Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process
Abstract
1. Introduction
2. Results and Discussion
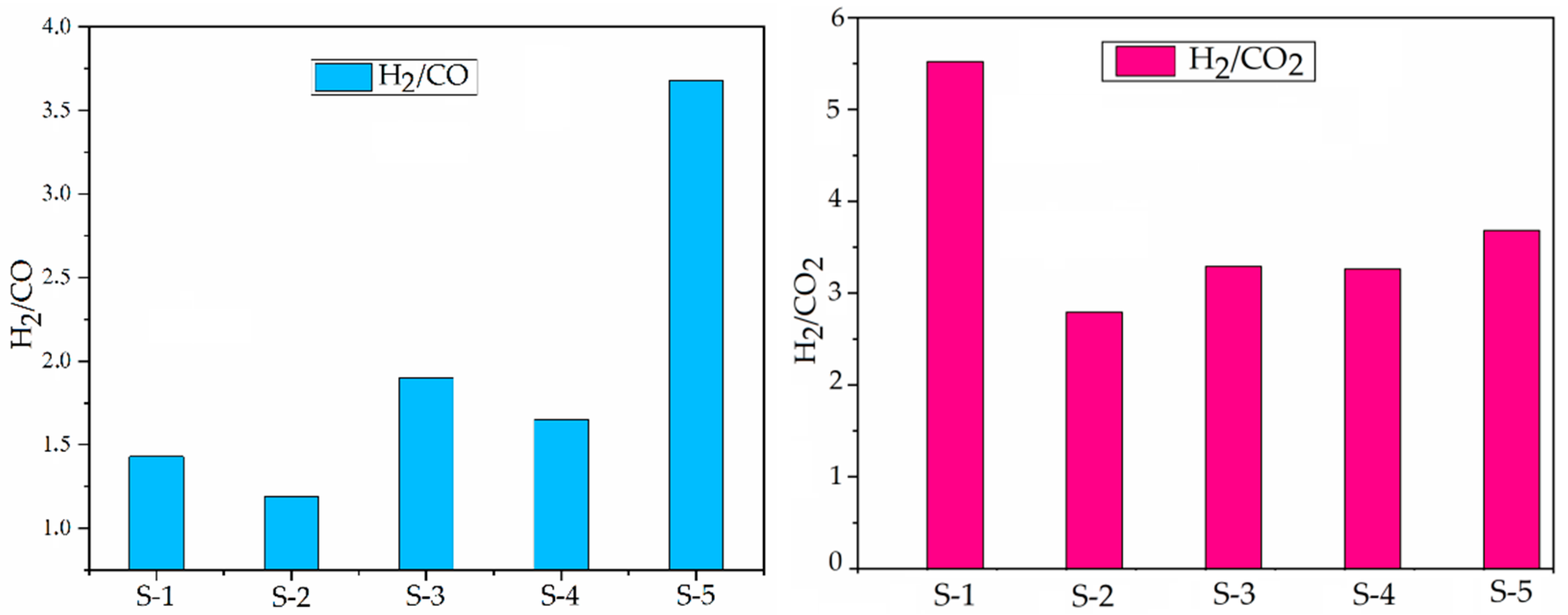
2.1. Hydrothermal Catalytic Effect on Gaseous Production in the Presence of Catalysts
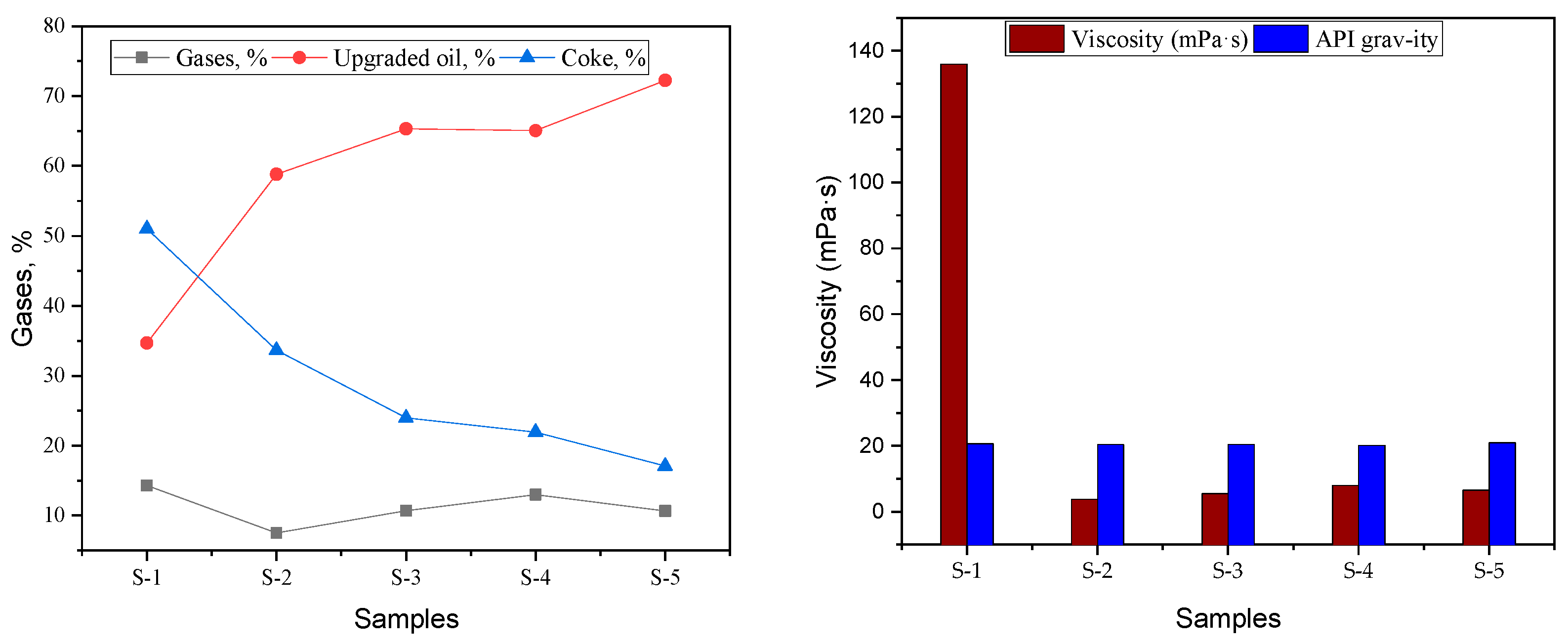
2.2. Product Conversion and Distribution in the Upgraded Products
2.3. SARA Analysis of Initial and Upgraded Oil
2.3.1. Saturate Fractions
2.3.2. Aromatic Fractions
2.3.3. Resin and Asphaltene Fractions
2.4. Elemental Analysis of Heavy Oil before and after Thermal, Hydrothermal, and Catalytic Upgrading
2.5. Aromatic Identification in the Upgraded Oil by GC–MS
3. Experimental Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthesis of Catalysts
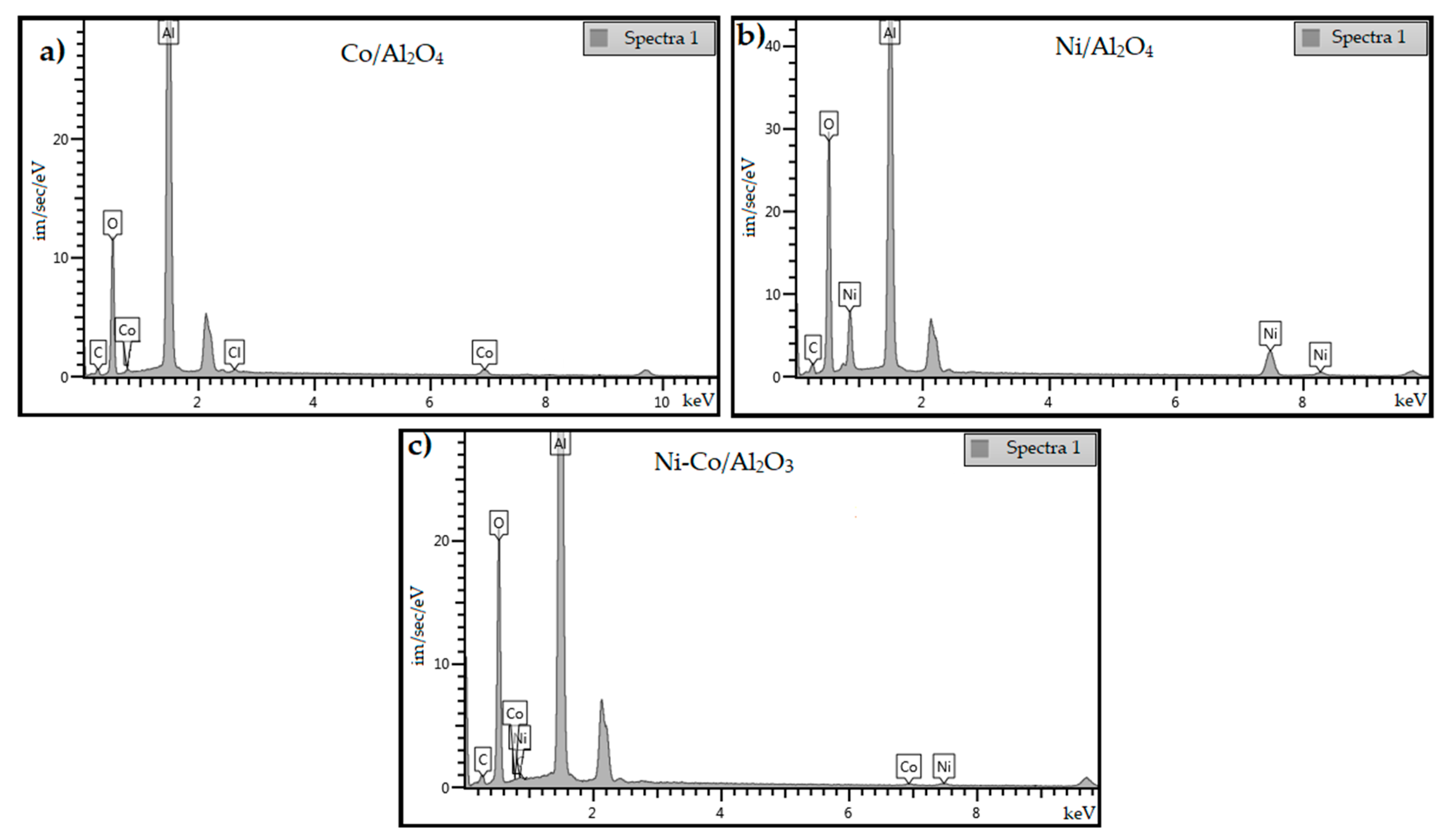
3.4. Catalyst Characterization
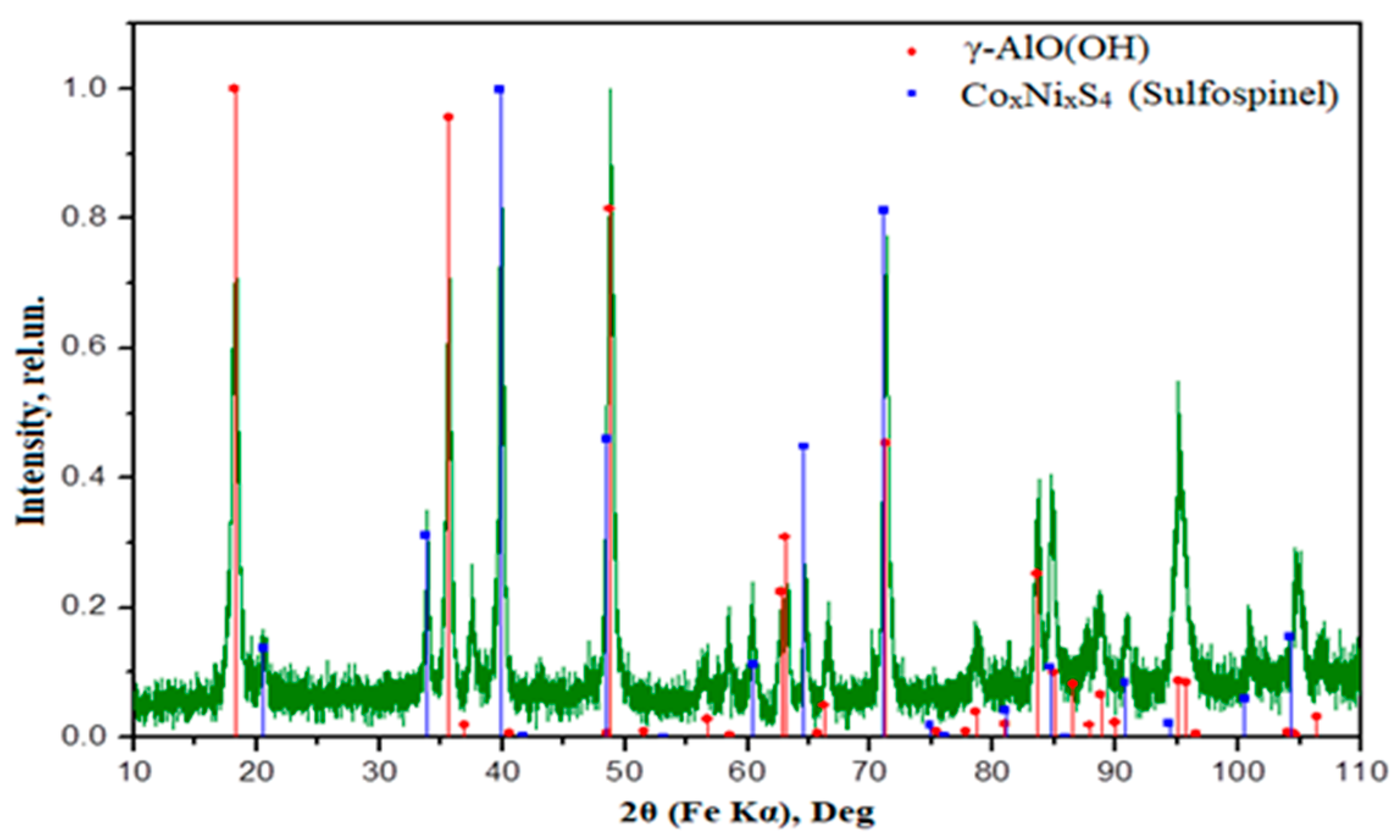
3.4.1. XRD Analysis
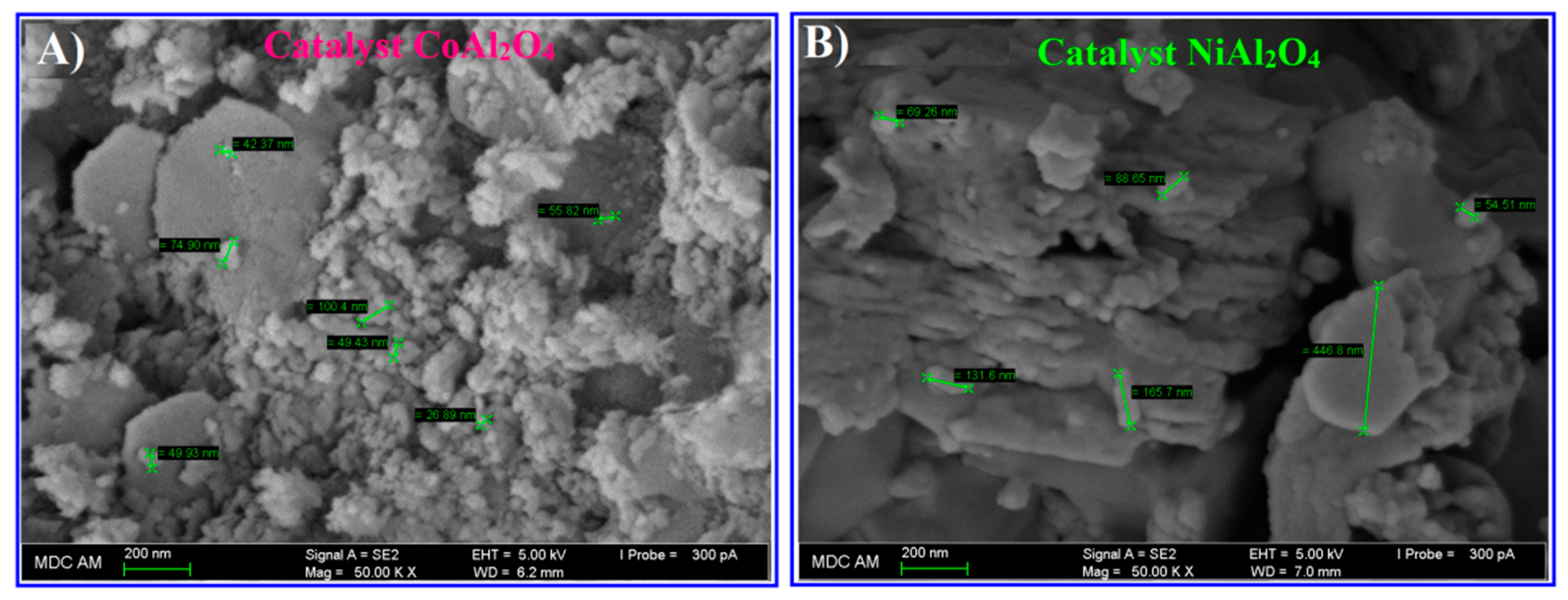
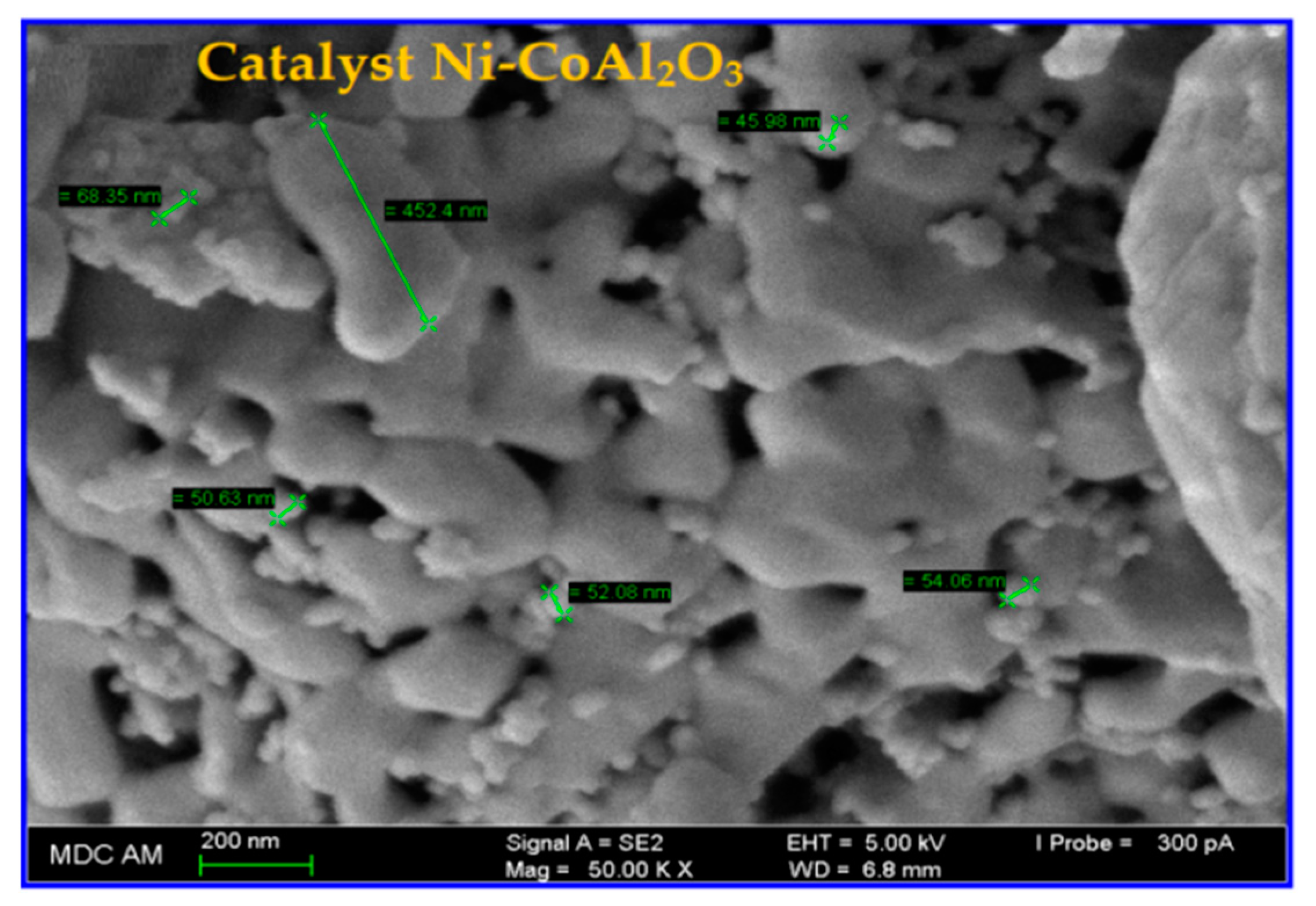
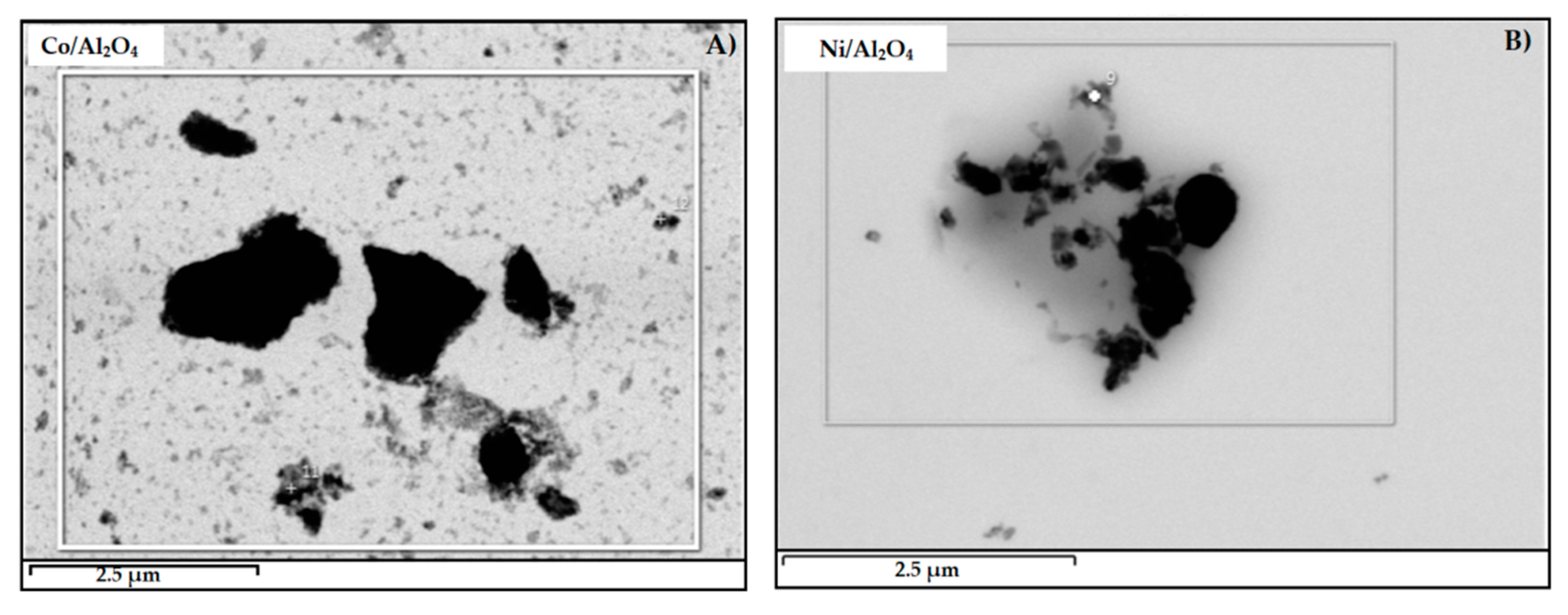
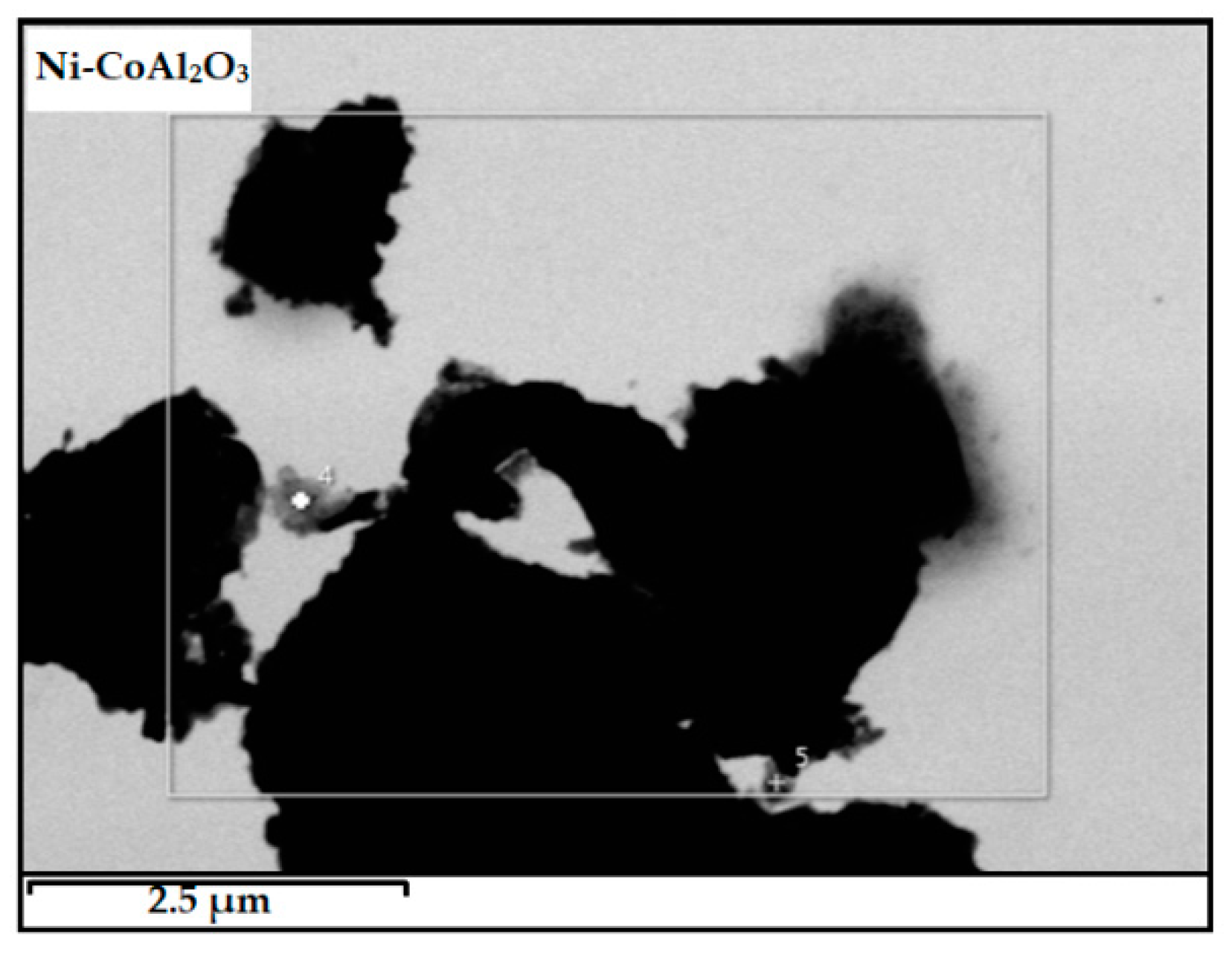
3.4.2. SEM/TEM Analyses
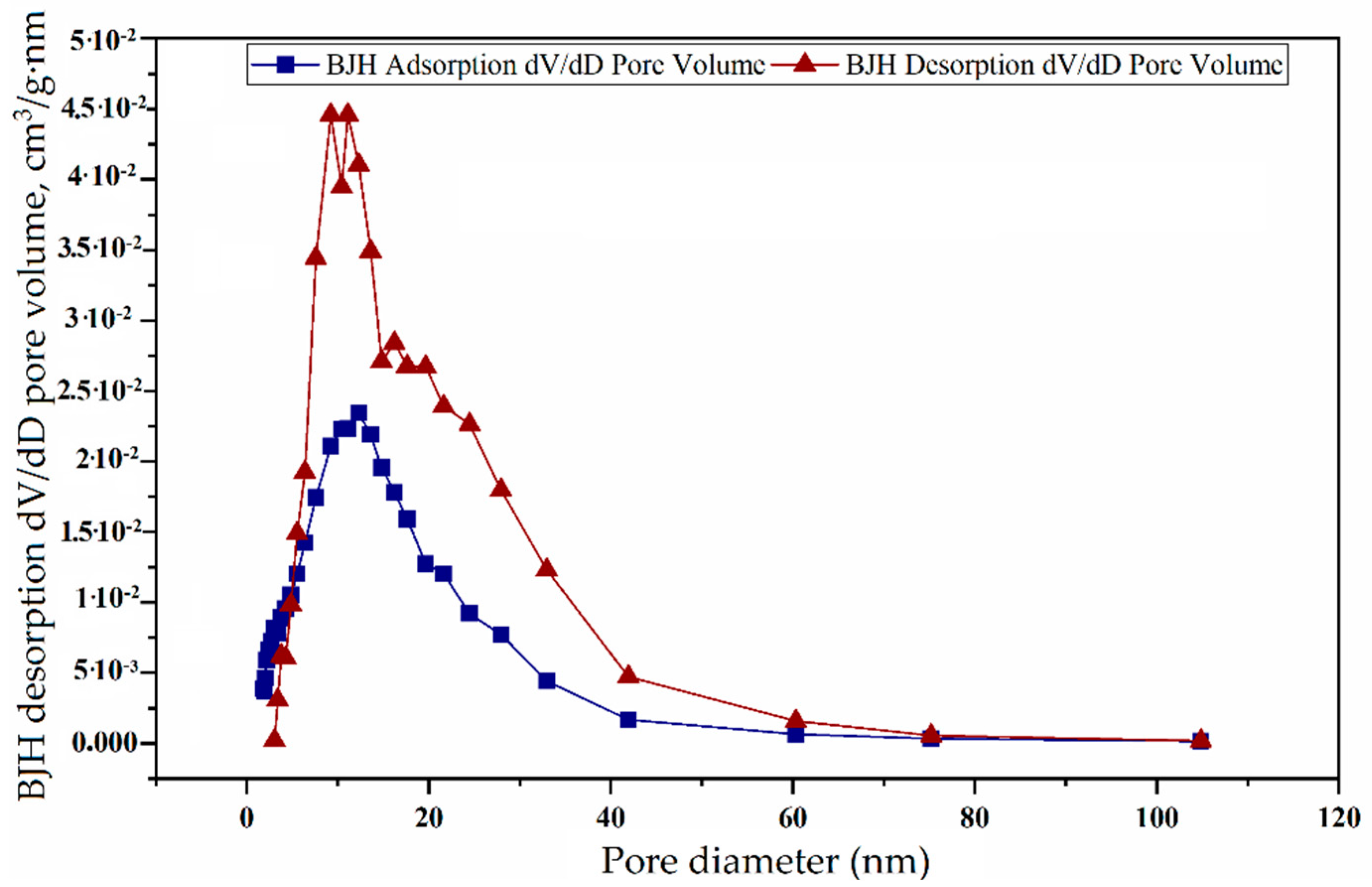
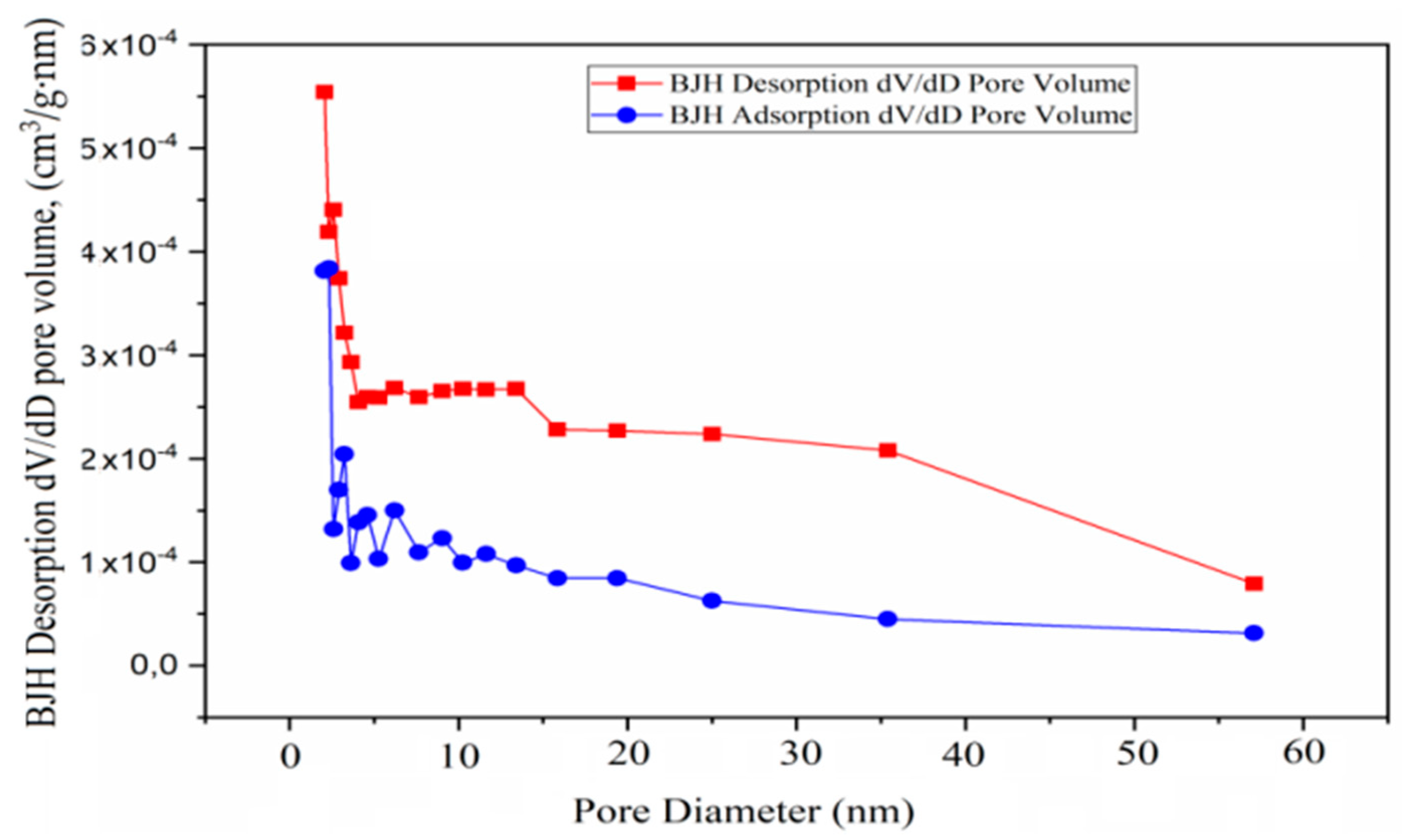
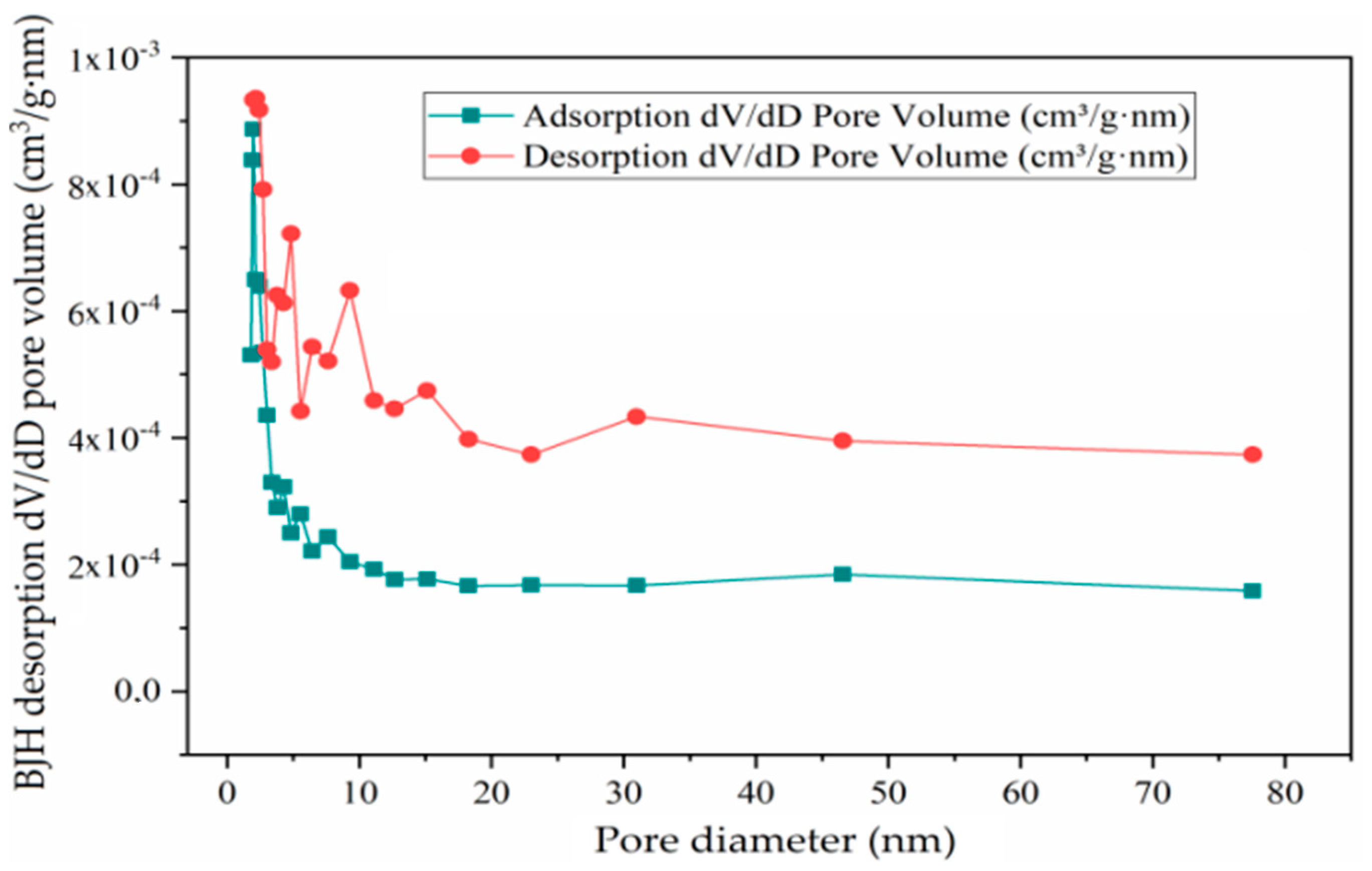
3.4.3. Analyses and Determination of Specific Surface Area and Pore Volume
3.4.4. BJH Adsorption and Desorption of Catalysts
3.5. Extra-Heavy Oil Hydrothermal Processing Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorin, F. World Energy Outlook 2006: The International Energy Agency (IEA) Report. Rev. Gen. Nucl. 2006, 6–8. [Google Scholar]
- IEA Global Hydrogen Review 2021; IEA: Paris, France, 2021.
- Brandon, N.P.; Kurban, Z. Clean Energy and the Hydrogen Economy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160400. [Google Scholar] [CrossRef] [PubMed]
- Qyyum, M.A.; Dickson, R.; Shah, S.F.A.; Niaz, H.; Khan, A.; Liu, J.J.; Lee, M. Availability, Versatility, and Viability of Feedstocks for Hydrogen Production: Product Space Perspective. Renew. Sustain. Energy Rev. 2021, 145, 110843. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. In Proceedings of the Conference Papers in Science; Hindawi: London, UK, 2013; Volume 2013. [Google Scholar]
- Song, C. Fuel Processing for Low-Temperature and High-Temperature Fuel Cells: Challenges, and Opportunities for Sustainable Development in the 21st Century. Catal. Today 2002, 77, 17–49. [Google Scholar] [CrossRef]
- McHugh, K.; Eisele, S.; Nestell, J. Hydrogen Production Methods; MPRWP-0001; MPR Associates. Inc.: Alexandria, VI, USA, 2005. [Google Scholar]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A Review on Perovskite Catalysts for Reforming of Methane to Hydrogen Production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Krummenacher, J.J.; West, K.N.; Schmidt, L.D. Catalytic Partial Oxidation of Higher Hydrocarbons at Millisecond Contact Times: Decane, Hexadecane, and Diesel Fuel. J. Catal. 2003, 215, 332–343. [Google Scholar] [CrossRef]
- Steinberg, M. A Highly Efficient Combined Cycle Fossil and Biomass Fuel Cell Power Generation and Hydrogen Production Plant with Zero CO2 Emission. In Proceedings of the International Conference on Fuel Cell Science, Engineering and Technology, Kyiv, Ukraine, 6–10 June 2004; Volume 41650, pp. 401–408. [Google Scholar]
- Chum, H.L.; Overend, R.P. Biomass and Renewable Fuels. Fuel Process. Technol. 2001, 71, 187–195. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion Characteristics of Different Biomass Fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Holladay, J.D.; Wang, Y.; Jones, E. Review of Developments in Portable Hydrogen Production Using Microreactor Technology. Chem. Rev. 2004, 104, 4767–4790. [Google Scholar] [CrossRef]
- Weingärtner, H.; Franck, E.U. Supercritical Water as a Solvent. Angew. Chemie Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ren, Y.; Ou, G.; Jin, H. Influence of Special Water Properties Variation on the Heat Transfer of Supercritical Water Flow around a Sphere. Chem. Eng. Sci. 2020, 222, 115698. [Google Scholar] [CrossRef]
- Fois, E.S.; Sprik, M.; Parrinello, M. Properties of Supercritical Water: An Ab Initio Simulation. Chem. Phys. Lett. 1994, 223, 411–415. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A Review on Subcritical and Supercritical Water Gasification of Biogenic, Polymeric and Petroleum Wastes to Hydrogen-Rich Synthesis Gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Fedyaeva, O.N.; Grin’ko, A.A.; Vostrikov, A.A. Structural Group Characteristics of Resins and Asphaltenes of High-Sulfur Natural Asphaltite and Products of Its Conversion in Supercritical Water. Pet. Chem. 2020, 60, 668–674. [Google Scholar] [CrossRef]
- Cocero, M.J.; Calvo, L. Supercritical Fluid Extraction of Sunflower Seed Oil with CO2-ethanol Mixtures. J. Am. Oil Chem. Soc. 1996, 73, 1573–1578. [Google Scholar] [CrossRef]
- Bruges, E.A.; Gibson, M.R. Dynamic Viscosity of Compressed Water to 10 Kilobar and Steam to 1500 C. J. Mech. Eng. Sci. 1969, 11, 189–205. [Google Scholar] [CrossRef]
- Yoshii, N.; Miura, S.; Okazaki, S. A Molecular Dynamics Study of Dielectric Constant of Water from Ambient to Sub-and Supercritical Conditions Using a Fluctuating-Charge Potential Model. Chem. Phys. Lett. 2001, 345, 195–200. [Google Scholar] [CrossRef]
- Redelius, P. Bitumen Solubility Model Using Hansen Solubility Parameter. Energy Fuels 2004, 18, 1087–1092. [Google Scholar] [CrossRef]
- Chham, A.; Khouya, E.; Abourriche, A.K.; Oumam, M.; Gmouh, S.; Mansouri, S.; El Harti, M.; Hannache, H. Supercritical Water Extraction and Characterization of Moroccan Shale Oil by Different Solvent. J. Mater. Environ. Sci. 2018, 9, 1771–1778. [Google Scholar]
- Duan, P.-G.; Yang, S.-K.; Xu, Y.-P.; Wang, F.; Zhao, D.; Weng, Y.-J.; Shi, X.-L. Integration of Hydrothermal Liquefaction and Supercritical Water Gasification for Improvement of Energy Recovery from Algal Biomass. Energy 2018, 155, 734–745. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Feoktistov, D.A.; Vakhin, A.V. Iron Oxide Nanoparticles Impact on Improving Reservoir Rock Minerals Catalytic Effect on Heavy Oil Aquathermolysis. Fuel 2022, 327, 124956. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.M.A.; Tajik, A.; Lapuk, S.E.S.E.; Rezaeisadat, M.; Eskin, A.A.A.A.; Rodionov, N.O.N.O.; Vakhin, A.V.A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Wu, Y.; Gao, J.; Zhang, Q. Advances in Metal–Organic Frameworks and Their Derivatives for Diverse Electrocatalytic Applications. Electrochem. Commun. 2021, 126, 107024. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F. Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes; Springer: Berlin/Heidelberg, Germany, 2010; Volume 33, ISBN 9048136962. [Google Scholar]
- Zalesskiy, S.S.; Ananikov, V.P. Pd2 (Dba) 3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis. Organometallics 2012, 31, 2302–2309. [Google Scholar] [CrossRef]
- Bahrami, F.; Panahi, F.; Daneshgar, F.; Yousefi, R.; Shahsavani, M.B.; Khalafi-Nezhad, A. Synthesis of New α-Aminophosphonate Derivatives Incorporating Benzimidazole, Theophylline and Adenine Nucleobases Using l-Cysteine Functionalized Magnetic Nanoparticles (LCMNP) as Magnetic Reusable Catalyst: Evaluation of Their Anticancer Properties. RSC Adv. 2016, 6, 5915–5924. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 Catalysts with High CO2 Methanation Activity and High CH4 Selectivity at Low Temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Gao, J.; Zhang, Q. Recent Advances in Vacancy Engineering of Metal-organic Frameworks and Their Derivatives for Electrocatalysis. SusMat 2021, 1, 66–87. [Google Scholar] [CrossRef]
- Hossain, M.M. Upgrading of Heavy Oil in Supercritical Water Using an Iron Based Multicomponent Catalyst. Int. J. Chem. React. Eng. 2017, 15, 161–167. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Upgrading of Crude Algal Bio-Oil in Supercritical Water. Bioresour. Technol. 2011, 102, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Remón, J.; Arauzo, J.; García, L.; Arcelus-Arrillaga, P.; Millan, M.; Suelves, I.; Pinilla, J.L. Bio-Oil Upgrading in Supercritical Water Using Ni-Co Catalysts Supported on Carbon Nanofibres. Fuel Process. Technol. 2016, 154, 178–187. [Google Scholar] [CrossRef]
- You, X.; Wang, X.; Ma, Y.; Liu, J.; Liu, W.; Xu, X.; Peng, H.; Li, C.; Zhou, W.; Yuan, P. Ni-Co/Al2O3 Bimetallic Catalysts for CH4 Steam Reforming: Elucidating the Role of Co for Improving Coke Resistance. ChemCatChem 2014, 6, 3377–3386. [Google Scholar] [CrossRef]
- Nyankson, E.; Efavi, J.K.; Yaya, A.; Manu, G.; Asare, K.; Daafuor, J.; Abrokwah, R.Y. Synthesis and Characterisation of Zeolite-A and Zn-Exchanged Zeolite-A Based on Natural Aluminosilicates and Their Potential Applications. Cogent. Eng. 2018, 5, 1440480. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Fatemi, S.; Ahmadi, S.J.; Morimoto, M.; Akizuki, M.; Oshima, Y.; Fumoto, E. The Synergistic Effect between Supercritical Water and Redox Properties of Iron Oxide Nanoparticles during In-Situ Catalytic Upgrading of Heavy Oil with Formic Acid. Isotopic Study. Appl. Catal. B Environ. 2018, 230, 91–101. [Google Scholar] [CrossRef]
- Dejhosseini, M.; Aida, T.; Watanabe, M.; Takami, S.; Hojo, D.; Aoki, N.; Arita, T.; Kishita, A.; Adschiri, T. Catalytic Cracking Reaction of Heavy Oil in the Presence of Cerium Oxide Nanoparticles in Supercritical Water. Energy Fuels 2013, 27, 4624–4631. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Reina, T.R.; Bulavchenko, O.A.; Saraev, A.A.; Gerasimov, E.Y.; Zaikina, O.O.; Bermúdez, J.M.; Arcelus-Arrillaga, P.; Yakovlev, V.A.; Millan, M. Phenanthrene Catalytic Cracking in Supercritical Water: Effect of the Reaction Medium on NiMo/SiO2 Catalysts. Catal. Today 2019, 329, 197–205. [Google Scholar] [CrossRef]
- Vogelaar, B.M.; Makkee, M.; Moulijn, J.A. Applicability of Supercritical Water as a Reaction Medium for Desulfurisation and Demetallisation of Gasoil. Fuel Process. Technol. 1999, 61, 265–277. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Timko, M.T.; Class, C.A.; Bonomi, R.E.; Kida, Y.; Hernandez, H.H.; Tester, J.W.; Green, W.H. Supercritical Water Desulfurization of Organic Sulfides Is Consistent with Free-Radical Kinetics. Energy Fuels 2013, 27, 6108–6117. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Martins, A.R.; Reyes, P.; Oportus, M.; Albonoz, A.; Vicentini, V.; do Carmo Rangel, M. Preparation and Characterization of Ru/MgO-Al2O3 Catalysts for Methane Steam Reforming. Catal. Today 2009, 142, 52–60. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the Economic and Environmental Impact of Combining Dry Reforming with Steam Reforming of Methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry Reforming of Methane Has No Future for Hydrogen Production: Comparison with Steam Reforming at High Pressure in Standard and Membrane Reactors. Int. J. Hydrog. Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Prakash, G.K.S. Bi-Reforming of Methane from Any Source with Steam and Carbon Dioxide Exclusively to Metgas (CO–2H2) for Methanol and Hydrocarbon Synthesis. J. Am. Chem. Soc. 2013, 135, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Sugimoto, Y.; Saotome, Y.; Sato, S.; Takanohashi, T. Effect of Supercritical Water on Upgrading Reaction of Oil Sand Bitumen. J. Supercrit. Fluids 2010, 55, 223–231. [Google Scholar] [CrossRef]
- Hart, A.; Omajali, J.B.; Murray, A.J.; Macaskie, L.E.; Greaves, M.; Wood, J. Comparison of the Effects of Dispersed Noble Metal (Pd) Biomass Supported Catalysts with Typical Hydrogenation (Pd/C, Pd/Al2O3) and Hydrotreatment Catalysts (CoMo/Al2O3) for in-Situ Heavy Oil Upgrading with Toe-to-Heel Air Injection (THAI). Fuel 2016, 180, 367–376. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D. Oil Dispersed Nickel-Based Catalyst for Catalytic Upgrading of Heavy Oil Using Supercritical Water. Fuel 2021, 313, 122702. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Suwaid, M.A.; Feoktistov, D.A.; Rakhmatullin, I.Z.; Milovankin, A.A.; Murzakhanov, F.; Morozov, V. Deep Insights into Heavy Oil Upgrading Using Supercritical Water by a Comprehensive Analysis of GC, GC–MS, NMR, and SEM–EDX with the Aid of EPR as a Complementary Technical Analysis. ACS Omega 2021, 6, 135–147. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntasser, A.A.; Suweid, M.A.; Osin Yu, N.; Diop, F.S.; Mustafina, A.N.; Garaeva, D.I. Upgrading of Heavy Oil under the Exposition of Supercritical Water at Different Temperatures. Oктябрь 2018. [Google Scholar] [CrossRef]
- Rakhmatullin, I.; Efimov, S.; Tyurin, V.; Gafurov, M.; Al-Muntaser, A.; Varfolomeev, M.; Klochkov, V. Qualitative and Quantitative Analysis of Heavy Crude Oil Samples and Their SARA Fractions with 13C Nuclear Magnetic Resonance. Processes 2020, 8, 995. [Google Scholar] [CrossRef]
- Yen, T.F.; Erdman, J.G.; Pollack, S.S. Investigation of the Structure of Petroleum Asphaltenes by X-Ray Diffraction. Anal. Chem. 1961, 33, 1587–1594. [Google Scholar] [CrossRef]




| Samples | Chemical Elements, wt.% | |||||
|---|---|---|---|---|---|---|
| C | H | S | N | (Me + O) * | H/C | |
| S-0 | 84.38 | 9.78 | 1.84 | 0.47 | 3.53 | 1.39 |
| S-1 | 87.06 | 9.82 | 1.42 | 0.92 | 0.78 | 1.35 |
| S-2 | 87.07 | 10.26 | 1.57 | 0.69 | 0.41 | 1.41 |
| S-3 | 86.40 | 10.83 | 1.53 | 0.71 | 0.53 | 1.50 |
| S-4 | 86.31 | 11.03 | 1.55 | 0.69 | 0.42 | 1.53 |
| S-5 | 85.85 | 11.69 | 1.35 | 0.68 | 0.43 | 1.63 |
| Compounds | S-0 | S-1 | S-2 | S-3 | S-4 | S-5 |
|---|---|---|---|---|---|---|
| Alkanes | 0.67 | 23.30 | 2.47 | 3.01 | 27.80 | 0.25 |
| Monoaromatics | 4.22 | 18.05 | 44.93 | 35.50 | 1.75 | 48.74 |
| Diaromatics | 55.61 | 9.653 | 35.50 | 50.58 | 9.13 | 34.75 |
| Polyaromatic | 37.46 | 48.98 | 13.28 | 10.32 | 61.32 | 9.09 |
| Viscosity (mPa·s) | API Gravity | SARA Fractions wt.% | Elemental Analysis wt.% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S | A | R | A | C | H | N | S | ||
| 61·106 a | 4.5 | 26.11 | 19.30 | 20.05 | 24.90 | 84.8 | 9.78 | 0.33 | 1.84 |
| Catalysts | Nickel | Cobalt | Aluminum Oxide | Oxygen |
|---|---|---|---|---|
| (Ni) | (Co) | (γ-Al2O3) | (O2) | |
| CoAl2O4 | 0.00 | 3.55 | 54.32 | 42.11 |
| NiAl2O4 | 12.15 | 0.00 | 35.52 | 52.31 |
| Ni-CoAl2O3 | 2.71 | 1.69 | 46.05 | 49.53 |
| Nomination | Co/Al2O4 | Ni/Al2O4 | Ni-CoAl2O3 |
|---|---|---|---|
| Average particle size, nm | 37.0355 | 944.33 | 874.8551 |
| TEM, nm | 620.55 | 1851 | 1071 |
| Micropore volume: cm3/g | 0.004882 | 0.000279 | 0.000377 |
| External surface area: m2/g | 147.9614 | 3.632 | 7.4253 |
| Total surface area (BET): m2/g | 162.006 | 3.039 | 6.8583 |
| Adsorption pore diameter (4V/A by BET), nm | 12.205 | 16.19 | 22.054 |
| Desorption pore diameter (4V/A by BET), nm | 11.758 | 7.999 | 9.457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djimasbe, R.; Ilyasov, I.R.; Kwofie, M.; Khelkhal, M.A.; Emelianov, D.A.; Al-Muntaser, A.A.; Suwaid, M.A.; Varfolomeev, M.A. Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process. Catalysts 2022, 12, 1183. https://doi.org/10.3390/catal12101183
Djimasbe R, Ilyasov IR, Kwofie M, Khelkhal MA, Emelianov DA, Al-Muntaser AA, Suwaid MA, Varfolomeev MA. Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process. Catalysts. 2022; 12(10):1183. https://doi.org/10.3390/catal12101183
Chicago/Turabian StyleDjimasbe, Richard, Ildar R. Ilyasov, Michael Kwofie, Mohammed A. Khelkhal, Dmitrii A. Emelianov, Ameen A. Al-Muntaser, Muneer A. Suwaid, and Mikhail A. Varfolomeev. 2022. "Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process" Catalysts 12, no. 10: 1183. https://doi.org/10.3390/catal12101183
APA StyleDjimasbe, R., Ilyasov, I. R., Kwofie, M., Khelkhal, M. A., Emelianov, D. A., Al-Muntaser, A. A., Suwaid, M. A., & Varfolomeev, M. A. (2022). Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process. Catalysts, 12(10), 1183. https://doi.org/10.3390/catal12101183







