Organocatalytic Asymmetric Michael Addition in Aqueous Media by a Hydrogen-Bonding Catalyst and Application for Inhibitors of GABAB Receptor
Abstract
:1. Introduction
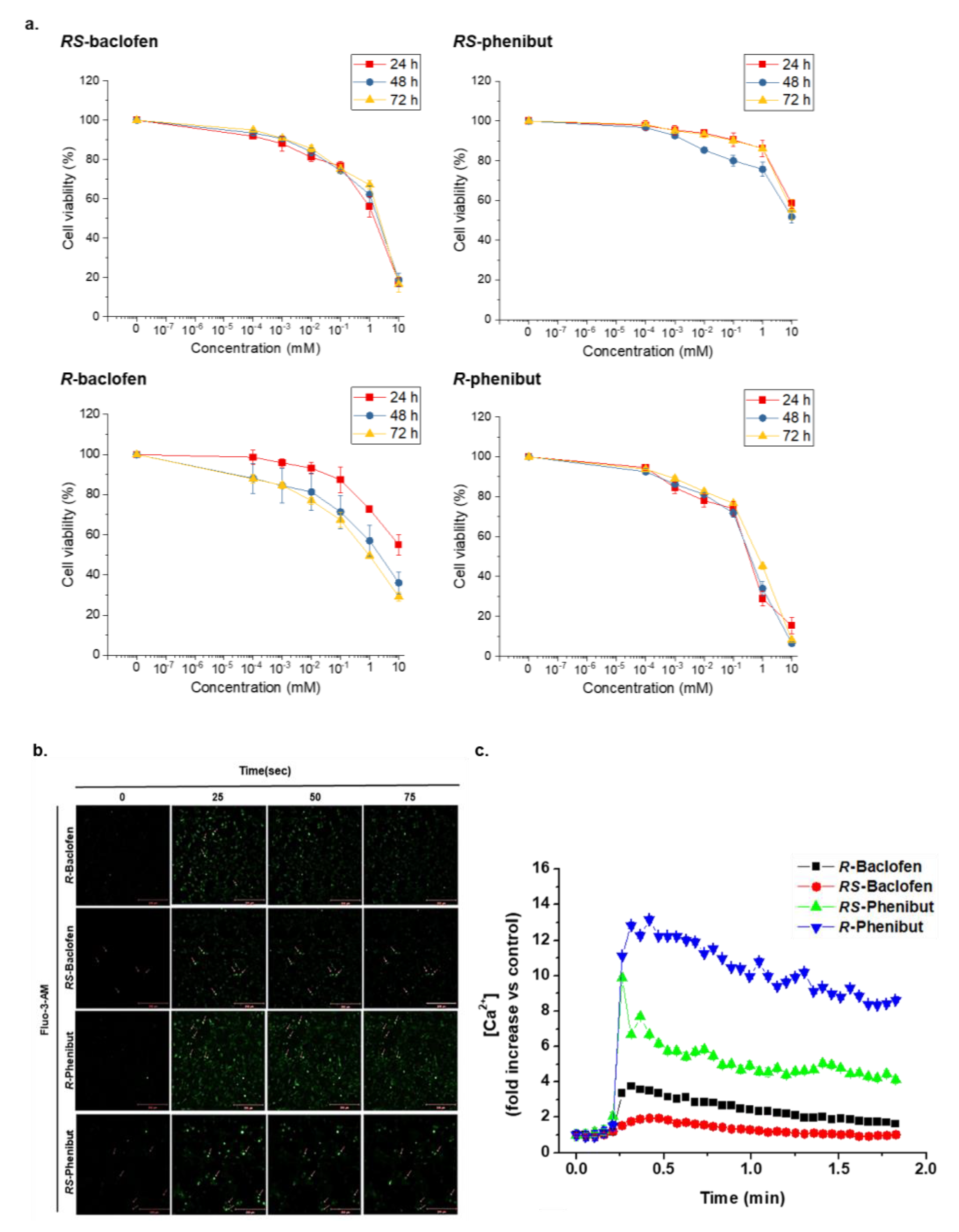
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Asymmetric Michael Reaction
3.2. Reagents
3.3. Cell Culture
3.4. Cytotoxicity Analysis
3.5. Intracellular Ca2+ Measurements Using Confocal Laser Scanning Microscopy (CLSM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. Engl. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef]
- Curti, C.; Rassu, G.; Zambrano, V.; Pinna, L.; Pelosi, G.; Sartori, A.; Battistini, L.; Zanardi, F.; Casiraghi, G. Bifunctional cinchona alkaloid/thiourea catalyzes direct and enantioselective vinylogous Michael addition of 3-alkylidene oxindoles to nitroolefins. Angew. Chem. Int. Ed. Engl. 2012, 51, 6200–6204. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Dudziński, K.; Łyżwa, D. Effect of high pressure on the organocatalytic asymmetric Michael reaction: Highly enantioselective synthesis of γ-nitroketones with quaternary stereogenic centers. Org. Lett. 2011, 13, 3624–3627. [Google Scholar] [CrossRef]
- Zhang, Y.; Matsuo, Y.; Nakamura, E. Regiocontrolled synthesis of 1,2-di(organo)fullerenes via copper-assisted 1,4-aryl migration from silicon to carbon. Org. Lett. 2011, 13, 6058–6061. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On Water”: Unique Reactivity of Organic Compounds in Aqueous Suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Marcus, R.A. On the nature of organic catalysis “on water”. J. Am. Chem. Soc. 2007, 129, 5492–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, T.J.; Janda, K.D. Aqueous aldol catalysis by a nicotine metabolite: Dickerson and K. D. Janda. J. Am. Chem. Soc. 2002, 124, 3220–3221. [Google Scholar] [CrossRef]
- Font, D.; Jimeno, C.; Pericàs, M.A. Polystyrene-supported hydroxyproline: An insoluble, recyclable organocatalyst for the asymmetric aldol reaction in water. Org. Lett. 2006, 8, 4653–4655. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yu, M.; Zhao, G.; Wang, S. Highly efficient and reusable dendritic catalysts derived from N-prolylsulfonamide for the asymmetric direct aldol reaction in water. Org. Lett. 2006, 8, 4417–4420. [Google Scholar] [CrossRef]
- Zu, L.; Wang, J.; Li, H.; Wang, W. A recyclable fluorous (S)-pyrrolidine sulfonamide promoted direct, highly enantioselective Michael addition of ketones and aldehydes to nitroolefins in water. Org. Lett. 2006, 8, 3077–3079. [Google Scholar] [CrossRef]
- Vishnumaya, V.; Singh, V.K. Highly enantioselective water-compatible organocatalyst for Michael reaction of ketones to nitroolefins. Org. Lett. 2007, 9, 1117–1119. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Tang, J.; Wang, X.; Zhou, J. Highly Efficient “On Water” Catalyst-Free Nucleophilic Addition Reactions Using Difluoroenoxysilanes: Dramatic Fluorine Effects. Angew. Chem. Int. Ed. 2014, 53, 9512–9516. [Google Scholar] [CrossRef]
- Giuffredi, G.T.; Gouverneur, V.; Bernet, B. Intramolecular OH⋅⋅⋅FC Hydrogen Bonding in Fluorinated Carbohydrates: CHF is a Better Hydrogen Bond Acceptor than CF2†. Angew. Chem. Int. Ed. 2013, 52, 10524–10528. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Huang, F.; Deng, H.; Schaffrath, C.; Spencer, J.B.; O’Hagan, D.; Naismith, J.H. Crystal struture and mechanism of a bacterial fluorinating enzyme. Nature 2004, 427, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Dalvit, C.; Vulpetti, A. Fluorine-protein interactions and ¹⁹F NMR isotropic chemical shifts: An empirical correlation with implications for drug design. ChemMedChem 2011, 6, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Dalvit, C.; Vulpetti, A. Intermolecular and intramolecular hydrogen bonds involving fluorine atoms: Implications for recognition, selectivity, and chemical properties. ChemMedChem 2012, 7, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.S.; Jacobsen, E.N. Schiff Base Catalysts for the Asymmetric Strecker Reaction Identified and Optimized from Parallel Synthetic Libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Hiemstra, H.; Wynberg, H. Addition of aromatic thiols to conjugated cycloalkenones, catalyzed by chiral beta.-hydroxy amines. A mechanistic study of homogeneous catalytic asymmetric synthesis. J. Am. Chem. Soc. 1981, 103, 417–430. [Google Scholar] [CrossRef]
- Dolling, U.H.; Davis, P.; Grabowski, E.J.J. Efficient catalytic asymmetric alkylations. 1. Enantioselective synthesis of (+)-indacrinone via chiral phase-transfer catalysis. J. Am. Chem. Soc. 1984, 106, 446–447. [Google Scholar] [CrossRef]
- McGilvra, J.D.; Unni, A.K.; Modi, K.; Rawal, V.H. Highly diastereo- and enantioselective Mukaiyama aldol reactions catalyzed by hydrogen bonding. Angew. Chem. Int. Ed. Engl. 2006, 45, 6130–6133. [Google Scholar] [CrossRef]
- Huang, Y.; Unni, A.K.; Thadani, A.N.; Rawal, V.H. Hydrogen bonding: Single enantiomers from a chiral-alcohol catalyst. Nature 2003, 424, 146. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Deb Majumdar, I.; Porco, J.A., Jr.; Devanabanda, A.; Fox, B.; Schwartzman, J.; Cong, H.; Porco, J.A., Jr.; Weber, H.C. Synthetic cyclohexenyl chalcone natural products possess cytotoxic activities against prostate cancer cells and inhibit cysteine cathepsins in vitro. Biochem. Biophys. Res. Commun. 2011, 416, 397–402. [Google Scholar] [CrossRef]
- Cleland, W.W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. Determining the geometries of transition States by use of antihydrophobic additives in water. Acc. Chem. Res. 2004, 37, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Weeks, J.D.; Zhou, R. Dewetting and hydrophobic interaction in physical and biological systems. Annu. Rev. Phys. Chem. 2009, 60, 85–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Water in Stereoselective Organocatalytic Reactions. Adv. Synth. Catal. 2009, 351, 33–57. [Google Scholar] [CrossRef]
- Mase, N.; Barbas, C.F., III. In water, on water, and by water: Mimicking nature’s aldolases with organocatalysis and water. Org. Biomol. Chem. 2010, 8, 4043–4050. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H.; Ha, D.C. Organocatalytic Asymmetric Michael Addition of Ketones to α, β-Unsaturated Nitro Compounds. Catalysts 2020, 10, 618. [Google Scholar] [CrossRef]
- Shim, J.H.; Ha, D.C. Enantioselective organocatalytic Michael reactions using chiral (R,R)-1,2-diphenylethylenedi-aminederived thioureas. RSC Adv. 2020, 10, 31808–31814. [Google Scholar] [CrossRef]
- Reznikov, A.N.; Golovin, E.V.; Klimochkin, Y.N. Enantioselective synthesis of γ-aminobutyric acid derivatives by Ni(II)-catalyzed reaction of diethyl malonate with nitroalkenes. Russ. J. Org. Chem. 2013, 49, 663–668. [Google Scholar] [CrossRef]
- Schreiner, P.R.; Wittkopp, A. H-Bonding Additives Act Like Lewis Acid Catalysts. Org. Lett. 2002, 4, 217–220. [Google Scholar] [CrossRef]
- Paraskar, A.S.; Sudalai, A. Co-catalyzed reductive cyclization of azido and cyano substituted α,β-unsaturated esters with NaBH4: Enantioselective synthesis of (R)-baclofen and (R)-rolipram. Tetrahedron 2006, 62, 4907–4916. [Google Scholar] [CrossRef]
- Hajra, S.; Aziz, S.M.; Maji, R. Organocatalytic enantioselective conjugate addition of nitromethane to alkylidenemalonates: Asymmetric synthesis of pyrrolidine-3-carboxylic acid derivatives. RSC Adv. 2013, 3, 10185. [Google Scholar] [CrossRef]
- Barnes, D.M.; Ji, J.; Fickes, M.G.; Fitzgerald, M.A.; King, S.A.; Morton, H.E.; Plagge, F.A.; Preskill, M.; Wagaw, S.H.; Wittenberger, S.J.; et al. Development of a catalytic enantioselective conjugate addition of 1,3-dicarbonyl compounds to nitroalkenes for the synthesis of endothelin-A antagonist ABT-546. Scope, mechanism, and further application to the synthesis of the antidepressant rolipram. J. Am. Chem. Soc. 2002, 124, 13097–13105. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. Enantio- and diastereoselective Michael reaction of 1,3-dicarbonyl compounds to nitroolefins catalyzed by a bifunctional thiourea. J. Am. Chem. Soc. 2005, 127, 119–125. [Google Scholar] [CrossRef]
- Karolina, Z.; Karolina, K. Application of β-Phosphorylated Nitroethenes in [3+2] Cycloaddition Reactions Involving Benzonitrile N-Oxide in the Light of a DFT Computational Study. Organics 2021, 2, 3. [Google Scholar] [CrossRef]
- Jasinski, R. β-Trifluoromethylated nitroethenes in Diels-Alder reaction with cyclopentadiene: A DFT computational study. J. Fluor. Chem. 2018, 206, 1–7. [Google Scholar] [CrossRef]
- Manabe, K.; Kobayashi, S. Catalytic asymmetric carbon-carbon bond-forming reactions in aqueous media. Chemistry 2002, 8, 4094–4101. [Google Scholar] [CrossRef]
- Bhowmick, S.; Bhowmick, K.C. Catalytic asymmetric carbon–carbon bond-forming reactions in aqueous media. Tetrahedron Asymmetry 2011, 22, 1945–1979. [Google Scholar] [CrossRef]
- Bae, H.Y.; Song, C.E. Unprecedented Hydrophobic Amplification in Noncovalent Organocatalysis “on Water”: Hydrophobic Chiral Squaramide Catalyzed Michael Addition of Malonates to Nitroalkenes. ACS Catal. 2015, 5, 3613–3619. [Google Scholar] [CrossRef]
- Butler, R.N.; Coyne, A.G. Organic synthesis reactions on-water at the organic-liquid water interface. Org. Biomol. Chem. 2016, 14, 9945–9960. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, W.; Liu, Y.; Chu, Y.; Lin, L.; Liu, X.; Feng, X. Asymmetric Cyanation of Activated Olefins with Ethyl Cyanoformate Catalyzed by a Modular Titanium Catalyst. Org. Lett. 2010, 12, 1280–1283. [Google Scholar] [CrossRef]
- Kimmel, K.L.; Weaver, J.D.; Lee, M.; Ellman, J.A. Catalytic enantioselective protonation of nitronates utilizing an organocatalyst chiral only at sulfur. J. Am. Chem. Soc. 2012, 134, 9058–9061. [Google Scholar] [CrossRef] [PubMed]
- Zvejniece, L.; Svalbe, B.; Veinberg, G.; Grinberga, S.; Vorona, M.; Kalvinsh, I.; Dambrova, M. Investigation into stereoselective pharmacological activity of phenotropil. Basic Clin. Pharmacol. Toxicol. 2011, 109, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, H.; Ishikawa, H.; Hayashi, Y. Diphenylprolinol silyl ether as catalyst of an asymmetric, catalytic, and direct Michael reaction of nitroalkanes with alpha, beta-unsaturated aldehydes. Org. Lett. 2007, 9, 5307–5309. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Xie, H.; Li, H.; Wang, J.; Wang, W. Highly Enantioselective Organocatalytic Conjugate Addition of Nitromethane to α,β-Unsaturated Aldehydes: Three-Step Synthesis of Optically Active Baclofen. Adv. Synth. Catal. 2007, 349, 2660–2664. [Google Scholar] [CrossRef]
- Dambrova, M.; Zvejniece, L.; Liepinsh, E.; Cirule, H.; Zharkova, O.; Veinberg, G.; Kalvinsh, I. Comparative pharmacological activity of optical isomers of phenibut. Eur. J. Pharmacol. 2008, 583, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Borowsky, B.; Tamm, J.A.; Craig, D.A.; Durkin, M.M.; Dai, M.; Yao, W.J.; Johnson, M.; Gunwaldsen, C.; Huang, L.Y.; et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 1998, 396, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Goodkin, D.E. Drugs used to treat spasticity. Drugs 2000, 59, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, L.; Liu, L.; Xia, Z.; Pin, J.P.; Nan, F.; Liu, J. A negative allosteric modulator modulates GABAB-receptor signalling through GB2 subunits. Biochem. J. 2016, 473, 779–787. [Google Scholar] [CrossRef]
- Addolorato, G.; Leggio, L.; Ferrulli, A.; Cardone, S.; Vonghia, L.; Mirijello, A.; Abenavoli, L.; D’Angelo, C.; Caputo, F.; Zambon, A.; et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: Randomised, double-blind controlled study. Lancet 2007, 370, 1915–1922. [Google Scholar] [CrossRef]
- Rolland, B.; Bordet, R.; Cottencin, O. Alcohol-dependence: The current French craze for baclofen. Addiction 2012, 107, 848–849. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, P.A.; Auffret, M.; Deheul, S.; Bordet, R.; Cottencin, O.; Rolland, B. Baclofen-induced manic symptoms: Case report and systematic review. Psychosomatics 2014, 55, 326–332. [Google Scholar] [CrossRef] [PubMed]

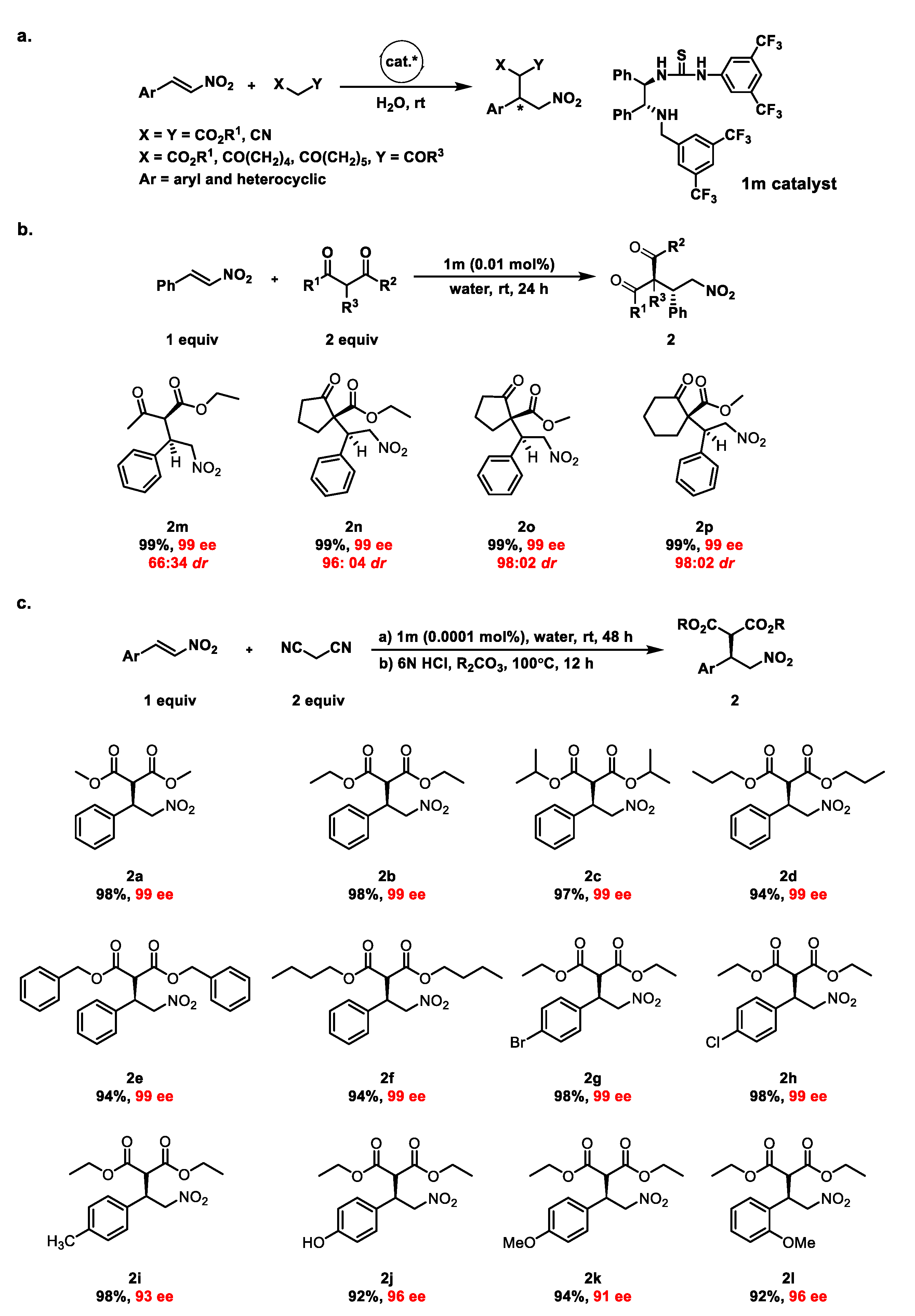

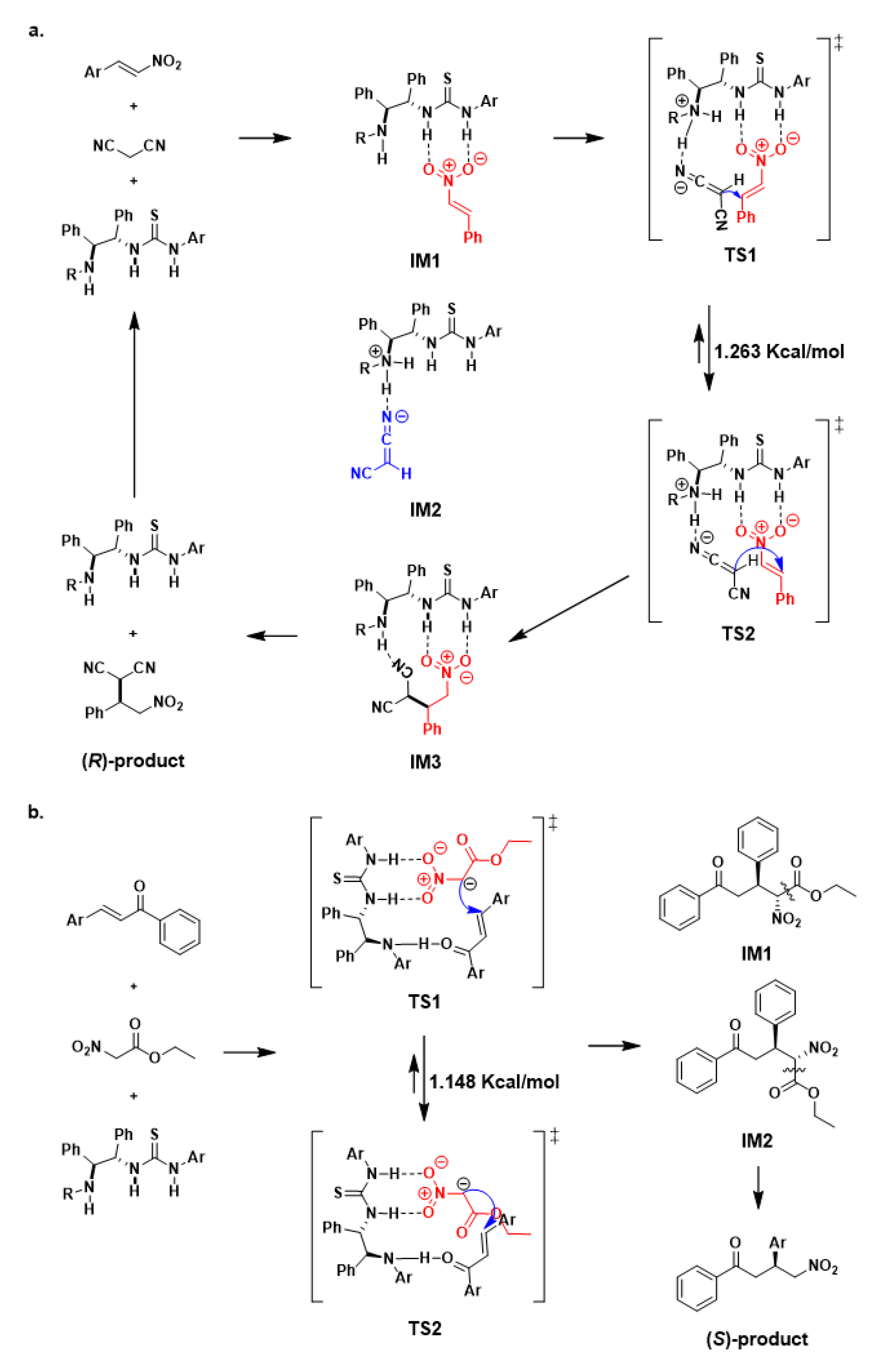
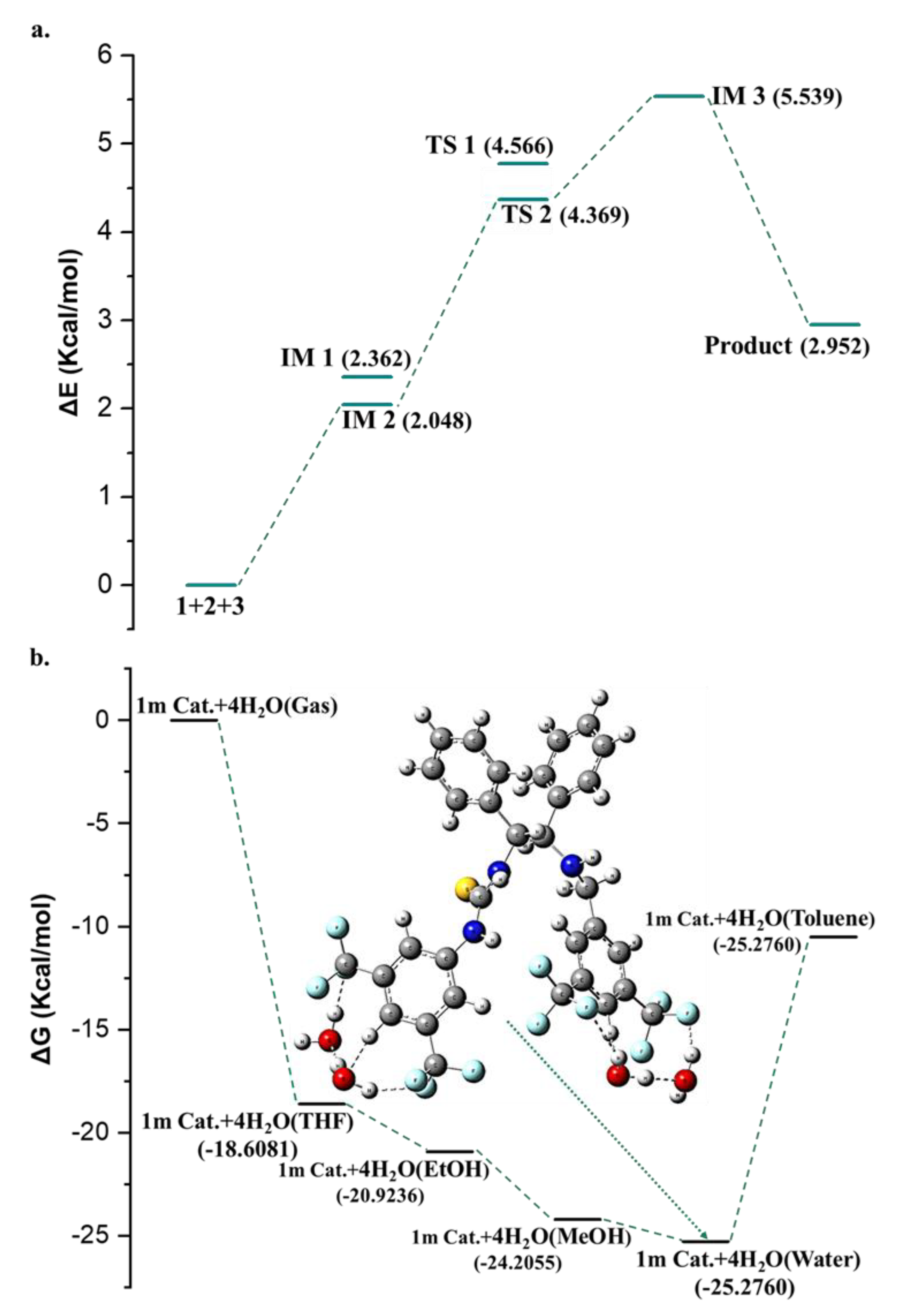
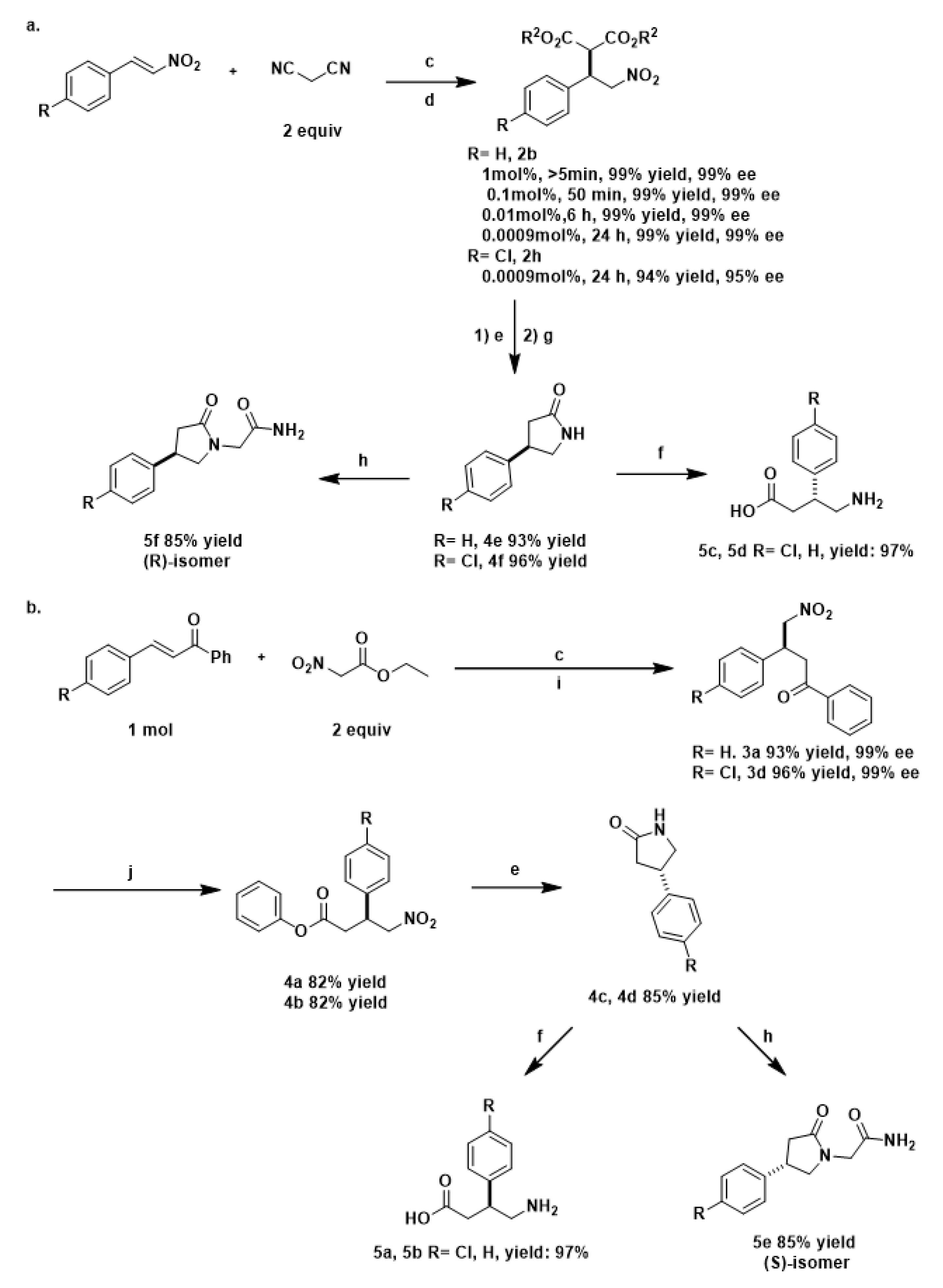
 | |||||
|---|---|---|---|---|---|
| Entry | R | Ar | Time (min) | Yield (%) b | ee (%) c |
| 1 | Me | Ph | 30 | 99 | 99 |
| 2 | Et | Ph | 40 | 99 | 99 |
| 3 d | Et | Ph | 10 | 99 | 99 |
| 4 e | Et | Ph | 10 | 99 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, J.H.; Hong, Y.; Kim, J.H.; Kim, H.S.; Ha, D.-C. Organocatalytic Asymmetric Michael Addition in Aqueous Media by a Hydrogen-Bonding Catalyst and Application for Inhibitors of GABAB Receptor. Catalysts 2021, 11, 1134. https://doi.org/10.3390/catal11091134
Shim JH, Hong Y, Kim JH, Kim HS, Ha D-C. Organocatalytic Asymmetric Michael Addition in Aqueous Media by a Hydrogen-Bonding Catalyst and Application for Inhibitors of GABAB Receptor. Catalysts. 2021; 11(9):1134. https://doi.org/10.3390/catal11091134
Chicago/Turabian StyleShim, Jae Ho, Yeonsun Hong, Ji Hae Kim, Hyeon Soo Kim, and Deok-Chan Ha. 2021. "Organocatalytic Asymmetric Michael Addition in Aqueous Media by a Hydrogen-Bonding Catalyst and Application for Inhibitors of GABAB Receptor" Catalysts 11, no. 9: 1134. https://doi.org/10.3390/catal11091134
APA StyleShim, J. H., Hong, Y., Kim, J. H., Kim, H. S., & Ha, D.-C. (2021). Organocatalytic Asymmetric Michael Addition in Aqueous Media by a Hydrogen-Bonding Catalyst and Application for Inhibitors of GABAB Receptor. Catalysts, 11(9), 1134. https://doi.org/10.3390/catal11091134






