Preparation of C3N4 Thin Films for Photo-/Electrocatalytic CO2 Reduction to Produce Liquid Hydrocarbons
Abstract
1. Introduction
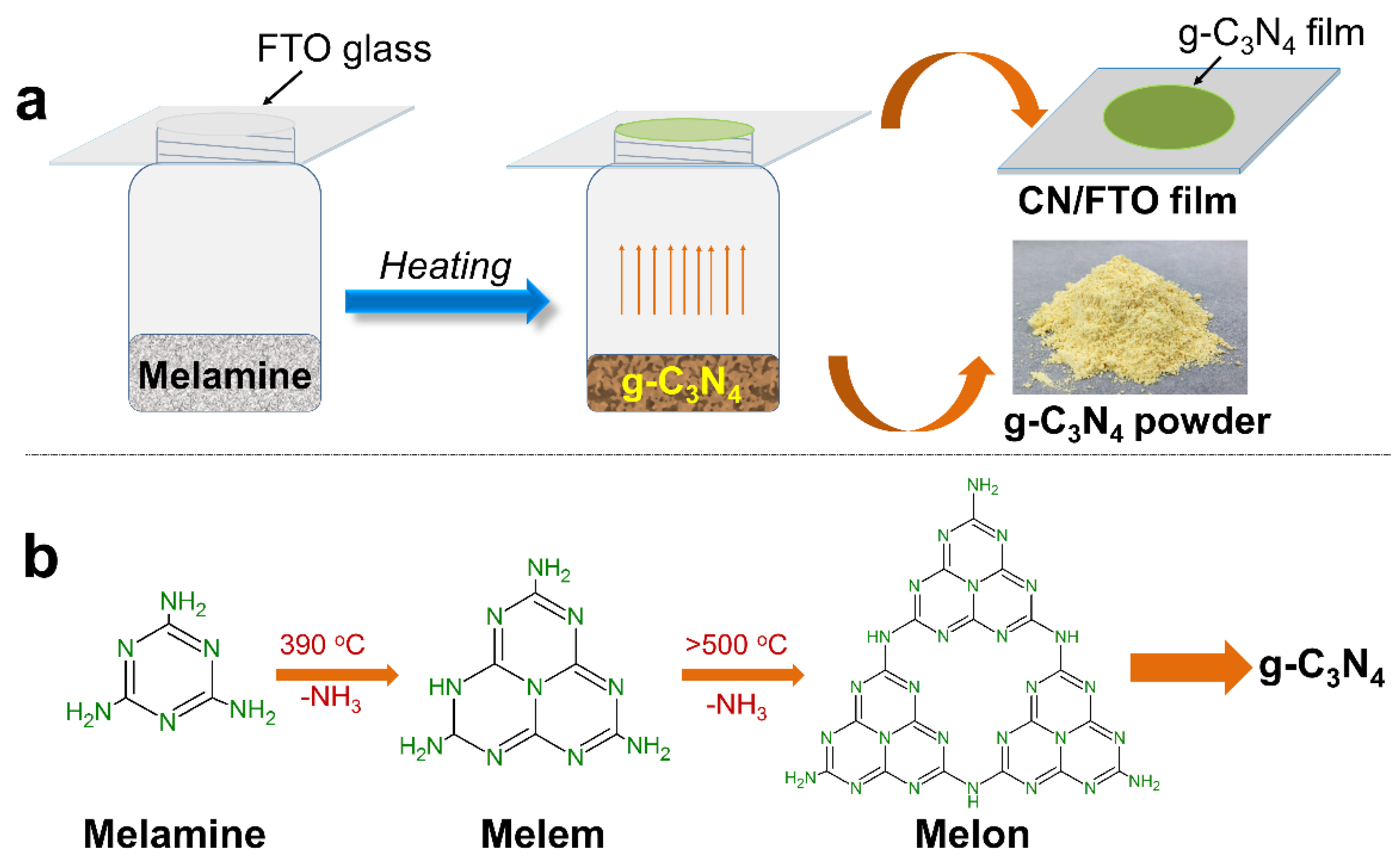
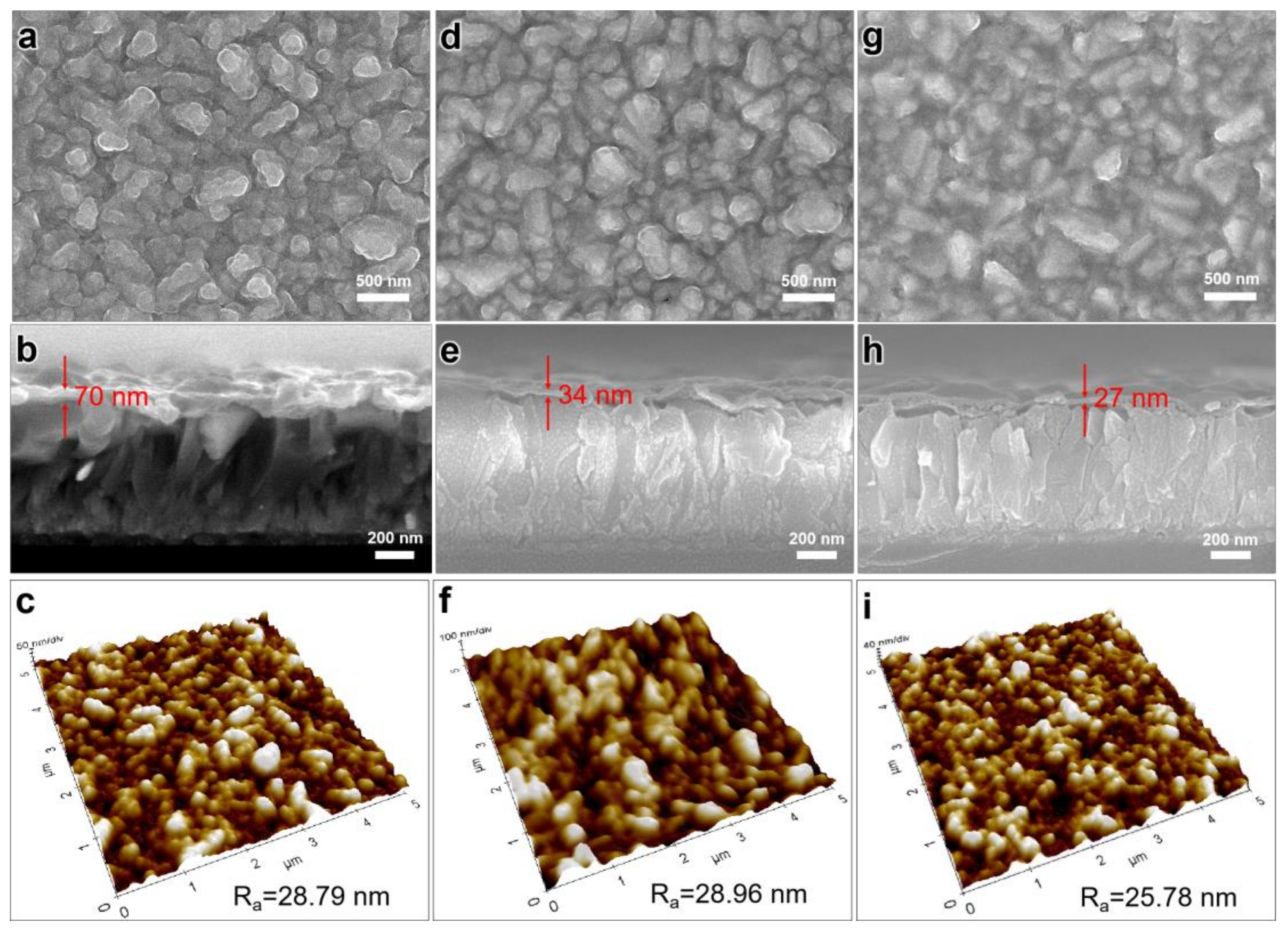
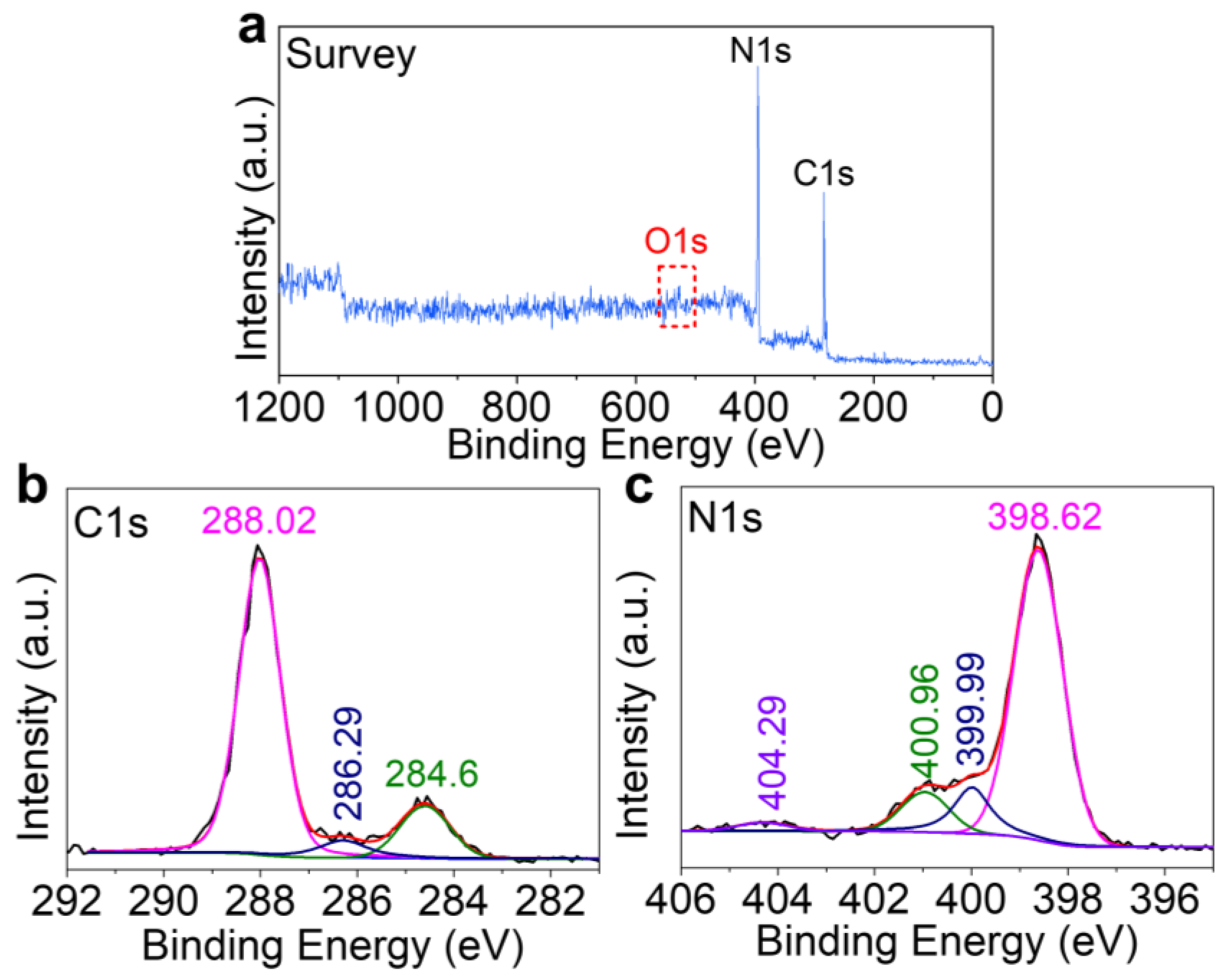
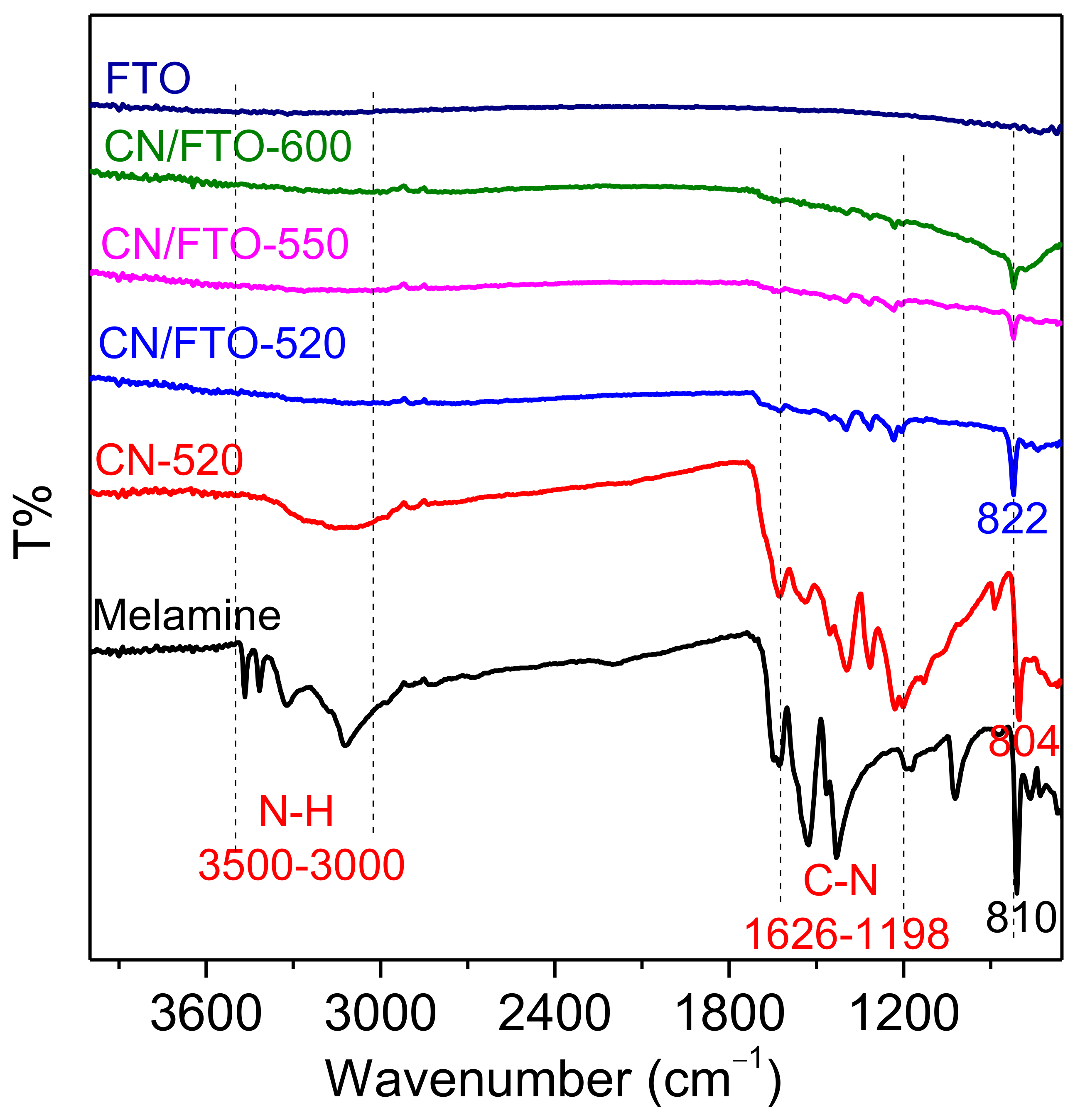
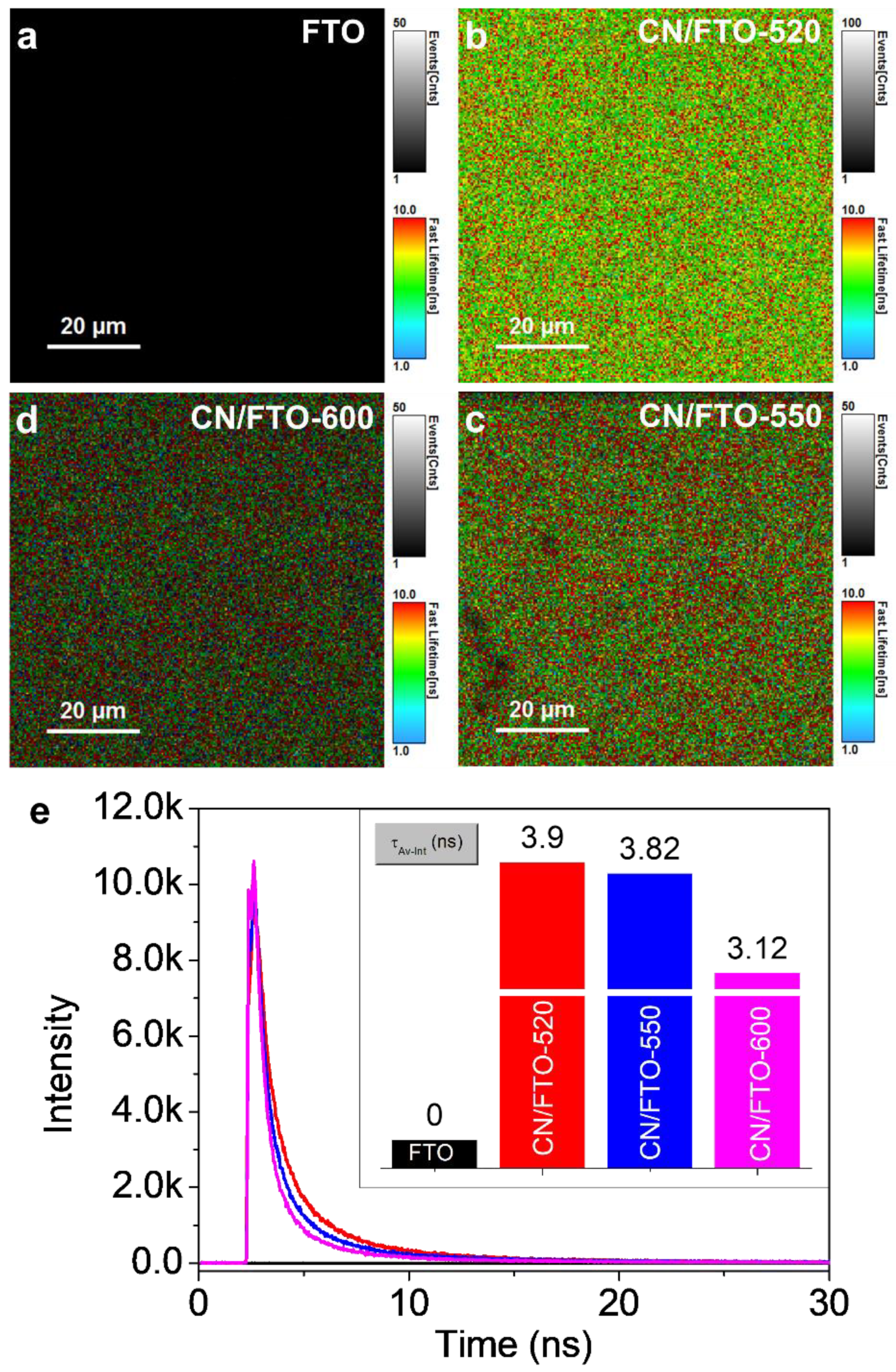
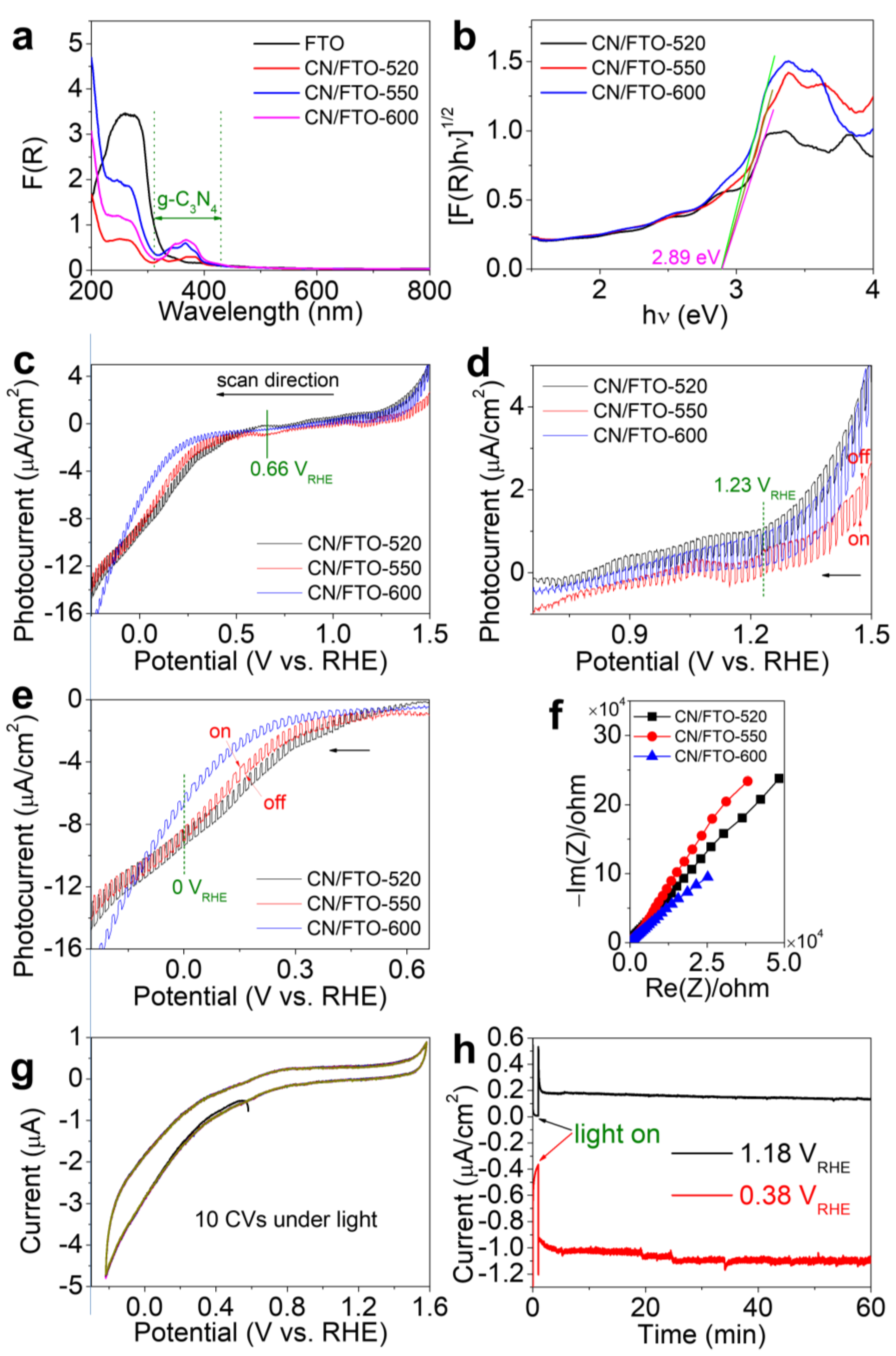
2. Results and Discussion
3. Materials and Experiments
3.1. Materials and Chemicals
3.2. Preparation of g-C3N4 Film on FTO (CN/FTO)
3.3. Characterization
3.4. Photoelectrochemical Measurements
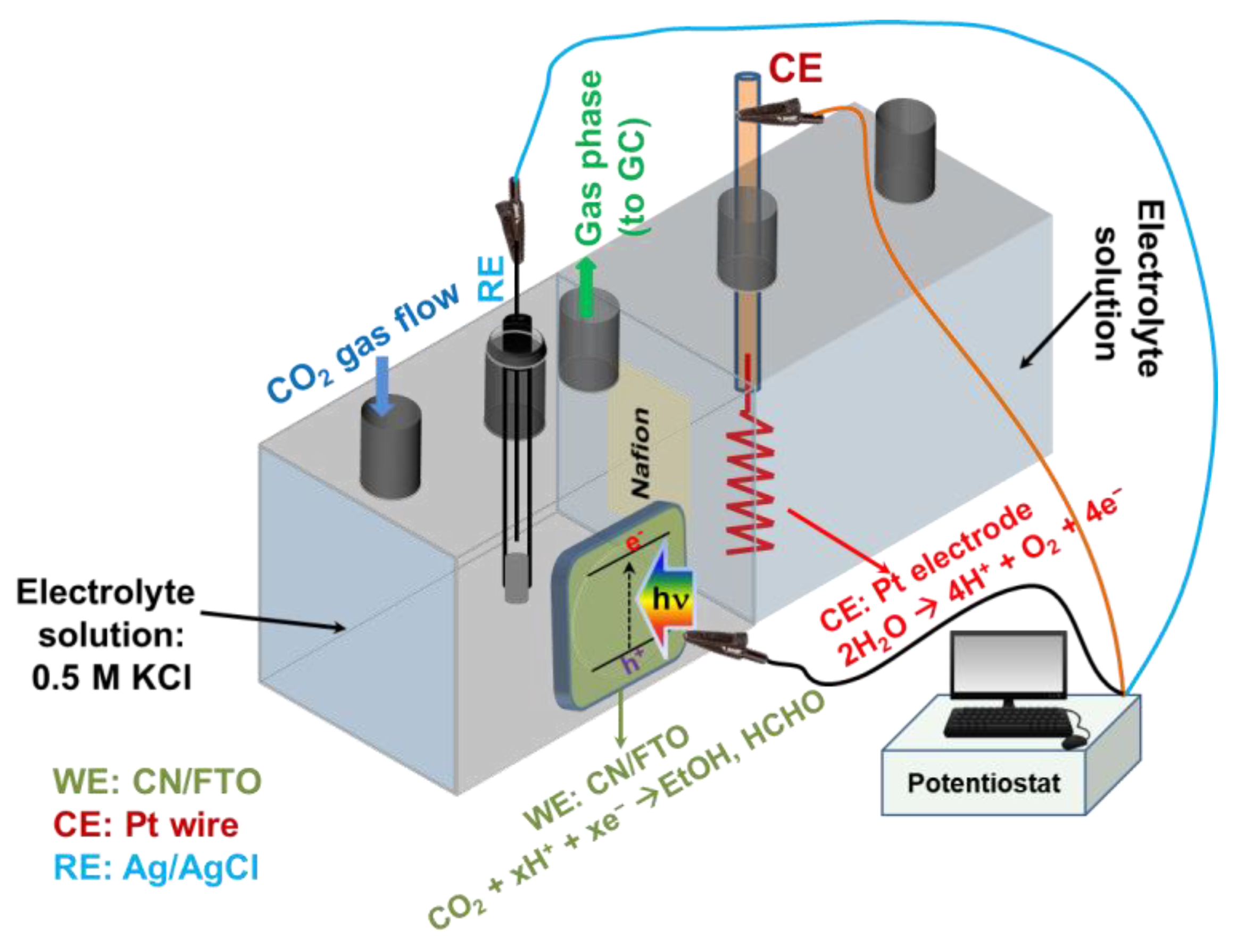
3.5. Electrochemical and Photoelectrochemical CO2 Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Duan, Y.H.; Zhang, J.F.; Tan, Y.S. Catalytic Conversion of CO2 into High Value-Added Hydrocarbons over Tandem Catalyst. J. Fuel Chem. Technol. 2022, 50, 538–563. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Tuci, G.; Sanson, A.; Peruzzini, M.; Giambastiani, G. Metal-Free Carbon-Based Materials for Electrocatalytic and Photo-Electrocatalytic CO2 Reduction. Rend. Lincei Sci. Fis. Nat. 2019, 30, 497–513. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Yang, L.; Zhang, Y.; Zhang, X.; Xiao, K.; Xu, J.; Liu, J. Graphitic Carbon Nitride Films: Emerging Paradigm for Versatile Applications. ACS Appl. Mater. Interfaces 2020, 12, 53571–53591. [Google Scholar] [CrossRef]
- Hasija, V.; Patial, S.; Singh, P.; Nguyen, V.-H.; Le, Q.V.; Thakur, V.K.; Hussain, C.M.; Selvasembian, R.; Huang, C.-W.; Thakur, S.; et al. Photocatalytic Inactivation of Viruses Using Graphitic Carbon Nitride-Based Photocatalysts: Virucidal Performance and Mechanism and Mechanism. Catalysts 2021, 11, 1448. [Google Scholar] [CrossRef]
- Ng, S.; Jie, F.; Ong, W. Solar-Powered Chemistry: Engineering Low-Dimensional Carbon Nitride-Based Nanostructures for Selective CO2 Conversion to C1-C2 Products. InfoMat 2022, 4, e12279. [Google Scholar] [CrossRef]
- Martin, D.J.; Reardon, P.J.T.; Moniz, S.J.A.; Tang, J. Visible Light-Driven Pure Water Splitting by a Nature-Inspired Organic Semiconductor-Based System. J. Am. Chem. Soc. 2014, 136, 12568–12571. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem.-Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. G-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-Free Catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A Fantastic Graphitic Carbon Nitride (g-C3N4) Material: Electronic Structure, Photocatalytic and Photoelectronic Properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Patnaik, S.; Martha, S.; Acharya, S.; Parida, K.M. An Overview of the Modification of G-C3N4 with High Carbon Containing Materials for Photocatalytic Applications. Inorg. Chem. Front. 2016, 3, 336–347. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- De Brito, J.F.; Corradini, P.G.; Silva, A.B.; Mascaro, L.H. Reduction of CO2 by Photoelectrochemical Process Using Non-Oxide Two-Dimensional Nanomaterials—A Review. ChemElectroChem 2021, 8, 4305–4320. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 Reduction by a P-Type Boron-Doped g-C3N4 Electrode under Visible Light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef]
- Pawar, A.U.; Pal, U.; Zheng, J.Y.; Kim, C.W.; Kang, Y.S. Thermodynamically Controlled Photo-Electrochemical CO2 Reduction at Cu/RGO/PVP/Nafion Multi-Layered Dark Cathode for Selective Production of Formaldehyde and Acetaldehyde. Appl. Catal. B Environ. 2022, 303, 120921. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Shen, Q.; Guo, C.; Zhao, G. A Biomimetic Photoelectrocatalyst of Co–Porphyrin Combined with a g-C3N4 Nanosheet Based on π–π Supramolecular Interaction for High-Efficiency CO2 Reduction in Water Medium. Green Chem. 2017, 19, 5900–5910. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Yang, J.; Han, B.; Nie, R.; Wang, J.; Dong, Y.; Yu, X.; Wang, J.; Jing, H. Highly Efficient Photoelectrocatalytic Reduction of CO2 on the Ti3C2/g-C3N4 Heterojunction with Rich Ti3+ and Pyri-N Species. J. Mater. Chem. A 2018, 6, 15213–15220. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yu, Z.; Jiang, X.; Chen, J.; Tao, L.; Wang, M.; Shen, Y. Artificial Photosynthesis of Ethanol Using Type-II g-C3N4/ZnTe Heterojunction in Photoelectrochemical CO2 Reduction System. Nano Energy 2019, 60, 827–835. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Jlassi, K.; Eldesoky, A.S.; Abdo, G.G. Precise Fabrication of Porous One-Dimensional GC3N4 Nanotubes Doped with Pd and Cu Atoms for Efficient CO Oxidation and CO2 Reduction. Inorg. Chem. Commun. 2019, 107, 107460. [Google Scholar] [CrossRef]
- Xia, X.; Hu, X.D.; Tarek, M.; Saravanan, P.; Alqadhi, R. Tailoring the Properties of G-C3N4 with CuO for Enhanced Photoelectrocatalytic CO2 Reduction to Methanol. J. CO2 Util. 2020, 40, 101222. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of Processing Temperature on Structure and Photocatalytic Properties of G-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Kim, S.; Bark, C.W. Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells. Energies 2020, 13, 585. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Kim, C.W.; Pawar, A.U.; Kang, Y.S. Fabrication of P-Cu2O/n-Bi-WO3 Heterojunction Thin Films: Optical and Photoelectrochemical Properties. New J. Chem. 2017, 41, 755–762. [Google Scholar] [CrossRef]
- Kumar, P.; Vahidzadeh, E.; Thakur, U.K.; Kar, P.; Alam, K.M.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.M.; Michaelis, V.K.; et al. C3N5: A Low Bandgap Semiconductor Containing an Azo-Linked Carbon Nitride Framework for Photocatalytic, Photovoltaic and Adsorbent Applications. J. Am. Chem. Soc. 2019, 141, 5415–5436. [Google Scholar] [CrossRef]
- Gashi, A.; Parmentier, J.; Fioux, P.; Marsalek, R. Tuning the C/N Ratio of C-Rich Graphitic Carbon Nitride (g-C3N4) Materials by the Melamine/Carboxylic Acid Adduct Route. Chem.-Eur. J. 2022, 28, e202103605. [Google Scholar] [CrossRef]
- Dong, G.; Zhao, K.; Zhang, L. Carbon Self-Doping Induced High Electronic Conductivity and Photoreactivity of g-C3N4. Chem. Commun. 2012, 48, 6178–6180. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, K.; Feng, Z.; Bao, Y.; Dong, B. Hierarchical Sheet-on-Sheet ZnIn2S4/g-C3N4 Heterostructure with Highly Efficient Photocatalytic H2 Production Based on Photoinduced Interfacial Charge Transfer. Sci. Rep. 2016, 6, 19221. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin Hexagonal SnS2 Nanosheets Coupled with G-C3N4 Nanosheets as 2D/2D Heterojunction Photocatalysts toward High Photocatalytic Activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Hong, J.; Van, T.K.; Pawar, A.U.; Kim, D.Y.; Kim, C.W.; Haider, Z.; Kang, Y.S. Facile Fabrication of WO3 Nanoplates Thin Films with Dominant Crystal Facet of (002) for Water Splitting. Cryst. Growth Des. 2014, 14, 6057–6066. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of Rhodamine B and Methyl Orange over Boron-Doped g-C3N4 under Visible Light Irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- Chen, L.; Yan, R.; Oschatz, M.; Jiang, L.; Antonietti, M.; Xiao, K. Ultrathin 2D Graphitic Carbon Nitride on Metal Films: Underpotential Sodium Deposition in Adlayers for Sodium-Ion Batteries. Angew. Chem.-Int. Ed. 2020, 59, 9067–9073. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient Synthesis of Polymeric G-C3N4 Layered Materials as Novel Efficient Visible Light Driven Photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S. Facile Fabrication of Highly Efficient G-C3N4/Ag2O Heterostructured Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2013, 5, 12533–12540. [Google Scholar] [CrossRef]
- Bian, J.; Li, Q.; Huang, C.; Li, J.; Guo, Y.; Zaw, M.; Zhang, R.Q. Thermal Vapor Condensation of Uniform Graphitic Carbon Nitride Films with Remarkable Photocurrent Density for Photoelectrochemical Applications. Nano Energy 2015, 15, 353–361. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, H.; Liu, Z. Liquid-Based Growth of Polymeric Carbon Nitride Films and Their Extraordinary Photoelectrocatalytic Activity. RSC Adv. 2016, 6, 81372–81377. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Z.P.; Moehwald, H.; Fiechter, S.; Van De Krol, R.; Wen, L.; Jiang, L.; Antonietti, M. Microcontact-Printing-Assisted Access of Graphitic Carbon Nitride Films with Favorable Textures toward Photoelectrochemical Application. Adv. Mater. 2015, 27, 712–718. [Google Scholar] [CrossRef]
- Lv, X.; Cao, M.; Shi, W.; Wang, M.; Shen, Y. A New Strategy of Preparing Uniform Graphitic Carbon Nitride Films for Photoelectrochemical Application. Carbon N. Y. 2017, 117, 343–350. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Shen, Y.; Zhou, Z.; Yu, J.; Li, Y.; Wei, W.; Liu, S.; Zhang, Y. Environment-Friendly Preparation of Porous Graphite-Phase Polymeric Carbon Nitride Using Calcium Carbonate as Templates, and Enhanced. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 5126–5131. [Google Scholar] [CrossRef]
- Zhang, Y.; Schnepp, Z.; Cao, J.; Ouyang, S.; Li, Y.; Ye, J.; Liu, S. Biopolymer-Activated Graphitic Carbon Nitride towards a Sustainable Photocathode Material. Sci. Rep. 2013, 3, 2163. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qiu, Y.; Duan, W.; Liu, Z. Cathodic and Anodic Photocurrents Generation from Melem and Its Derivatives. RSC Adv. 2015, 5, 26675–26679. [Google Scholar] [CrossRef]
- Ye, L.; Chen, S. Fabrication and High Visible-Light-Driven Photocurrent Response of G-C3N4 Film: The Role of Thiourea. Appl. Surf. Sci. 2016, 389, 1076–1083. [Google Scholar] [CrossRef]
- Jeong, H.; Kang, M.J.; Jung, H.; Kang, Y.S. Electrochemical CO2 Reduction with Low Overpotential by a Poly(4-Vinylpyridine) Electrode for Application to Artificial Photosynthesis. Faraday Discuss. 2017, 198, 409–418. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Li, F.; Easton, C.D.; Li, J.; Bond, A.M.; Zhang, J. Direct Detection of Electron Transfer Reactions Underpinning the Tin-Catalyzed Electrochemical Reduction of CO2 Using Fourier-Transformed Ac Voltammetry. ACS Catal. 2017, 7, 4846–4853. [Google Scholar] [CrossRef]
- Chen, Y.; Kanan, M.W. Tin Oxide Dependence of the CO2 Reduction Efficiency on Tin Electrodes and Enhanced Activity for Tin/Tin Oxide Thin-Film Catalysts. J. Am. Chem. Soc. 2012, 134, 1986–1989. [Google Scholar] [CrossRef]
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.Y.; Pawar, A.U.; Kang, Y.S. Preparation of C3N4 Thin Films for Photo-/Electrocatalytic CO2 Reduction to Produce Liquid Hydrocarbons. Catalysts 2022, 12, 1399. https://doi.org/10.3390/catal12111399
Zheng JY, Pawar AU, Kang YS. Preparation of C3N4 Thin Films for Photo-/Electrocatalytic CO2 Reduction to Produce Liquid Hydrocarbons. Catalysts. 2022; 12(11):1399. https://doi.org/10.3390/catal12111399
Chicago/Turabian StyleZheng, Jin You, Amol Uttam Pawar, and Young Soo Kang. 2022. "Preparation of C3N4 Thin Films for Photo-/Electrocatalytic CO2 Reduction to Produce Liquid Hydrocarbons" Catalysts 12, no. 11: 1399. https://doi.org/10.3390/catal12111399
APA StyleZheng, J. Y., Pawar, A. U., & Kang, Y. S. (2022). Preparation of C3N4 Thin Films for Photo-/Electrocatalytic CO2 Reduction to Produce Liquid Hydrocarbons. Catalysts, 12(11), 1399. https://doi.org/10.3390/catal12111399







