LaNiO3 Perovskite Synthesis through the EDTA–Citrate Complexing Method and Its Application to CO Oxidation
Abstract
1. Introduction
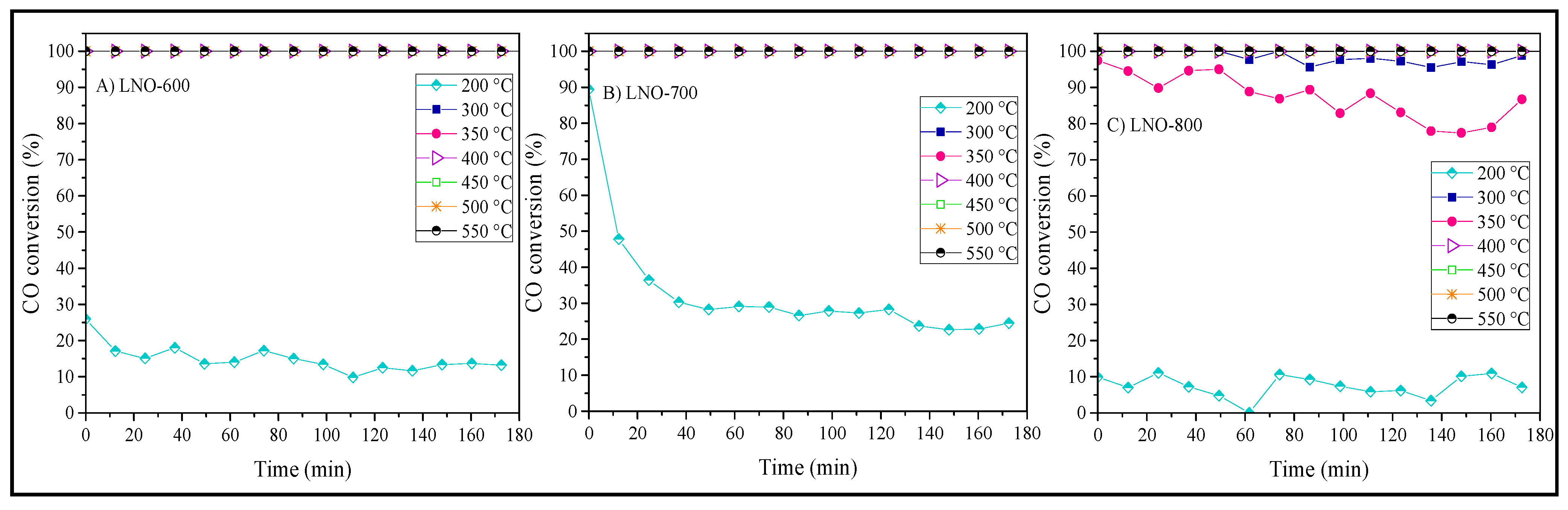
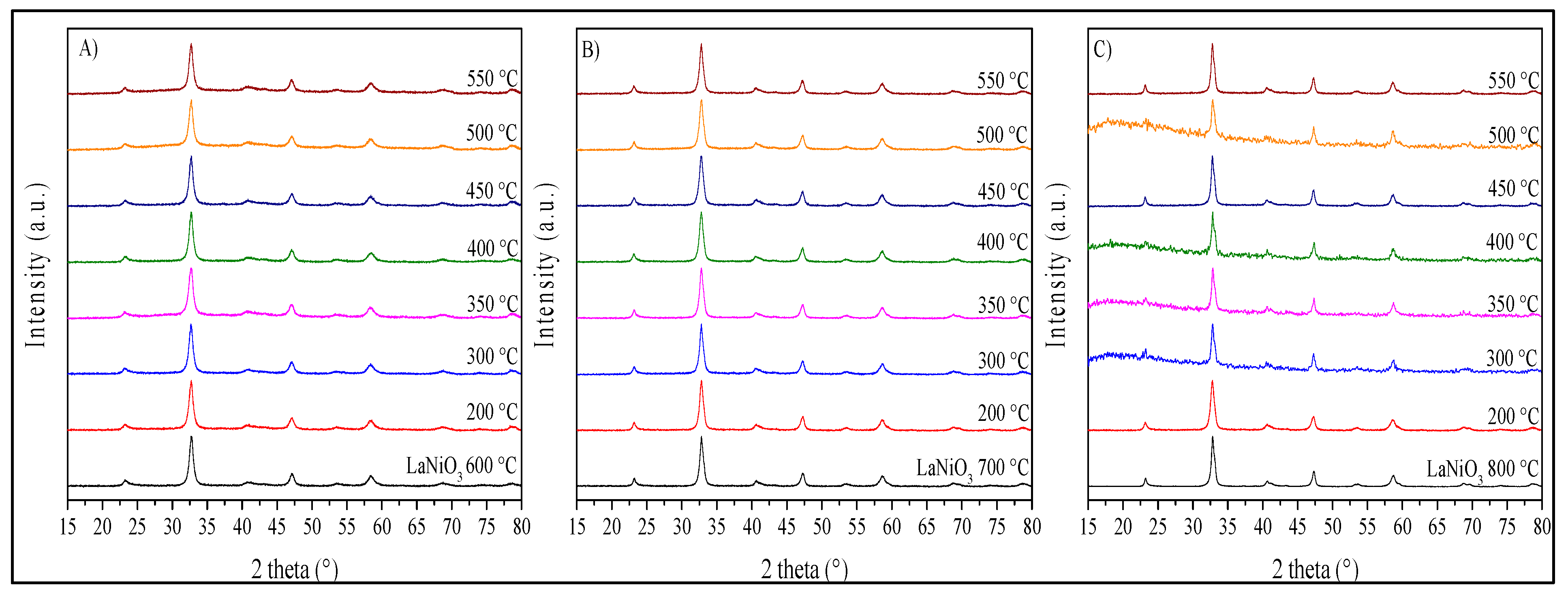
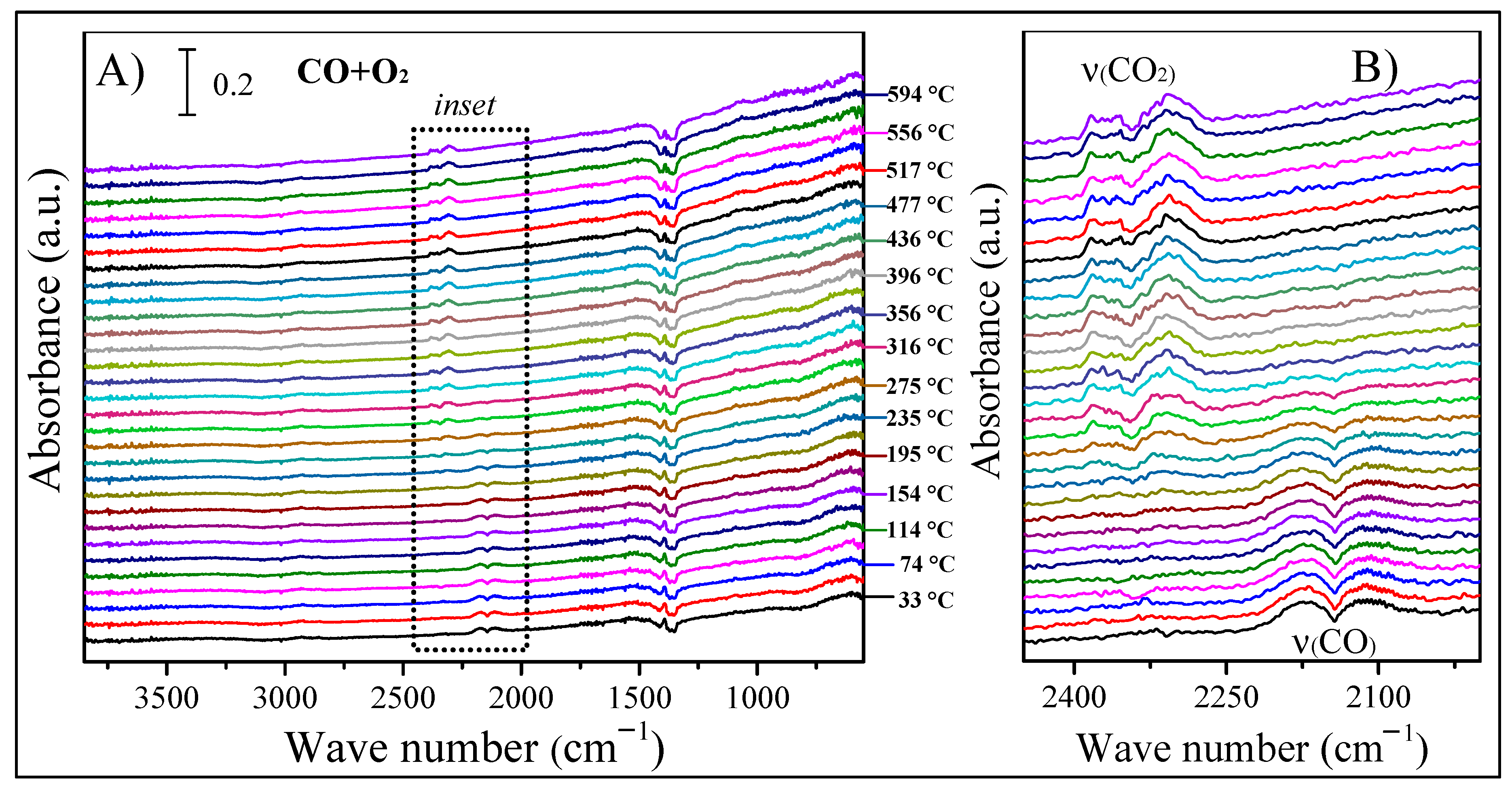
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Park, S.J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Chen, R.; Pan, G.; Zhang, Y.; Xu, Q.; Zeng, G.; Xu, X.; Chen, B.; Kan, H. Ambient carbon monoxide and daily mortality in three chinese cities: The China air pollution and health effects study. Sci. Total Environ. 2011, 409, 4923–4928. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater. Res. Technol. 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- Zhang, L.; Song, H.; Xu, G.; Wang, W.; Yang, L. MOFs derived mesoporous Co3O4 polyhedrons and plates for CO oxidation reaction. J. Solid State Chem. 2019, 276, 87–92. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Liu, Y.; Deng, J.; Jing, L.; Pei, W.; Zhang, X.; Hou, Z.; Rezaei, M.; Dai, H. Influence of preparation method on catalytic performance of three-dimensionally ordered macroporous NiO–CuO for CO oxidation. J. Solid State Chem. 2021, 297, 122091. [Google Scholar] [CrossRef]
- van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Oxidation of carbon monoxide over various nickel oxide catalysts in different conditions: A review. Chem. Eng. J. Adv. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, G.; Yao, X.; Liu, B. A comparison of NiO–CuO–CeO2 composite catalysts prepared via different methods for CO oxidation. J Solid State Chem. 2020, 292, 121697. [Google Scholar] [CrossRef]
- Ovalle-Encinia, O.; Sánchez-Camacho, P.; González-Varela, D.; Pfeiffer, H. Development of new bifunctional dense ceramic-carbonate membrane reactors for gas mixtures separation, through CO oxidation and subsequent CO2 permeation. ACS Appl. Energy Mater. 2019, 2, 1380–1387. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Cong, Y.; Yang, W. Ce0.85Sm0.15O1.925−Sm0.6Sr0.4Al0.3Fe0.7O3 dual-phase membranes: One-pot synthesis and stability in a CO2 atmosphere. Solid State Ionics 2013, 253, 57–63. [Google Scholar] [CrossRef]
- Angel, S.; Tapia, J.D.; Gallego, J.; Hagemann, U.; Wiggers, H. Spray-flame synthesis of LaMnO3+δ nanoparticles for selective CO oxidation (SELOX). Energy Fuels 2021, 35, 4367–4376. [Google Scholar] [CrossRef]
- Kostyukhin, E.M.; Kustov, A.L.; Evdokimenko, N.V.; Bazlov, A.I.; Kustov, L.M. Hydrothermal microwave-assisted synthesis of LaFeO3 catalyst for N2O decomposition. J. Am. Ceram. Soc. 2021, 104, 492–503. [Google Scholar] [CrossRef]
- Bak, J.; Bae, H.B.; Oh, C.; Son, J.; Chung, S.Y. Effect of lattice strain on the formation of Ruddlesden−Popper faults in heteroepitaxial LaNiO3 for Oxygen Evolution Electrocatalysis. J. Phys. Chem. Lett. 2020, 11, 7253–7260. [Google Scholar] [CrossRef] [PubMed]
- Tietz, F.; Arul Raj, I.; Ma, Q.; Baumann, S.; Mahmoud, A.; Hermann, R.P. Material properties of perovskites in the quasi-ternary system LaFeO3–LaCoO3–LaNiO3. J. Solid State Chem. 2016, 237, 183–191. [Google Scholar] [CrossRef]
- Abe, Y.; Satoh, I.; Saito, T.; Kan, D.; Shimakawa, Y. Oxygen reduction reaction catalytic activities of pure Ni-based perovskite-related structure oxides. Chem. Mater. 2020, 32, 8694–8699. [Google Scholar] [CrossRef]
- Mosinska, M.; Maniukiewicz, W.; Szynkowska-Jozwik, M.; Miercznski, P. Influence of NiO/La2O3 catalyst preparation method on its reactivity in the oxy-steam reforming of LNG process. Catalysts 2021, 11, 1174. [Google Scholar] [CrossRef]
- Wang, H.W.; Wu, J.X.; Wang, X.Y.; Wang, H.; Liu, J.R. Formation of perovskite-type LaNiO3 on La-Ni/Al2O3-ZrO2 catalysts and their performance for CO methanation. J. Fuel Chem. Technol. 2021, 49, 186–197. [Google Scholar] [CrossRef]
- Liu, J.; Jia, E.; Stoerzinger, K.A.; Wang, L.; Wang, Y.; Yang, Z.; Shen, D.; Engelhard, M.H.; Bowden, M.E.; Zhu, Z.; et al. Dynamic Lattice Oxygen Participation on Perovskite LaNiO3 during Oxygen Evolution Reaction. J. Phys. Chem. C 2020, 124, 15386–15390. [Google Scholar] [CrossRef]
- Islam, M.; Jeong, M.G.; Oh, I.H.; Nam, K.W.; Jung, H.G. Role of strontium as doping agent in LaMn0.5Ni0.5O3 for oxygen electro-catalysis. J. Ind. Eng. Chem. 2020, 85, 94–101. [Google Scholar] [CrossRef]
- Lu, Y.; Akbar, M.; Xia, C.; Mi, Y.; Ma, L.; Wang, B.; Zhu, B. Catalytic membrane with high ion–electron conduction made of strongly correlated perovskite LaNiO3 and Ce0.8Sm0.2O2-d for fuel cells. J. Catal. 2020, 386, 117–125. [Google Scholar] [CrossRef]
- Rakshit, S.; Gopalakrishnan, P.S. Oxygen Nonstoichiometry and Its Effect on the Structure of LaNiO3. J. Solid State Chem. 1994, 110, 28–31. [Google Scholar] [CrossRef]
- Djani, F.; Omari, M.; Martínez-Arias, A. Synthesis, characterization and catalytic properties of La(Ni,Fe)O3–NiO nanocomposites. J. Sol-Gel Sci. Technol. 2016, 78, 1–10. [Google Scholar] [CrossRef]
- Phumuen, P.; Kumnorkaew, P.; Srepusharawoot, P.; Klangtakai, P.; Pimanpang, S.; Amornkitbamrung, V. Ball Milling Modification of Perovskite LaNiO3 Powders for Enhancing Electrochemical Pseudocapacitor. Surf. Interfaces 2021, 25, 101282. [Google Scholar] [CrossRef]
- Pereñíguez, R.; González-delaCruz, V.M.; Holgado, J.P.; Caballero, A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B Environ. 2010, 93, 346–353. [Google Scholar] [CrossRef]
- Talaie, N.; Sadr, M.H.; Aghabozorg, H.; Zare, K. Synthesis and Application of LaNiO3 Perovskite-Type Nanocatalyst with Zr for Carbon Dioxide Reforming of Methane. Oriental J. Chem. 2016, 32, 2723–2730. [Google Scholar] [CrossRef][Green Version]
- Komarala, E.P.; Komissarov, I.; Rosen, B.A. Effect of Fe and Mn Substitution in LaNiO3 on Exsolution, Activity, and Stability for Methane Dry Reforming. Catalysts 2020, 10, 27. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Gao, L.; Guo, L. Synthesis and characterization of doped LaCrO3 perovskite prepared by EDTA-citrate complexing method. J. Alloys Compd. 2008, 458, 346–350. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Dong, B.; Li, H.L. Template induced sol–gel synthesis of highly ordered LaNiO3 nanowires. J. Solid State Chem. 2005, 178, 1157–1164. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to form a Capacitor. US Patent 3 330 697, 1964. [Google Scholar]
- Zhou, W.; Shao, Z.; Jin, W. Synthesis of nanocrystalline conducting composite oxides based on a non-ion selective combined complexing process for functional applications. J. Alloys Compd. 2006, 426, 368–374. [Google Scholar] [CrossRef]
- Ringbom, A. Complexation in Analytical Chemistry; Interscience: New York, NY, USA, 1963. [Google Scholar]
- Chanaud, P.; Julbe, A.; Vaija, P.; Persin, M.; Cot, L. Study of lanthanum-based colloidal sols formation. J. Mater. Sci. 1994, 29, 4244–4251. [Google Scholar] [CrossRef]
- Wu, W.C.; Huang, J.T.; Chiba, A. Synthesis and properties of samaria-doped ceria electrolyte for IT-SOFCs by EDTA-citrate complexing method. J. Power Sources. 2010, 195, 5868–5874. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids ans Powders: Surface Area, Pore Size and Density; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Rida, K.; Peña, M.A.; Sastre, E.; Martínez-Arias, A. Effect of calcination temperature on structural properties and catalytic activity in oxidation reactions of LaNiO3 perovskite prepared by Pechini method. J. Rare Earths. 2012, 30, 210–216. [Google Scholar] [CrossRef]
- Hyeok-Yang, E.; Moon, D.J. Synthesis of LaNiO3 perovskite using an EDTA-cellulose method and comparison with the conventional Pechini method: Application to steam CO2 reforming of methane. RSC Adv. 2016, 6, 112885–112898. [Google Scholar] [CrossRef]
- Skoog, C.S.; Douglas, A.; James, H.F. Principles of Instrumental Analysis, 6th ed.; CENGAGE Learning: Boston, MA, USA, 2008. [Google Scholar]
- He, A.; Zhou, F.; Ye, F.; Zhang, Y.; He, X.; Zhang, X.; Guo, R.; Zhao, X.; Sun, Y.; Huang, M.; et al. Preparation and characterization of lanthanum carbonate octahydrate for the treatment of hyperphosphatemia. J. Spectr. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Dreyer, M.; Krebs, M.; Najafishirtari, S.; Rabe, A.; Ortega, K.F.; Behrens, M. The effect of Co incorporation on the CO oxidation activity of LaFe1−xCoxO3 perovskites. Catalysts 2021, 11, 550. [Google Scholar] [CrossRef]
- Shahnazi, A.; Firoozi, S. Improving the catalytic performance of LaNiO3 perovskite by manganese substitution via ultrasonic spray pyrolysis for dry reforming of methane. J. CO2 Util. 2021, 45, 101455. [Google Scholar] [CrossRef]
- Mickevičius, S.; Grebinskij, S.; Bondarenka, V.; Vengalis, B.; Šliužiene, K.; Orlowski, B.A.; Osinniy, V.; Drube, W. Investigation of epitaxial LaNiO3-x thin films by high-energy XPS. J. Alloys Compd. 2006, 423, 107–111. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Direct observation of Ni3+ and Ni2+ in correlated LaNiO3-δ films. IOP Sci. 2011, 93, 57002. [Google Scholar]
- Che, W.; Wei, M.; Sang, Z.; Ou, Y.; Liu, Y.; Liu, J. Perovskite LaNiO3-δ oxide as an anion-intercalated pseudocapacitor electrode. J. Alloys Compd. 2018, 731, 381–388. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M. The influence of partial substitution of alkaline earth with la in the LaNiO3 perovskite catalyst. Catal. Commun. 2012, 26, 169–172. [Google Scholar] [CrossRef]
- Lombardo, E.A.; Ulla, M.A. Perovskite oxides in catalysis: Past, present and future. Res. Chem. Intermed. 1998, 24, 581–592. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Gallego, G.A.S.; Mondragon, F.; Barrault, J.; Tatibouët, J.M. CO2 reforming of methane over LaNiO3 as precursor material. Catal. Today 2005, 107–108, 474–480. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klo, B.; et al. In Situ-Determined Catalytically Active State of LaNiO3 in Methane Dry Reforming. ACS Catal. 2020, 10, 1102–1112. [Google Scholar] [CrossRef]
- Tian, F.X.; Zhu, M.; Liu, X.; Tu, W.; Han, Y.F. Dynamic structure of highly disordered manganese oxide catalysts for low-temperature CO oxidation. J. Catal. 2021, 401, 115–128. [Google Scholar] [CrossRef]
- Gou, Y.; Liang, X.; Chen, B. Porous Ni–Co bimetal oxides nanosheets and catalytic properties for CO oxidation. J. Alloys Comp. 2013, 574, 181–187. [Google Scholar] [CrossRef]
- Wang, D.; Xu, R.; Wang, X.; Li, Y. NiO nanorings and their unexpected catalytic property for CO oxidation. Nanotechnology 2006, 17, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, K.; Wan, C.; Zhu, J.; Li, X.; Tong, S.; Zhao, Y. Comparison of the nickel addition patterns on the catalytic performances of LaCoO3 for low-temperature CO oxidation. Catal. Today 2017, 281, 534–541. [Google Scholar] [CrossRef]
- Vaz, T.; Salker, A.V. Preparation, characterization and catalytic CO oxidation studies on LaNi1−xCoxO3 system. Mater. Sci. Eng. B 2007, 143, 81–84. [Google Scholar] [CrossRef]

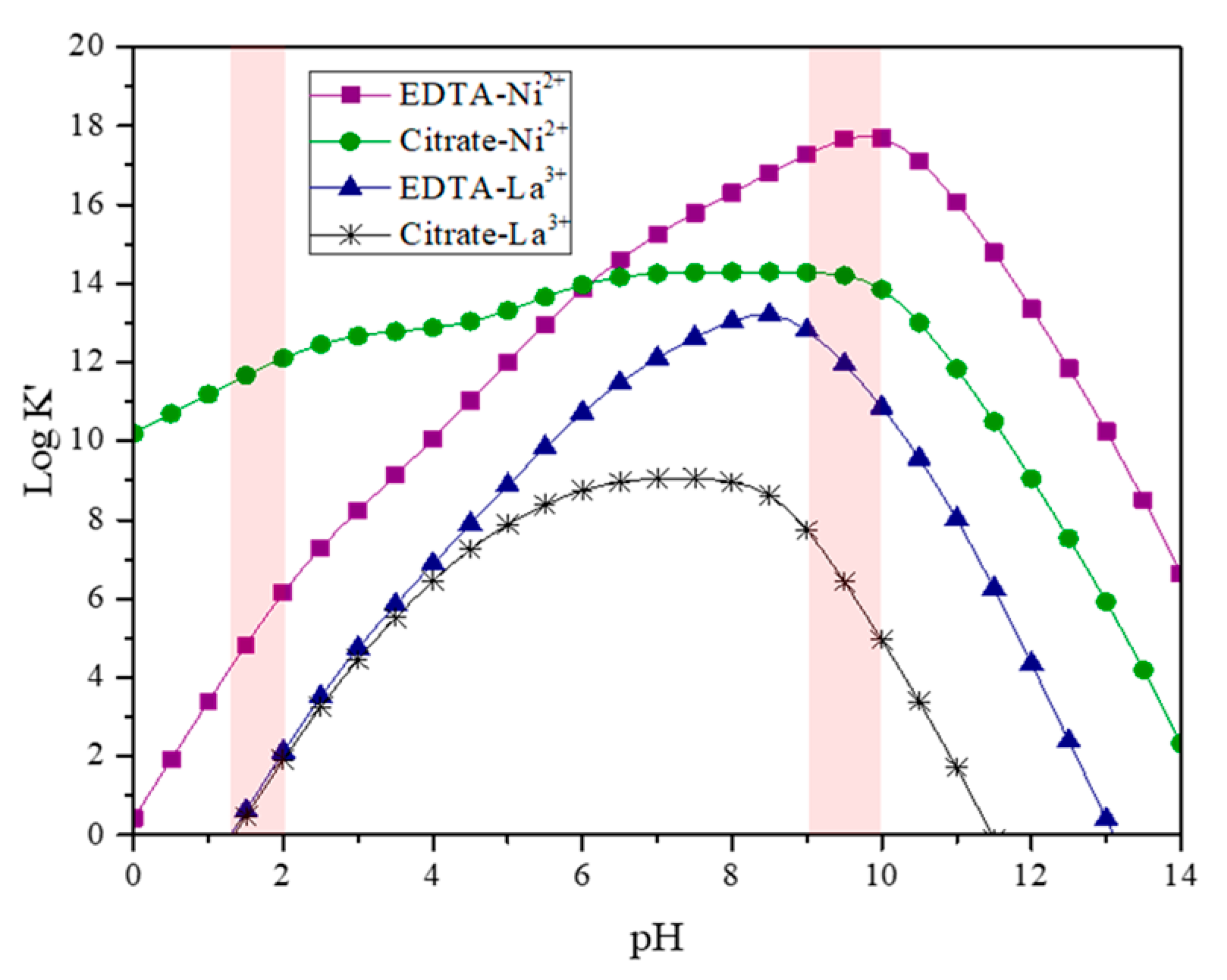
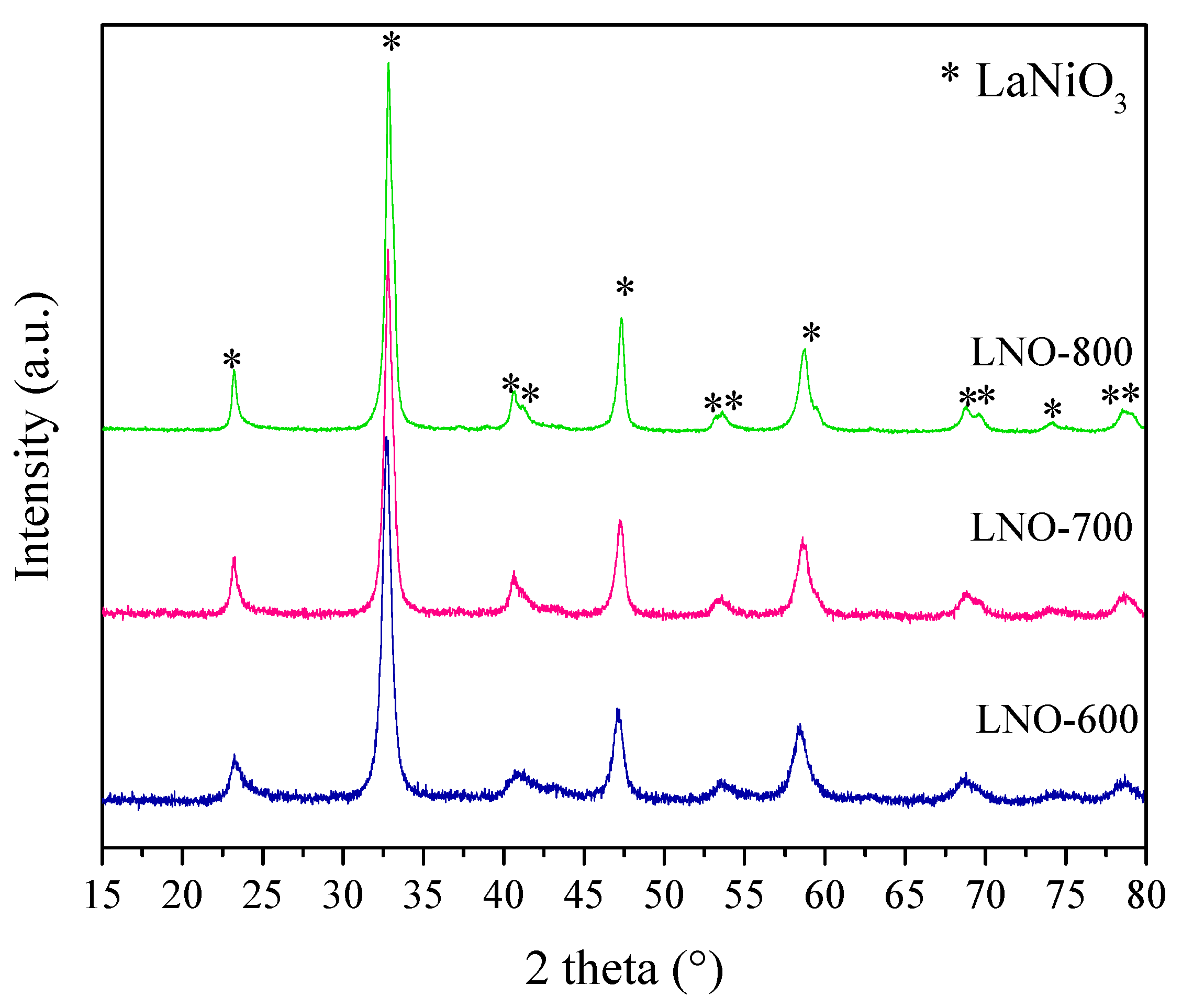
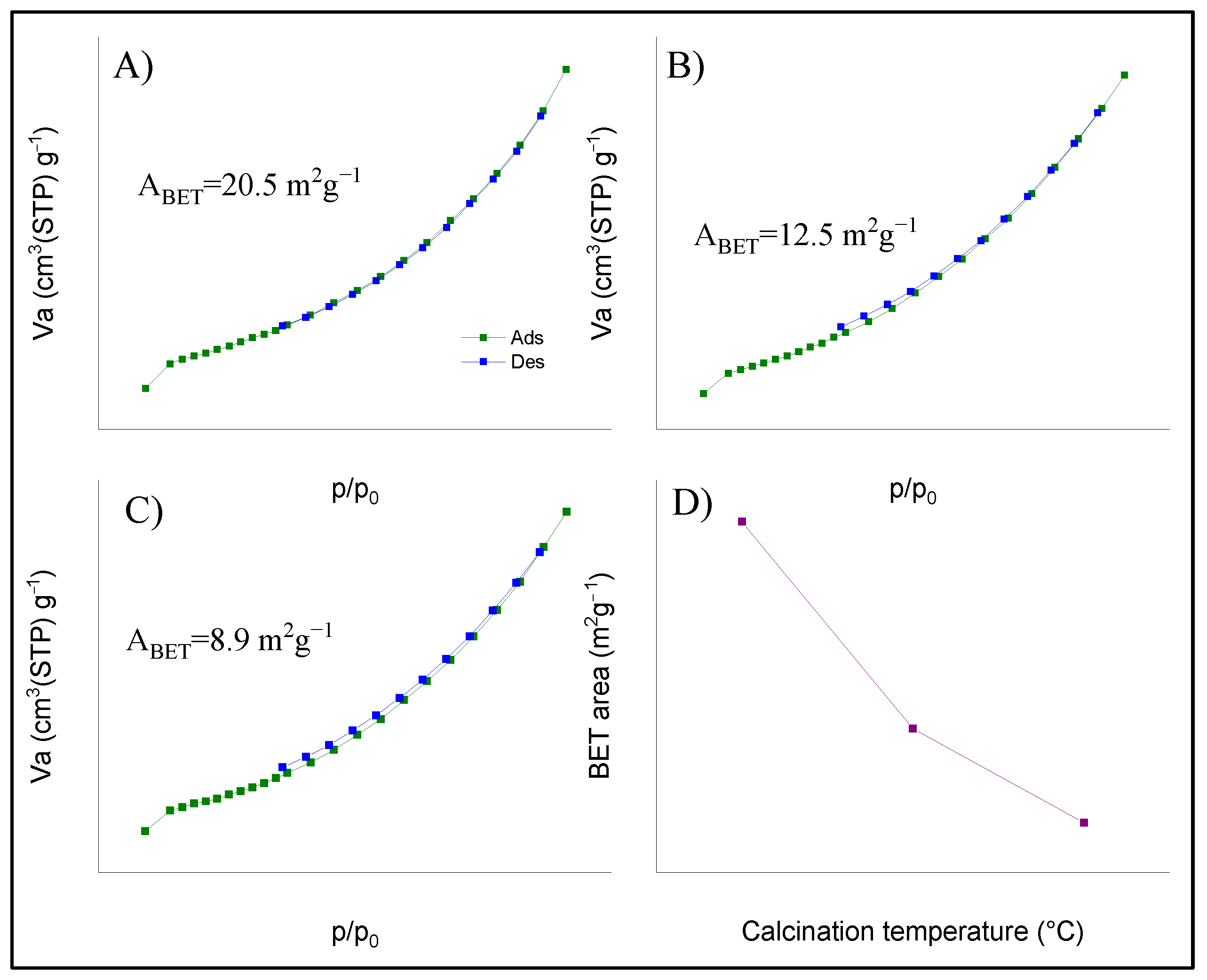
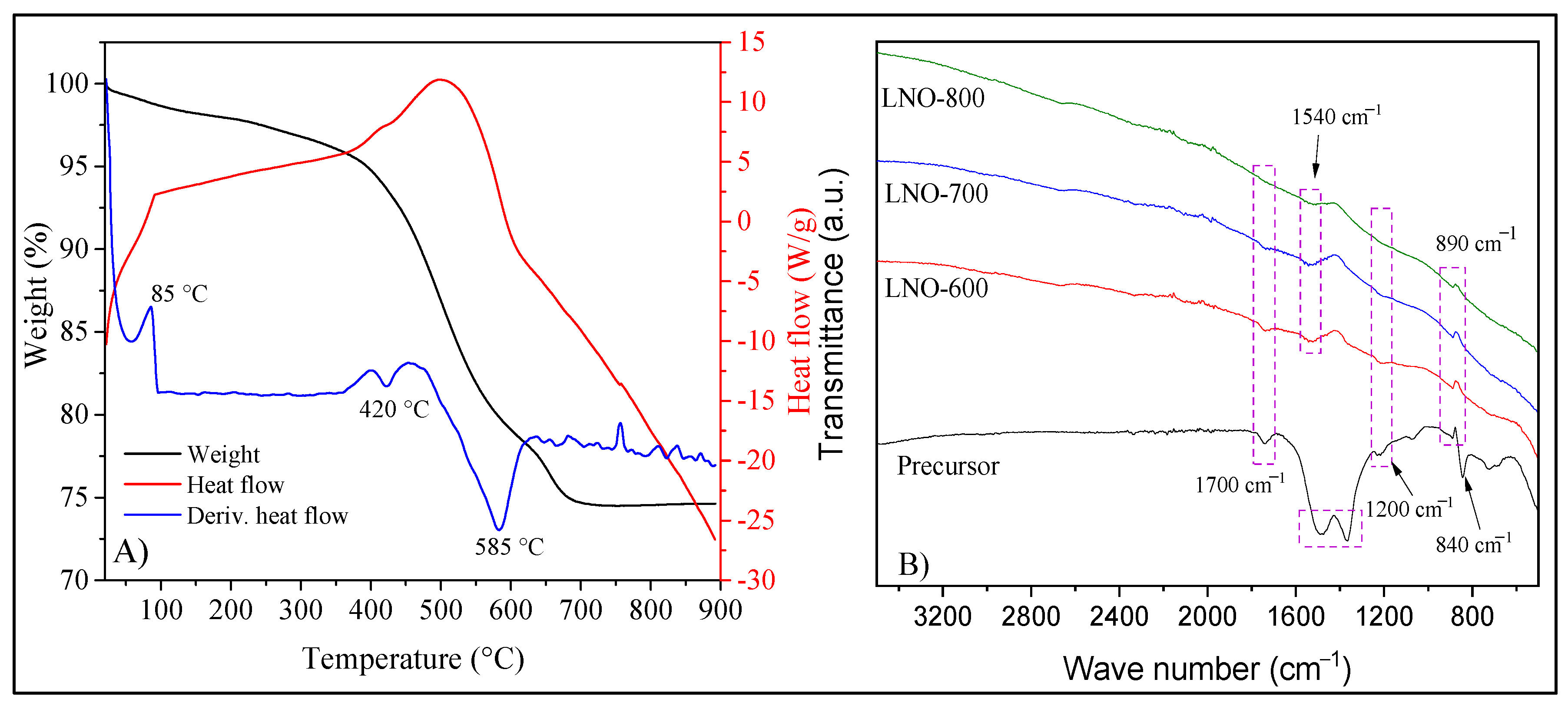
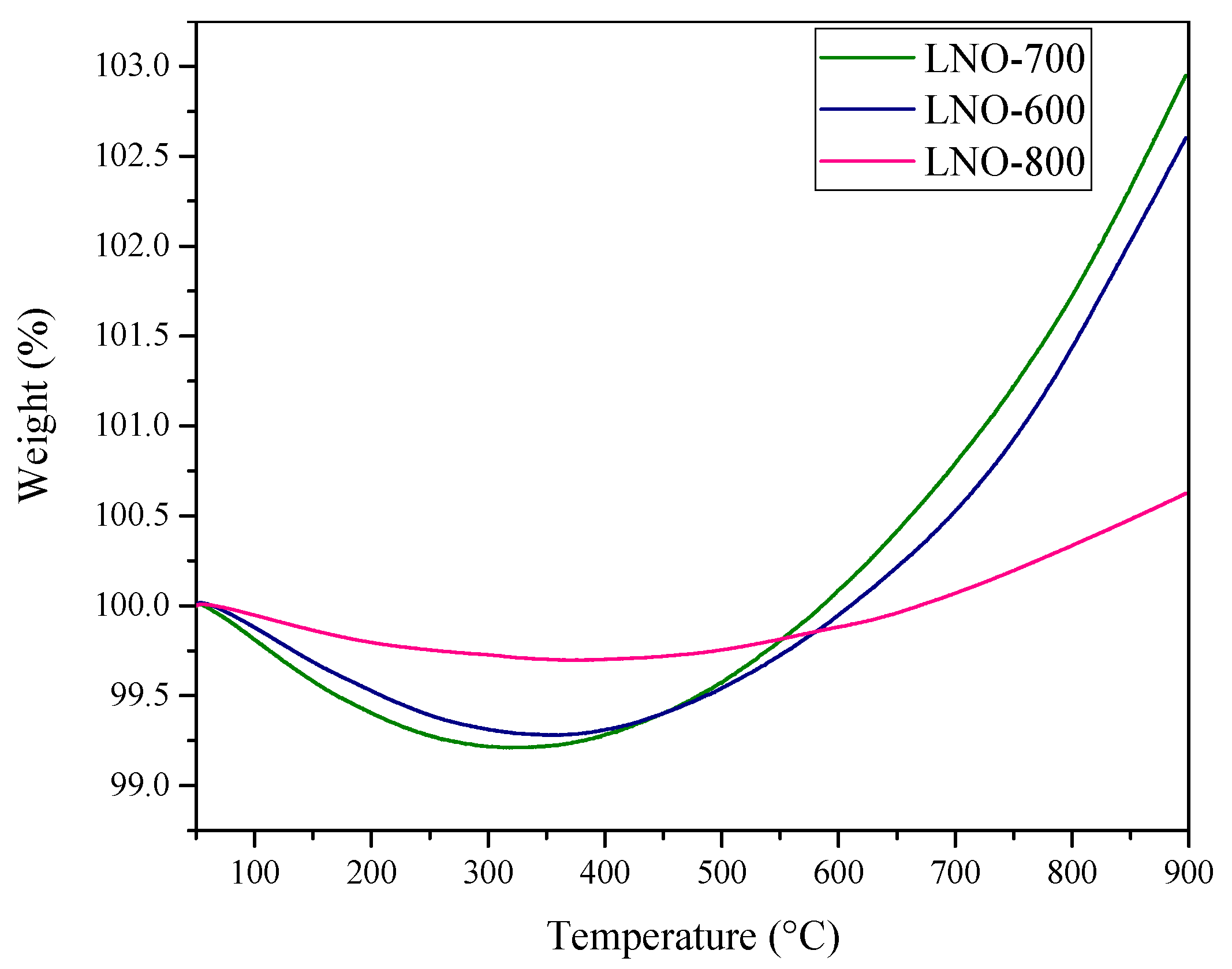
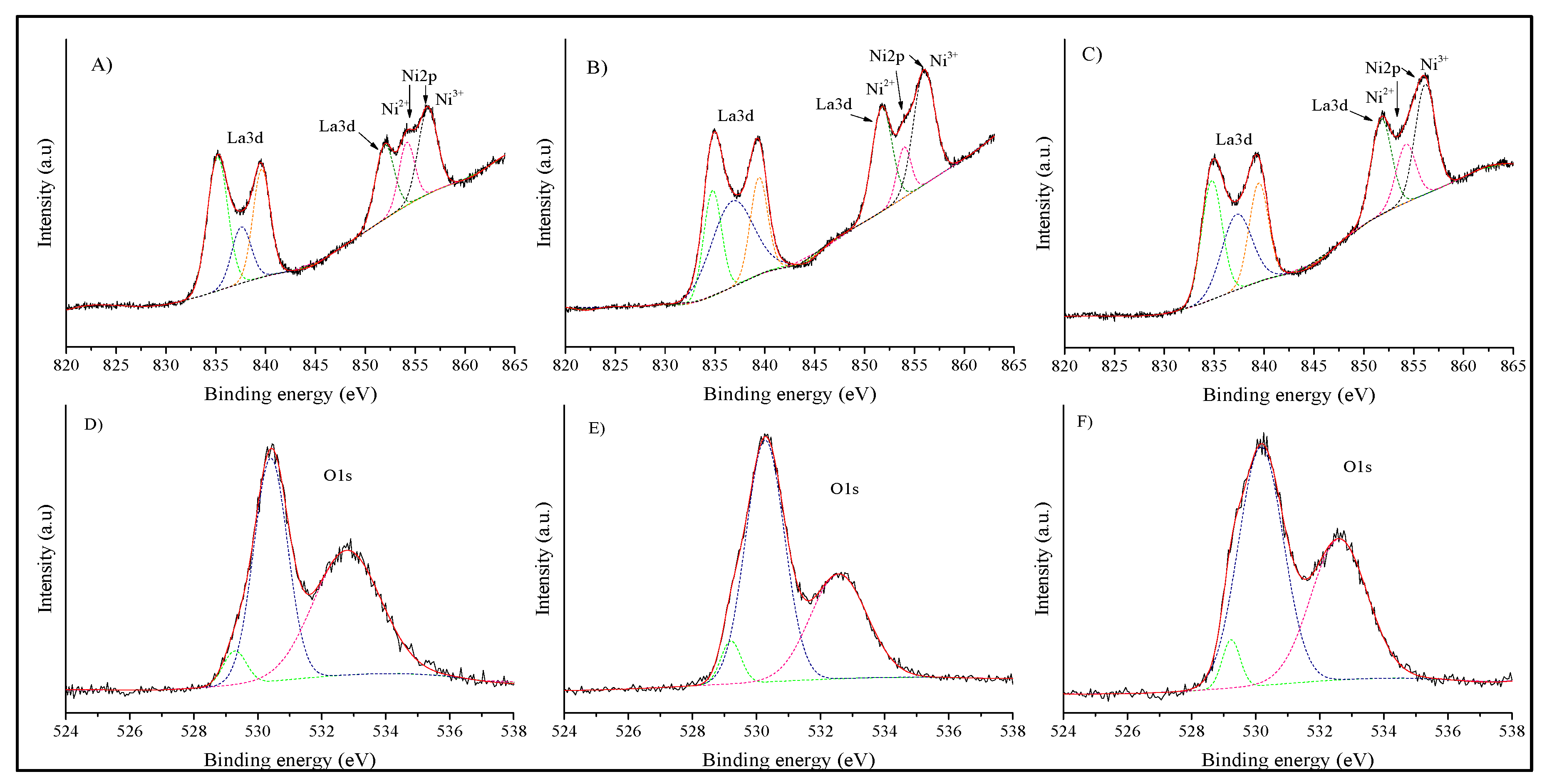
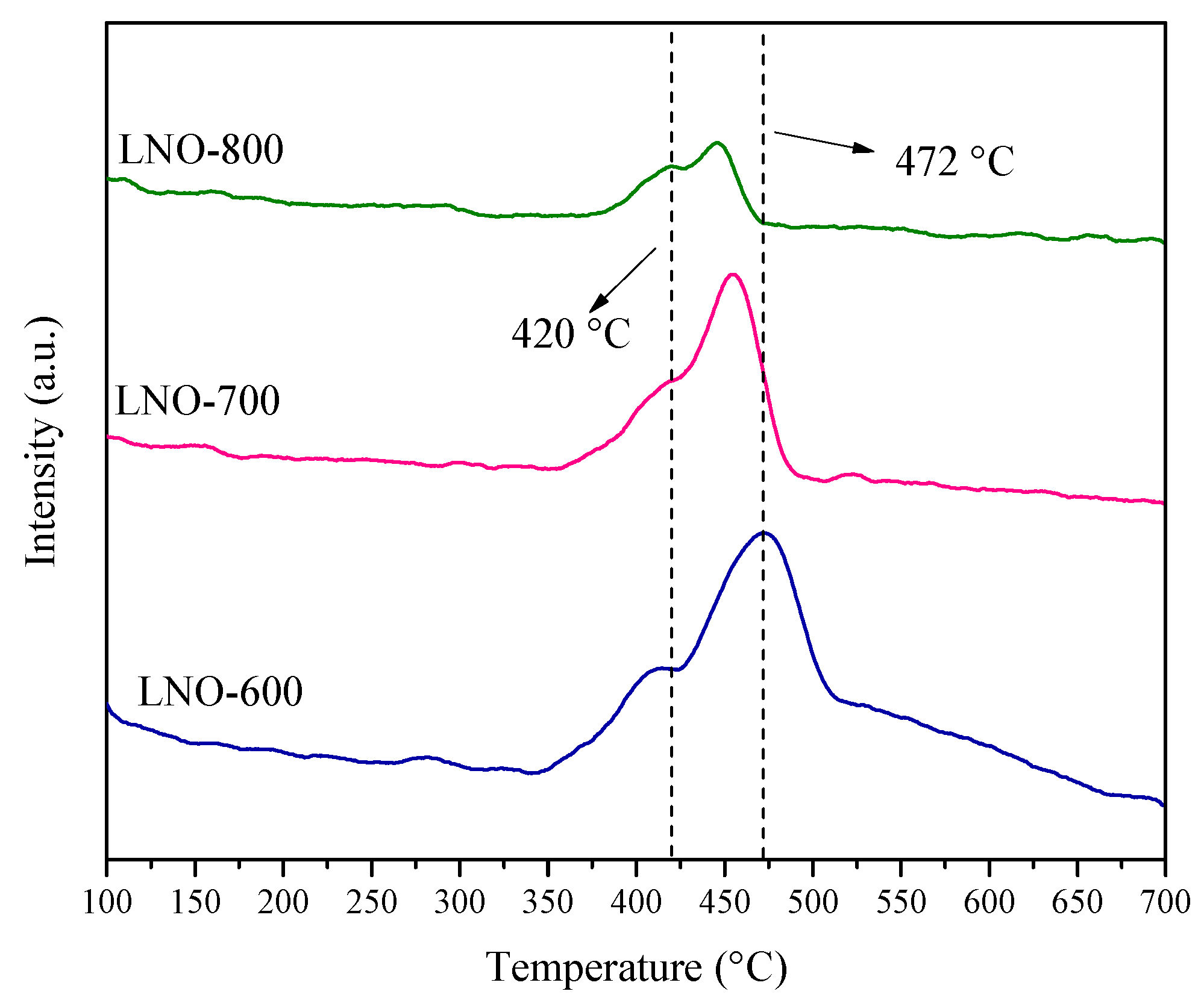

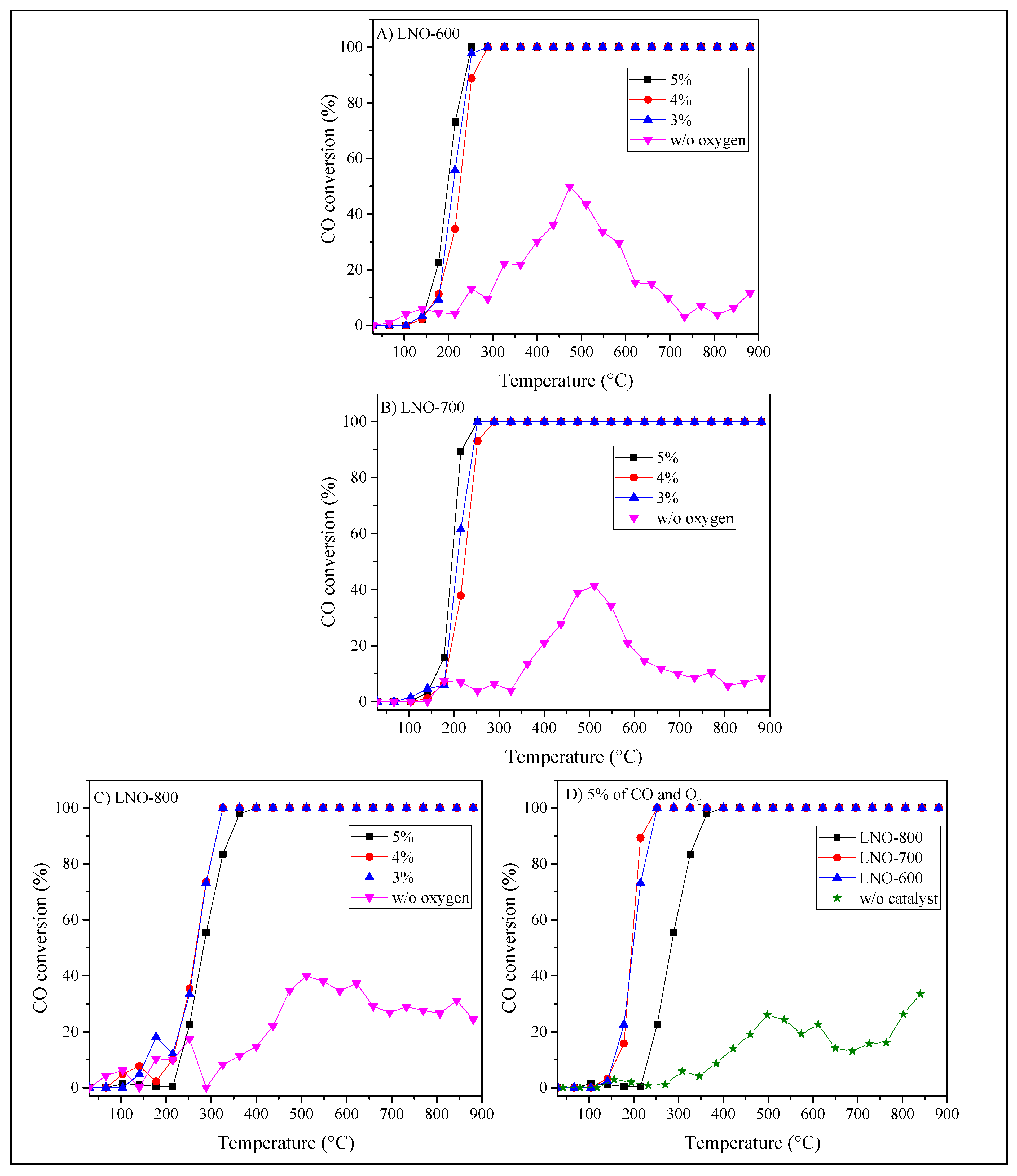

| LNO-600 | LNO-700 | LNO-800 | Peak identification | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | RA (%) | R2 | BE (eV) | RA (%) | R2 | BE (eV) | RA (%) | R2 | Specie |
| 529.3 | 0.7 | 0.9947 | 529.2 | 0.7 | 0.998 | 529.2 | 0.6 | 0.9964 | O2− |
| 530.4 | 6.6 | 0.9947 | 530.3 | 7.1 | 0.998 | 530.2 | 7.5 | 0.9964 | O2− |
| 532.8 | 7.3 | 0.9947 | 532.6 | 4.5 | 0.998 | 532.6 | 5.5 | 0.9964 | OH1− |
| 835.2 | 24.1 | 0.9961 | 834.8 | 13.3 | 0.9981 | 834.8 | 17.8 | 0.9972 | La(OH)3 |
| 837.6 | 9.6 | 0.9961 | 836.7 | 25.6 | 0.9981 | 837.3 | 16.5 | 0.9972 | La2O3 |
| 839.6 | 16.5 | 0.9961 | 839.4 | 11.8 | 0.9981 | 839.5 | 13.6 | 0.9972 | La(OH)3 |
| 851.9 | 12.3 | 0.9961 | 851.7 | 14.9 | 0.9981 | 851.7 | 14.1 | 0.9972 | La2O3 |
| 854.1 | 8.7 | 0.9961 | 853.9 | 5.0 | 0.9981 | 854.2 | 8.2 | 0.9972 | NiO |
| 856.2 | 14.3 | 0.9961 | 855.9 | 17.2 | 0.9981 | 856.1 | 16.1 | 0.9972 | Ni2O3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Varela, D.; Araiza, D.G.; Díaz, G.; Pfeiffer, H. LaNiO3 Perovskite Synthesis through the EDTA–Citrate Complexing Method and Its Application to CO Oxidation. Catalysts 2022, 12, 57. https://doi.org/10.3390/catal12010057
González-Varela D, Araiza DG, Díaz G, Pfeiffer H. LaNiO3 Perovskite Synthesis through the EDTA–Citrate Complexing Method and Its Application to CO Oxidation. Catalysts. 2022; 12(1):57. https://doi.org/10.3390/catal12010057
Chicago/Turabian StyleGonzález-Varela, Daniela, Daniel G. Araiza, Gabriela Díaz, and Heriberto Pfeiffer. 2022. "LaNiO3 Perovskite Synthesis through the EDTA–Citrate Complexing Method and Its Application to CO Oxidation" Catalysts 12, no. 1: 57. https://doi.org/10.3390/catal12010057
APA StyleGonzález-Varela, D., Araiza, D. G., Díaz, G., & Pfeiffer, H. (2022). LaNiO3 Perovskite Synthesis through the EDTA–Citrate Complexing Method and Its Application to CO Oxidation. Catalysts, 12(1), 57. https://doi.org/10.3390/catal12010057







