Site-Specific Covalent Immobilization of Methylobacterium extorquens Non-Blue Laccse Melac13220 on Fe3O4 Nanoparticles by Aldehyde Tag
Abstract
1. Introduction
2. Results and Discussion
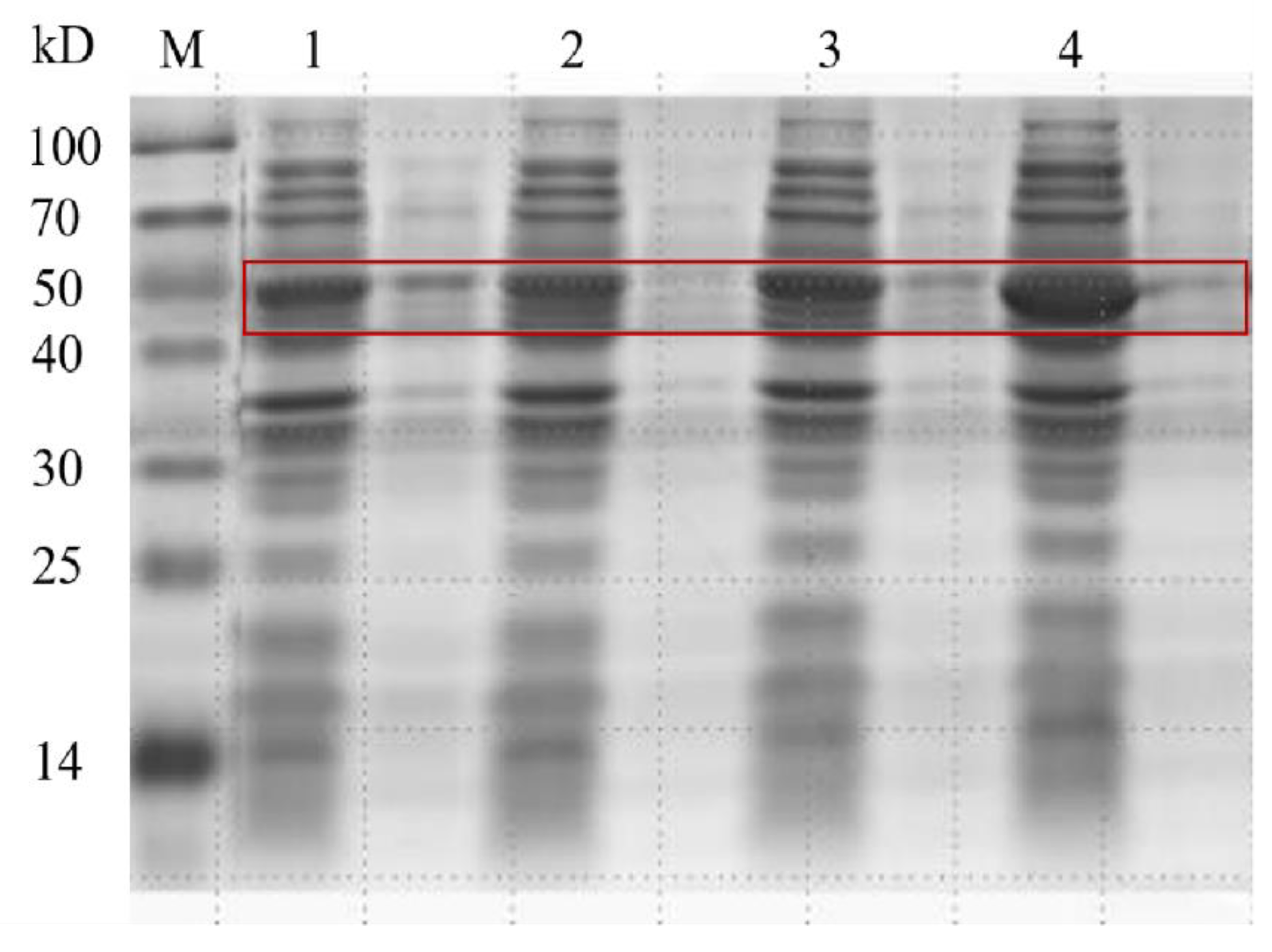
2.1. Expression of the Aldehyde Tag Protein
2.2. Optimization of Conditions for Immobilization
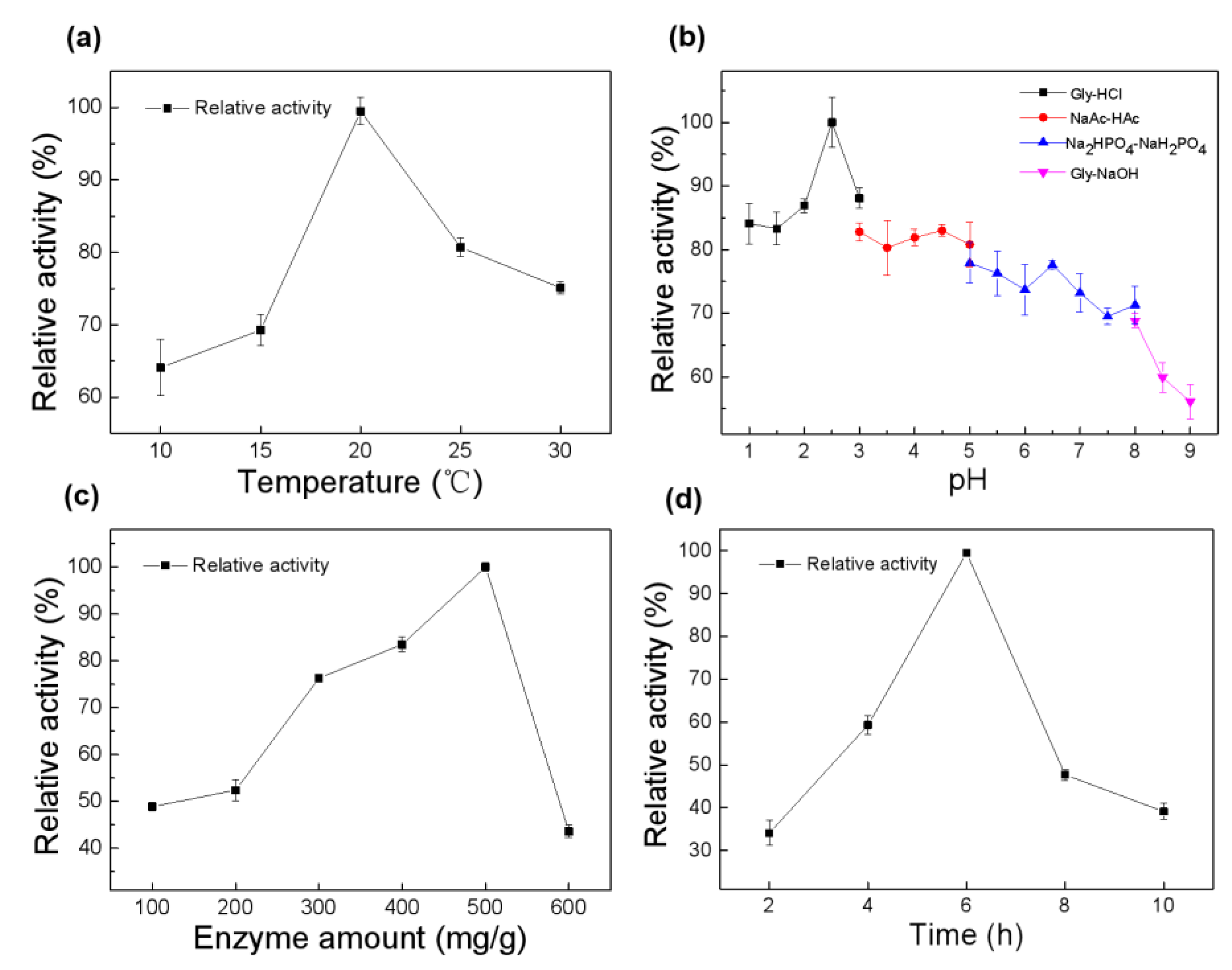
2.2.1. Optimization of Conditions for Site-Specific Covalent Immobilization
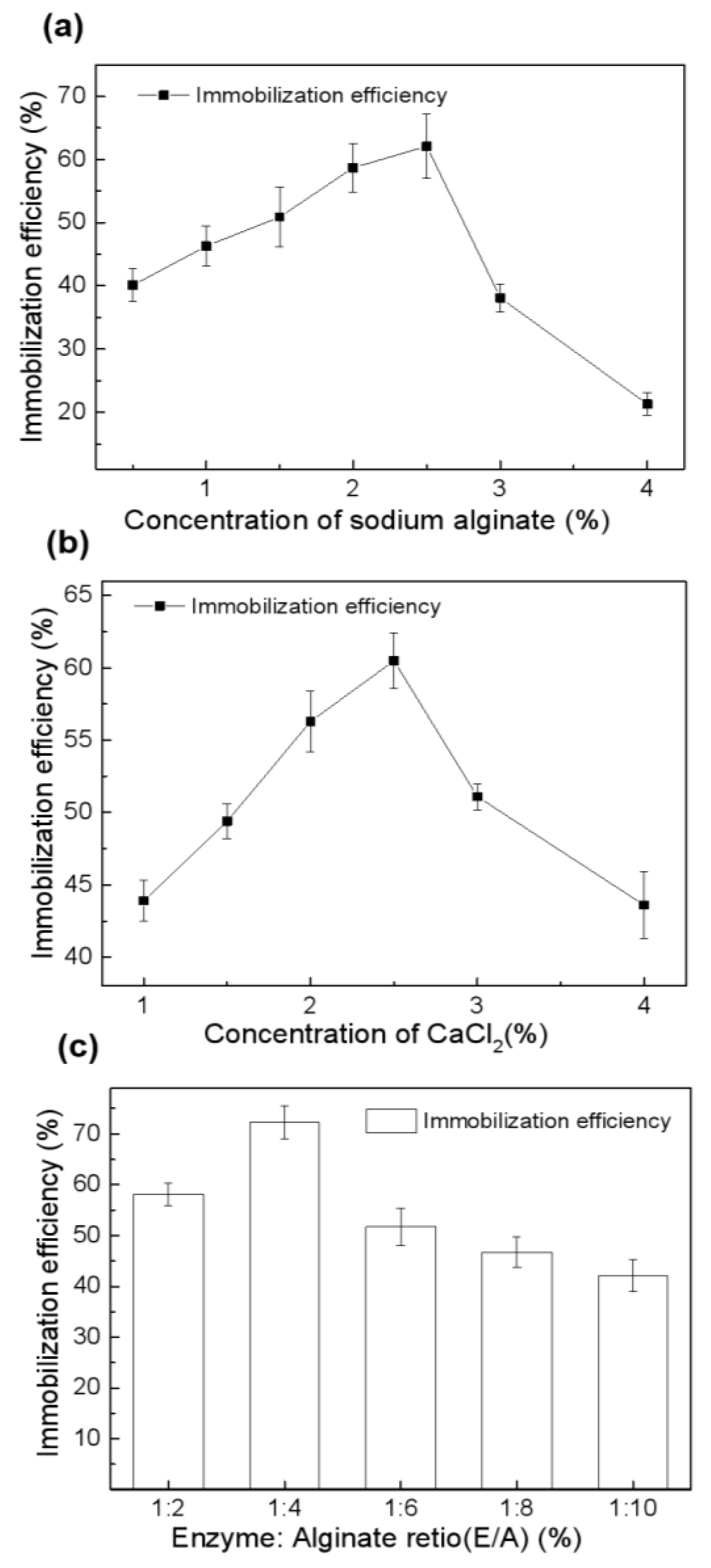
2.2.2. Optimization of Entrapment Conditions for Melac13220
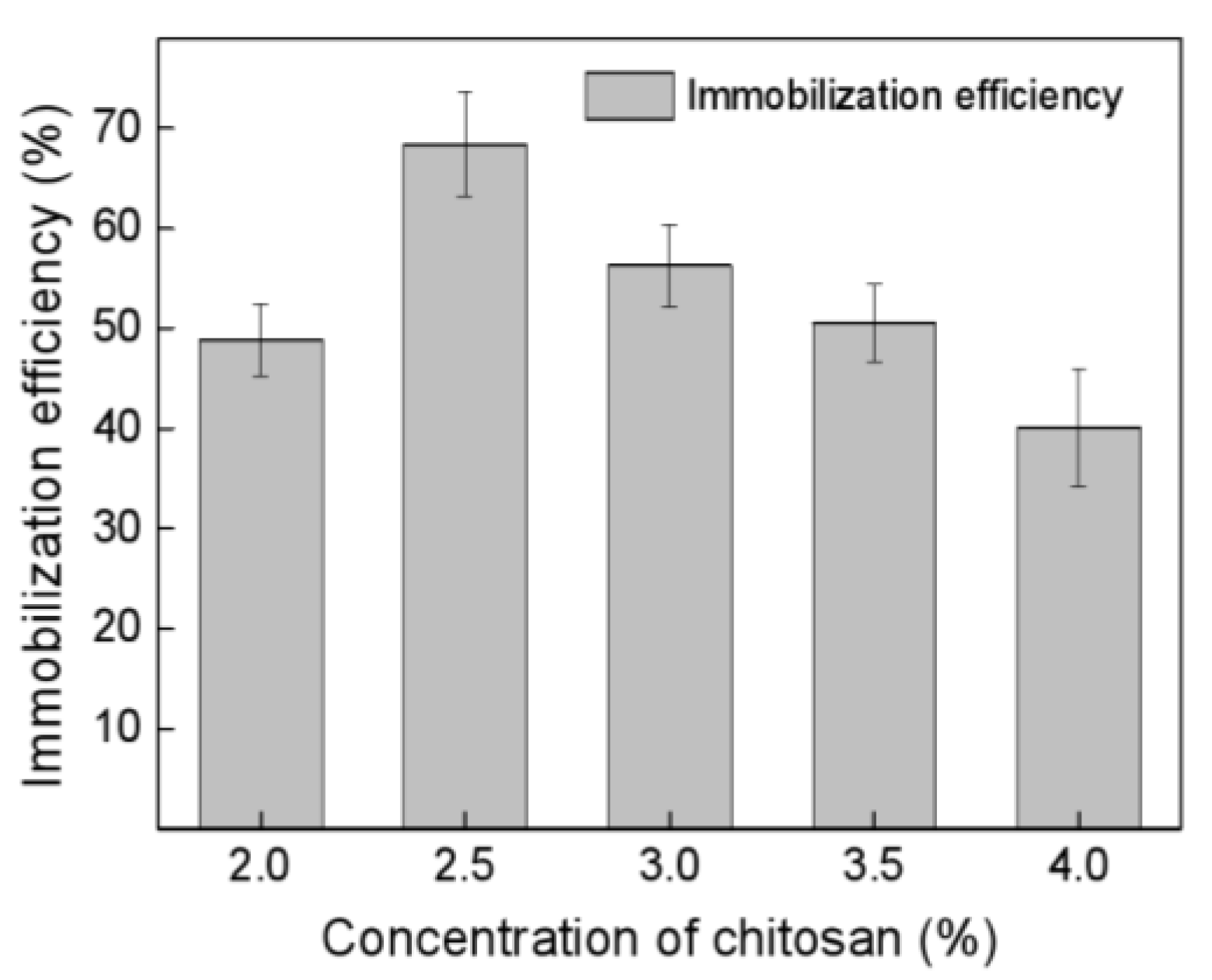
2.2.3. Optimization of Crosslinking Conditions for Melac13220
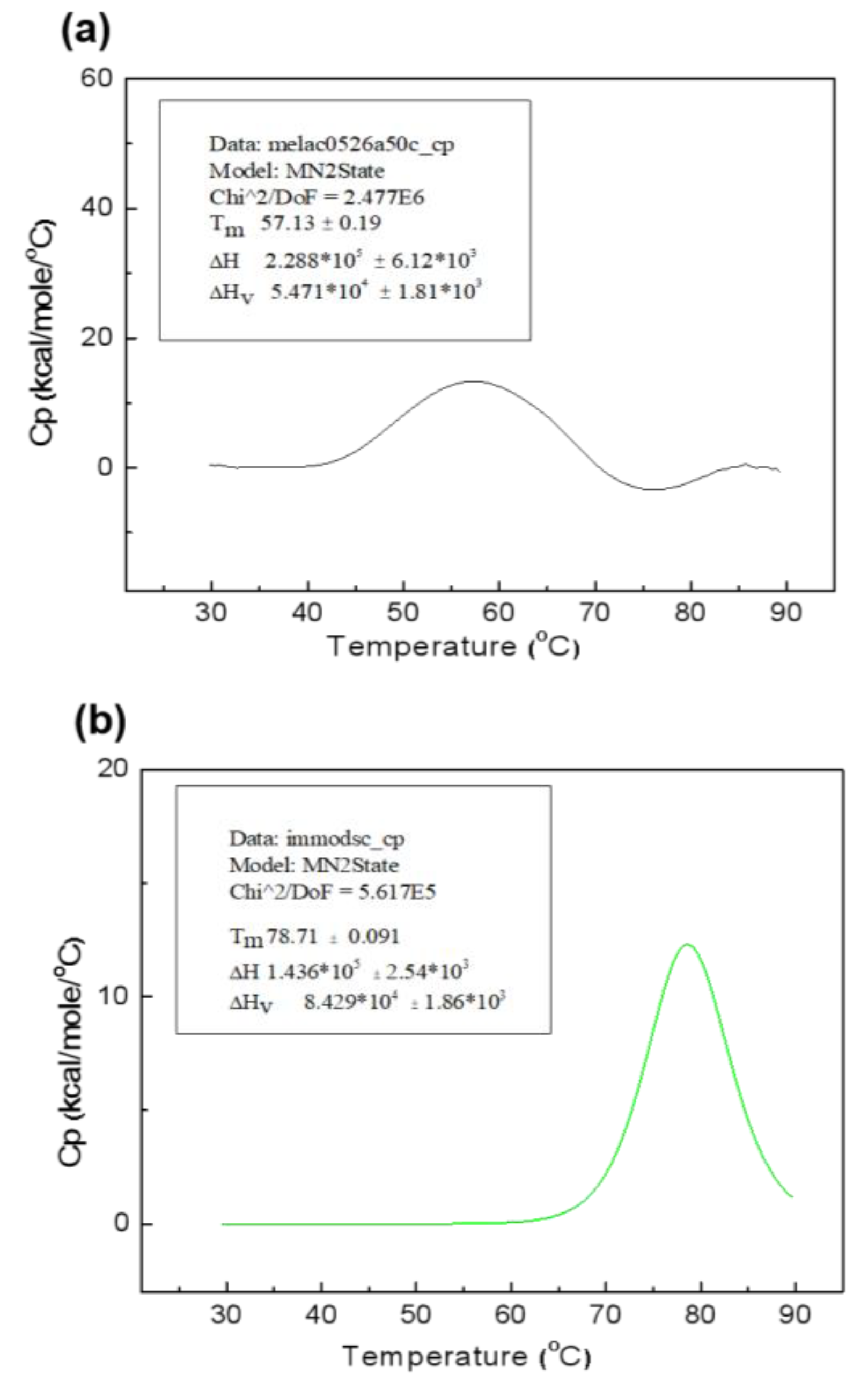
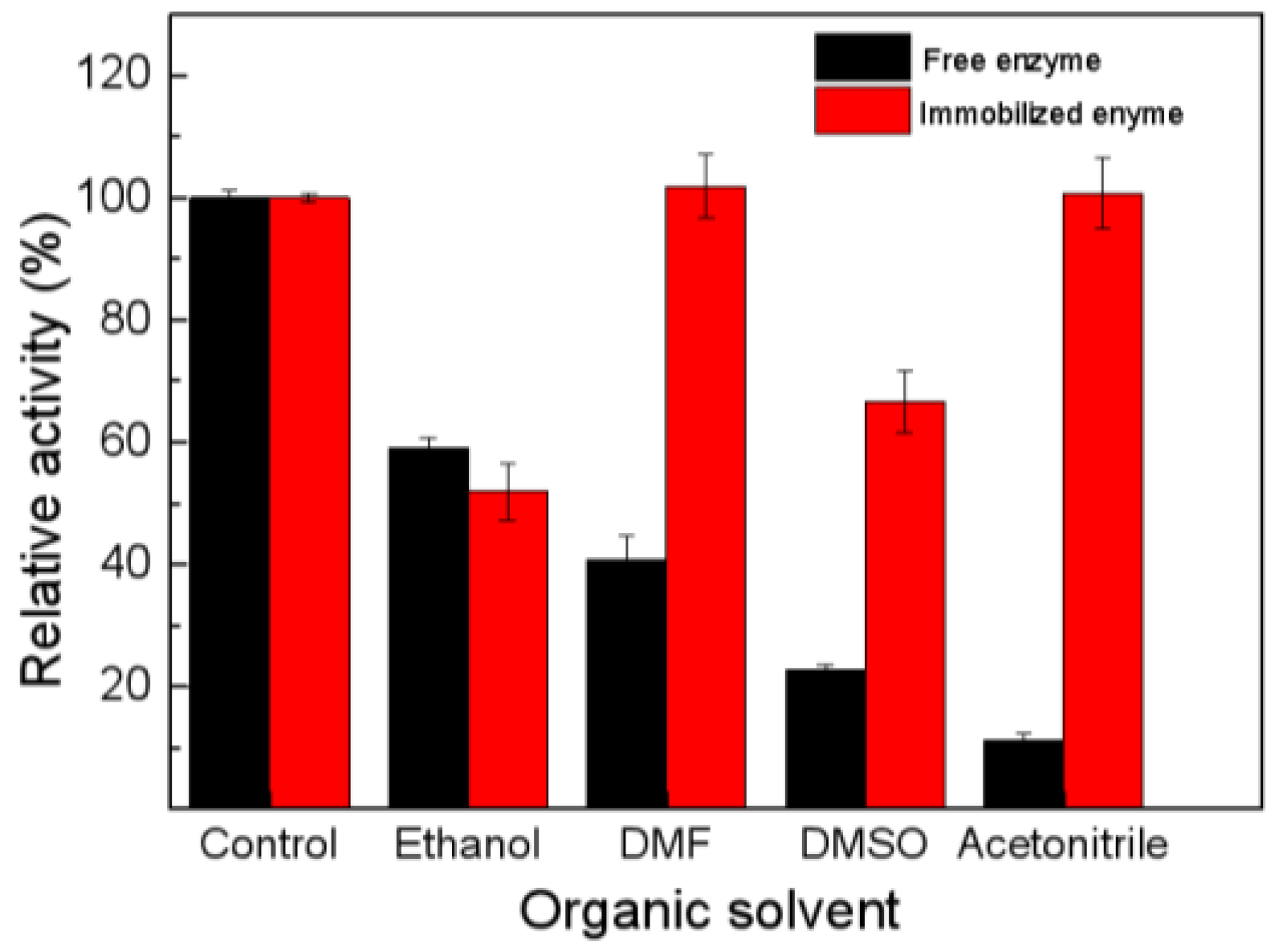
2.3. Characterization of Three Immobilized Enzymes
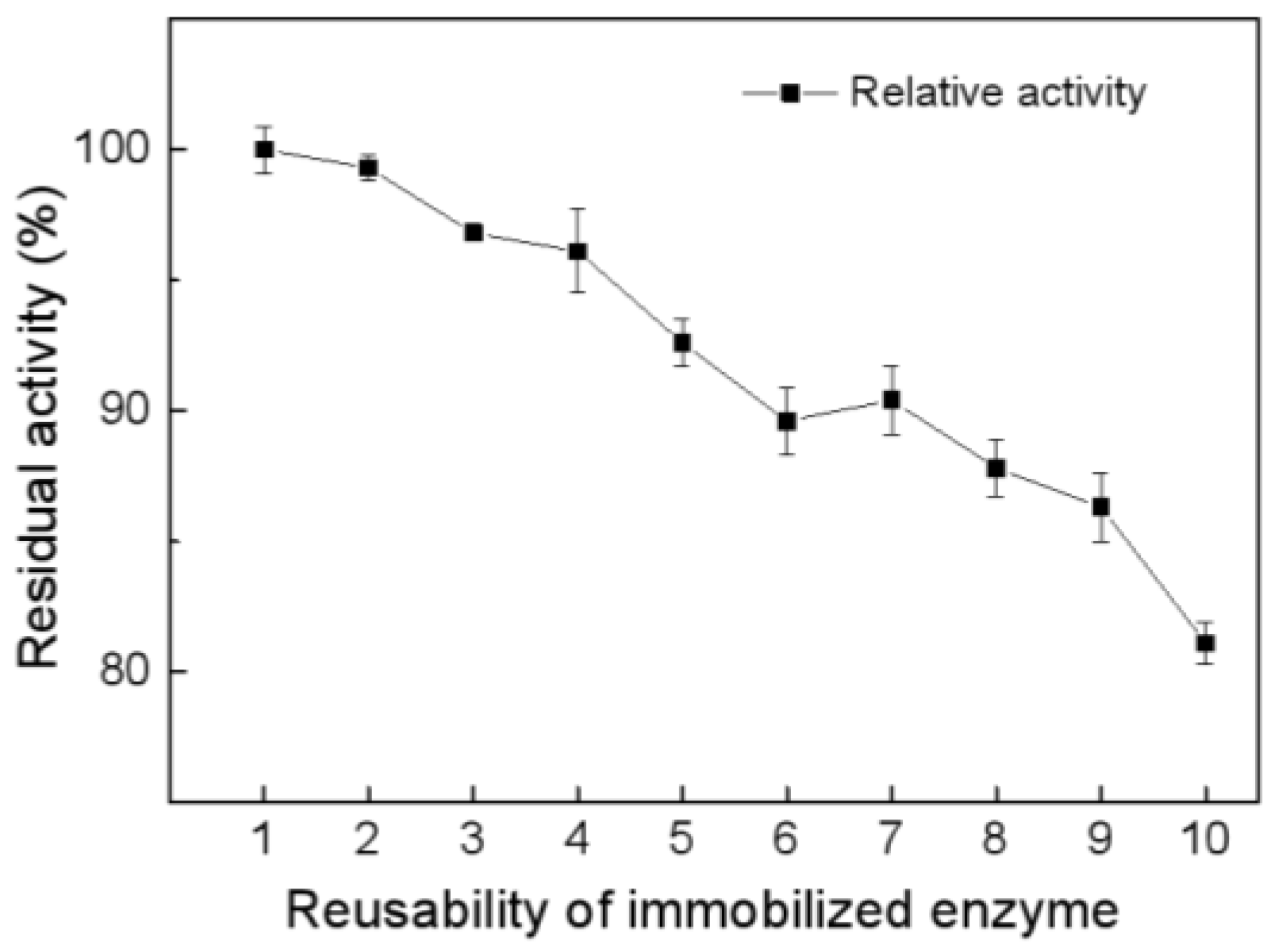
2.4. Recycling of Immobilized Melac13220
2.5. Application of Immobilized Melac13220 in Dye Decolorization
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup
3.2.1. Acquisition of Melac13220 with Aldehyde Tags
3.2.2. Enzyme Assay
3.2.3. Immobilization of Melac13220
3.2.4. Characterizations of the Immobilized Melac13220
3.2.5. Recycling of Immobilized Melac13220
3.2.6. Application of Immobilized Melac13220 in Dyes Decolorization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572–136592. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. Exploring current tendencies in techniques and materials for immobilization of laccases—A review. Int. J. Biol. Macromol. 2021, 181, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Iark, D.; Buzzo, A.J.D.R.; Garcia, J.A.A.; Correa, V.G.; Helm, C.V.; Correa, R.C.G.; Peralta, R.A.; Moreira, R.D.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655–121661. [Google Scholar] [CrossRef] [PubMed]
- Osma, J.F.; Toca-herrera, J.L.; Rodriguez-Couto, S. Uses of Laccases in the Food Industry. Enzyme Res. 2010, 2010, 918761–918769. [Google Scholar] [CrossRef]
- Kudanga, T.; Nemadziva, B.; Roes-Hill, M.L. Laccase catalysis for the synthesis of bioactive compounds. Appl. Microbiol. Biotechnol. 2017, 101, 13–33. [Google Scholar] [CrossRef]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal Laccases and Their Applications in Bioremediation. Enzyme Res. 2014, 2014, 163242–163263. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.C.; Sung, J.S.; Kadam, A.A. Anti-proliferative applications of laccase immobilized on super-magnetic chitosan-functionalized halloysite nanotube. Int. J. Biol. Macromol. 2018, 118, 228–237. [Google Scholar] [CrossRef]
- Jørgensen, H.; Pinelo, M. Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2017, 11, 150–167. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Lin, T.; Feng, Y.; Jia, S. Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chem. Eng. J. 2018, 343, 629–637. [Google Scholar] [CrossRef]
- Olajuyigbekki, F.M.; Adetuyi, O.Y.; Fatokun, C.O. Characterization of free and immobilized laccase from Cyberlindnera fabianii and application in degradation of bisphenol A. Int. J. Biol. Macromol. 2019, 125, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K. A breakthrough in enzyme technology to fight penicillin resistance—Industrial application of penicillin amidase. Appl. Microbiol. Biotechnol. 2016, 100, 3825–3839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Ren, D.J.; Yu, H.Y.; Zhang, S.Q.; Zhang, X.Q.; Chen, W.S. Preparation optimization and stability comparison study of alkali-modified biochar immobilized laccase under multi-immobilization methods. Biochem. Eng. J. 2022, 181, 108401–108411. [Google Scholar] [CrossRef]
- Yu, C.C.; Kuo, Y.Y.; Liang, C.F.; Chien, W.T.; Wu, H.T.; Chang, T.C.; Jan, F.D.; Lin, C.C. Site-specific Immobilization of Enzymes on Magnetic Nanoparticles and Their Use in Organic Synthesis. Bioconjug. Chem. 2012, 23, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Wang, Y.; Bai, Y.; Li, R.; Gao, R. Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Fow Reactors. Molecules 2016, 21, 895. [Google Scholar] [CrossRef]
- Lyu, J.; Li, Z.; Men, J.; Jiang, R.; Tang, G.; Zhou, Y.; Gao, R. Covalent immobilization of Bacillus subtilis lipase A on Fe3O4 nanoparticles by aldehyde tag: An ideal immobilization with minimal chemical modification. Process Biochem. 2019, 81, 63–69. [Google Scholar] [CrossRef]
- Huang, B.C.B.; Kim, Y.C.; Bañas, S.; Barfield, R.M.; Drake, P.M.; Rupniewski, I.; Haskins, W.E.; Rabuka, D. Antibody-drug conjugate library prepared by scanning insertion of the aldehyde tag into IgG1 constant regions. MAbs 2018, 10, 1182–1189. [Google Scholar] [CrossRef]
- Daronch, N.A.; Kelbert, M.; Pereira, C.S.; Hermes de Araújo, P.H.; Oliveira, D. Elucidating the choice for a precise matrix for laccase immobilization: A review. Chem. Eng. J. 2020, 397, 125506–125521. [Google Scholar] [CrossRef]
- Robinson, P.J.; Dunnill, P.; Lilly, M.D. The properties of magnetic supports in relation to immobilized enzyme reactors. Biotechnol. Bioeng. 1973, 15, 603–606. [Google Scholar] [CrossRef]
- Kharazmi, S.; Kafrani, A.T.; Soozanipour, A. Efficient immobilization of pectinase on trichlorotriazine-functionalized polyethylene glycol-grafted magnetic nanoparticles: A stable and robust nanobiocatalyst for fruit juice clarification. Food Chem. 2020, 32, 126890–126896. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501–023545. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.P.; Zou, Y.; Xu, Y.; Liu, X.; Zhang, H. Fe3O4@MoS2@PEI-facilitated enzyme tethering for efficient removal of persistent organic pollutants in water. Chem. Eng. J. 2019, 375, 121947–121954. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Ainiwaer, A.; Liang, Y.; Ye, X.; Gao, R. Characterization of a Novel Fe2+ Activated Non-Blue Laccase from Methylobacterium extorquens. Int. J. Mol. Sci. 2022, 23, 9804. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.P.; Witte, M.D.; Theile, C.S.; Bozkurt, G.; Kundrat, L.; Blom, A.E.M.; Ploegh, H.L. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc. 2013, 8, 1787–1799. [Google Scholar] [CrossRef]
- Brugnari, T.; Braga, D.M.; Santos, C.S.A.d.; Torres, B.H.C.; Modkovski, T.A.; Haminiuk, C.W.I.; Maciel, G.M. Laccases as green and versatile biocatalysts: From lab to enzyme market—An overview. Bioresour. Bioprocess. 2021, 8, 131–159. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Anwar, M.Z.; Kim, D.J.; Kumar, A.; Patel, S.K.S.; Otari, S.; Mardina, P.; Jeong, J.H.; Sohn, J.H.; Kim, J.H.; Park, J.T.; et al. SnO2 hollow nanotubes: A novel and efficient support matrix for enzyme immobilization. Sci. Rep. 2017, 7, 15333–15344. [Google Scholar] [CrossRef]
- Cao, S.L.; Huang, Y.M.; Li, X.H.; Xu, P.; Wu, H.; Li, N.; Lou, W.Y.; Zong, M.H. Preparation and Characterization of Immobilized lipase from Pseudomonas Cepacia onto Magnetic Cellulose Nanocrystals. Sci. Rep. 2016, 6, 20420–20432. [Google Scholar] [CrossRef]
- Li, K.; Fan, Y.; He, Y.; Zeng, L.; Han, X.; Yan, Y. Burkholderia cepacia lipase immobilized on heterofunctional magnetic nanoparticles and its application in biodiesel synthesis. Sci. Rep. 2017, 7, 16473–16490. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.S.; Al-Salamah, A.A.; El-Toni, A.M.; Almaary, K.S.; El-Tayeb, M.A.; Elbadawi, Y.B.; Antranikian, G. Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres. Int. J. Mol. Sci. 2016, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Deska, M.A.; Kończak, B. Laccase Immobilization on Biopolymer Carriers—Preliminary Studies. J. Ecol. Eng. 2022, 23, 235–249. [Google Scholar] [CrossRef]
- Wang, A.; Du, F.; Wang, F.; Shen, Y.; Gao, W.; Zhang, P. Convenient one-step purification and immobilization of lipase using a genetically encoded aldehyde tag. Biochem. Eng. J. 2013, 73, 86–92. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme Stability and Activity in Non-Aqueous Reaction Systems: A Mini Review. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, H. Enhanced decolorization of malachite green by a magnetic graphene oxide-CotA laccase composite. Int. J. Biol. Macromol. 2019, 138, 1–12. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Han, J.; Wu, J.; Li, C.; Ni, L.; Wang, Y. Improving laccase activity and stability by HKUST-1 with cofactor via one-pot encapsulation and its application for degradation of bisphenol A. J. Hazard Mater. 2020, 383, 121130–121140. [Google Scholar] [CrossRef]
- Dai, J.; Wang, H.; Chi, H.; Wang, Y.; Zhao, J. Immobilization of laccase from Pleurotus ostreatus on magnetic separable SiO2 support and excellent activity towards azo dye decolorization. J. Environ. Chem. Eng. 2016, 4, 2585–2591. [Google Scholar] [CrossRef]
- Iriarte-Mesa, C.; Díaz-Castañón, S.; Abradelo, D.G. Facile immobilization of Trametes versicolor laccase on highly monodisperse superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2019, 181, 470–479. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Lin, Y.; Ng, T.B.; Ye, X.; Lin, J. Immobilized Cerrena sp. laccase: Preparation, thermal inactivation, and operational stability in malachite green decolorization. Sci. Rep. 2017, 7, 16429–16438. [Google Scholar] [CrossRef]
- Amin, R.; Khorshidi, A.; Shojaei, A.F.; Rezaei, S.; Faramarzi, M.A. Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int. J. Biol. Macromol. 2018, 114, 106–113. [Google Scholar] [CrossRef] [PubMed]

| Substrate | Enzyme Specific Activity (U/mg) |
|---|---|
| Melac13220 | 515.52 |
| Melac13220-DQ | 121.20 |
| Melac13220-NQ | 183.03 |
| Melac13220-CQ | 602.87 |
| Glutaraldehyde (%) | Activation Time (h) | Immobilization (%) |
|---|---|---|
| 0.0 | 3.0 | 51.0 ± 3.16 |
| 6.0 | 48.2 ± 1.87 | |
| 1.0 | 3.0 | 57.4 ± 2.66 |
| 6.0 | 58.0 ± 3.15 | |
| 2.0 | 3.0 | 74.7 ± 2.78 |
| 6.0 | 69.4 ± 3.29 | |
| 3.0 | 3.0 | 66.3 ± 2.69 |
| 6.0 | 62.0 ± 1.58 | |
| 4.0 | 3.0 | 60.2 ± 3.18 |
| 6.0 | 56.0 ± 2.19 |
| Substrate | Immobilization Efficiency(%) | Activity Recovery Rate (%) |
|---|---|---|
| SiO2-Fe3O4-Melac13220-CQ | 89.93 | 76.18 |
| E-Melac13220 | 78.53 | 50.30 |
| C-Melac13220 | 74.70 | 37.81 |
| Km (mM) | Vmax (mM s−1) | |
|---|---|---|
| Free Melac13220-CQ | 7.39 × 10−2 | 0.378 |
| Immobilized Melac13220 | 9.33 × 10−2 | 0.439 |
| Dyes | λmax (nm) | Laccase | Decolorization Rate (%) |
|---|---|---|---|
| Remazol Brilliant Blue R | 595 | Immobilized | 41.3 ± 1.6 |
| Free | 13.9 ± 0.7 | ||
| Crystal Violet | 590 | Immobilized | 51.4 ± 1.1 |
| Free | 10.7 ± 1.1 | ||
| Indigo Carmine | 610 | Immobilized | 87.9 ± 1.3 |
| Free | 54.0 ± 0.9 | ||
| Congo Red | 488 | Immobilized | 100 ± 0.9 |
| Free | 99 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ainiwaer, A.; Li, A.; Zhao, X.; Xu, Y.; Han, S.; Gao, R. Site-Specific Covalent Immobilization of Methylobacterium extorquens Non-Blue Laccse Melac13220 on Fe3O4 Nanoparticles by Aldehyde Tag. Catalysts 2022, 12, 1379. https://doi.org/10.3390/catal12111379
Ainiwaer A, Li A, Zhao X, Xu Y, Han S, Gao R. Site-Specific Covalent Immobilization of Methylobacterium extorquens Non-Blue Laccse Melac13220 on Fe3O4 Nanoparticles by Aldehyde Tag. Catalysts. 2022; 12(11):1379. https://doi.org/10.3390/catal12111379
Chicago/Turabian StyleAiniwaer, Abidan, Ao Li, Xingwang Zhao, Yujiao Xu, Siping Han, and Renjun Gao. 2022. "Site-Specific Covalent Immobilization of Methylobacterium extorquens Non-Blue Laccse Melac13220 on Fe3O4 Nanoparticles by Aldehyde Tag" Catalysts 12, no. 11: 1379. https://doi.org/10.3390/catal12111379
APA StyleAiniwaer, A., Li, A., Zhao, X., Xu, Y., Han, S., & Gao, R. (2022). Site-Specific Covalent Immobilization of Methylobacterium extorquens Non-Blue Laccse Melac13220 on Fe3O4 Nanoparticles by Aldehyde Tag. Catalysts, 12(11), 1379. https://doi.org/10.3390/catal12111379







