, W.; Liu, H.; Huo, C. Preparation of Iron Carbides Formed by Iron Oxalate Carburization for Fischer–Tropsch Synthesis. Catalysts 2019, 9, 347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).