Abstract
CO2 resourceful utilization contributes to the goal of carbon neutrality. Chemical Looping Dry Reforming (CLDR) has attracted significant attention as a method for converting CO2 to CO. NiFe2O4 oxygen carrier (OC) is found to be a potential material for CLDR. However, the migration process of lattice oxygen, which are critical for the conversion of CO2 to CO, was not extensively investigated. In this study, the reduction and oxidation degrees of the NiFe2O4 were finely modulated in a thermogravimetric analyzer. The lattice oxygen migration mechanism of the NiFe2O4 in redox cycles was characterized by means of X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and in-situ Raman. The novelty of this paper is clarifying the release-uptake paths of lattice oxygen during CO2 resourceful utilization. The result indicates that the concentration gradient between the surface and the bulk drives the diffusion of lattice oxygen. The stabilization of surface lattice oxygen content is attributed to the rapid migration of O anion, which is closely associated with the movement process of Ni particles inward and outward through the spinel bulk. In addition, a highly reactive chemical reaction interface consisting of lattice oxygen and the corresponding metal atoms is always present on the surface of the oxygen carrier and is confirmed by an in-situ Raman and XPS during the whole process of CLDR. The results of this paper offer reference and basis for further development and design of CLDR using spinel OC.
1. Introduction
Excessive emission of greenhouse gases (mainly CO2) by human beings is considered the primary factor leading to global warming. The CO2 resource utilization, an effective pathway to reduce greenhouse gas emissions, contributes to the goal of global carbon neutrality [1,2]. The chemical looping dry reforming (CLDR) has attracted much attention for converting CO2 to CO with the aid of oxygen carriers (OC) [3,4]. The CLDR separates a reforming process into three stages: (1) oxygen carrier reduction stage with a hydrocarbon fuel; (2) CO2 oxidation stage; (3) air oxidation stage. At the reduction stage, the OC is reduced to a metallic state (Me) and meanwhile partially oxidizes the hydrocarbon fuel into high-quality syngas; At the CO2 oxidation stage, the reduced oxygen carrier in the metallic state (Me) is oxidized by CO2 to replenish a part of its lattice oxygen to form a metal oxide with lower oxygen content (MeO1−x) and meanwhile CO2 is converted to CO; At the air oxidation stage, the partially oxygen-recovered OC (MeO1−x) is fully oxidized into its initial state (MeO), as shown in Figure 1. In the reduction stage, many fuels including but not limited to methane can be used as long as the oxygen carrier exhibits enough reactivity with the fuels in the CLDR process such as H2, CO, pyrolysis gas, etc. Due to the separation of the redox process, CLDR has a few of potential advantages such as inherent CO2 separation, highly efficient activation of CO2 with a minimal energy penalty, and low operating cost [5,6,7,8].
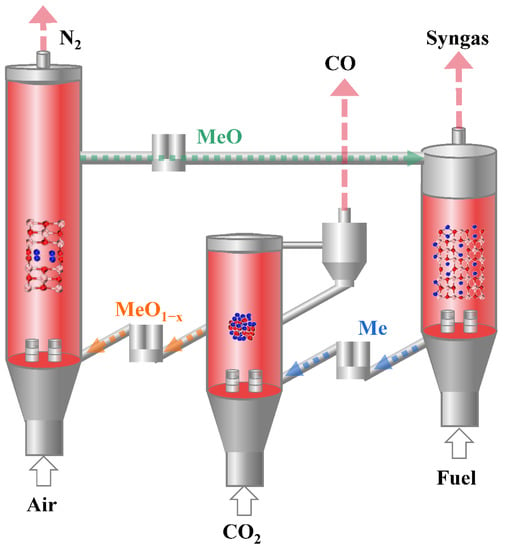
Figure 1.
Conceptual illustration of CLDR.
The OC is viewed as the cornerstone of the chemical looping dry reforming [9]. Due to the considerably steady molecule structure and low free energy characteristic of CO2, the capacity of oxygen carriers to maintain enough high reactivity toward CO2 reforming is regarded as the most critical issue [10]. Single metal (Co, Ce, Fe, etc.) oxides have been evaluated as oxygen carriers for the CLDR process [11,12]. Among them, Fe-based OC showed the highest CO2 reducibility. Tt is also abundant, low-cost, has more oxygen capacity, and is environmentally benign [10]. Thus, Fe-based OCs are considered excellent OC candidates for CLDR [13,14]. In addition, in order to further improve the reactivity of Fe-based oxygen carriers, the use of compound metal oxides (e.g., Ni/Fe, Mn/Fe, Co/Fe, Cu/Fe, and Ca/Fe) as oxygen carrier candidates has been proposed with promising prospect [15,16,17]. CoFeOy OC formed from Co-doped Fe-based oxygen carriers exhibited a high syngas yield and maintained stability for 10 cycles in a chemical looping reaction [18]. The synergistic effect of Co doping in ferrite materials to promote Fe reduction. Cu-doped Fe2O3 led to the formation of oxygen vacancies, which reduced the reaction activation energy [19], and its reaction rate remained high after 15 cycles. For the methane-carbon dioxide reaction system in CLDR, MnFe2O4 OC exhibits the maximum conversion of CH4 and CO2 are 63% and 99% [20]. Sun et al. designed a Ca2Fe2O5 OC [21], and the cyclic performance of reduced OC is much better than that of Fe2O3. Furthermore, several literatures indicate that the different metals form some unique crystalline structures, such as spinel (AB2O4) [22,23] and perovskites (ABO3) [24,25,26], can also obtain some excellent reactivity in CLDR.
In our group, we found that NiFe2O4 spinel OC is a potential OC for the CLDR of methane [27]. The capability of CO2 reduction of reduced NiFe2O4 OC is up to 238 mL/g, which is 2.14 times that of reduced Fe2O3 + NiO OC, which gives an average of 185 mL of CO was generated in a single cycle in the 10 successive redox cycles, indicating that the NiFe2O4 spinel OC shows a relatively stable and good recyclability [28]. As well, the NiFe2O4 OCs evidently improve the reactivity of Fe-based OCs.
It is known that all the reactions in the three stages of the CLDR involve the migration of lattice oxygen, therefore it is considered the key factor determining the reactivity of OC [29]. However, a lack of fundamental understanding of lattice oxygen migration paths in oxygen carriers limits high-performance oxygen carriers design and development. To date, some exploratory investigations on oxygen ion migration in oxygen carriers have been performed. Chen et al. [30] studied the role of oxygen species in cerium-based oxygen carriers at 450 °C using CH3SH as a model compound by XPS. they found that the improvement in catalytic stability is closely related to faster migration of bulk phase lattice oxygen. Liu et al. [31] studied the evolution of the active components of copper-iron-based oxygen carriers in the chemical looping combustion of CO, and the results of XPS analysis combined with DFT theory calculation indicated that the bulk phase lattice oxygen tends to migrate outward rather than CO diffusion inward into the particle. Zhao et al. [32] investigated the reaction mechanism of La1.6Sr0.4FeCoO6 oxygen carriers in chemical looping methane steam reforming using XRD and XPS. As well, the results showed that the reaction boundary was fixed on the surface of the oxygen carriers. The oxygen vacancies formed on the surface promoted the migration of lattice oxygen from the bulk phase to the surface. In addition, there are several literatures to discuss the oxygen transfer mechanisms, especially using the density functional theory (DFT) method [33,34]. But there is still a lack of in-depth and systematic understanding of lattice oxygen migration in the bulk of oxygen carriers. As well, experimental investigations on the lattice oxygen transfer in spinel OCs were not extensively investigated. It’s rarely found the studies on migration mechanism of lattice oxygen in CLDR to achieve CO2 resource utilization. Thus, the investigations on release-recovery paths of lattice oxygen are of great significance for understanding the oxidation–reduction reaction mechanisms of OCs.
In order to clarify the release-uptake paths of lattice oxygen during the CLDR process, a thermogravimetric analyzer (TGA) is used to control the reduction degree and oxidation degree of OCs. The migration of lattice oxygen and the evolution of active components during chemical looping reactions were studied by means of XPS, XRD, TEM-EDS, and in-situ Raman. The results of this paper offer reference and basis for further development and design of CLDR using spinel OC.
2. Results and Discussion
2.1. XRD Analysis
2.1.1. Crystalline Phase Evolution of the OC in Reduction
The changes in XRD patterns of OC in the reduction stage are shown in Figure 2. The XRD patterns of the as-synthesized OC showed 12 characteristic peaks, which were found to be in perfect agreement with the standard NiFe2O4 card 00-054-0964 indicating that the as-synthesized OC by the sol-gel method is pure NiFe2O4 spinel. When the oxygen carrier was reduced by CO for 5 min, two small Ni peaks (card: 03-065-0380, marked as C) are observed. It indicates that NiO in the NiFe2O4 is firstly reduced by releasing lattice oxygen and separated from the spinel structure to form free metallic Ni. Another phenomenon was observed in that the rest of the characteristic peaks were slightly broadened and weakened due to a part of NiFe2O4 converted to Fe3-xO4 (card: 01-086-1355, marked as B). NiFe2O4 and Fe3-xO4 are both spinel structures, and the XRD spectra of them are very consistent.
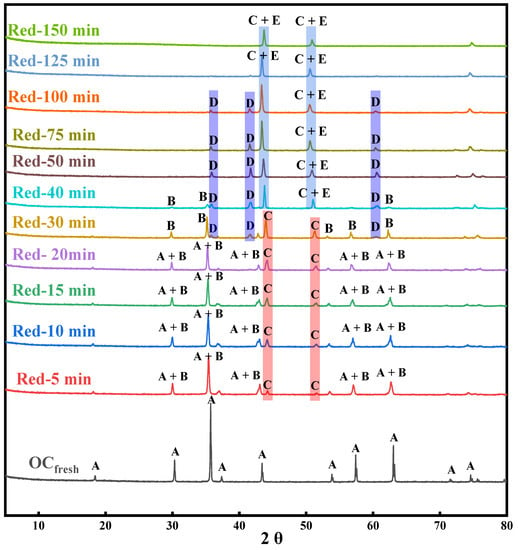
Figure 2.
XRD patterns of NiFe2O4 OC at different reduction times. (A: NiFe2O4, B: Fe3−xO4, C: Ni, D: Fe1−xO, E: Fe).
As the reduction degree deepens, three characteristic peaks of Fe1−xO (card: 01-089-0687) are observed (marked as D). It means that the spinel structure of NiFe2O4 and Fe3−xO4 gradually collapsed due to Fe3+ ions being continuously reduced. Finally, when reduction time is above 100 min, almost Ni and Fe cations are completely reduced into metallic Ni and Fe (card: 96-901-4712).
2.1.2. Crystalline Phase Evolution of the Reduced OC in Oxidation
The metallic Fe and Ni, as the completely reduced product of NiFe2O4 OC, show a stepwise oxidation behavior under a CO2 atmosphere. As well, no apparent carbon deposits were found during the CO2 oxidation process. As shown in Figure 3.
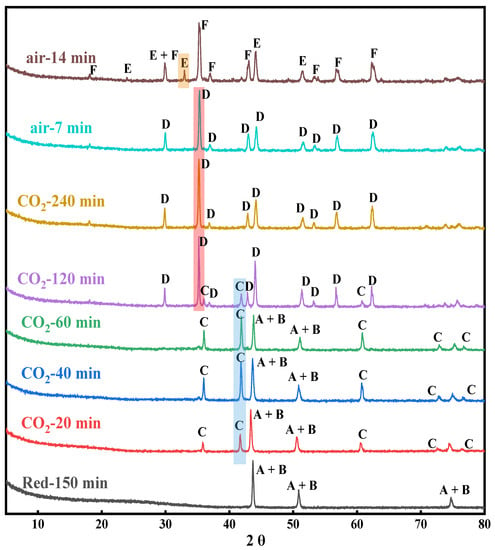
Figure 3.
XRD patterns of reduced OC at different oxidation times. (A: Ni, B: Fe, C: Fe1−xO, D: Fe3−xO4, E: Fe2O3, F: NiFe2O4).
When the oxygen carrier was oxidized by CO2 for 5 min, a part of metallic Fe is oxidized recovering a fraction of lattice oxygen to form the Fe1−xO phase (marked as C). As the oxidation degree deepens, the characteristic peaks of Fe3−δO4 are observed (marked as D). It is worth noting that the Ni atoms still exist in the form of metallic Ni due to thermodynamic limitations. It is difficult to replenish all the lattice oxygen under the CO2 atmosphere owing to the weak oxidation performance of CO2. Thus, in order to recover all the lattice oxygen, an air oxidation step is necessary to achieve a whole chemical looping cycle. The OC after CO2 oxidation is further oxidized by air to recover all the lattice oxygen to form the NiFe2O4 phase (marked as F) with spinel structure and a small amount of Fe2O3 phase (card: 00-004-0755, marked as E). Although it is difficult to re-oxidize the reduced OC back into its original state, it still exhibits good performance after multi-cycles during the CLDR [27,35].
2.2. XPS Analysis
2.2.1. The Release of Lattice Oxygen
The nature of the oxygen species and the transformation of metal species (Fe and Ni) on the near-surface of OC with reaction time were explored in detail using XPS combined with XRD to further clarify the mechanism of the migration of lattice oxygen. The experimental results are shown in Figure 4 and Figure 5 (the samples analyzed by XPS and XRD are consistent). And the XPS analysis of the O element and the Fe, Ni elements will form a corroboration relation, which strongly supports the migration mechanisms of lattice oxygen during the reduction of NiFe2O4 OC.
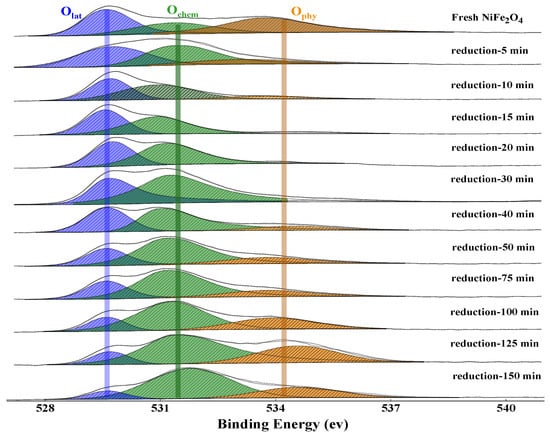
Figure 4.
XPS of O1s at different reduction times. (Blue: lattice oxygen, green: chemisorbed oxygen, yellow: physiosorbed oxygen).
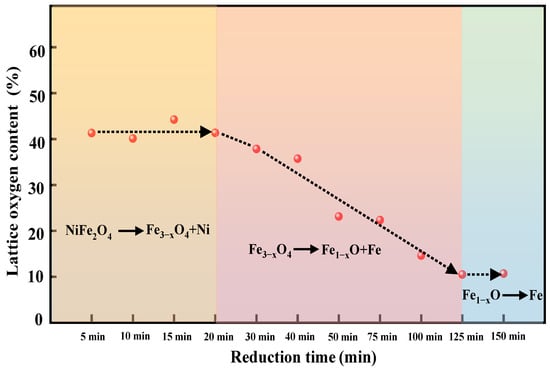
Figure 5.
The relative content of lattice oxygen on the surface of OC at different reduction times combined with the evolution of the crystal phase.
The oxygen species on the oxygen carrier surface mainly include lattice oxygen, chemisorbed oxygen, and physiosorbed oxygen [30]. The binding energy of lattice oxygen, chemisorbed oxygen, and physiosorbed oxygen are 529.0–530.0 eV, 530.0–532.0 eV, and >532.0 eV, respectively [36,37,38,39,40]. Previous literature reported that the different oxygen species can be transformed into each other as follows: O2(g)↔O2−(ads)↔O22−(ads)↔2O−(ads)↔2O2−(ads)↔2O2−(lat) [41].
As shown in Figure 4, the XPS spectra shows that three oxygen species (lattice oxygen; chemisorbed oxygen; and physiosorbed oxygen) can be found on the near-surface of the fresh NiFe2O4 oxygen carrier particles. At the initial reduction stage (5–20 min), the relative content of physiosorbed oxygen gradually decreases with the increase of reduction time (between 5–20 min), which decreases from ~15% at 5 min to ~0% at 20 min. On the contrary, the relative content of chemisorbed oxygen gradually increases with reduction time, which increases from ~40% at 5 min to ~60% at 30 min. It is worth noting that the relative content of lattice oxygen tends to stabilize at ~40% for the first 20 min at the reduction stage (Figure 5). Meanwhile, the result of XRD shows that the OC maintains the spinel structure and the main phase is Ni0.6Fe2.4O4 and Fe3−xO4 during the first 20 min (Figure 5). As well, the oxygen carrier exhibits a fast reduction rate at the initial stage. The results of XRD combined with XPS indicate that the physiosorbed oxygen was first consumed from the surface of the oxygen carrier due to the weak adsorption capacity for CO2/CO. As well, the surface lattice oxygen is easily converted into chemically adsorbed oxygen due to being highly active in the chemical reaction interface [41]. Thus, the relative content of physiosorbed oxygen decreased whereas chemisorbed oxygen increased. It also indicates that the conversion rate of the surface lattice oxygen to the chemisorbed oxygen is less than the rate of bulk lattice oxygen migration to the surface, which causes the relative content of surface lattice oxygen to be stable. In other words, three reaction rates determine the changing trend of different oxygen species, namely the consumption rate of chemisorbed oxygen bound to fuel, the conversion rate of surface lattice oxygen to chemisorbed oxygen, and the migration rate of bulk lattice oxygen to the surface, respectively. In the initial stage of the reduction reaction, the migration rate of bulk phase lattice oxygen to the surface is fairly rapid due to the presence of a large amount of lattice oxygen in the bulk of OC and the concentration gradient between the surface and the bulk. This process is accompanied by a part of the Ni element in OC being reduced into free metallic nickel and divorces from the spinel lattice of the NiFe2O4. As well, it also caused the gas-solid reaction interface of was fixed at the surface of the oxygen carrier. Hence, the reaction pathway can be described as follows: CO is firstly adsorbed at the active sites on the surface of the OC particles and then it combines with chemically adsorbed oxygen at the reaction interface to form CO2 or CO32−. The surface lattice oxygen is rapidly and continuously converted into the chemically adsorbed oxygen, meanwhile, the bulk lattice oxygen rapidly migrates to the surface of the spinel structure to replace the consumed lattice oxygen at the initial stage of the reduction reaction. Thus, we inferred that as Ni migrates outward to the particle surface, promoting oxygen vacancies formation and the diffusion of lattice oxygen. It finally leads to a fast rate and the stabilization of the surface lattice oxygen content at the initial reduction stage.
In the intermediate stage of the reduction reaction (20–125 min), the relative content of lattice oxygen gradually decreases with the increase of reduction time, which decreases from ~40% at 20 min (Main phase: Fe3−xO4) to ~10% at 125 min (Main phase: metallic Fe and metallic Ni), and then it gradually tends to stabilize (Figure 5). Whereas, the content of physiosorbed oxygen gradually increases from ~0% at 20 min (Main phase: Fe3−xO4) to ~25% at 125 min (Main phase: metallic Fe and metallic Ni), and then it is gradually stabilized. It indicates that with the collapse of the spinel structure, the bulk lattice oxygen of OC is continuously consumed. In other words, the rate of migration of bulk-phase lattice oxygen to the surface slows down due to the concentration of the lattice oxygen decreasing gradually.
Interestingly, the XRD and XPS analysis show that even if the NiFe2O4 OC is completely reduced to the metallic Ni and Fe phases, which means the bulk lattice oxygen is almost fully consumed, and a fraction of lattice oxygen always appears at the surface of the reduced OC (Figure 5). At the same time, the physiosorbed oxygen content of the fully reduced OC (125–150 min) was significantly higher than those of the OC in the initial reduction stage (0–20 min). It indicates that the completely reduced OC, namely the metallic Fe and Ni phase, easily adsorbs CO2. As CO2 is adsorbed on the surface of the reduced OC, a CO2-splitting reaction occurs on the active metallic Fe. The O anions extracted from CO2 transform into surface adsorbed oxygen. Subsequently, the adsorbed oxygen further transforms into lattice oxygen induced by the oxygen vacancies on the surface. These two reactions are represented as R (1) [35] and R (2) [41]. Eventually, the relative concentrations of each oxygen species remain unchanged.
Fe + CO2→Feδ+ + [O−ads] + CO R
[O−ads]↔[O−lat] R
Therefore, an active interface consisting of lattice oxygen and the corresponding metallic ions is maintained during the whole reduction process.
The valence evolution of metal cations has a corroborative relationship with the surface oxygen species, thereby shedding light on the migration mechanism of lattice oxygen. It was reported that the peaks with binding energy at around ~856.2 eV, ~854 eV, and ~852.6 eV are assigned to Ni2+, Ni0 (metallic Ni), and Ni0 (Fe-Ni), respectively [42,43]. As shown in Figure 6, only Ni2+ peaks can be observed in the XPS spectra of the fresh NiFe2O4 OC. After reduction for 5 min, a peak for Ni0 appears in the Ni 2p spectra, indicating that the metallic Ni phase is formed on the surface at the initial reduction stage. This is consistent with the XRD observations. In the reduction time from 5 to 15 min, the relative content of Ni2+ ions decreased significantly reaching zero till 20 min, whereas the Ni0 content increased rapidly. Combined with the O1s XPS spectra (see Figure 4), it can be observed that physiosorbed oxygen decrease is accompanied by the initial reduction stage. Thus, the presence of metallic Ni0 on the surface probably has no effect on the adsorption capacity of OC for CO2/CO. It was probably due to thermodynamic limitations that metallic Ni0 cannot react with CO2 [22]. After 15 min, the peak of Ni2+ disappears. All Ni2+ is converted to Ni0 on the surface.
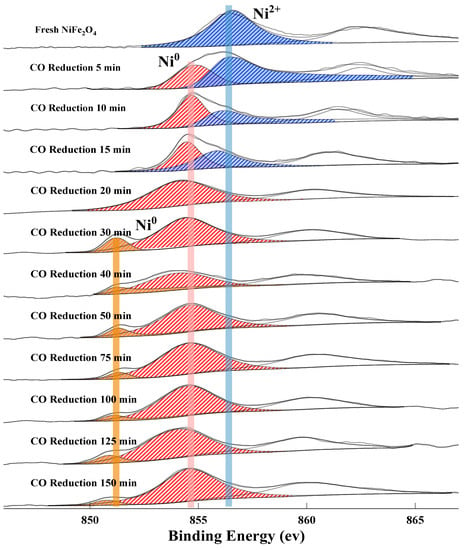
Figure 6.
XPS spectrum of Ni 2p 3/2 at different reduction times.
At 30 min, a peak for Fe-Ni alloy appears, corresponding to the formation of metallic Fe with the decomposition of Fe3-xO4 to Fe1-xO and Fe. In the reduced NiFe2O4 OC, the presence of metallic Fe may create an effect on the electronic structure of metallic Ni. It further indicates that the complete reduction product of OC, is not a simple mixture of Ni and Fe, but a composite with complex space structure, which performance is better than that of the complete reduction product of NiO + Fe2O3 OC in CLDR [35].
The XPS spectrum of Fe appears in the form of double peaks. As shown in Figure 7. The fresh OC has two Fe3+ peaks located at the binding energy of 713.2 eV and 715.5 eV assigned to the spinel NiFe2O4 with the structure of the tetrahedron and octahedron structure, respectively. With the reaction proceeding, Fe3+ is continuously reduced to Fe2+ or Fe0. Thus, the Fe0, Fe2+ and Fe3+ peaks appear at ~706.7 eV, ~710 eV and ~713–715 eV, respectively [44]. It is worth noting that the Fe0 peak cannot be observed on the surface of OC at an initial reduction of 30min. However, the XRD analysis of the crystal phase and the XPS analysis of Ni is similarly observed in the presence of metallic Fe0 at 30 min. It indicated that Fe0 is firstly formed inside of the OC particles. In other words, the lattice oxygen tends to migrate to the surface at high temperatures, therefore metallic phase begins to be formed from inside the OC bulk. Similar results have been obtained in other literatures [31,45]. Hence, the metal ions migration pathway can be described as follow: With the release of lattice oxygen, Ni2+ was firstly migrated to the surface and reduced to Ni0 due to the higher reactivity of Ni. Then the lower active Fe2+ and Fe3+ are subsequently reduced thereby providing lattice oxygen to the fuel on the surface. As the reduction degree deepens, Fe cations begin to be reduced into metallic Fe0. The presence of metallic Fe in the bulk creates an effect on the electronic structure of metallic Ni. The surface Fe/Ni ratio of the OC will be analyzed via EDS combined with HRTEM, to further clarify the phenomenon of the migration and enrichment of Fe and Ni atoms during the reduction process. In addition, it was observed that even if OC is completely reduced to the metal phase, the Fe element on the surface is still dominated by Fe2+ and Fe3+ and only a small amount of Fe0. It is due to the strong surface adsorption of the metallic Fe in complete reduced OC, the reaction R (1) is dominated in the later stage of the reaction, thus the relative concentration of surface Fe species remains stable. As well, compared to the initial stage of the reaction, the relative content of physiosorbed oxygen gradually increases. The analysis of different elements forms a good corroboration relation, which strongly supports the migration mechanisms of lattice oxygen during the reduction of NiFe2O4 OC.
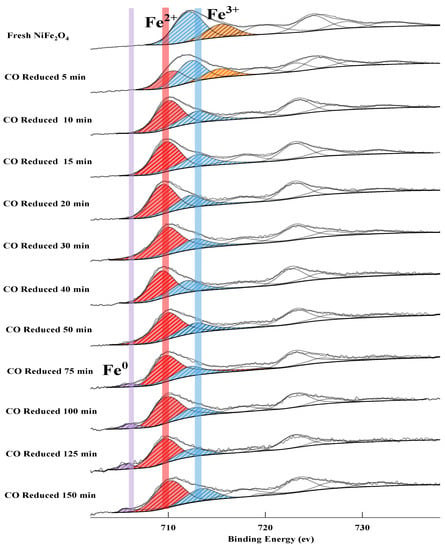
Figure 7.
XPS spectrum of Fe 2p 3/2 at different reduction times.
2.2.2. The Uptake of Lattice Oxygen
The O 1s XPS spectra of the oxygen species of the reduced OC re-oxidized in the CO2-air atmosphere at different oxidation times are shown in Figure 8. It was observed that the lattice oxygen content gradually increases from ~10% at 0 min to ~37% at 240 min of CO2-oxidization (see Figure 9). However, the physiosorbed oxygen content gradually decreases from ~23% at 0 min to ~0% at 120 min. It is noted that the physiosorbed oxygen reappeared at oxidization for 240 min. These results should be attributed to a fact that the chemical reaction interface is fixed on the surface of the OC particle during the CO2 oxidation. If the reaction interface migrates from the surface to the inside. The surface lattice oxygen will reach a maximum at the initial stage of the reaction and then tends to stabilize, rather than gradually increases. In other words, if the surface metal restores all lattice oxygen at the initial stage of the reaction, CO2 molecules will penetrate through the oxide layer to the interior of the particle to react with metallic Fe. Thus, it indicates that the rate of conversion of the chemisorbed oxygen to the surface lattice oxygen is slightly quicker than the rate of surface lattice oxygen migration to bulk, and then the surface lattice oxygen gradually accumulates. The oxygen concentration gradient between the surface and the bulk continuously drives the lattice oxygen inward transportation to the bulk. In addition, with the recovery of lattice oxygen, the adsorption capacity of oxygen carriers for physisorbed oxygen gradually weakens, which is consistent with the analysis of the reduction process. However, at the end of CO2 oxidation, a large amount of chemisorbed oxygen can be still observed due to the slower rate of CO2 splitting and the prolonged contact between CO2 and oxygen carriers. Because of the weak oxidizability of CO2, it is very difficult to recover the entire lattice oxygen for the reduced OC. Thus, an air oxidation process is required to recover the full lattice oxygen of the OC.
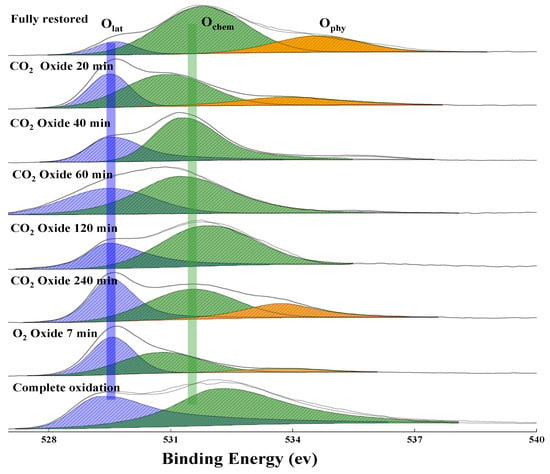
Figure 8.
XPS of O1s at different oxidation times. (Blue: lattice oxygen, green: chemisorbed oxygen, yellow: physiosorbed oxygen).
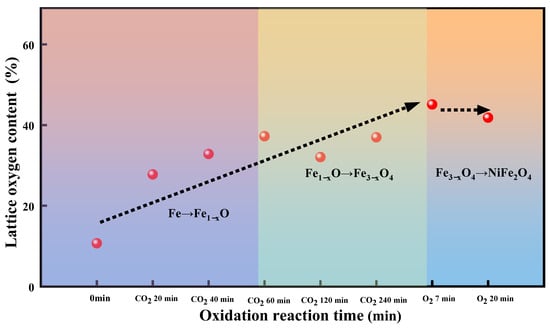
Figure 9.
The relative content of lattice oxygen on the surface of OC at different oxidation times combined with the evolution of the crystal phase.
Figure 9 illustrates the lattice contents as a function of oxidation reaction time in air oxidation of the reduced OC. It was observed that the relative content of lattice oxygen increases, which increases from ~37% (at 240 min of CO2-oxidization) to ~45%, and it tends to stabilize. The increase in the relative content of lattice oxygen is due to metallic Ni0 recovery lattice oxygen on the surface, namely the formation of NiFe2O4 species. The stability of the relative content of lattice oxygen is due to the rate of conversion of the chemisorbed oxygen to the surface lattice oxygen is much quicker than the rate of surface lattice oxygen migration to bulk. There is a concentration gradient of lattice oxygen between the surface and the bulk, thus the lattice oxygen is continuously diffused from the surface to the bulk to fill the oxygen vacancies and a relatively stable equilibrium is attained. Thus, the concentrations of lattice oxygen remain stable. Through the above analysis, we infer that the concentration gradient between the surface and the bulk drives the transmission of lattice oxygen to achieve the reduction or oxidation of OC. The migration rate of lattice oxygen is associated with the movement process of Ni particles into and out of spinel bulk.
The Ni 2p XPS spectra (Figure 10) show that the nickel element on the surface of the OC is kept in form of Ni0 in the course of CO2 oxidation due to metallic Ni cannot be oxidized in thermodynamics. The peak of two kinds of Ni0 was observed, namely metallic Ni (Ni-Ni) and other kinds of Ni0 that the presence of Fe creates an effect on the electronic structure of metallic Ni. The relative content of the two kinds of Ni0 fluctuates due to elemental Fe gradually restoring lattice oxygen. During the air oxidation stages, the peak of Ni2+ was observed. The result is very consistent with the analysis of oxygen species. In addition, the signal of the Ni element on the surface disappears at 60 min due to the Fe0 quickly recovering the lattice oxygen to form FexO and the generated oxide (FexO) can form an oxide film on the surface. The surface Ni atoms are covered by the Fe atoms. As well, the signal of the Ni element is related to the thickness of the surface oxide (FexO). Two peaks of Ni2+ were observed for fully oxidized oxygen carriers, which is probably due to the presence of different spatial structures of Fe2O3 and NiFe2O4.
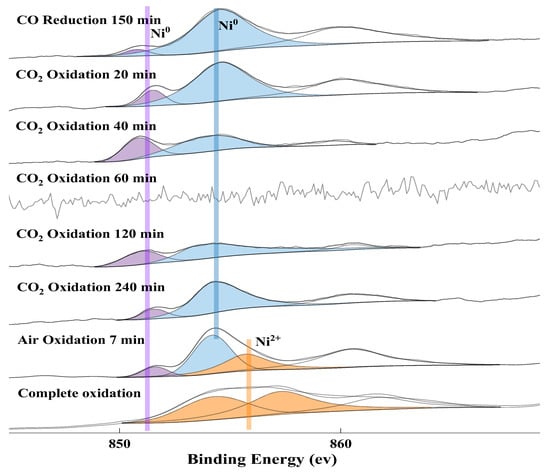
Figure 10.
XPS spectrum of Ni 2p 3/2 at different oxidation times.
As shown in Figure 7 and Figure 11, the Fe element in the completely reduced OC is mainly in form of Fe2+, Fe3+, and a minor amount of Fe0. As the reduced OC is oxidized, the Fe0 and Fe2+ are continuously oxidized into Fe3+. When the OC is oxidized by CO2 for above 20 min, the XPS peak of Fe0 disappears. After CO2-oxidation for 240 min, the Fe element in the reduced NiFe2O4 OC can be reoxidized into Fe3O4 by such a weak oxidant, CO2. In the completely oxidized OC, the surface Fe element of OC has two Fe3+ peaks due to the complex spatial structure of the NiFe2O4 and Fe2O3 particles.
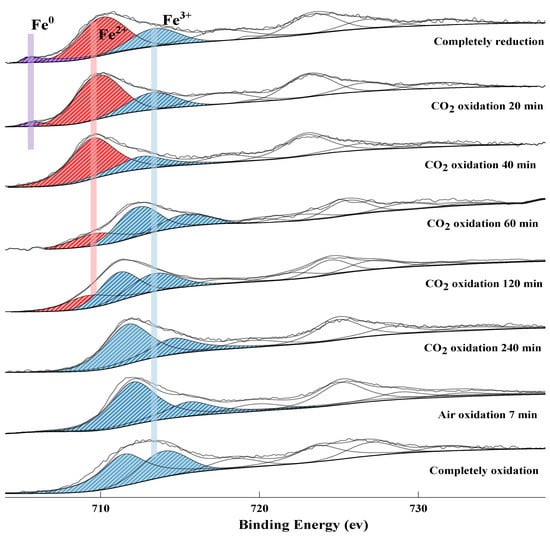
Figure 11.
XPS spectrum of Fe 2p 3/2 at different oxidation times.
2.3. In Situ Raman Analysis
The vibrations changes of the chemical bonding in the course of redox reactions on the surface of the OC were studied via in-situ Raman. Compared to non-in situ characterization, in-situ characterization makes it easier to obtain the real results of the materials. As shown in Figure 12, Raman characterizations show five peaks attributed to NiFe2O4 [46]. The strongest peak at ~695cm−1 is attributed to the symmetric stretching vibration of the O atom along the Fe-O bond direction. The peak at 481 cm−1 is attributed to the vibration of the Ni-O bond [47]. The peak at 330 cm−1 is attributed to the symmetric bending vibration of the Fe-O bond [48,49].
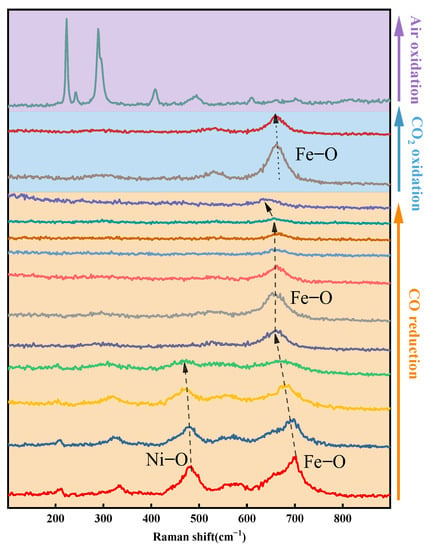
Figure 12.
Raman spectra of the redox process of OC.
At the initial stage of the CO-reduction reaction, the peak of Ni-O (481 cm−1) and Fe-O (695 cm−1) display a red shift and gradually becomes weaker, which is due to the formation of oxygen vacancy during lattice oxygen release. Then a very important process is found that the peak of Fe-O (695 cm−1) changes from weak to strong. Meanwhile, the peak of Ni-O (481 cm−1) disappeared. It indicates that the element Ni underwent Ni2+ to Ni0 transition on the surface and Fe atoms combined with remaining lattice oxygen to form a new structure. With the reaction proceeding, the peak of Fe-O gradually becomes weaker from the new structure. However, no matter how long the reduction reaction time lasts, even if the XRD analysis show OC completely reduced to the metal phase, there will always be a peak of Fe-O. The in-situ Raman analysis and the XPS analysis are consistent.
For the OC oxidized by CO2, the peak of Fe-O changes from weak to strong. As well, no Ni-O vibration peaks were found. The Raman spectra of the oxygen carrier after air oxidation are different from the fresh samples. But it is very similar to the Raman bands of the pure Fe2O3 [49]. It is due to the formation of Fe2O3 on the surface of oxygen carriers [50].
2.4. TEM Analysis
The morphology of the fresh NiFe2O4 OC, completely reduced OC, reoxidized OC by CO2, and completely reoxidized OC by air was investigated by HRTEM, as shown in Figure 13. It is observed that the Ni, Fe, and O elements distribute uniformly throughout the particle in the fresh oxygen carrier. The lattice spacing of the TEM under high magnification was also performed. The crystal forms of the fresh oxygen carrier, ~2.49 Å of the lattice fringe spacing ascribed to (311) plane of NiFe2O4, can be observed, which are located in the exogenous parts of the oxygen carrier particle. The ratio of surface Fe/Ni~1.83 is very consistent with the theoretical value (Table 1).
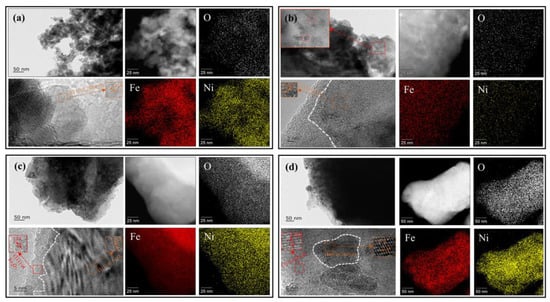
Figure 13.
HRTEM and EDS mapping: (a) (Fresh NiFe2O4 OC); (b) (Completely reduced OC); (c) (Completely oxidized OC by CO2); (d) (Completely oxidized OC by Air).

Table 1.
The relative content of Fe and Ni from EDS mapping.
From Figure 13b, it can be found that the particle size of samples shows an increase due to the collapse of the spinel structure and the enrichment of metallic phases. Small circular particles were observed in distribution inside of the oxygen carrier, which is presumed to be enriched Ni0. Under high magnification, a fuzzy boundary located in the exogenous parts of the oxygen carrier particle, and ~2.02 Å of the lattice fringe spacing ascribed to (111) plane of metallic Fe0, can be observed. A fuzzy boundary probably has a relationship with the highly reactive chemical reaction interface, which consists of lattice oxygen and the corresponding metallic ions. The EDS mapping shows the ratio of surface Fe/Ni~6.28, which indicates that the surface Ni atoms are covered by the Fe atoms.
As shown in Figure 13c, many small circular particles were still observed distributed inside of the oxygen carrier. It is probably due to Ni0 cannot be oxidized by CO2. The lattice spacing of Fe3O4(311) and a boundary are observed. From Figure 13d, in the middle of the Fe2O3(311), there is a layer with the lattice fringe spacing of ~1.47 Å related to the (440) plane of NiFe2O4. The EDS mapping shows that the ratio of surface Fe/Ni did not return to the original state. It indicates that Fe2O3 exists on the surface of the completely oxidized oxygen carrier [50].
According to the above analysis, the potential reaction paths and mechanism of NiFe2O4 in the CLDR process can be proposed as shown in Figure 14. It can observe that the process of CLDR of methane consists of a successive three-step chemical looping scheme, where NiFe2O4 OC is supposed to: (i) convert various fuels (e.g., CH4, biomass) to value-added products (e.g., heat, syngas), (ii) reduce CO2 to produce CO, and (iii) consume oxygen from the air to replenish full lattice oxygen meanwhile sustaining the thermal balance of the system. The chemical reaction interface is fixed on the surface of OC particles (Figure 14). During the oxygen carrier reduction process, the bulk lattice oxygen migrated to the particle surface and converted to adsorbed oxygen, which is consumed by fuel. During the oxygen carrier oxidation process, CO2 is adsorbed on the particle surface and dissociates into O ions, which are converted from adsorbed oxygen to lattice oxygen and migrate to the bulk. The diffusion of oxygen anions maintains the overall charge neutrality due to the oxygen chemical potential gradient. It is worth noting that the phase segregation caused by the outward diffusion of metal cations is not conducive to the cyclic stability of oxygen carriers. Adding an inert component to control the movement of active metal atoms is one of the directions worthy of our efforts in the future to achieve better performance. Through the above analysis, we infer that utilizing or controlling the reversible dynamic changes behavior of particles will be an effective means to enhance the reactivity and stability of oxygen carriers in future chemical looping applications.
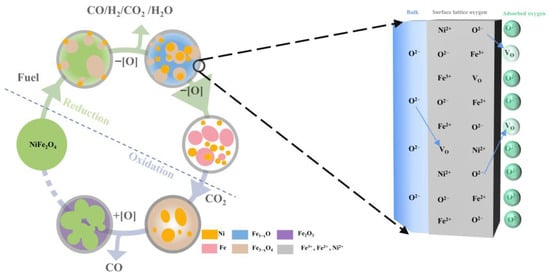
Figure 14.
Redox mechanism description in CLDR process.
3. Materials and Methods
3.1. Preparation of Oxygen Carrier
The sol−gel methods were applied to prepare NiFe2O4 nanoparticles. Nickel nitrate hexahydrate [Ni(NO3)2·6H2O] (Aladdin, ≥99.99%) and iron nitrate nonahydrate [Fe(NO3)3·9H2O] (Aladdin, ≥99.99%) were used as raw materials and dissolved in deionized water, forming 1 M of Ni(NO3)2 solution and 2 M of Fe(NO3)3 solution, respectively. The citric acid (Macklin, ≥99.5%) with the equivalent molar Ni(NO3)2 was added to the original mixed solution. Subsequently, obtain a pH of 7.5. Finally, the solution was heated at 65–75 °C to evaporate 2/3 of the water, and the gel formed when the solution was cooled to room temperature. The precursors were dried in a vacuum evaporator at 120 °C and calcined for the formation of the ferrite phase in a muffle furnace under an air atmosphere according to the same temperature program. The dried materials were calcined by increasing the temperature from 25 °C to 400 °C with a ramp of 8 °C/min and keeping at 400 °C for 2 h. Then the sample was increased to 1200 °C at a heating rate of 4 °C/min, and kept at 1200 °C for 2 h [46].
3.2. Thermo-Gravimetric (TG) Analysis
The three stages of the CLDR experiment are carried out by the STA-409/PC thermogravimetric analyzer, which is produced by NETZSCH, Selb, Germany. The three different gases (CO, CO2, and air) flow rates are all set to 100 mL/min. The experimental procedure is as follows: NiFe2O4 OC (m = 150 mg ± 0.1 mg) was added to the thermogravimetric reactor. the reaction temperature is heated up to 950 °C at a heating rate of 10 °C/min in an inert atmosphere. Then, CO is introduced for the reduction reaction. After the reduction experiment, CO2 and air is introduced for oxidation reaction, respectively. Obtain 19 samples with different reaction times for subsequent analysis, which are recorded as OCfresh, reduction-5 min, reduction-10 min, reduction-15 min, reduction-20 min, reduction-30 min, reduction-40 min, reduction-50 min, reduction-75 min, reduction-100 min, reduction-125 min, reduction-150 min, CO2-oxidation-20 min, CO2-oxidation-40 min, CO2-oxidation-60 min, CO2-oxidation-120 min, CO2-oxidation-240 min, air-oxidation-7 min, air-oxidation-14 min, respectively.
3.3. Characterization
3.3.1. X-ray Photoelectron Spectroscopy (XPS)
An X-ray photoelectron spectroscopy technology (XPS, Thermo Fisher Scientific ESCALAB 250Xi, Waltham, MA, USA) was carried out to investigate the valence state of variable metal ions and oxygen species of OCs, with an Al Ka X-ray source (1486.6 eV photons) at an operating voltage of 20 kV and a current of 10 mA, respectively.
3.3.2. X-ray Diffraction (XRD)
An X-ray diffractometer (XRD) using 40 kV, 40 mA Cu Kα radiation was used to analyze the crystal structure of fresh and reacted samples. XRD measurement scanning rate is 2 °/min from 2θ = 5–80° with a step size of 0.02°.
3.3.3. In Situ Raman
A Laser Confocal Micro-Raman Spectral System (LabRAM HR800, HORIBA Scientific, Piscataway, NJ, USA) was used to analyze the surface structure of the oxygen carrier. Laser full range of 50 mw, selected a laser probe with a wavelength of 432.521 cm−1. Added NiFe2O4 oxygen carrier mass of 100 mg in the in-situ stage, under 20 mL/min inert atmosphere, warmed up at a rate of 50 K/min to 950 °C. When the temperature rises to 950 °C, a gas (the CO reduction and CO2-air oxidation) is continuously introduced into the reactor, respectively. A total of 20 mL/min CO gas (10 vol%CO, N2 equilibrium) for reduction. Then switch to CO2 (10 vol%CO2, N2 equilibrium) with a flow rate of 20 mL/min for oxidation reaction after the end of reduction. Finally, pass air (20 mL/min) for strong oxidation.
3.3.4. Transmission Electron Microscopy (TEM)
The microstructure of the sample was analyzed using a high-resolution field emission transmission electron microscope (JEOL 125 JEM-2100F, Tokyo, Japan). EDS energy spectrometer manufactured by American Thermoelectric Noran (Waltham, MA, USA).
4. Conclusions
In this study, we elaborated on the reversible phase transition law of NiFe2O4 using the sol-gel method for the preparation of the chemical looping CO2 splitting process. Then valence evolution of active components of the oxygen carriers was analyzed in detail by XPS. As well, we explained the chemical mechanism of the change in the relative content of different oxygen species. A large number of evidence about bulk lattice oxygen migration was obtained by different characterizations.
A fact was confirmed by an in-situ Raman and XPS that a highly reactive chemical reaction interface consisting of lattice oxygen and the corresponding metal atoms is always present on the surface of the oxygen carrier during the CLDR. In summary, the diffusion of oxygen anions maintains the overall charge neutrality due to the oxygen chemical potential gradient. Oxygen anions move from a high concentration area to a low concentration area, namely the surface lattice oxygen is converted to chemisorbed oxygen and forms oxygen vacancies. The bulk lattice oxygen to the surface and healed the surface vacancy via outward diffusion. The diffusion of metallic Fe and metallic Ni changes the morphology and crystalline structure of OC. The extraordinary performance of NiFe2O4 for CLDR is due to the movement of Ni particles into and out of spinel bulk, which promote oxygen migration and diffusion. In the reduction period under CO, a part of the Ni element in OC is first separated from the spinel structure to become the free metallic Ni. It was confirmed that exsolved Ni nanoparticles were not redissolved into the spinel in the oxidation under CO2, and the active phase (Ni) was dissolved into the matched spinel under an air atmosphere. This understanding of the migration of active metals and lattice oxygen is of great significance for material design for the applications of CLDR, especially through structure design and utilizing the dynamic phase change to stabilize the metal particles. As well, establishing the relationship between the dynamic behavior and performance of the metal is one of the directions worthy of our efforts in the future to achieve better performance of OC in CLDR; it also can promote the process of CO2 resource utilization and carbon neutrality goals.
Author Contributions
Conceptualization, D.S. and Y.L.; methodology, Z.H.; software, D.S.; validation, D.S., Z.H. and F.H.; formal analysis, K.Z. and F.H.; investigation, D.S.; resources, Y.X.; data curation, D.S.; writing—original draft preparation, D.S.; writing—review and editing, F.H.; visualization, Z.H.; supervision, Z.H.; project administration, Y.L.; funding acquisition, Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52006224, 52076209, 22179027), the Foundation and Applied Foundation Research of Guangdong Province (2019B1515120022, 2019A1515110828, 2020A1515110138, 2021A1515010459, 2022B1515020045), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (2021021), Guangxi Natural Science Foundation (2018GXNSFDA281005), Youth Innovation Promotion Association, CAS (2019341).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donat, F.; Müller, C.R. CO2-free conversion of CH4 to syngas using chemical looping. Appl. Catal. B Environ. 2020, 278. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Kim, J.; Johnson, T.A.; Miller, J.E.; Stechel, E.B.; Maravelias, C.T. Fuel production from CO2 using solar-thermal energy: System level analysis. Energy Environ. Sci. 2012, 5, 8417–8429. [Google Scholar] [CrossRef]
- Ekström, C.; Schwendig, F.; Biede, O.; Franco, F.; Haupt, G.; de Koeijer, G.; Papapavlou, C.; Røkke, P.E. Techno-Economic Evaluations and Benchmarking of Pre-combustion CO2 Capture and Oxy-fuel Processes Developed in the European ENCAP Project. Energy Procedia 2009, 1, 4233–4240. [Google Scholar] [CrossRef]
- Meng, W.X.; Banerjee, S.; Zhang, X.; Agarwal, R.K. Process simulation of multi-stage chemical-looping combustion using Aspen Plus. Energy 2015, 90, 1869–1877. [Google Scholar] [CrossRef]
- Ksepko, E.; Lysowski, R. Reactivity Study of Bimetallic Fe-Mn Oxides with Addition of TiO2 for Chemical Looping Combustion Purposes. Catalysts 2021, 11, 1437. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent CO2 separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Tang, M.C.; Xu, L.; Fan, M.H. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. CeO2-Modified Fe2O3 for CO2 Utilization via Chemical Looping. Ind. Eng. Chem. Res. 2013, 52, 8416–8426. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping for CO2 conversion to CO. Appl. Catal. B Environ. 2015, 164, 184–191. [Google Scholar] [CrossRef]
- Song, T.; Shen, T.; Shen, L.; Xiao, J.; Gu, H.; Zhang, S. Evaluation of hematite oxygen carrier in chemical-looping combustion of coal. Fuel 2013, 104, 244–252. [Google Scholar] [CrossRef]
- Linderholm, C.; Knutsson, P.; Schmitz, M.; Markström, P.; Lyngfelt, A. Material balances of carbon, sulfur, nitrogen and ilmenite in a 100 kW CLC reactor system. Int. J. Greenh. Gas Control 2014, 27, 188–202. [Google Scholar] [CrossRef]
- Cocchi, S.; Mari, M.; Cavani, F.; Millet, J.-M.M. Chemical and physical behavior of CoFe2O4 in steam–iron process with methanol. Appl. Catal. B Environ. 2014, 152, 250–261. [Google Scholar] [CrossRef]
- Siriwardane, R.; Tian, H.; Simonyi, T.; Poston, J. Synergetic effects of mixed copper–iron oxides oxygen carriers in chemical looping combustion. Fuel 2013, 108, 319–333. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Ma, J.-L.; Chin, C.-P.; Kuo, Y.-L.; Ku, Y. Preparation of composite nickel–iron oxide as highly reactive oxygen carrier for chemical-looping combustion process. J. Taiwan Inst. Chem. Eng. 2014, 45, 174–179. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, S.; Cui, D.; Li, M.; Zeng, J.; Zeng, D.; Xiao, R. Enhanced hydrogen production performance at intermediate temperatures through the synergistic effects of binary oxygen carriers. Appl. Energy 2019, 252, 113454. [Google Scholar] [CrossRef]
- Qin, L.; Guo, M.; Liu, Y.; Cheng, Z.; Fan, J.A.; Fan, L.-S. Enhanced methane conversion in chemical looping partial oxidation systems using a copper doping modification. Appl. Catal. B Environ. 2018, 235, 143–149. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, Q.; Wei, G.; Zhang, Y.; Hou, Y.; Huang, Z.; Zheng, A.; Zhao, Z.; Li, H. Reaction performance and lattice oxygen migration of MnFe2O4 oxygen carrier in methane-carbon dioxide reaction system. Int. J. Hydrogen Energy 2020, 45, 30254–30266. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Russell, C.K.; Dyar, M.D.; Sklute, E.C.; Toan, S.; Fan, M.; Duan, L.; Xiang, W. Synergistic enhancement of chemical looping-based CO2 splitting with biomass cascade utilization using cyclic stabilized Ca2Fe2O5 aerogel. J. Mater. Chem. A 2018, 7, 1216–1226. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; Wei, G.; He, F.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Exploration of Reaction Mechanisms on Hydrogen Production through Chemical Looping Steam Reforming Using NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 11621–11632. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G. Identifying the roles of MFe2O4 (M=Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon Dioxide Conversion by Reverse Water–Gas Shift Chemical Looping on Perovskite-Type Oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Bork, A.H.; Moser, T.; Sediva, E.; Hood, Z.D.; Rupp, J.L.M. Modifying La0.6Sr0.4MnO3 Perovskites with Cr Incorporation for Fast Isothermal CO2-Splitting Kinetics in Solar-Driven Thermochemical Cycles. Adv. Energy Mater. 2019, 9, 13. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, H.; He, F.; Chen, D.; Wei, G.; Zhao, K.; Zheng, A.; Feng, Y.; Zhao, Z.; Li, H. Evaluation of multi-cycle performance of chemical looping dry reforming using CO2 as an oxidant with Fe–Ni bimetallic oxides. J. Energy Chem. 2016, 25, 62–70. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Huang, Z.; Liu, M.; Wei, G.; Zhao, Z.; Li, H.; Fang, Y. Chemical looping gasification coupled with steam reforming of biomass using NiFe2O4: Kinetic analysis of DAEM-TI, thermodynamic simulation of OC redox, and a loop test. Chem. Eng. J. 2020, 395, 125046. [Google Scholar] [CrossRef]
- Liu, W. Controlling lattice oxygen activity of oxygen carrier materials by design: A review and perspective. React. Chem. Eng. 2021, 6, 1527–1537. [Google Scholar] [CrossRef]
- Chen, D.; He, D.; Lu, J.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B Environ. 2017, 218, 249–259. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.; Xuan, G.; Zhang, F.; Yang, L. Spatial evolution characteristics of active components of copper-iron based oxygen carrier in chemical looping combustion. Fuel 2021, 306, 121650. [Google Scholar] [CrossRef]
- Zhao, K.; Zheng, A.; Li, H.; He, F.; Huang, Z.; Wei, G.; Shen, Y.; Zhao, Z. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6. Appl. Catal. B Environ. 2017, 219, 672–682. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, S.X.; Xia, M.; Wang, M.K. A Theoretical Study of the Oxygen Release Mechanisms of a Cu-Based Oxygen Carrier during Chemical Looping with Oxygen Uncoupling. Catalysts 2022, 12, 332. [Google Scholar] [CrossRef]
- Cheng, Z.; Baser, D.S.; Nadgouda, S.G.; Qin, L.; Fan, J.A.; Fan, L.-S. C2 Selectivity Enhancement in Chemical Looping Oxidative Coupling of Methane over a Mg–Mn Composite Oxygen Carrier by Li-Doping-Induced Oxygen Vacancies. ACS Energy Lett. 2018, 3, 1730–1736. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Chen, D.; Zhao, K.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Investigation on reactivity of iron nickel oxides in chemical looping dry reforming. Energy 2016, 116, 53–63. [Google Scholar] [CrossRef]
- Cao, J.-L.; Wang, Y.; Yu, X.-L.; Wang, S.-R.; Wu, S.-H.; Yuan, Z.-Y. Mesoporous CuO–Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. B Environ. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Cao, W.; Tan, O.; Pan, J.; Zhu, W.; Reddy, C.V.G. XPS characterization of xα-Fe2O3–(1−x)ZrO2 for oxygen gas sensing application. Mater. Chem. Phys. 2002, 75, 67–70. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Wang, D.; Jin, L.; Li, Y.; Hu, H. Partial oxidation of vacuum residue over Al and Zr-doped α-Fe2O3 catalysts. Fuel 2017, 210, 803–810. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. XPS study on the correlation between chemical state and oxygen-sensing properties of an iron oxide thin film. Appl. Surf. Sci. 2007, 253, 9476–9482. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Li, K. Ce-Fe-O mixed oxide as oxygen carrier for the direct partial oxidation of methane to syngas. J. Rare Earths 2010, 28, 560–565. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Zhao, Z.; Felix, L.G.; Slimane, R.B.; Choi, C.W.; Ozkan, U.S. Olivine catalysts for methane- and tar-steam reforming. Appl. Catal. B Environ. 2008, 81, 14–26. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wang, D.; Rosynek, M.P.; Lunsford, J.H. Conversion of Methane to Benzene over Transition Metal Ion ZSM-5 Zeolites: II. Catalyst Characterization by X-ray Photoelectron Spectroscopy. J. Catal. 1998, 175, 347–351. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, Z.; Fan, J.A.; Fan, L.-S.; Gong, J. Metal oxide redox chemistry for chemical looping processes. Nat. Rev. Chem. 2018, 2, 349–364. [Google Scholar] [CrossRef]
- Liu, S.; He, F.; Huang, Z.; Zheng, A.; Feng, Y.; Shen, Y.; Li, H.; Wu, H.; Glarborg, P. Screening of NiFe2O4 Nanoparticles as Oxygen Carrier in Chemical Looping Hydrogen Production. Energy Fuels 2016, 30, 4251–4262. [Google Scholar] [CrossRef]
- Yang, H.; Gong, L.; Wang, H.; Dong, C.; Wang, J.; Qi, K.; Liu, H.; Guo, X.; Xia, B.Y. Preparation of nickel-iron hydroxides by microorganism corrosion for efficient oxygen evolution. Nat. Commun. 2020, 11, 5075. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-Film Ni–Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Ma, Z.; Zeng, D.; Zhang, S.; Xiao, R. Effect of Supports on the Redox Performance of NiFe2O4 in a Chemical Looping Process. Energy Technol. 2019, 7, 1900374. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).