A Remarkable Photocatalyst Filter for Indoor Air Treatment
Abstract
1. Introduction
2. Results and Discussion
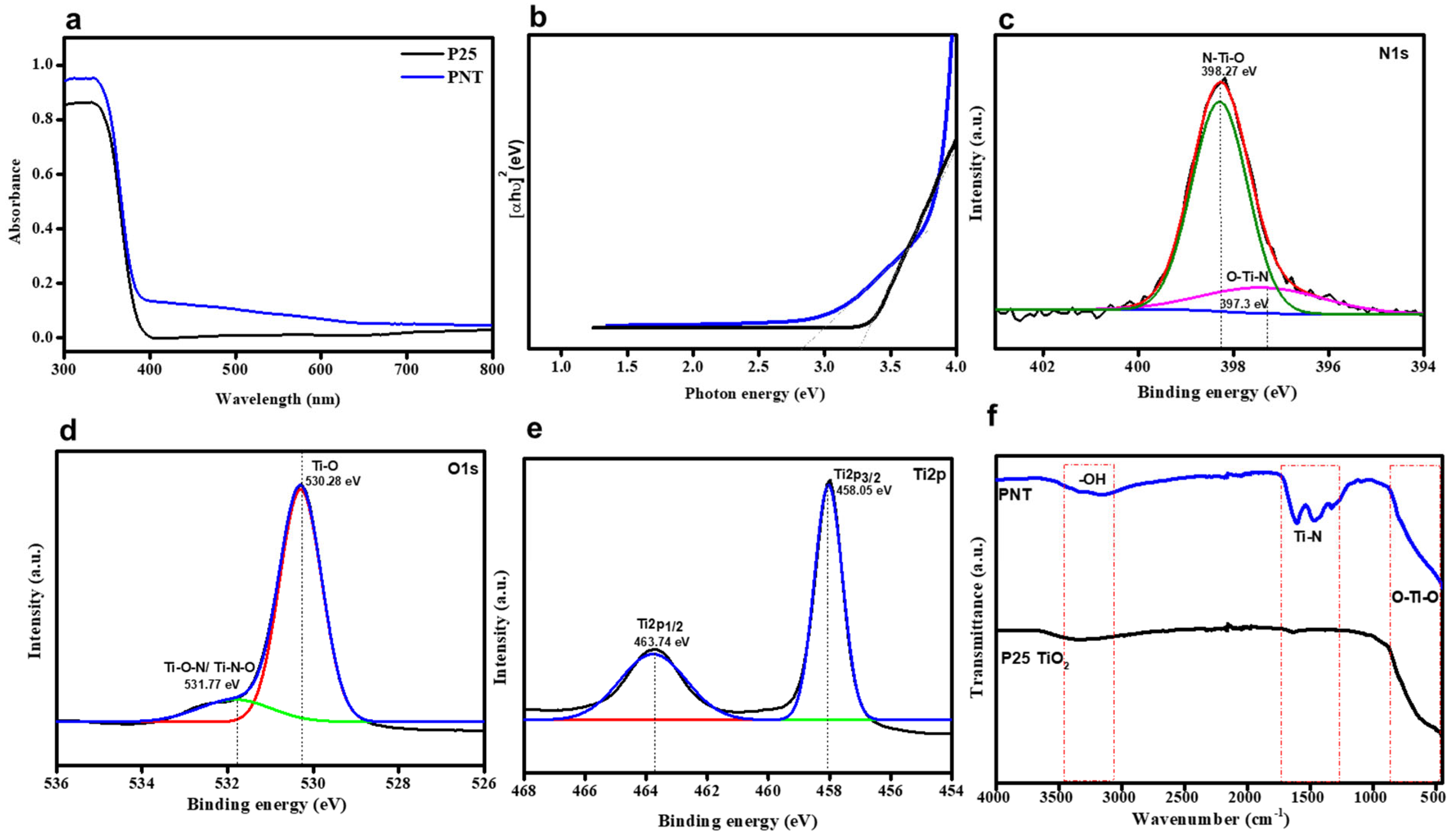
2.1. Characterization of PNT
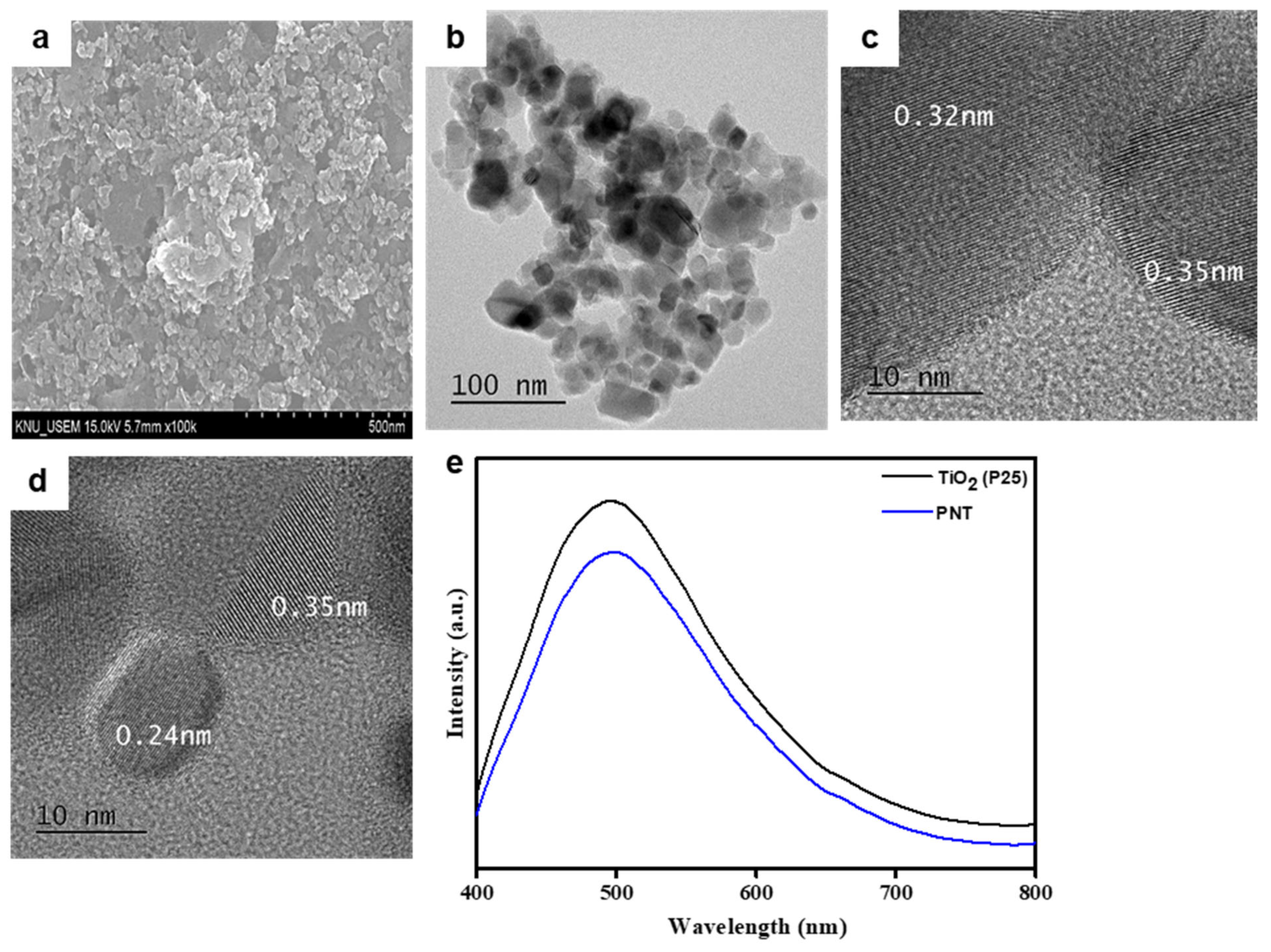
2.2. Morphology and Photoluminescence Spectra of PNT
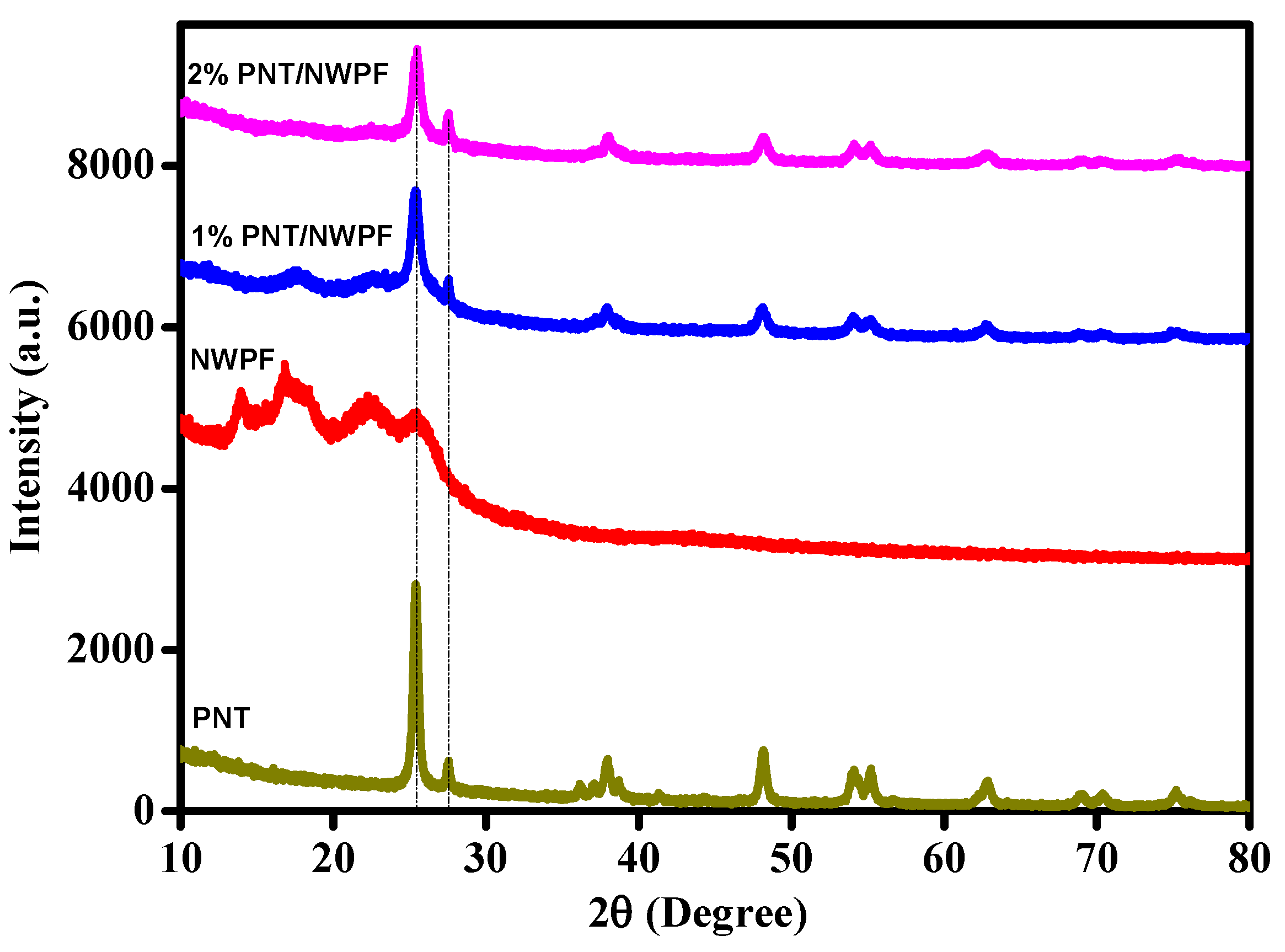
2.3. Crystallinity of PNT/NWPF
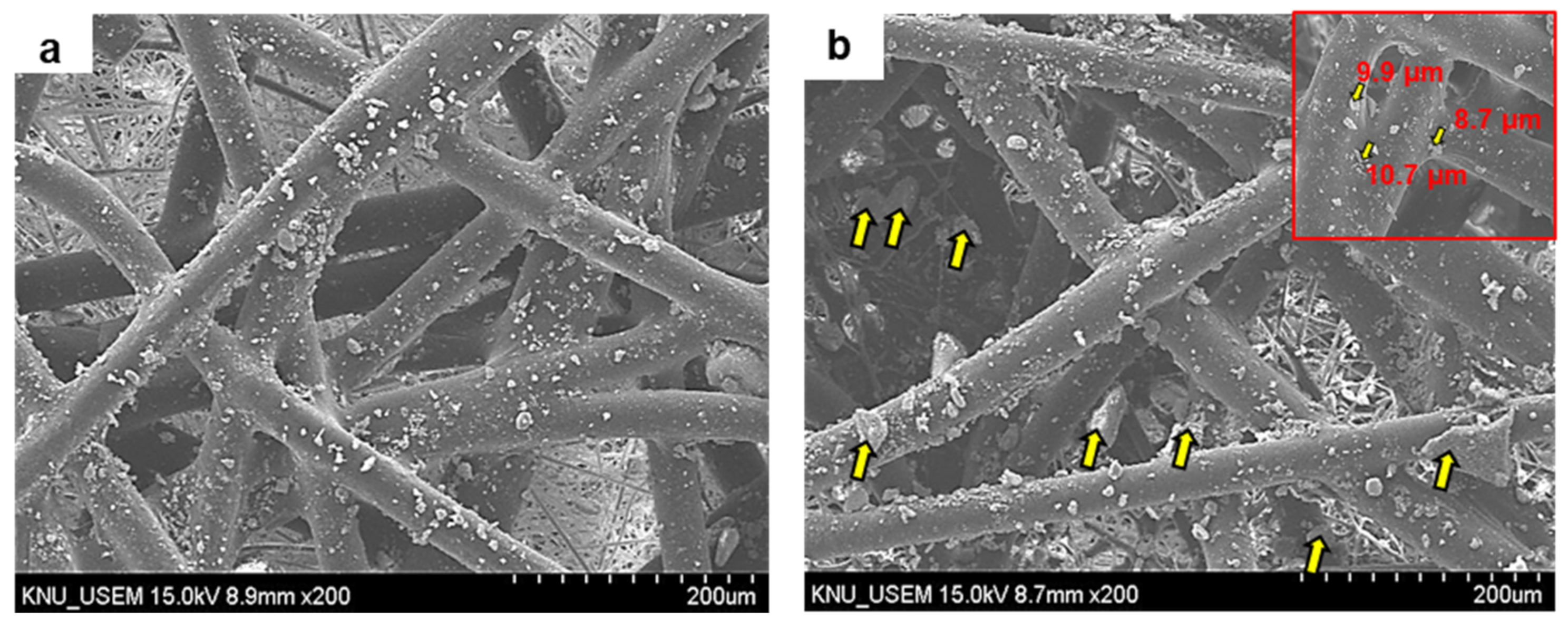
2.4. Morphology of PNT Coated on NWPF
2.5. Coating Stability of PNT/NWPF
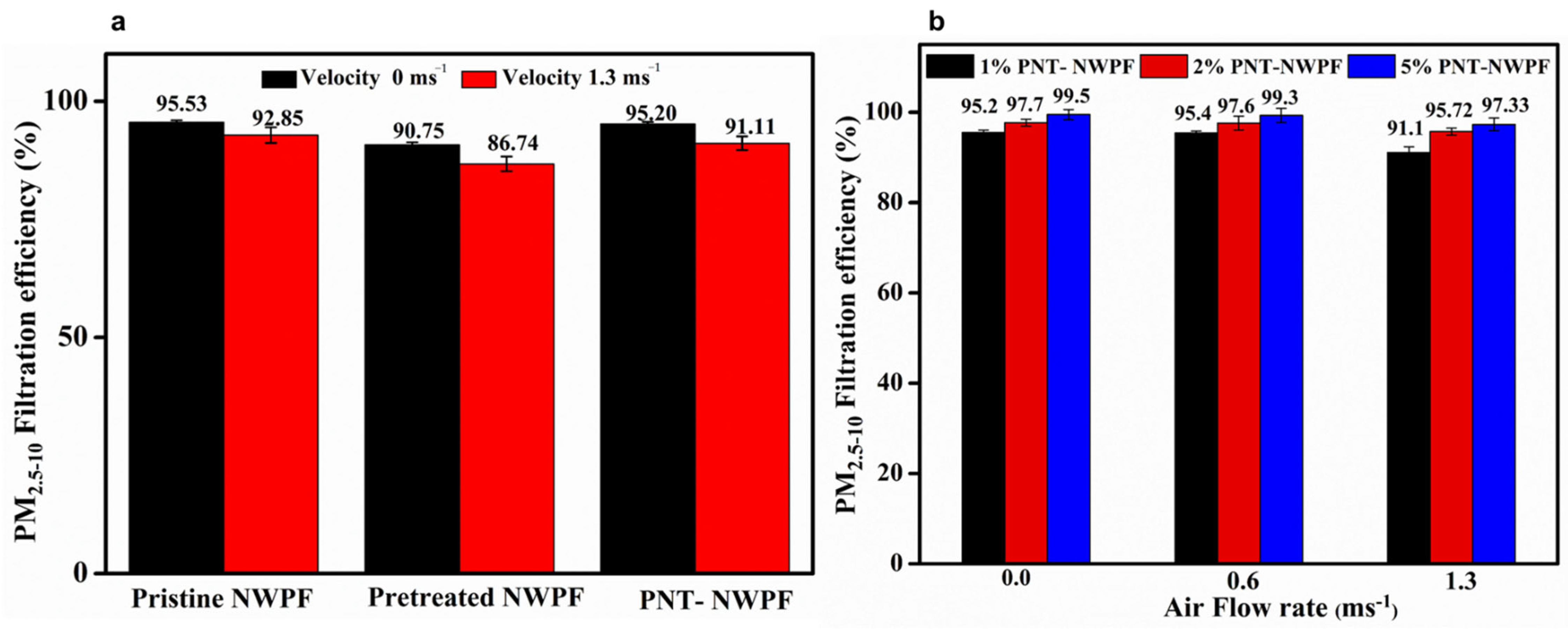
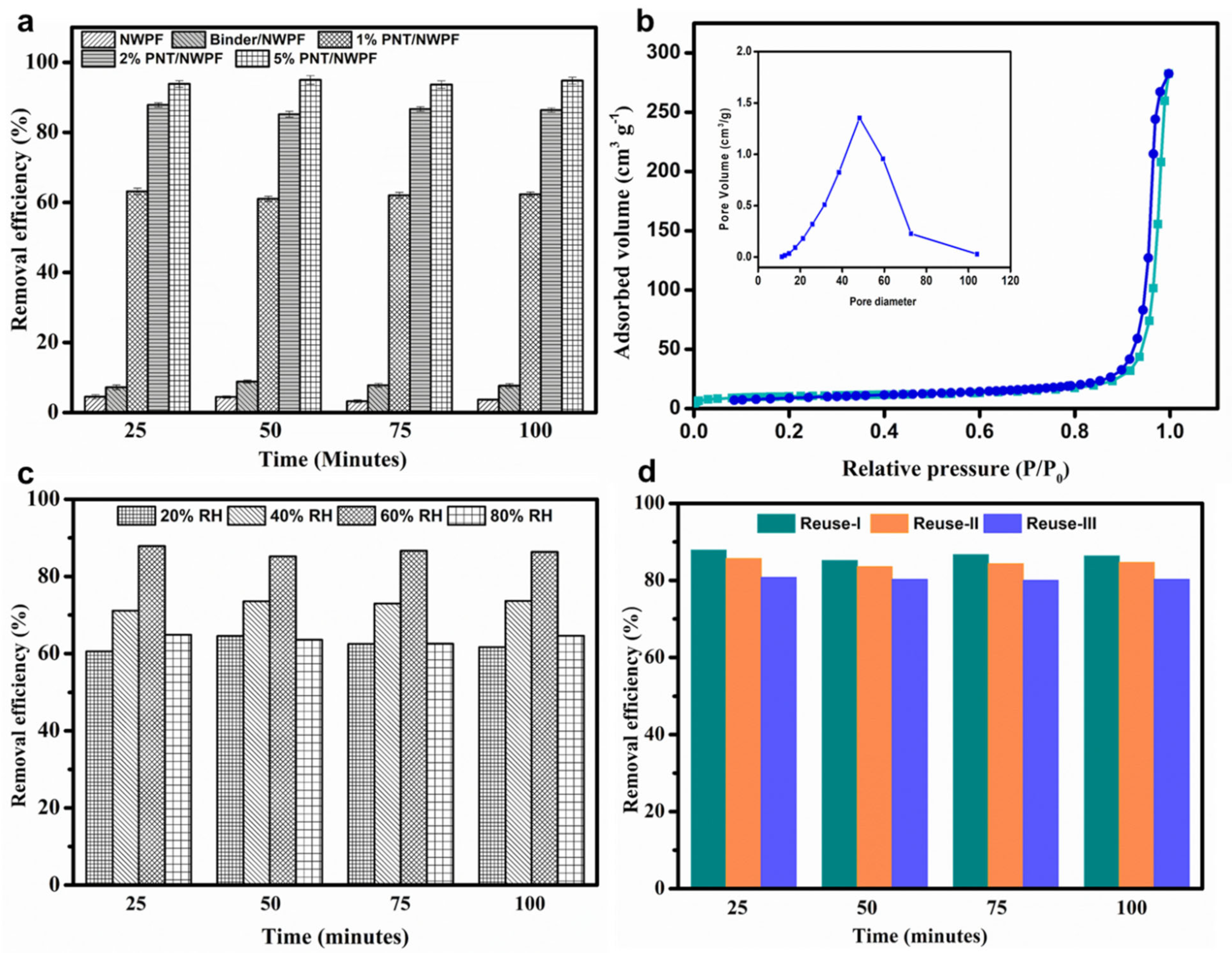
2.6. Dust Capture Efficiency of PNT Decorated NWPF
2.7. VOC Removal Efficiency of PNT/NWPF
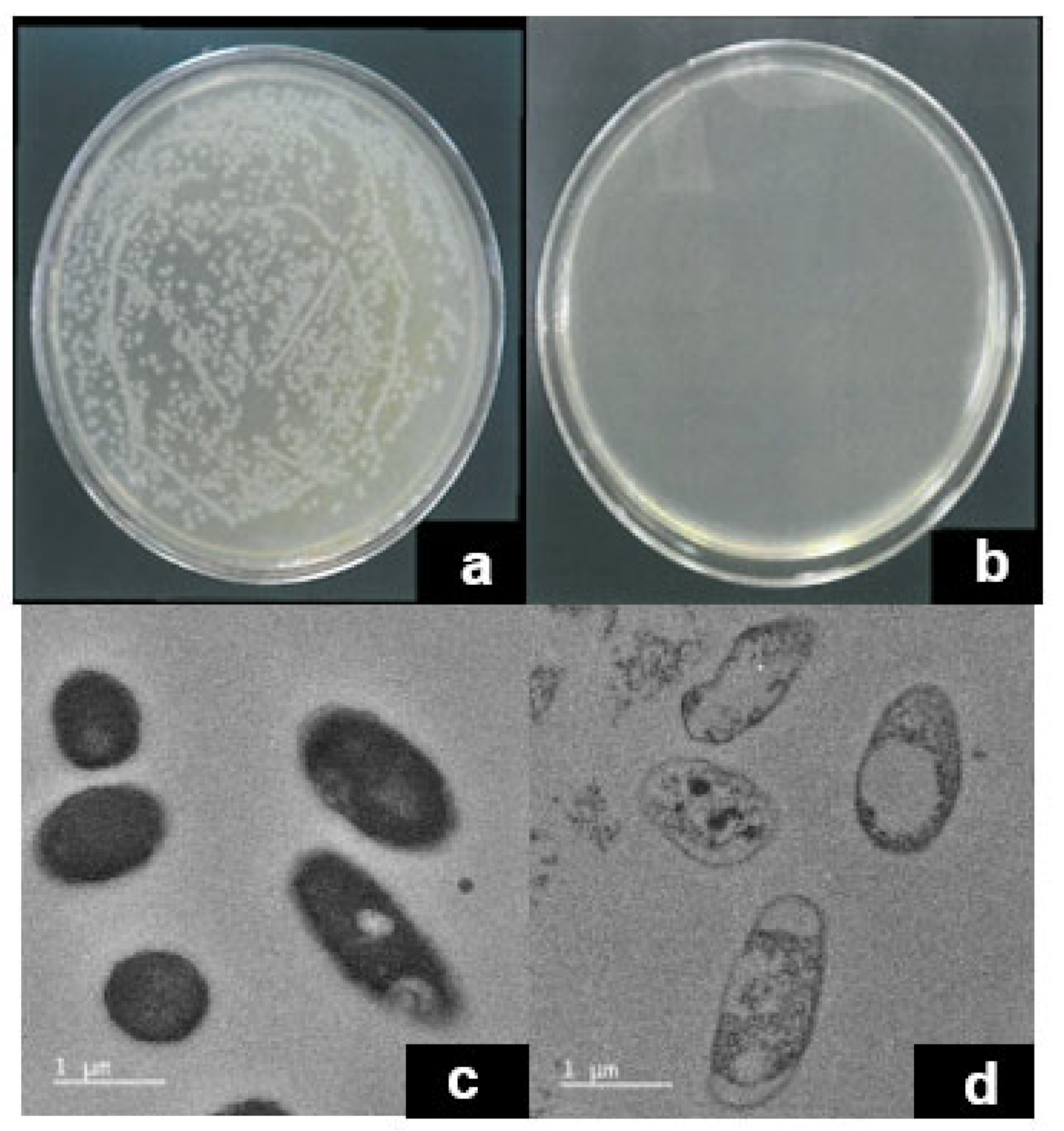
2.8. Antibacterial Activity of PNT/NWPF
3. Materials and Methods
3.1. Materials
3.2. Preparation of PNT
3.3. Fabrication of PNT/NWPF Air Filter
3.4. Dust Capture (PM2.5–10) Experiment
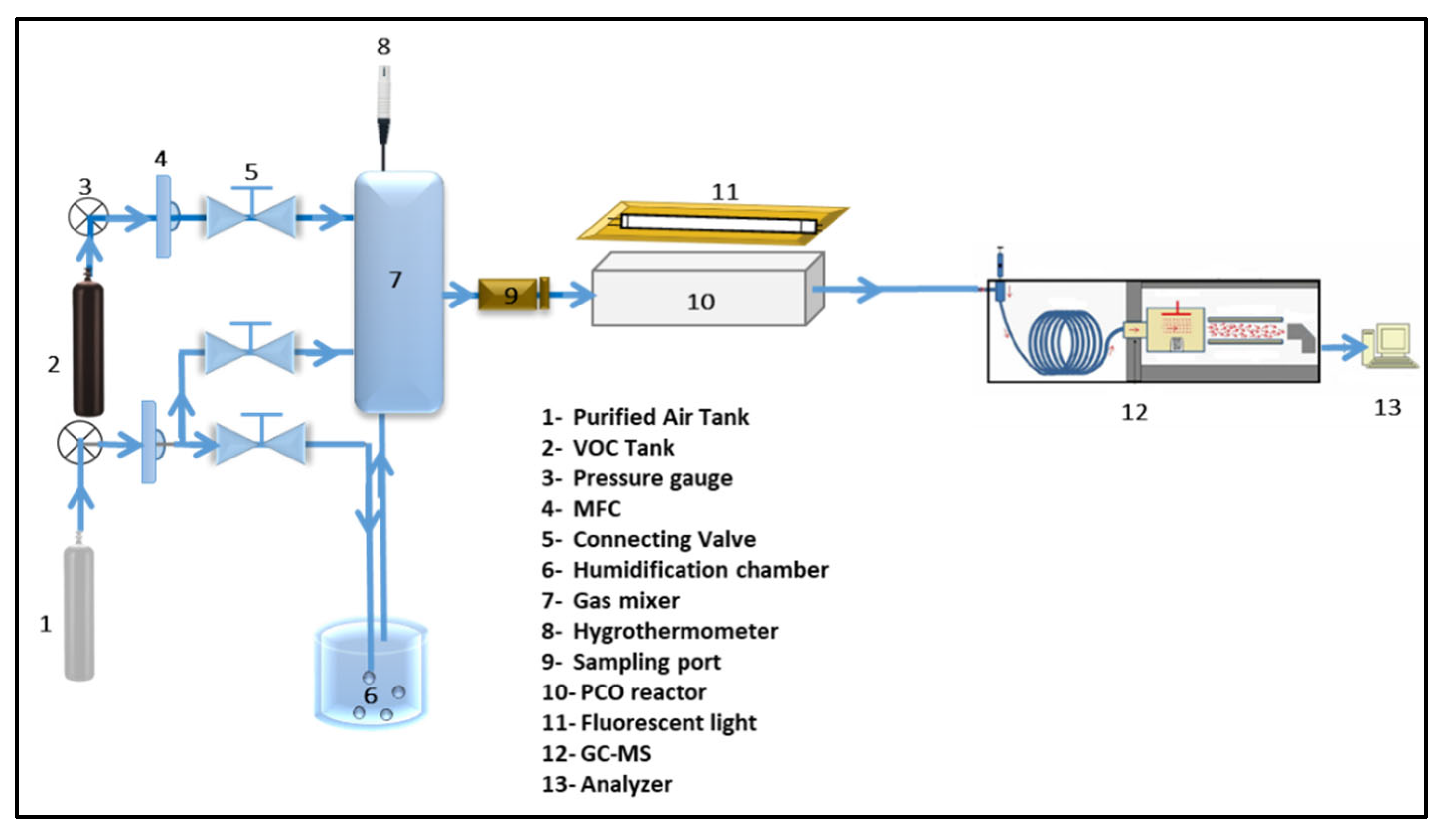
3.5. Experiment for Photocatalytic Oxidation
3.6. Antibacterial Application of PNT/NWPF
3.7. Materials Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hargreaves, M.; Parappukkaran, S.; Morawska, L.; Hitchins, J.; He, C.; Gilbert, D. A pilot investigation into associations between indoor airborne fungal and non-biological particle concentrations in residential houses in Brisbane, Australia. Sci. Total Environ. 2003, 312, 89–101. [Google Scholar] [CrossRef]
- Zhang, J.F.; Smith, K.R. Indoor air pollution: A global health concern. Br. Med. Bull. 2003, 68, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Maggos, T.; Binas, V.; Siaperas, V.; Terzopoulos, A.; Panagopoulos, P.; Kiriakidis, G. A Promising Technological Approach to Improve Indoor Air Quality. Appl. Sci. 2019, 9, 4837. [Google Scholar] [CrossRef]
- Suryadhi, M.A.H.; Abudureyimu, K.; Kashima, S.; Yorifuji, T. Effects of Household Air Pollution From Solid Fuel Use and Environmental Tobacco Smoke on Child Health Outcomes in Indonesia. J. Occup. Environ. Med. 2019, 61, 335–339. [Google Scholar] [CrossRef]
- Wickliffe, J.K.; Stock, T.H.; Howard, J.L.; Frahm, E.; Simon-Friedt, B.R.; Montgomery, K.; Wilson, M.J.; Lichtveld, M.Y.; Harville, E. Increased long-term health risks attributable to select volatile organic compounds in residential indoor air in southeast Louisiana. Sci. Rep. 2020, 10, 21649. [Google Scholar] [CrossRef]
- Guo, P.; Yokoyama, K.; Piao, F.; Sakai, K.; Khalequzzaman, M.; Kamijima, M.; Nakajima, T.; Kitamura, F. Sick building syndrome by indoor air pollution in Dalian, China. Int. J. Environ. Res. Public Health 2013, 10, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Todaka, E.; Seto, H.; Saito, I.; Hanazato, M.; Watanabe, M.; Mori, C. Correlating the symptoms of sick-building syndrome to indoor VOCs concentration levels and odour. Indoor Built Environ. 2014, 23, 804–813. [Google Scholar] [CrossRef]
- Todaka, E.; Nakaoka, H.; Hanazato, M.; Seto, H.; Mori, C. Sick building syndrome and total volatile organic compounds. Toxicol. Lett. 2012, 211, S94. [Google Scholar] [CrossRef]
- Im, J.; Kim, B.; Kim, H.; Lee, M.; Jeon, D.; Ryu, J.; Yun, D.; Jang, Y.; Lee, C. A Study on the Characteristics of Hazardous Pollutant Emissions in Korea from 2007 to 2016. Int. J. Environ. Res. 2020, 14, 335–346. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Luo, Y.; Yang, G.; Zhou, T. Joint action and lethal levels of toluene, ethylbenzene, and xylene on midge (Chironomus plumosus) larvae. Environ. Sci. Pollut. Res. 2013, 20, 957–966. [Google Scholar] [CrossRef]
- Sriprapat, W.; Suksabye, P.; Areephak, S.; Klantup, P.; Waraha, A.; Sawattan, A.; Thiravetyan, P. Uptake of toluene and ethylbenzene by plants: Removal of volatile indoor air contaminants. Ecotoxicol. Environ. Saf. 2014, 102, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Bahadar, H.; Maqbool, F.; Abdollahi, M. A review of environmental and occupational exposure to xylene and its health concerns. EXCLI J. 2015, 14, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.-H.; Oh, I.; Kim, S.; You, S.; Kim, Y.; Kwon, H.-J.; Kim, G.-B. Modeling the effects of pollutant emissions from large industrial complexes on benzene, toluene, and xylene (BTX) concentrations in urban areas. Environ. Health Toxicol. 2017, 32, e2017022. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, N.; Rao, L.N. COVID-19 and importance of air filtration. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102183. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Rohra, H.; Taneja, A. Assessing effectiveness of air purifiers (HEPA) for controlling indoor particulate pollution. Heliyon 2021, 7, e07976. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chan, K.H.; Lam, K.B.H.; Qiu, H.; Li, Z.; Yim, S.H.L.; Ho, K.-F. Effectiveness of indoor air purification intervention in improving cardiovascular health: A systematic review and meta-analysis of randomized controlled trials. Sci. Total Environ. 2021, 789, 147882. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Hu, S.; Jin, L.; Si, W.; Wang, B.; Zhu, M. Sulfur Vacancies Enriched 2D ZnIn2S4 Nanosheets for Improving Photoelectrochemical Performance. Catalysts 2022, 12, 400. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Yang, J.; Zhu, M. Efficient ozone decomposition over bifunctional Co3Mn-layered double hydroxide with strong electronic interaction. Chin. Chem. Lett. 2022, 33, 4679–4682. [Google Scholar] [CrossRef]
- Taranto, J.; Frochot, D.; Pichat, P. Photocatalytic air purification: Comparative efficacy and pressure drop of a TiO2-coated thin mesh and a honeycomb monolith at high air velocities using a 0.4 m3 close-loop reactor. Sep. Purif. Technol. 2009, 67, 187–193. [Google Scholar] [CrossRef]
- Andersen, B.M.; Bånrud, H.; Bøe, E.; Bjordal, O.; Drangsholt, F. Comparison of UV C light and chemicals for disinfection of surfaces in hospital isolation units. Infect. Control Hosp. Epidemiol. 2006, 27, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Quan, X.; Xiang, J.; Huang, Y.; Xu, Y. Photocatalytic degradation of bisphenol A using an integrated system of a new gas-liquid-solid circulating fluidized bed reactor and micrometer Gd-doped TiO2 particles. J. Environ. Sci. 2012, 24, 1317–1326. [Google Scholar] [CrossRef]
- Xi, G.; Ouyang, S.; Ye, J. General Synthesis of Hybrid TiO2 Mesoporous “French Fries” Toward Improved Photocatalytic Conversion of CO2 into Hydrocarbon Fuel: A Case of TiO2/ZnO. Chemistry 2011, 17, 9057–9061. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, T.; Zhang, P.; Zhang, J.; Li, A.; Gong, J. Enhanced Surface Reaction Kinetics and Charge Separation of p–n Heterojunction Co3O4/BiVO4 Photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.J.; Liu, G.; Moniz, S.J.A.; Bi, Y.; Beale, A.M.; Ye, J.; Tang, J. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef]
- Ma, X.; Lv, Y.; Xu, J.; Liu, Y.; Zhang, R.; Zhu, Y. A Strategy of Enhancing the Photoactivity of g-C3N4 via Doping of Nonmetal Elements: A First-Principles Study. J. Phys. Chem. C 2012, 116, 23485–23493. [Google Scholar] [CrossRef]
- Khan, T.T.; Bari, G.A.R.; Kang, H.J.; Lee, T.G.; Park, J.W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Kim, T.H.; Go, G.-M.; Cho, H.-B.; Song, Y.; Lee, C.-G.; Choa, Y.-H. A Novel Synthetic Method for N Doped TiO2 Nanoparticles Through Plasma-Assisted Electrolysis and Photocatalytic Activity in the Visible Region. Front. Chem. 2018, 6, 458. [Google Scholar] [CrossRef]
- Kong, X.; Peng, Z.; Jiang, R.; Jia, P.; Feng, J.; Yang, P.; Chi, Q.; Ye, W.; Xu, F.; Gao, P. Nanolayered Heterostructures of N-Doped TiO2 and N-Doped Carbon for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 1373–1381. [Google Scholar] [CrossRef]
- He, Q.; Sun, Z.; Shi, X.; Wu, W.; Cheng, J.; Zhuo, R.; Zhang, Z.; Wang, J. Electrochemical Performance Enhancement of Nitrogen-Doped TiO2 for Lithium-Ion Batteries Investigated by a Film Electrode Model. Energy Fuels 2021, 35, 2717–2726. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Yu, H.; Wang, H.; Yang, J. Synthesis and characterization of substitutional and interstitial nitrogen-doped titanium dioxides with visible light photocatalytic activity. J. Solid State Chem. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- Ji, T.; Liu, Q.; Zou, R.; Zhang, Y.; Wang, L.; Sang, L.; Liao, M.; Hu, J. Enhanced UV-visible light photodetectors with a TiO2/Si heterojunction using band engineering. J. Mater. Chem. C 2017, 5, 12848–12856. [Google Scholar] [CrossRef]
- Batalović, K.; Bundaleski, N.; Radaković, J.; Abazović, N.; Mitrić, M.; Silva, R.A.; Savić, M.; Belošević-Čavor, J.; Rakočević, Z.; Rangel, C.M. Modification of N-doped TiO2 photocatalysts using noble metals (Pt, Pd)—A combined XPS and DFT study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Li, J.-F.; Wang, X.-L.; Qin, J.-Q.; Shi, W.-J.; Liu, Y.-F.; Gao, H.-P.; Mao, Y.-L. Enhancement of Perovskite Solar Cells Efficiency using N-Doped TiO2 Nanorod Arrays as Electron Transfer Layer. Nanoscale Res. Lett. 2017, 12, 43. [Google Scholar] [CrossRef]
- Le, P.H.; Hieu, L.T.; Lam, T.-N.; Hang, N.T.N.; Truong, N.V.; Tuyen, L.T.C.; Phong, P.T.; Leu, J. Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process. Micromachines 2018, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E.N. Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Alexiou, V.F.; Mathioudakis, G.N.; Andrikopoulos, K.S.; Soto Beobide, A.; Voyiatzis, G.A. Poly(ethylene Terephthalate) Carbon-Based Nanocomposites: A Crystallization and Molecular Orientation Study. Polymers 2020, 12, 2626. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, N.; Zeng, Q.; Liu, J.; Zhang, X.; Ge, M.; Zhang, W.; Li, S.; Fu, Y.; Zhang, Y. Design of Polypropylene Electret Melt Blown Nonwovens with Superior Filtration Efficiency Stability through Thermally Stimulated Charging. Polymers 2020, 12, 2341. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, L.; Hwang, Y.; Yu, J.; Lee, J.; Liu, Y.; Wang, H.; Kim, J.; Song, H.Y.; Lee, H. Binder-free TiO2 hydrophilic film covalently coated by microwave treatment. Mater. Chem. Phys. 2021, 258, 123884. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, T.; Cui, J.; Keshari Samal, S.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 119623. [Google Scholar] [CrossRef]
- Magnone, E.; Kim, M.-K.; Lee, H.J.; Park, J.H. Testing and substantial improvement of TiO2/UV photocatalysts in the degradation of Methylene Blue. Ceram. Int. 2019, 45, 3359–3367. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Zheng, X.; Bondarenko, V.; Fava, G.; Ruello, M.L. Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite. Environments 2019, 6, 87. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Yang, S.; Lee, G.W. Electrostatic enhancement of collection efficiency of the fibrous filter pretreated with ionic surfactants. J. Air Waste Manag. Assoc. 2005, 55, 594–603. [Google Scholar] [CrossRef]
- Yang, S.; Lee, G. Filtration characteristics of a fibrous filter pretreated with anionic surfactants for monodisperse solid aerosols. J. Aerosol Sci. 2005, 36, 419–437. [Google Scholar] [CrossRef]
- Chuaybamroong, P.; Chotigawin, R.; Supothina, S.; Sribenjalux, P.; Larpkiattaworn, S.; Wu, C.Y. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air 2010, 20, 246–254. [Google Scholar] [CrossRef]
- Limmongkon, Y.; Johns, J.; Charerntanyarak, L. Preparation of a TiO2 -coated photocatalytic air filter for use with an electrostatic air filter pack for xylene removal. Scienceasia 2013, 39, 284. [Google Scholar] [CrossRef][Green Version]
- Mukhopadhyay, A. Composite nonwovens in filters: Applications. In Composite Non-Woven Materials; Woodhead Publishing: Sawston, UK, 2014; pp. 164–210. [Google Scholar] [CrossRef]
- Han, Z.; Chang, V.; Wang, X.; Lim, T.; Hildemann, L. Experimental study on visible-light induced photocatalytic oxidation of gaseous formaldehyde by polyester fiber supported photocatalysts. Chem. Eng. J. 2013, 218, 9–18. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, B.; Wang, F.; Zhang, X.; Zhao, Z.; Si, M.; Guo, S. Enhanced photocatalytic degradation of xylene by blackening TiO2 nanoparticles with high dispersion of CuO. J. Hazard. Mater. 2020, 391, 121642. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Yu, Y.-H.; Nguyen, V.-H.; Lu, K.-T.; Wu, J.C.-S.; Chang, L.-M.; Kuo, C.-W. Enhanced xylene removal by photocatalytic oxidation using fiber-illuminated honeycomb reactor at ppb level. J. Hazard. Mater. 2013, 262, 717–725. [Google Scholar] [CrossRef]
- Binas, V.; Stefanopoulos, V.; Kiriakidis, G.; Papagiannakopoulos, P. Photocatalytic oxidation of gaseous benzene, toluene and xylene under UV and visible irradiation over Mn-doped TiO2 nanoparticles. J. Mater. 2019, 5, 56–65. [Google Scholar] [CrossRef]
- Pichat, P. Some views about indoor air photocatalytic treatment using TiO2: Conceptualization of humidity effects, active oxygen species, problem of C1–C3 carbonyl pollutants. Appl. Catal. B Environ. 2010, 99, 428–434. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Zhong, L.; Brancho, J.J.; Batterman, S.; Bartlett, B.M.; Godwin, C. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation. Appl. Catal. B Environ. 2017, 216, 122–132. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-specific mineralization and deactivation behaviors of TiO2 as an air-cleaning photocatalyst. Appl. Catal. B Environ. 2020, 275, 119145. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-J.; Lin, H.-I.; Chou, C.-M.; Hsieh, P.-Y.; Hsiao, C.-H.; Shi, Z.-Y.; He, J.-L. Inactivation of Staphylococcus aureus and Escherichia coli under various light sources on photocatalytic titanium dioxide thin film. Surf. Coat. Technol. 2009, 203, 1081–1085. [Google Scholar] [CrossRef]
- Javid, A.; Kumar, M.; Ashraf, M.; Lee, J.H.; Han, J.G. Photocatalytic antibacterial study of N-doped TiO2 thin films synthesized by ICP assisted plasma sputtering method. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 106, 187–193. [Google Scholar] [CrossRef]
- Li, Q.; Page, M.A.; Mariñas, B.J.; Shang, J.K. Treatment of Coliphage MS2 with Palladium-Modified Nitrogen-Doped Titanium Oxide Photocatalyst Illuminated by Visible Light. Environ. Sci. Technol. 2008, 42, 6148–6153. [Google Scholar] [CrossRef] [PubMed]
- Thunyasirinon, C. Enhancement of Air Filter with TiO2 Photocatalysis for Mycobacterium Tuberculosis Removal. Aerosol Air Qual. Res. 2015, 15, 600–610. [Google Scholar] [CrossRef]


| Sample | Total Filter Weight after Coating Visible Active Photocatalyst | Weight Loss (%) | ||
|---|---|---|---|---|
| Area Density before Shaking Test (g/m2) | Area Density after Shaking Test (g/m2) | Difference in Weight Loss (g/m2) | ||
| Sample-1 | 45.8 | 45.6 | 0.2 | 0.43 |
| Sample-2 | 64 | 63.58 | 0.42 | 0.65 |
| Sample-3 | 90.1 | 89.1 | 1 | 1.1 |
| Photocatalyst | Area Density (g/m2) | Filtration Efficiency (%) | Pressure Drop (Pa−1) | Quality factor (Pa−1) |
|---|---|---|---|---|
| 1% PNT/NWPF | 45.8 | 95.4 | 78.36 | 0.0393 |
| 2% PNT/NWPF | 64 | 97.6 | 96.62 | 0.0403 |
| 5% PNT/NWPF | 90.1 | 99.3 | 128.12 | 0.0387 |
| Photocatalyst | Pollutant | Light Source | Removal Efficiency (%) | Reference |
|---|---|---|---|---|
| 5% (wt) Degussa-TiO2 coated on high efficiency particulate air filter (HEPA) | xylene | 36 W UV lamp | 49% | [51] |
| Blackened TiO2 | xylene | UV+ Visible light (365–375 nm + 470–618 nm) | 35.64% | [54] |
| TiO2, Mn-doped TiO2 on Ceramic Honeycomb substrate | xylene | UV light UV254+185 nm | 33.5% 94% | [55] |
| Degussa-TiO20.1% Mn-TiO2 | xylene | 300 W UV light 300 W UV, 500 W Halogen light | 58% 58% 22% | [56] |
| 1% (wt) PNT/NWPF 2% (wt) PNT/NWPF 5% (wt) PNT/NWPF | xylene | 24 W Fluorescent lamp | 62.1% 86.5% 94.2% | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasuraman, V.; Sekar, P.P.; Lee, H.; Sheraz, M.; Lee, W.R.; Park, T.Y.; Kim, S. A Remarkable Photocatalyst Filter for Indoor Air Treatment. Catalysts 2022, 12, 1433. https://doi.org/10.3390/catal12111433
Parasuraman V, Sekar PP, Lee H, Sheraz M, Lee WR, Park TY, Kim S. A Remarkable Photocatalyst Filter for Indoor Air Treatment. Catalysts. 2022; 12(11):1433. https://doi.org/10.3390/catal12111433
Chicago/Turabian StyleParasuraman, Vijayarohini, Parasuraman Perumalswamy Sekar, Hojae Lee, Mahshab Sheraz, Woo Ram Lee, Tae Young Park, and Seungdo Kim. 2022. "A Remarkable Photocatalyst Filter for Indoor Air Treatment" Catalysts 12, no. 11: 1433. https://doi.org/10.3390/catal12111433
APA StyleParasuraman, V., Sekar, P. P., Lee, H., Sheraz, M., Lee, W. R., Park, T. Y., & Kim, S. (2022). A Remarkable Photocatalyst Filter for Indoor Air Treatment. Catalysts, 12(11), 1433. https://doi.org/10.3390/catal12111433






