A Review of Synergistic Catalytic Removal of Nitrogen Oxides and Chlorobenzene from Waste Incinerators
Abstract
1. Introduction
2. Types of Catalysts for NO and CB Co-Removal
2.1. Non-Loaded Transition Metal Oxide Catalysts
| Catalyst | Reaction Conditions | Conversion | Reference |
|---|---|---|---|
| FeVO4-Fe2O3 | NOx = 500 ppm, CB = 50 ppm, NH3 = 515 or 485 ppm, O2 = 10 vol%, H2O = 5 vol%, SO2 = 100 ppm, N2 as balance gas GHSV = 60,000 h−1 | NOx: ~100% (200 °C) CB: ~90% (275 °C) | [39] |
| MnNb0.4Ce0.2O2 | NOx = 600 ppm, CB = 50 ppm, NH3 = 600 ppm, O2 = 12 vol%, H2O = 7 vol%, N2 as balance gas GHSV = 30,000 h−1 | NOx: ~100% (170 °C) CB: ~90% (250 °C) | [40] |
| MnOx(0.4)-CeO2 | NO = 500 ppm, CB = 50 ppm, NH3 = 500 ppm, O2 = 10 vol%, N2 as balance gas GHSV = 60,000 h−1 | NOx: ~100% (200 °C) CB: ~90% (270 °C) | [55] |
| Al0.1-CeO2 | NO = 500 ppm, CB = 500 ppm, NH3 = 500 ppm, O2 = 10 vol%, H2O = 5 vol%, N2 as balance gas GHSV = 40,000 h−1 | NOx: ~100% (300 °C) CB: ~90% (300 °C) | [58] |
| MnFe0.7 | NO = 500 ppm, NH3 = 550 ppm, O2 = 2 vol%, or CB = 250 ppm, O2 = 2 vol%, N2 as balance gas GHSV = 100,000 h−1 | NOx: >90% (150 °C) CB: ∼40% (275 °C) | [59] |
| SO42–0.10Fe–MnOx | NO = 500 ppm, CB = 100 ppm, NH3 = 500 ppm, O2 = 3 vol%, N2 as balance gas GHSV = 30,000 h−1 | NOx: >90% (140 °C) CB: ∼90% (200 °C) | [60] |
2.2. Loaded Transition Metal Oxide Catalysts
| Catalyst | Reaction Conditions | Conversion | Reference |
|---|---|---|---|
| WZrOx/TiCeMnOx | NO = 500 ppm, CB = 100 ppm, NH3 = 500 ppm, O2 = 10 vol%, N2 as balance gas GHSV = 30,000 h−1 | NOx: ~100% (250 °C) CB: ~100% (400 °C) | [76] |
| V/Ti | NO = 500 ppm, CB = 50 ppm, NH3 = 500 ppm, O2 = 3.5 vol%, N2 as balance gas GHSV = 60,000 h−1 | NOx: ~100% (250 °C) CB: ~20% (300 °C) | [80] |
| V-Ce/Ti | NO = 500 ppm, CB = 50 ppm, NH3 = 500 ppm, O2 = 3.5 vol%, N2 as balance gas GHSV = 60,000 h−1 | NOx: ~100% (300 °C) CB: ~20% (300 °C) | [80] |
| V-Mn/Ti | NO = 500 ppm, CB = 50 ppm, NH3 = 500 ppm, O2 = 3.5 vol%, N2 as balance gas GHSV = 60,000 h−1 | NOx: ~100% (300 °C) CB: ~5% (300 °C) | [80] |
| V-W/Ti | NO = 600 ppm, CB = 100 ppm, NH3 = 600 ppm, O2 = 5 vol%, N2 as balance gas GHSV = 40,000 h−1 | NOx: ~100% (275 °C) CB: ∼100% (350 °C) | [81] |
| V-Mo/Ti | NO = 500 ppm, CB = 100 ppm, NH3 = 500 ppm, O2 = 10 vol%, N2 as balance gas GHSV = 30,000 h−1 | NOx: ~100% (250 °C) CB: ~100% (350 °C) | [81] |
| V-Mo/Ti | NO = 500 ppm, CB = 100 ppm, NH3 = 500 ppm, O2 = 10 vol%, N2 as balance gas GHSV = 30,000 h−1 | NOx: ~100% (200 °C) CB: ~100% (300 °C) | [82] |
| Pd-V/Ti | NO = 600 ppm, CB = 100 ppm, NH3 = 600 ppm, O2 = 10 vol%, N2 as balance gas GHSV = 30,000 h−1 | NOx: ~100% (250 °C) CB: ~100% (400 °C) | [83] |
3. Mechanism of Synergistic Multi-Reactant Removal Interaction
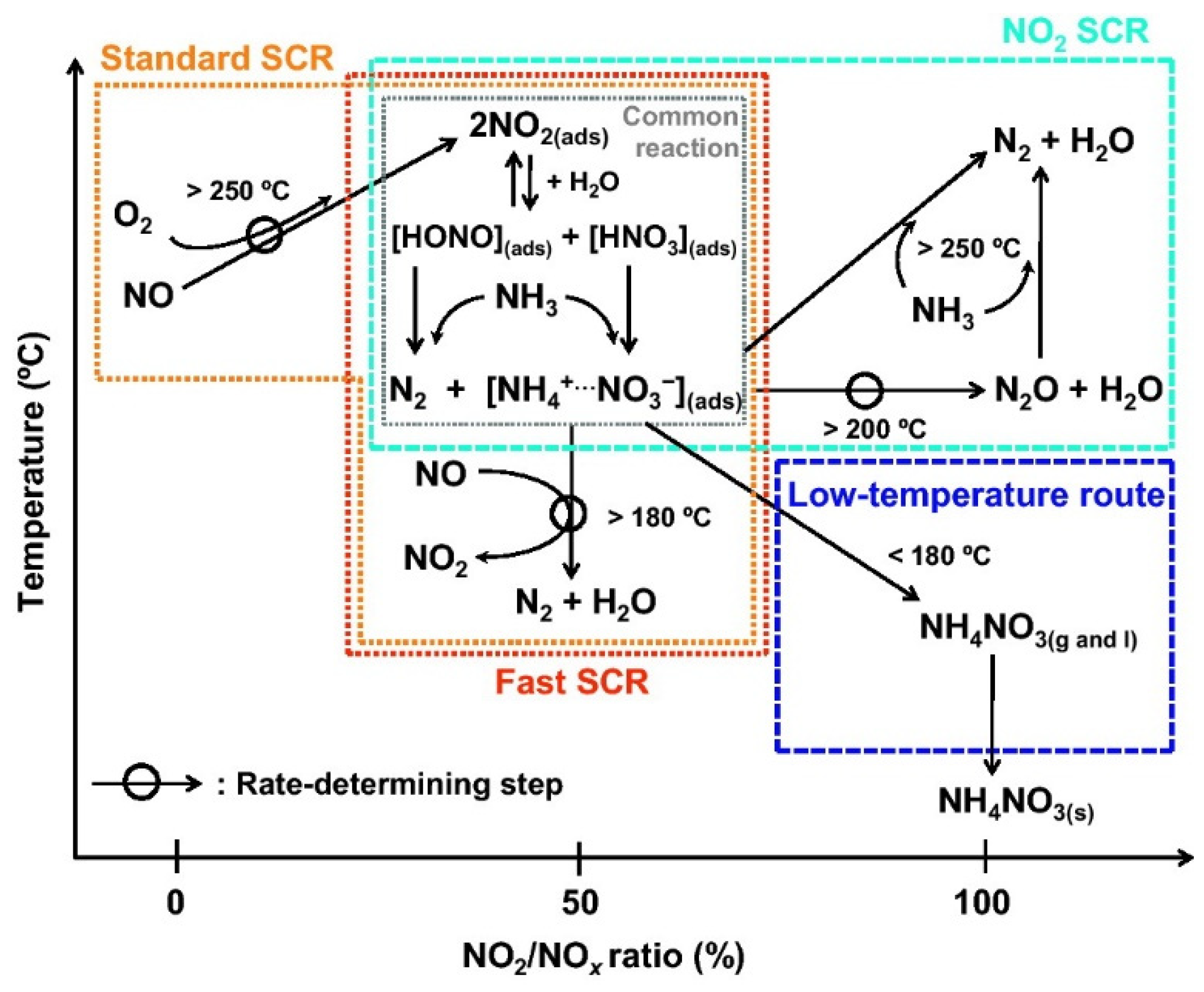
3.1. Effect of NH3-SCR on CBCO
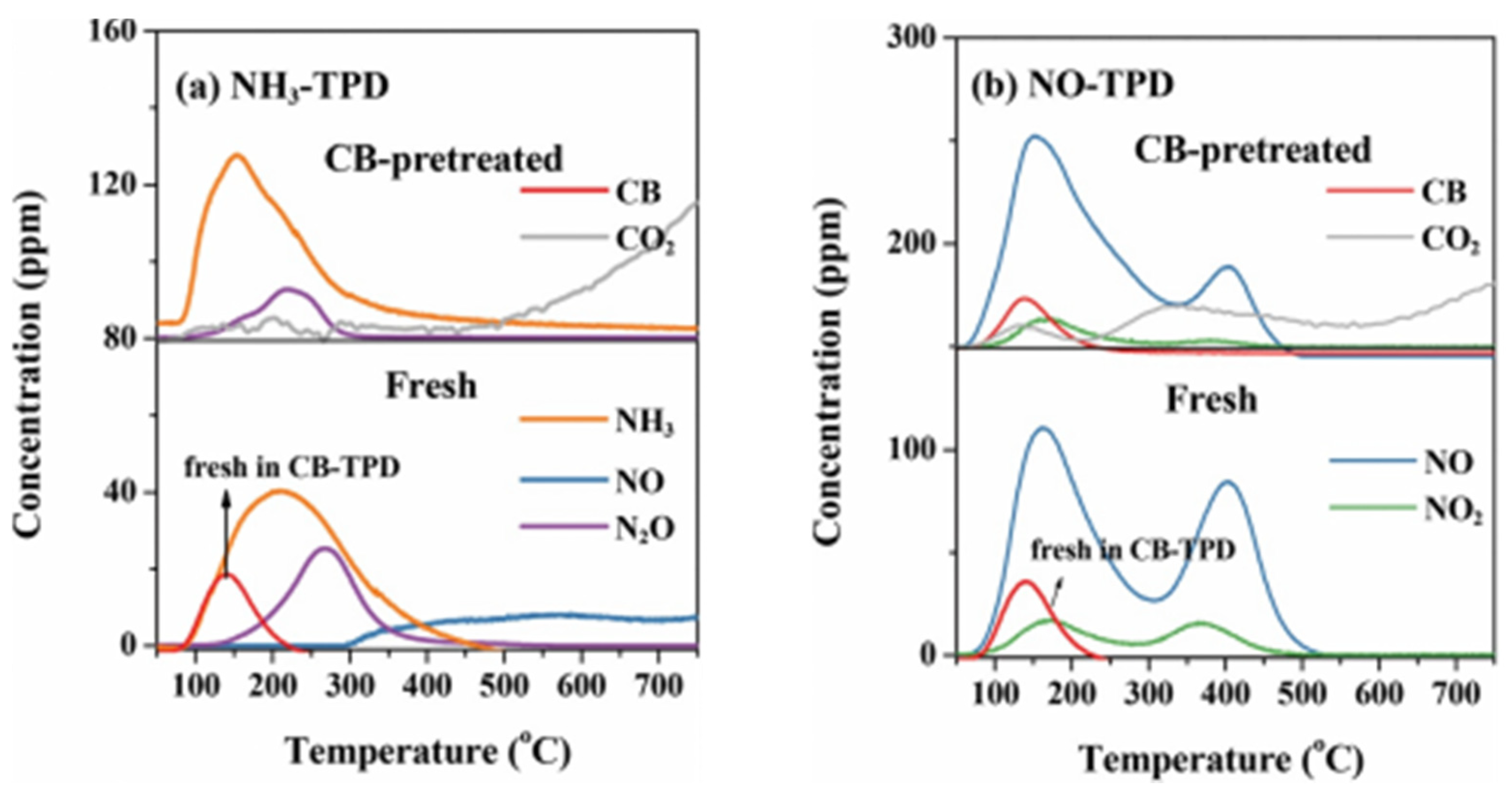
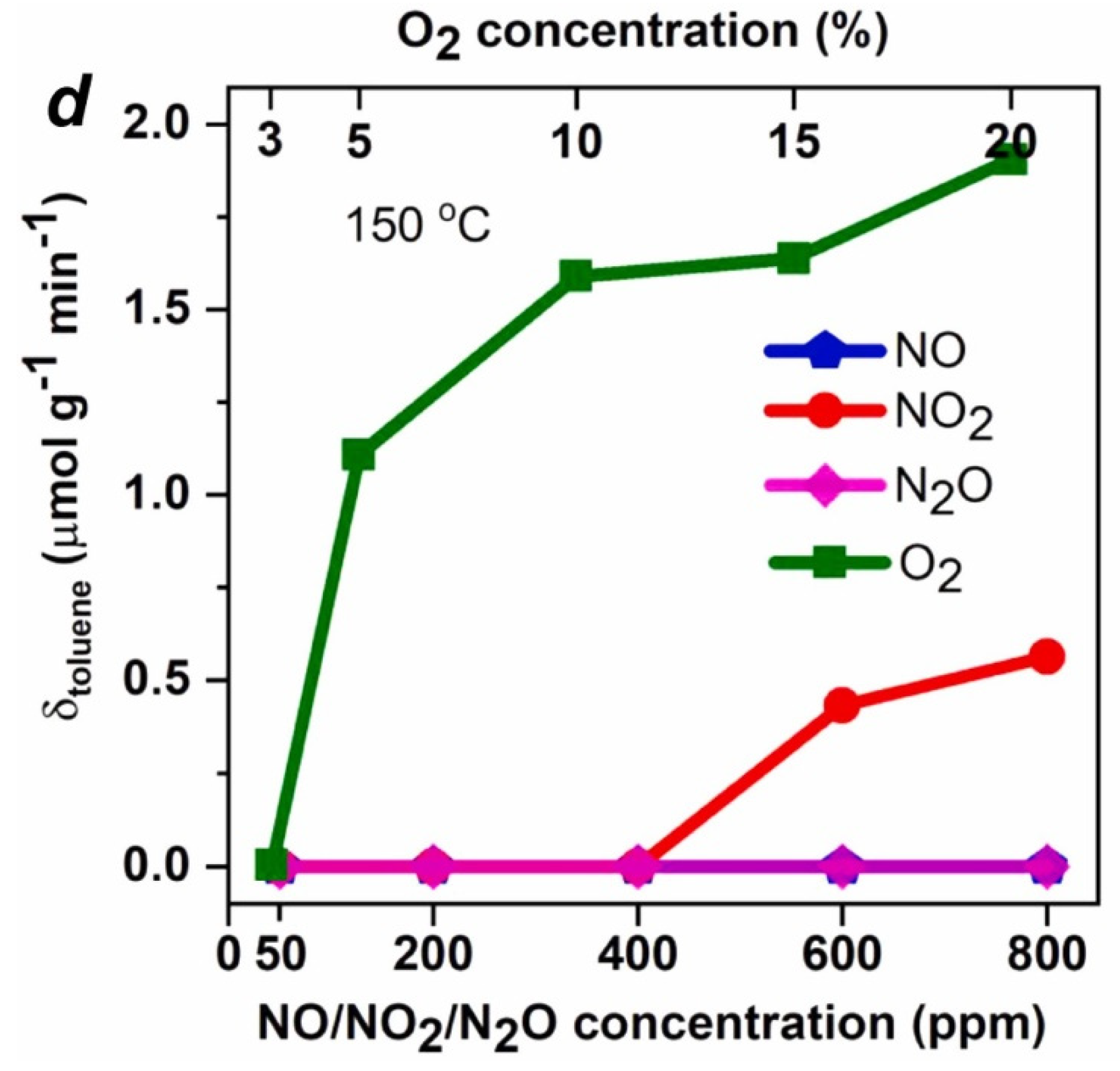
3.2. Effect of CBCO on NH3-SCR
4. Catalyst Deactivation
5. Conclusions
- Effect of NH3-SCR on CBCO. NH3 competes with CB for adsorption, and NH3 has significantly stronger adsorption performance than CB, resulting in lower CBCO low-temperature activity. NO2 is produced in the NH3-SCR process. NO2 has a stronger oxidation performance than O2 and can promote CB conversion. However, this promotion effect is related to the morphology of NO2, which can improve the CB conversion efficiency and reduce the CB ignition point when NO2 is present in the adsorbed form in the reaction system.
- Effect of CB on NH3-SCR. The effect of CB on the amount of NH3 adsorbed was not significant; so, there was no significant inhibition of the NH3-SCR reaction by CB at low temperatures. The decomposition of CB at high-temperature and the generated Cl- provided additional acid sites to increase the NH3 adsorption and improve the NO conversion efficiency. The generated Cl- reacts with NH3 to form inert NH4Cl, which regulates the redox performance of the catalyst, effectively inhibits the C-O reaction, and widens the SCR temperature window.
Author Contributions
Funding
Conflicts of Interest
References
- Mavridis, S.; Voudrias, E.A. Using biogas from municipal solid waste for energy production: Comparison between anaerobic digestion and sanitary Landfilling. Energy Convers. Manag. 2021, 247, 114613. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Ayoub, N. Comparative study of MSW heat treatment processes and electricity generation. J. Energy Inst. 2018, 91, 481–488. [Google Scholar] [CrossRef]
- Shi, W.; Dong, Q.; Saleem, M.; Wu, X.; Wang, N.; Ding, S.; Huang, J.; Wang, X.; Zhou, B.; Gao, Z. Microbial-based detonation and processing of vegetable waste for high-quality compost production at low temperatures. compost production at low temperatures. J. Clean. Prod. 2022, 369, 133276. [Google Scholar] [CrossRef]
- Beylot, A.; Hochar, A.; Michel, P.; Descat, M.; Ménard, Y.; Villeneuve, J. Municipal solid waste incineration in France: An overview of air pollution control techniques, emissions, and energy efficiency. J. Ind. Ecol. 2018, 22, 1016–1026. [Google Scholar] [CrossRef]
- Lau, W.W.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Thabit, Q.; Nassour, A.; Nelles, M. Flue Gas Composition and Treatment Potential of a Waste Incineration Plant. Appl. Sci. 2022, 12, 5236. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic materials for deNox selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct decomposition of NOx over TiO2 supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Qu, W.; Liu, R.; Xu, D.; Ma, Z.; Tang, X. Sulfur-resistant Ceria-based low-temperature SCR catalysts with the non-bulk electronic states of Ceria. states of Ceria. Environ. Sci. Technol. 2021, 55, 5435–5441. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, H.; Iwasaki, K.; Terada, A.; Hosomi, M. Utilization of recycled charcoal as a thermal source and adsorbent for the treatment of PCDD/Fs contaminated sediment. J. Hazard. Mater. 2012, 225–226, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Hay, A. Dioxin as a health hazard. Nature 1980, 283, 229–230. [Google Scholar] [CrossRef]
- GB 18485-2014; Standard for Pollution Control on the Municipal Solid Waste Incineration. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2014.
- Li, B.; Song, C.; Lv, G.; Chen, K.; Cao, X. Impact of soot on NOx adsorption over Cu-modified hydrotalcite-derived lean NOx trap catalyst. Langmuir 2017, 33, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhao, Y.; Yang, J.; Zhang, S.; Zhang, J.; Zheng, C. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energy Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Li, X. Catalytic oxidation of nitric oxide (NO) over different catalysts: An overview. Catal. Sci. Technol. 2017, 7, 3440–3452. [Google Scholar] [CrossRef]
- Javed, M.T.; Irfan, N.; Gibbs, B.M. Control of combustion-generated nitrogen oxides by selective non-catalytic reduction. J. Environ. Manag. 2007, 83, 251–289. [Google Scholar] [CrossRef]
- Tayyeb Javed, M.; Nimmo, W.; Mahmood, A.; Irfan, N. Effect of oxygenated liquid additives on the urea based SNCR process. J. Environ. Manag. 2009, 90, 3429–3435. [Google Scholar] [CrossRef]
- Weng, X.; Dai, X.; Zeng, Q.; Liu, Y.; Wu, Z. Drift studies on promotion mechanism of H3PW12O40 in selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2016, 461, 9–14. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn-Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Xiong, S.-C.; Li, B.; Gan, L.-N.; Lu, C.-M.; Chen, J.-J.; Ma, Y.-L.; Li, J.-H. De-reducibility mechanism of titanium on maghemite catalysts for the SCR reaction: An in situ DRIFTS and quantitative kinetics study. Appl. Catal. B Environ. 2018, 221, 556–564. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.-T.; Liu, S.-M.; Wang, S.-X.; Pan, W.-G.; Li, M.-Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Shinjoh, H. A comparative study of “standard”, “fast” and “NO2” SCR reactions over Fe/zeolite catalyst. Appl. Catal. A Gen. 2010, 390, 71–77. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Madia, G.; Koebel, M.; Elsener, M.; Wokaun, A. The effect of an oxidation precatalyst on the NOx reduction by ammonia SCR. Ind. Eng. Chem. Res. 2002, 41, 3512–3517. [Google Scholar] [CrossRef]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of the state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Perlatti, B.; da Silva, M.F.; Fernandes, J.B.; Forim, M.R. Biodegradation of 1,2,3,4-tetrachlorodibenzo-p-dioxin in liquid broth by brown-rot fungi. Bioresour. Technol. 2013, 148, 624–627. [Google Scholar] [CrossRef]
- Lin, W.C.; Chang-Chien, G.P.; Kao, C.M.; Newman, L.; Wong, T.Y.; Liu, J.K. Biodegradation of polychlorinated dibenzo-ρ-dioxins by strain NSYSU. J. Environ. Qual. 2014, 43, 349–357. [Google Scholar] [CrossRef]
- Binh, N.D.; Oanh, N.T.; Parkpian, P. Photodegradation of dioxin in contaminated soil in the presence of solvents and nanoscale TiO2 particles. Environ. Technol. 2014, 35, 1121–1132. [Google Scholar] [CrossRef]
- Niu, J.; Dai, Y.; Yin, L.; Shang, J.; Crittenden, J.C. Photocatalytic reduction of triclosan on Au-Cu2O nanowire arrays as plasmonic photocatalysts under visible light irradiation. Phys. Chem. Phys. 2015, 17, 17421–17428. [Google Scholar] [CrossRef]
- Zhan, M.X.; Fu, J.Y.; Ji, L.J.; Deviatkin, I.; Lu, S.Y. Comparative analyses of catalytic degradation of PCDD/Fs in the laboratory vs. industrial conditions. Chemosphere 2018, 191, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.F.; Lin, X.Q.; Li, X.D.; Yan, M.; Prabowo, B.; Li, W.W.; Chen, T.; Yan, J.H. Catalytic destruction of PCDD/Fs over vanadium oxide-based catalysts. Environ. Sci. Pollut. Res. Int. 2016, 23, 16249–16258. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.S.; Ren, Y.; Buekens, A.; Chen, T.; Lu, S.Y.; Cen, K.F.; Li, X.D. Treating PCDD/Fs by combined catalysis and activated carbon adsorption. Chemosphere 2014, 102, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Ikeguchi, T.; Yagi, Y.; Tamade, Y.; Omori, K. A research on dioxin generation from the industrial waste incineration. Chemosphere 2002, 46, 1309–1319. [Google Scholar] [CrossRef]
- Lichtenberger, J. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts. J. Catal. 2004, 223, 296–308. [Google Scholar] [CrossRef]
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over Mnx Ce1-x O2 /H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, X.; Zhu, T.; Guo, Y.; Qi, H. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts: The effects of chlorine substituents. Catal. Today 2015, 241, 92–99. [Google Scholar] [CrossRef]
- Yin, R.; Chen, J.; Mi, J.; Liu, H.; Yan, T.; Shan, L.; Lang, J.; Li, J. Breaking the Activity-Selectivity Trade-Off for Simultaneous Catalytic Elimination of Nitric Oxide and Chlorobenzene via FeVO4-Fe2O3 Interfacial Charge Transfer. ACS Catal. 2022, 12, 3797–3806. [Google Scholar] [CrossRef]
- Yang, B.; Jin, Q.; Huang, Q.; Chen, M.; Xu, L.; Shen, Y.; Xu, H.; Zhu, S.; Li, X. Synergetic catalytic removal of chlorobenzene and NO from waste incineration exhaust over MnNb0.4Ce0.2Ox catalysts: Performance and mechanism study. J. Rare Earths 2020, 38, 1178–1189. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Uemichi, Y.; Ayame, A. Low-temperature hydrodechlorination mechanism of chlorobenzenes over platinum-supported and palladium-supported alumina catalysts. Appl. Catal. A Gen. 2005, 287, 89–97. [Google Scholar] [CrossRef]
- Lu, Y.; Dai, Q.; Wang, X. Catalytic combustion of chlorobenzene on modified LaMnO3 catalysts. Catal. Commun. 2014, 54, 114–117. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Rivas, J.A.; Amiridis, M.D. Catalytic Oxidation of 1,2-Dichlorobenzene over Supported Transition Metal Oxides. J. Catal. 2000, 193, 264–272. [Google Scholar] [CrossRef]
- De Jong, V.; Cieplik, M.K.; Louw, R. Formation of Dioxins in the Catalytic Combustion of Chlorobenzene and a Micropollutant-like Mixture on Pt/γ-Al2O3. Environ. Sci. Technol. 2004, 38, 5217–5223. [Google Scholar] [CrossRef]
- Poplawski, K.; Lichtenberger, J.; Keil, F.J.; Schnitzlein, K.; Amiridis, M.D. Catalytic oxidation of 1, 2-dichlorobenzene over ABO3-type perovskites. Catal. Today 2000, 62, 329–336. [Google Scholar] [CrossRef]
- Yu, S.; Niu, X.; Song, Z.; Huang, X.; Peng, Y.; Li, J. Improvement of Al2 O3 on the multi-pollutant control performance of NOx and chlorobenzene in vanadia- based catalysts. Chemosphere 2022, 289, 133156. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Zhang, J.; Jiang, L.; Wang, Y. Al2O3-modified CuO-CeO2 catalyst for simultaneous removal of NO and toluene at wide temperature range. Chem. Eng. J. 2020, 397, 125419. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, F.; Zhou, J.; Zhang, J.; Jin, J. Effect of HCl and o-DCBz on NH3-SCR of NO over MnO/TiO2 and MnO-CeO2/TiO2 catalysts. Appl. Catal. A Gen. 2020, 605, 117801. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Mei, C.; Zhang, Y.; Zeng, Y. Catalytic removal of NO and dioxins over W-Zr-Ox/Ti-Ce-Mn-Ox from flue gas: Performance and mechanism study. Catal. Today 2022, 388–389, 372–382. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Wang, D.; Peng, Y.; Li, J. The effect of additives and intermediates on vanadia-based catalyst for multi-pollutant control. Catal. Sci. Technol. 2020, 10, 323–326. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.; Lu, G. Low-temperature catalytic combustion of trichloroethylene over cerium oxide and catalyst deactivation. Appl. Catal. B Environ. 2008, 81, 192–202. [Google Scholar] [CrossRef]
- Wang, X.; Kang, Q.; Dao, L. Catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts. Appl. Catal. B Environ. 2009, 86, 166–175. [Google Scholar] [CrossRef]
- Ding, S.; Liu, F.; Shi, X.; He, H. Promotional effect of Nb additive on the activity and hydrothermal stability for the selective catalytic reduction of NO with NH3 over CeZrO2 catalyst. Appl. Catal. B Environ. 2016, 180, 766–774. [Google Scholar] [CrossRef]
- Long, G.; Chen, M.; Li, Y.; Ding, J.; Sun, R.; Zhou, Y.; Huang, X.; Han, G.; Zhao, W. One-pot synthesis of monolithic Mn-Ce-Zr ternary mixed oxides catalyst for the catalytic combustion of chlorobenzene. Chem. Eng. J. 2019, 360, 964–973. [Google Scholar] [CrossRef]
- Gan, L.; Li, K.; Xiong, S.; Zhang, Y.; Chen, J.; Peng, Y.; Li, J. MnOx-CeO2 catalysts for effective NO reduction in the presence of chlorobenzene. Catal. Commun. 2018, 117, 1–4. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Shen, Y.; He, J.; Qu, W.; Deng, J.; Han, L.; Chen, A.; Zhang, D. Synergistic catalytic elimination of NOx and chlorinated organics: Cooperation of acid sites. Environ. Sci. Technol. 2022, 56, 3719–3728. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S.; Bai, B.; Li, L.; Kang, Y.; Hu, X.; Liao, Z.; He, C. Biotemplate fabrication of hollow tubular CexSr1–xTiO3 with regulable surface acidity and oxygen mobility for efficient destruction of chlorobenzene: Intrinsic synergy effect and reaction mechanism. Environ. Sci. Technol. 2022, 56, 5796–5807. [Google Scholar] [CrossRef]
- Wei, L.; Liu, Y.; Dai, H.; Cui, S.; Wang, C.; Hsi, H.-C.; Duan, E.; Peng, Y.; Deng, J. Electronic structure tailoring of Al3+-and Ta5+-doped CeO2 for the synergistic removal of NO and chlorinated organics. synergistic removal of NO and chlorinated organics. Appl. Catal. B Environ. 2022, 304, 120939. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Peng, Y.; Duan, R.; Hu, F.; Jing, Q.; Chen, J.; Li, J. Fe-Doped alpha-MnO2 nanorods for the catalytic removal of NOx and chlorobenzene: The relationship between lattice distortion and catalytic redox properties. Phys. Chem. Phys. 2019, 21, 25880–25888. [Google Scholar] [CrossRef]
- Gong, P.; Cao, R.; Yu, Y.; Zhang, J. Promotion effect of SO42−/Fe2O3 modified MnOx catalysts for simultaneous control of NO and CVOCs. Surf. Interfaces 2022, 33, 102253. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, B.; Hao, R.; Zhao, Y.; Wang, X. A critical review on the method of simultaneous removal of multi-air-pollutant in flue gas. Chem. Eng. J. 2019, 378, 122155. [Google Scholar] [CrossRef]
- Martín-Martín, J.A.; Sánchez-Robles, J.; González-Marcos, M.P.; Aranzabal, A.; González-Velasco, J.R. Effect of preparation procedure and composition of catalysts based on Mn and Ce oxides in the simultaneous removal of NO composition of catalysts based on Mn and Ce oxides in the simultaneous removal of NOx and o-DCB. Mol. Catal. 2020, 495, 111152. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Grégoire, C.; Gaigneaux, E.M. Systematic investigation of supported transition metal oxide based formulations for the catalytic oxidative elimination of (chloro)-aromatics: Part II: Influence of the nature and addition protocol of secondary phases to VOx/TiO2. Appl. Catal. B Environ. 2006, 66, 10–22. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Si, Z.; Weng, D. Influences of impregnation procedure on the SCR activity and alkali resistance of V2O5-WO3/TiO2 catalyst. Appl. Surf. Sci. 2013, 283, 209–214. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Xu, B.; Wang, X. Engineering nanointerfaces for nanocatalysis. Chem. Soc. Rev. 2014, 43, 7870–7886. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Aranzabal, A.; Boukha, Z.; González-Marcos, J.A.; González-Velasco, J.R.; Martínez-Huerta, M.V.; Bañares, M.A. Role of surface vanadium oxide coverage support on titania for the simultaneous removal of o-dichlorobenzene and NOx from waste incinerator flue gas. surface vanadium oxide coverage support on titania for the simultaneous removal of o-dichlorobenzene and NOx from waste incinerator flue gas. Catal. Today 2015, 254, 2–11. [Google Scholar] [CrossRef]
- Zhai, S.; Su, Y.; Weng, X.; Li, R.; Wang, H.; Wu, Z. Synergistic Elimination of NOx and Chlorinated Organics over VOx/TiO2 Catalysts: A Combined Experimental and DFT Study for Exploring Vanadate Domain Effect. Experimental and DFT Study for Exploring Vanadate Domain Effect. Environ. Sci. Technol. 2021, 55, 12862–12870. [Google Scholar] [CrossRef]
- Albonetti, S.; Mengou, J.E.; Trifirò, F. Polyfunctionality of DeNOx catalysts in other pollutant abatement. Catal. Today 2007, 119, 295–300. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Ha, H.P.; Hong, S.C. The role of molybdenum on the enhanced performance and SO2 resistance of V/Mo-Ti catalysts for NH3 -SCR. Appl. Surf. Sci. 2019, 481, 1167–1177. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total. Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Liu, S. SnOx-MnOx-TiO2 catalysts with high resistance to chlorine poisoning for low-temperature chlorobenzene oxidation. Appl. Catal. A Gen. 2014, 482, 363–369. [Google Scholar] [CrossRef]
- Khaleel, A.; Al-Nayli, A. Supported and mixed oxide catalysts based on iron and titanium for the oxidative decomposition of chlorobenzene. Appl. Catal. B Environ. 2008, 80, 176–184. [Google Scholar] [CrossRef]
- Ma, X.; Shen, J.; Pu, W.; Sun, H.; Pang, Q.; Guo, J.; Zhou, T.; Cao, H. Water-resistant Fe-Ca-Ox/TiO2 catalysts for low temperature 1,2-dichlorobenzene oxidation. Appl. Catal. A Gen. 2013, 466, 68–76. [Google Scholar] [CrossRef]
- Chen, N.; Yang, S.; Liu, M.; Lee, J.; Chang, J. Pellet Vanadia Catalysts for Oxidative Destruction of 1,2-Dichlorobenzene: Roles of the Grafted TiO2 in Vanadia Morphology and Catalytic Reaction. Catal. Surv. Asia 2015, 19, 38–56. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Wei, Z.; Xin, Q.; Ying, P.; Li, C. Catalytic oxidation of chlorobenzene on supported manganese oxide catalysts. Appl. Catal. B Environ. 2001, 29, 61–67. [Google Scholar] [CrossRef]
- Jin, Q.; Xu, M.; Lu, Y.; Yang, B.; Ji, W.; Xue, Z.; Dai, Y.; Wang, Y.; Shen, Y.; Xu, H. Simultaneous catalytic removal of NO, mercury and chlorobenzene over WCeMnOx/TiO2-ZrO2: Performance study of microscopic morphology and phase composition. Chemosphere 2022, 295, 133794. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Aranzabal, A.; González-Marcos, J.A.; González-Velasco, J.R. Metal-loaded ZSM5 zeolites for catalytic purification of dioxin/furans and NOx containing exhaust gases from MWI plants: Effect of different metal cations. Appl. Catal. B Environ. 2016, 184, 238–245. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Peng, Y.; Si, W.; Li, X.; Li, B.; Li, J. Dechlorination of chlorobenzene on vanadium-based catalysts for low-temperature SCR. Chem. Commun. (Camb.) 2018, 54, 2032–2035. [Google Scholar] [CrossRef]
- Long, Y.; Su, Y.; Xue, Y.; Wu, Z.; Weng, X. V2O5-WO3/TiO2 Catalyst for Efficient Synergistic Control of NOx and Chlorinated Organics: Insights into the Arsenic Effect. Environ. Sci. Technol. 2021, 55, 9317–9325. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Yang, Q.; Peng, Y.; Li, J. Multi-pollutant control (MPC) of NO and chlorobenzene from industrial furnaces using a vanadia-based SCR catalyst. catalyst. Appl. Catal. B Environ. 2021, 285, 119835. [Google Scholar] [CrossRef]
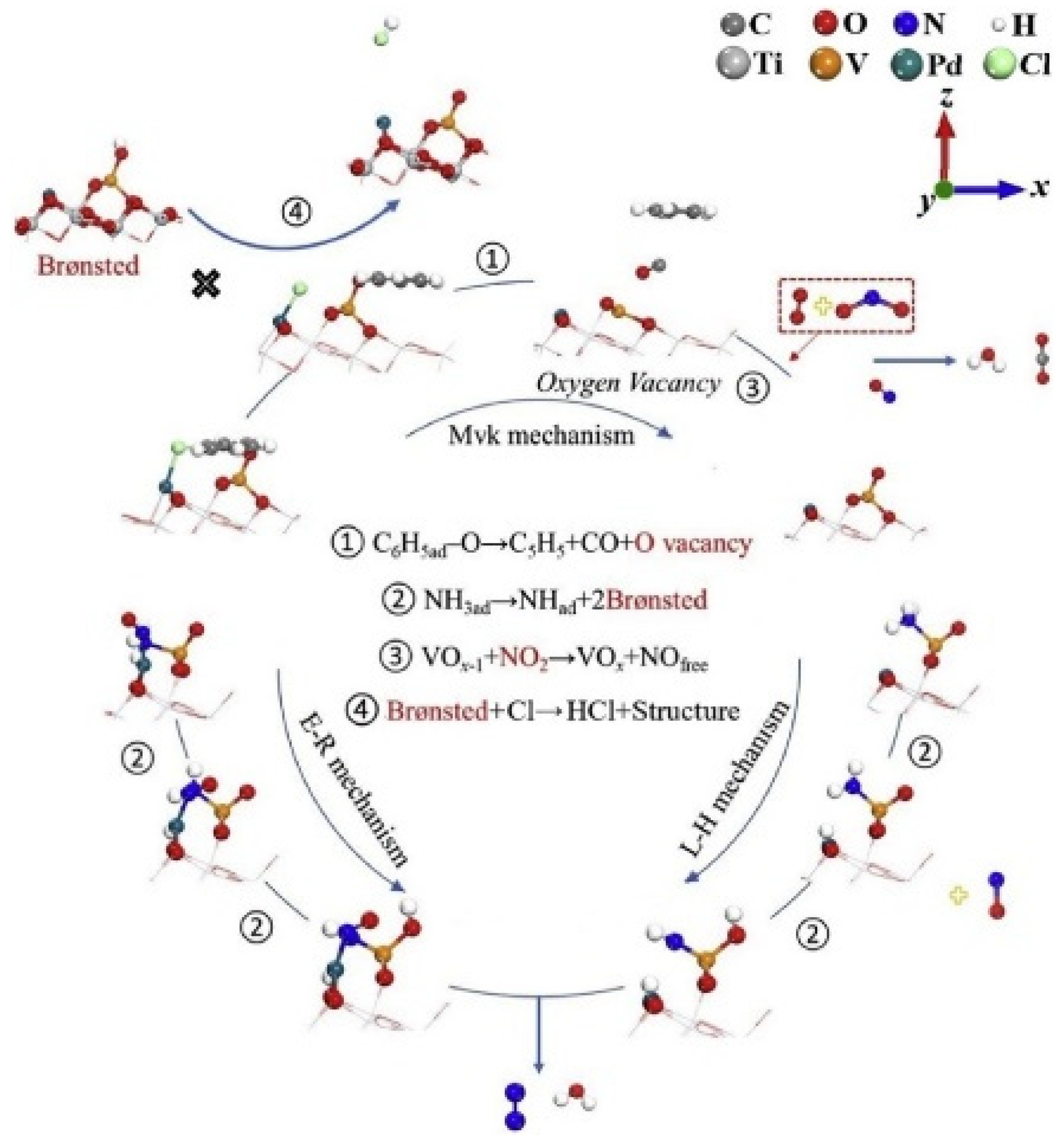
- Li, G.; Shen, K.; Wang, L.; Zhang, Y.; Yang, H.; Wu, P.; Wang, B.; Zhang, S. Synergistic degradation mechanism of chlorobenzene and NO over the multi- active center catalyst: The role of NO active center catalyst: The role of NO2, Brønsted acidic site, oxygen vacancy. Appl. Catal. B Environ. 2021, 286, 119865. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, Y.; Bi, F.; Sun, P.; Weng, X.; Wu, Z. Synergistic Elimination of NOx and Chloroaromatics on a Commercial V2O5-WO3/TiO2 Catalyst: Byproduct Analyses and the SO2 Effect. Environ. Sci. Technol. 2019, 53, 12657–12667. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Lu, P.; Peng, Y.; Li, J.; Huang, H. Impact of NOx and NH3 addition on toluene oxidation over MnOx-CeO2 catalyst. J. Hazard. Mater. 2021, 416, 125939. [Google Scholar] [CrossRef] [PubMed]
- Bertinchamps, F.; Treinen, M.; Blangenois, N.; Mariage, E.; Gaigneaux, E. Positive effect of NO on the performances of VO/TiO-based catalysts in the total oxidation abatement of chlorobenzene. J. Catal. 2005, 230, 493–498. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Treinen, M.; Eloy, P.; Dos Santos, A.M.; Mestdagh, M.M.; Gaigneaux, E.M. Understanding the activation mechanism induced by NOx on the performances of VOx/TiO2 based catalysts in the total oxidation of chlorinated VOCs. performances of VOx/TiO2 based catalysts in the total oxidation of chlorinated VOCs. Appl. Catal. B Environ. 2007, 70, 360–369. [Google Scholar] [CrossRef]
- Gan, L.; Wang, Y.; Chen, J.; Yan, T.; Li, J.; Crittenden, J.; Peng, Y. The synergistic mechanism of NOx and chlorobenzene degradation in municipal solid waste incinerators. Catal. Sci. Technol. 2019, 9, 4286–4292. [Google Scholar] [CrossRef]
- Gao, C.; Yang, G.; Huang, X.; Yang, Q.; Li, B.; Wang, D.; Peng, Y.; Li, J.; Lu, C.; Crittenden, J. Key intermediates from simultaneous removal of NOx and chlorobenzene over a V2O5-WO3/TiO2 catalyst: A combined experimental and DFT study. Catal. Sci. Technol. 2021, 11, 7260–7267. [Google Scholar] [CrossRef]
- Gan, L.; Shi, W.; Li, K.; Chen, J.; Peng, Y.; Li, J. Synergistic Promotion Effect between NOx and Chlorobenzene Removal on MnOx-CeO2 Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 30426–30432. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Krocher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium -Based Catalysts. Angew. Chem. Int. Ed. Engl. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Li, G.; Shen, K.; Wu, P.; Zhang, Y.; Hu, Y.; Xiao, R.; Wang, B.; Zhang, S. SO2 Poisoning Mechanism of the Multi-active Center Catalyst for Chlorobenzene and NOx Synergistic Degradation at Dry and Humid Environments. Environ. Sci. Technol. 2021, 55, 13186–13197. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3 -SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]

| Catalyst Type | Operating Temperature (°C) | Main Features |
|---|---|---|
| High-Temperature Catalyst | 345–595 | High NOx conversion rate; less leakage of NH3; strong resistance to SO2 poisoning above 425 °C |
| Medium-temperature catalyst | 260–425 | Wide application and high denitrification efficiency |
| Low-temperature catalyst | 150–300 | Low operating temperature, low energy consumption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Bian, Y.; Shi, Q.; Wang, J.; Yuan, P.; Shen, B. A Review of Synergistic Catalytic Removal of Nitrogen Oxides and Chlorobenzene from Waste Incinerators. Catalysts 2022, 12, 1360. https://doi.org/10.3390/catal12111360
Kang D, Bian Y, Shi Q, Wang J, Yuan P, Shen B. A Review of Synergistic Catalytic Removal of Nitrogen Oxides and Chlorobenzene from Waste Incinerators. Catalysts. 2022; 12(11):1360. https://doi.org/10.3390/catal12111360
Chicago/Turabian StyleKang, Dongrui, Yao Bian, Qiqi Shi, Jianqiao Wang, Peng Yuan, and Boxiong Shen. 2022. "A Review of Synergistic Catalytic Removal of Nitrogen Oxides and Chlorobenzene from Waste Incinerators" Catalysts 12, no. 11: 1360. https://doi.org/10.3390/catal12111360
APA StyleKang, D., Bian, Y., Shi, Q., Wang, J., Yuan, P., & Shen, B. (2022). A Review of Synergistic Catalytic Removal of Nitrogen Oxides and Chlorobenzene from Waste Incinerators. Catalysts, 12(11), 1360. https://doi.org/10.3390/catal12111360






