Solid–Waste–Derived Geopolymer–Type Zeolite–like High Functional Catalytic Materials Catalyze Efficient Hydrogenation of Levulinic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Geopolymer–Type Zeolite–Like Products
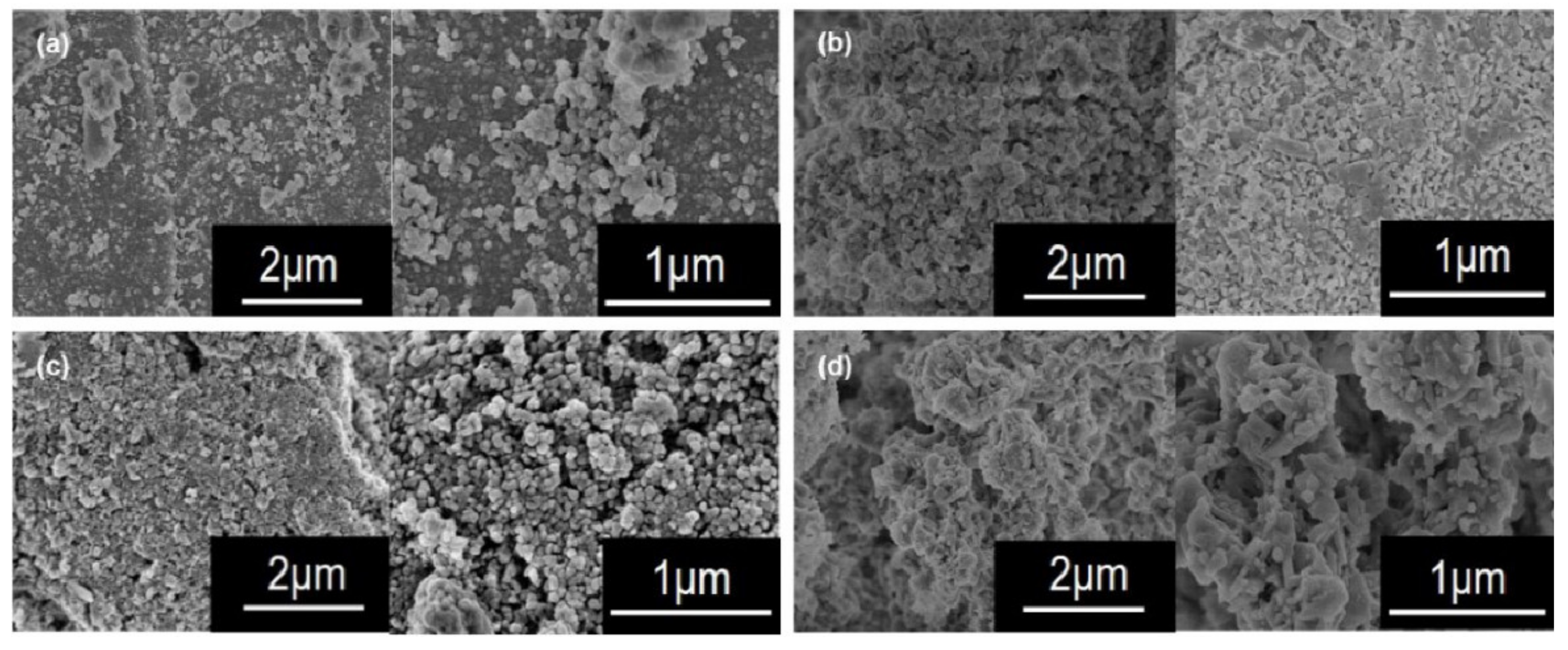
2.2. Catalyst Performance Characterization
2.3. Catalyst Performance Evaluation
2.4. Catalyst Repeatability Test
2.5. Metal Leaching Test in Reaction Solution
3. Materials and Methods
3.1. Materials
3.2. Material Preparation
3.3. Catalyst Preparation
3.4. Characterization Test
3.5. Catalytic Reaction Experiments
4. Conclusions
- Raw materials with a high content of silicon, aluminum, or calcium, such as waste incineration fly ash, are ideal raw materials for synthesizing geopolymer zeolite.
- The optimal synthesis conditions are as follows: the proportion of alkali activator is 40 wt% KOH solution (11 mol/L) and 70~80 wt% K2SiO3 solution (99%); the proportion of foaming agent is 3%. The total amount accounts for about 350% of the total amount of raw materials, and the mixing ratio of hydrogen peroxide (3%) and the foam stabilizer (pure oleic acid solution) is about 6.5:1 (w/w).
- The geopolymer–type zeolite synthesized under the above conditions possesses good structural properties and is suitable as a carrier to prepare a nickel catalyst. Ni/NiO–GC–WIFA can achieve a 100% LA conversion and a 94% GVL yield, with the GVL yield only decreasing by 1.23% after five cycles.
- The characterization test results show that the geopolymer–type zeolite–like product prepared using this method possesses excellent pore and surface structure, and high stability and durability. The catalytic effect of the catalyst prepared with the geopolymer–type zeolite–like product as a carrier is similar to that of the catalyst prepared with a typical commercial carrier. In the future, this material is also expected to be used in various high–value fields, such as electrocatalysis and adsorption.
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: A review. Environ. Chem. Lett. 2020, 19, 1433–1456. [Google Scholar] [CrossRef]
- Kaya-Özkiper, K.; Uzun, A.; Soyer-Uzun, S. Red mud- and metakaolin-based geopolymers for adsorption and photocatalytic degradation of methylene blue: Towards self-cleaning construction materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Huang, B.B.; Gan, M.; Ji, Z.Y.; Fan, X.H.; Zhang, D.; Chen, X.L.; Sun, Z.Q.; Huang, X.X.; Fan, Y. Recent progress on the thermal treatment and resource utilization technologies of municipal waste incineration fly ash: A review. Process Saf. Environ. Prot. 2022, 159, 547–565. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.L.; Bai, H.Y.; Kang, S.C.; Zhang, T.T.; Song, S.X. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Missengue, R.N.M.; Losch, P.; Sedres, G.; Musyoka, N.M.; Fatoba, O.O.; Louis, B.; Pale, P.; Petrik, L.F. Transformation of South African coal fly ash into ZSM-5 zeolite and its application as an MTO catalyst. C. R. Chim. 2017, 20, 78–86. [Google Scholar] [CrossRef]
- Vichaphund, S.; Wimuktiwan, P.; Sricharoenchaikul, V.; Atong, D. In Situ catalytic pyrolysis of Jatropha wastes using ZSM-5 from hydrothermal alkaline fusion of fly ash. J. Anal. Appl. Pyrolysis 2018, 139, 156–166. [Google Scholar] [CrossRef]
- De Oliveira, L.B.; de Azevedo, A.R.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of geopolymers with industrial waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Javed, M.F.; Akbar, A.; Shah, M.I.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Ni, T.; Wang, Q.; Li, H.; Colombo, P. Porosity, mechanical and insulating properties of geopolymer foams using vegetable oil as the stabilizing agent. J. Eur. Ceram. Soc. 2018, 38, 799–805. [Google Scholar] [CrossRef]
- Kaewmee, P.; Song, M.; Iwanami, M.; Tsutsumi, H.; Takahashi, F. Porous and reusable potassium-activated geopolymer adsorbent with high compressive strength fabricated from coal fly ash wastes. J. Clean. Prod. 2020, 272, 122617. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Liu, C.; Han, Y.; Ji, N. Synthesis of γ-valerolactone from different biomass-derived feedstocks: Recent advances on reaction mechanisms and catalytic systems. Renew. Sustain. Energy Rev. 2019, 112, 140–157. [Google Scholar] [CrossRef]
- Omoruyi, U.; Page, S.; Hallett, J.; Miller, P.W. Homogeneous Catalyzed Reactions of Levulinic Acid: To γ-Valerolactone and Beyond. ChemSusChem 2016, 9, 2037–2047. [Google Scholar] [CrossRef]
- Xu, W.P.; Chen, X.F.; Guo, H.J.; Li, H.L.; Zhang, H.R.; Xiong, L.; Chen, X.D. Conversion of levulinic acid to valuable chemicals: A review. J. Chem. Technol. Biotechnol. 2021, 96, 3009–3024. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.-P.; Fan, X.; Liu, Z.-Q.; Wang, R.-Y.; Wei, X.-Y. Catalytic Hydrogenation of Levulinic Acid into Gamma-Valerolactone over Ni/HZSM-5 Catalysts. Catal. Surv. Asia 2018, 22, 129–135. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Hengne, A.M.; Kadu, B.S.; Biradar, N.S.; Chikate, R.C.; Rode, C.V. Transfer hydrogenation of biomass-derived levulinic acid to γ-valerolactone over supported Ni catalysts. RSC Adv. 2016, 6, 59753–59761. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, Y.; Feng, J.; Huang, C.; Ma, T.; Pan, H. Highly efficient g-C3N4 supported ruthenium catalysts for the catalytic transfer hydrogenation of levulinic acid to liquid fuel γ-valerolactone. Renew. Energy 2021, 177, 652–662. [Google Scholar] [CrossRef]
- Chen, C.-B.; Chen, M.-Y.; Zada, B.; Ma, Y.-J.; Yan, L.; Xu, Q.; Li, W.-Z.; Guo, Q.-X.; Fu, Y. Effective conversion of biomass-derived ethyl levulinate into γ-valerolactone over commercial zeolite supported Pt catalysts. RSC Adv. 2016, 6, 112477–112485. [Google Scholar] [CrossRef]
- Sosa, L.F.; da Silva, V.T.; de Souza, P.M. Hydrogenation of levulinic acid to γ-valerolactone using carbon nanotubes supported nickel catalysts. Catal. Today 2021, 381, 86–95. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Nguyen, T.; Kashani, A.; van Deventer, J.S. Pore characteristics in one-part mix geopolymers foamed by H2O2: The impact of mix design. Mater. Des. 2017, 130, 381–391. [Google Scholar] [CrossRef]
- Falliano, D.; De Domenico, D.; Ricciardi, G.; Gugliandolo, E. Key factors affecting the compressive strength of foamed concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 431, 062009. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y. Influence of foaming agent on cement and foam concrete. Constr. Build. Mater. 2021, 280, 122399. [Google Scholar] [CrossRef]
- Su, Y.; Wei, B.; Sun, X.; Zhang, H. Factors affecting the stability of bubble in autoclaved aerated concrete. ce/papers 2018, 2, 181–186. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S. Review on characteristics of metakaolin-based geopolymer and fast setting. J. Korean Ceram. Soc. 2020, 57, 368–377. [Google Scholar] [CrossRef]
- Kovalchuk, G.; Fernández-Jiménez, A.; Palomo, A. Alkali-activated fly ash: Effect of thermal curing conditions on mechanical and microstructural development—Part II. Fuel 2007, 86, 315–322. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Song, S.; Yao, S.; Cao, J.; Di, L.; Wu, G.; Guan, N.; Li, L. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B Environ. 2017, 217, 115–124. [Google Scholar] [CrossRef]
- Zhong, H.; Li, Q.; Liu, J.; Yao, G.; Wang, J.; Zeng, X.; Huo, Z.; Jin, F. New Method for Highly Efficient Conversion of Biomass-Derived LevuliNic Acid to γ-Valerolactone in Water without Precious Metal Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 6517–6523. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Carvalho, H.W.P.; Lu, C.; Kleist, W.; Grunwaldt, J.-D. Synthesis of γ-valerolactone by hydrogenation of levuliNic acid over supported Nickel catalysts. Appl. Catal. A General 2015, 502, 18–26. [Google Scholar] [CrossRef]
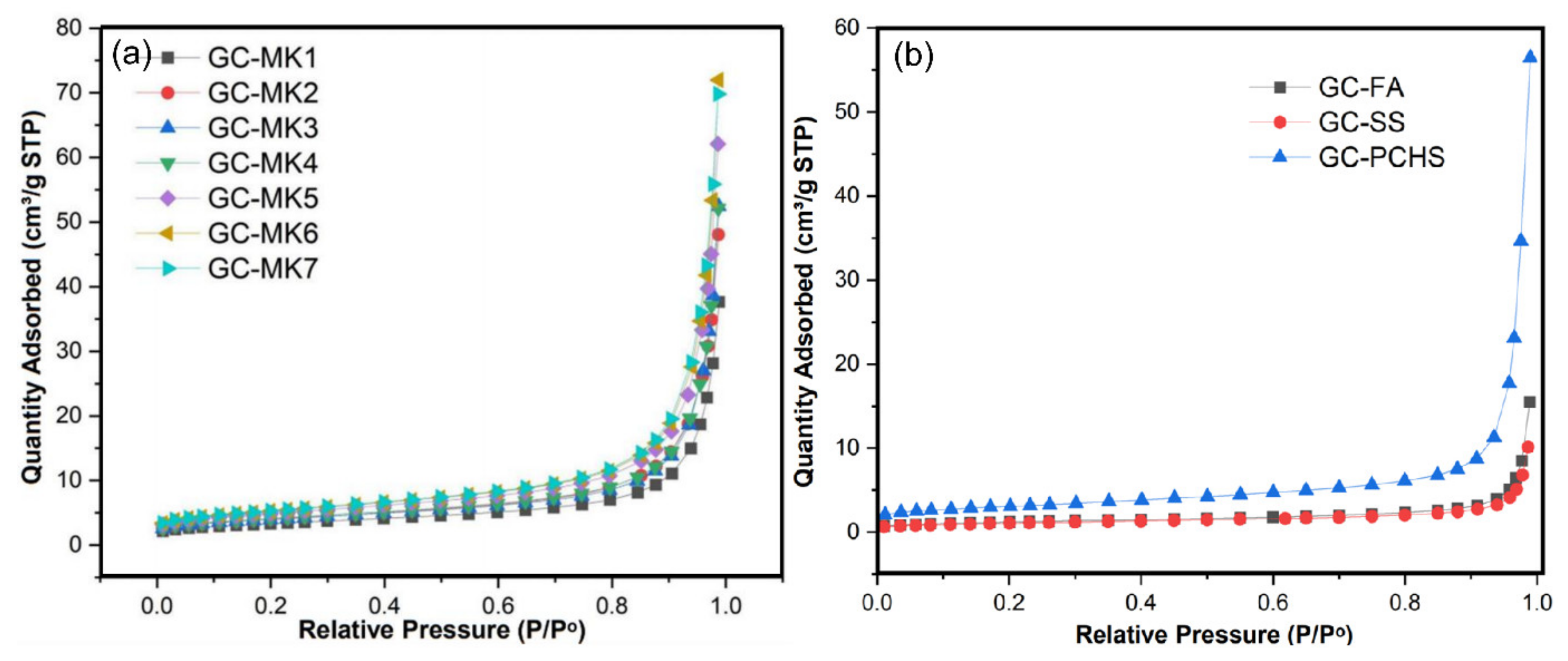
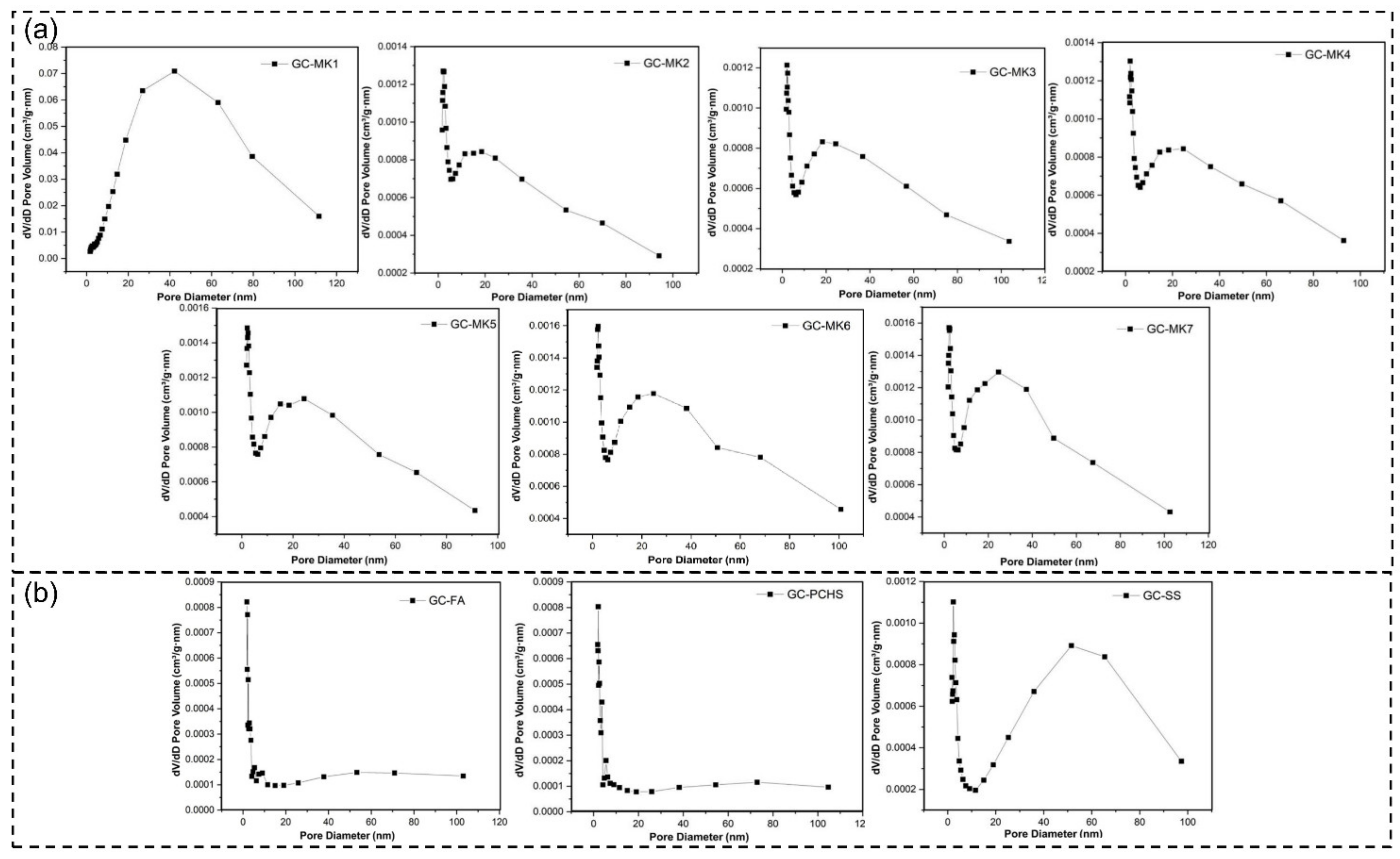
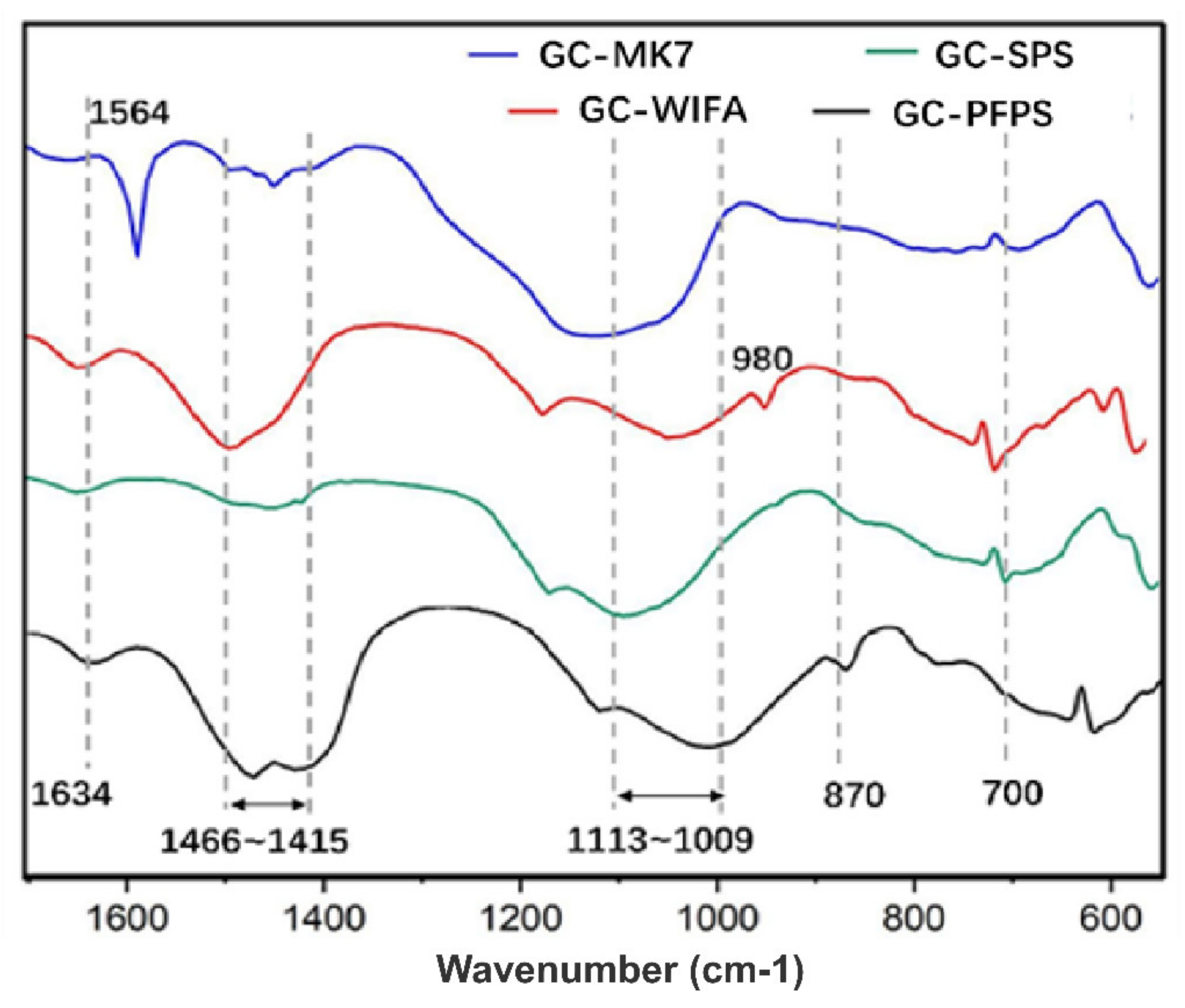
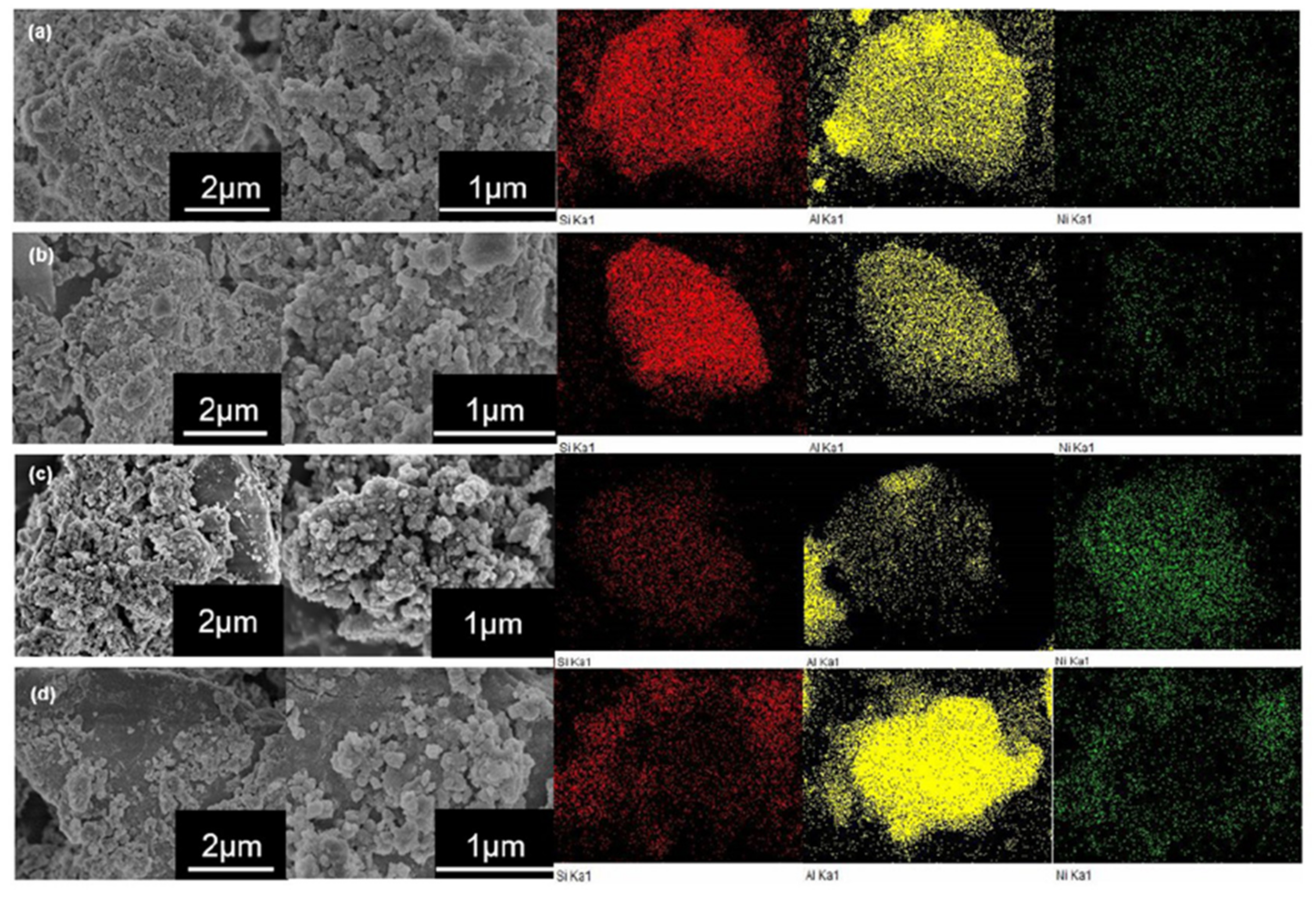
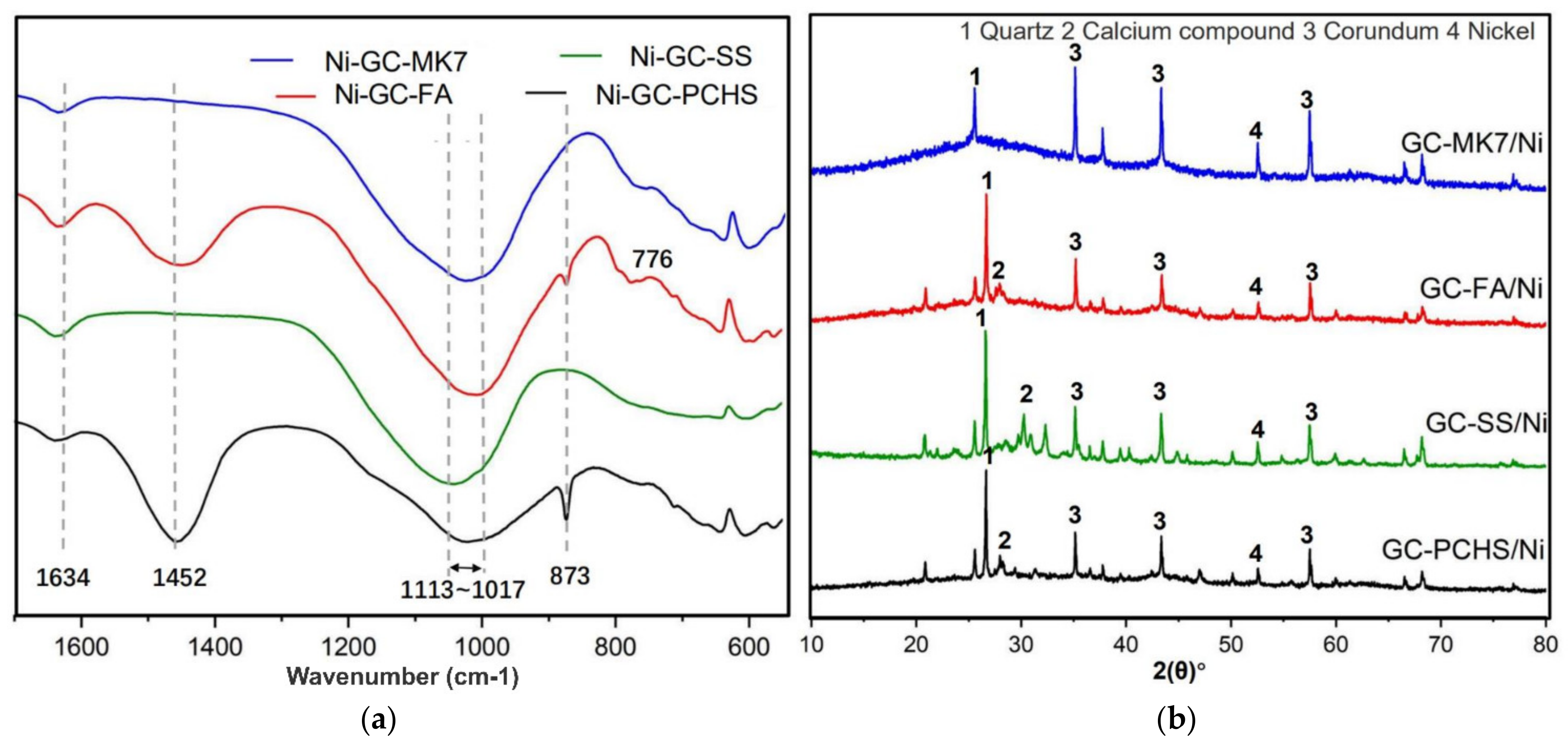

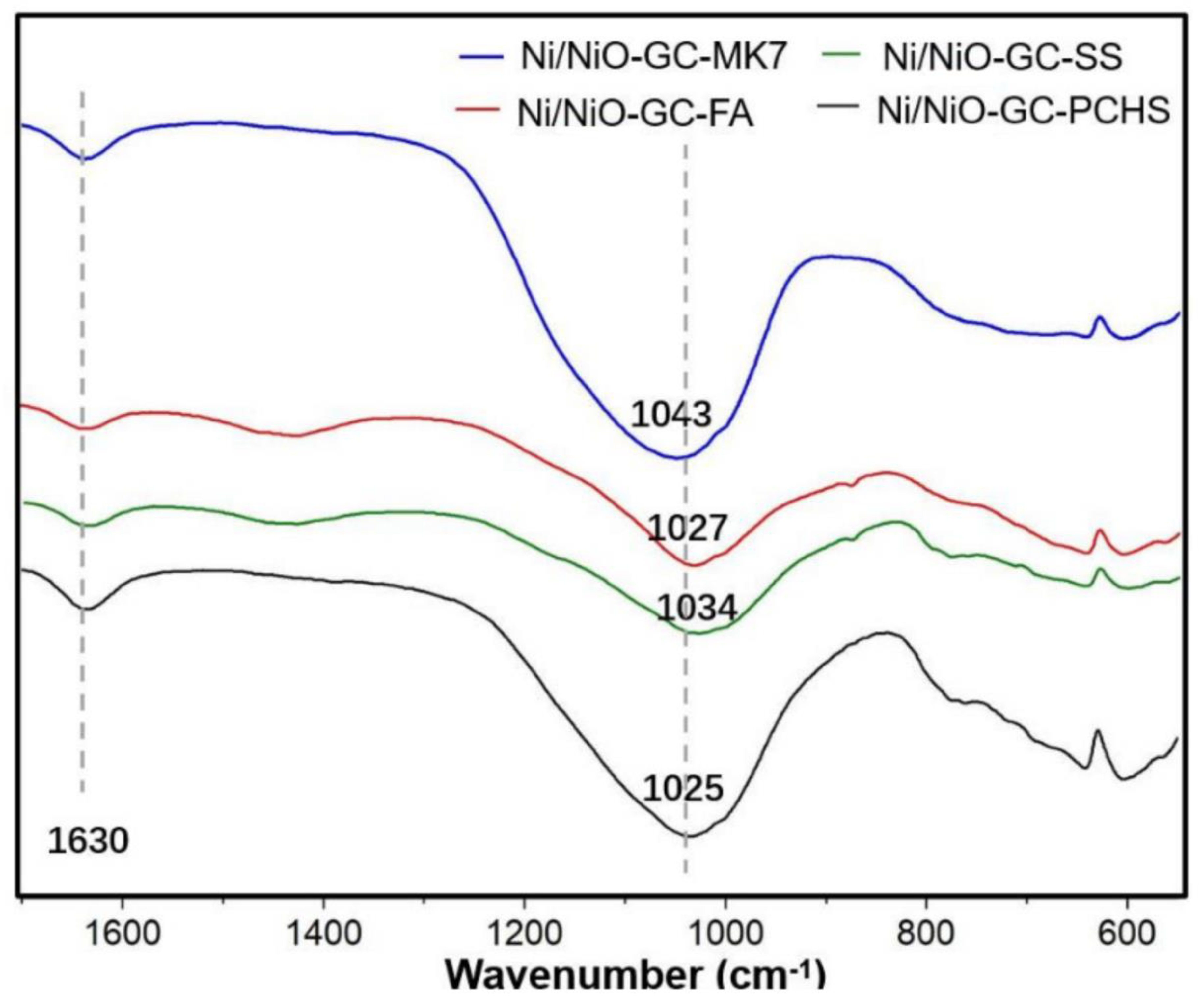
| GC–MK1 | GC–MK2 | GC–MK3 | GC–MK4 | GC–MK5 | GC–MK6 | GC–MK7 | GC–WIFA | GC–SPS | GC–PFPS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Specific surface area (m2/g) | 11.53 | 13.92 | 13.81 | 14.44 | 17.08 | 18.38 | 18.56 | 4.29 | 10.72 | 3.80 |
| Pore size (nm) | 18.64 | 20.24 | 20.24 | 21.38 | 21.85 | 22.89 | 22.64 | 22.27 | 32.61 | 16.48 |
| Pore volume (cm3/g) | 0.054 | 0.070 | 0.076 | 0.077 | 0.093 | 0.110 | 0.110 | 0.024 | 0.087 | 0.015 |
| Name | SiO2 | Al2O3 | Fe2O 3 | CaO | MgO | K | Na | S | Cl |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| WIFA | 7.58 | 3.07 | 2.46 | 30.12 | 1.75 | 9.68 | 9.01 | 3.82 | 32.79 |
| SPS | 33.52 | 15.89 | 10.64 | 9.21 | 3.32 | 4.16 | 1.38 | 8.25 | 0.53 |
| PFPS | 38.39 | 11.07 | 6.73 | 13.27 | 2.72 | 2.28 | 0.64 | 2.09 | 0.11 |
| MK | 54.00 | 43.00 | 1.00 | – | – | – | – | – | – |
| Support | Active Metal | Reaction Conditions | Yield | Reference |
|---|---|---|---|---|
| None | Ni | 250 °C, 150 min | 93% | [33] |
| None | Ni/NiO | 120 °C, 120 min | >99% | [32] |
| γ–Al2O3 | Ni (15 wt%) | 200 °C, 240min | 92% | [34] |
| SiO2 | Ni (50%) | 200 °C, 60 min | 40% | [20] |
| HZSM–5 | Ni (5 wt%) | 210 °C, 120 min | 100% | [18] |
| Carbon nanotubes | Ni (10 wt%) | 180 °C, 360 min | >90% | [23] |
| Zeolite materials | Ni (10 wt%) | 200 °C, 180 min | 94% | this work (maximum) |
| Zeolite materials | Ni/NiO (10 wt%) | 200 ℃, 240 min | 98% | this work (maximum) |
| Catalyst | Reaction Conditions | Conversion | Yield |
|---|---|---|---|
| Ni/NiO–GC–MK7 | 200 °C, 240 min | 100.00% | 94.18% |
| Ni/NiO–GC–WIFA | 200 °C, 240 min | 100.00% | 91.77% |
| Ni/NiO–GC–SPS | 200 °C, 240 min | 100.00% | 81.71% |
| Ni/NiO–GC–PFPS | 200 °C, 240 min | 100.00% | 58.50% |
| Test Element | WIFA | PFPS | SPS | Standard Limit Value (Class Ⅰ Standard of GB8978–1996) |
|---|---|---|---|---|
| Cu | 0.045 | 0.26 | 0.027 | ≤0.5 |
| Zn | 0.348 | 1.988 | 0.149 | ≤2 |
| Pb | 0.151 | 0.097 | 0.004 | ≤1 |
| Cd | – | – | 0.001 | ≤0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Lu, X.; Xiong, J.; Yu, Z.; Wang, Y.; Cui, J.; Zhang, R.; Weng, R. Solid–Waste–Derived Geopolymer–Type Zeolite–like High Functional Catalytic Materials Catalyze Efficient Hydrogenation of Levulinic Acid. Catalysts 2022, 12, 1361. https://doi.org/10.3390/catal12111361
Feng W, Lu X, Xiong J, Yu Z, Wang Y, Cui J, Zhang R, Weng R. Solid–Waste–Derived Geopolymer–Type Zeolite–like High Functional Catalytic Materials Catalyze Efficient Hydrogenation of Levulinic Acid. Catalysts. 2022; 12(11):1361. https://doi.org/10.3390/catal12111361
Chicago/Turabian StyleFeng, Wenli, Xuebin Lu, Jian Xiong, Zhihao Yu, Yilin Wang, Jianguo Cui, Rui Zhang, and Rengui Weng. 2022. "Solid–Waste–Derived Geopolymer–Type Zeolite–like High Functional Catalytic Materials Catalyze Efficient Hydrogenation of Levulinic Acid" Catalysts 12, no. 11: 1361. https://doi.org/10.3390/catal12111361
APA StyleFeng, W., Lu, X., Xiong, J., Yu, Z., Wang, Y., Cui, J., Zhang, R., & Weng, R. (2022). Solid–Waste–Derived Geopolymer–Type Zeolite–like High Functional Catalytic Materials Catalyze Efficient Hydrogenation of Levulinic Acid. Catalysts, 12(11), 1361. https://doi.org/10.3390/catal12111361