Ultrafine TaOx/CB Oxygen Reduction Electrocatalyst Operating in Both Acidic and Alkaline Media
Abstract
1. Introduction
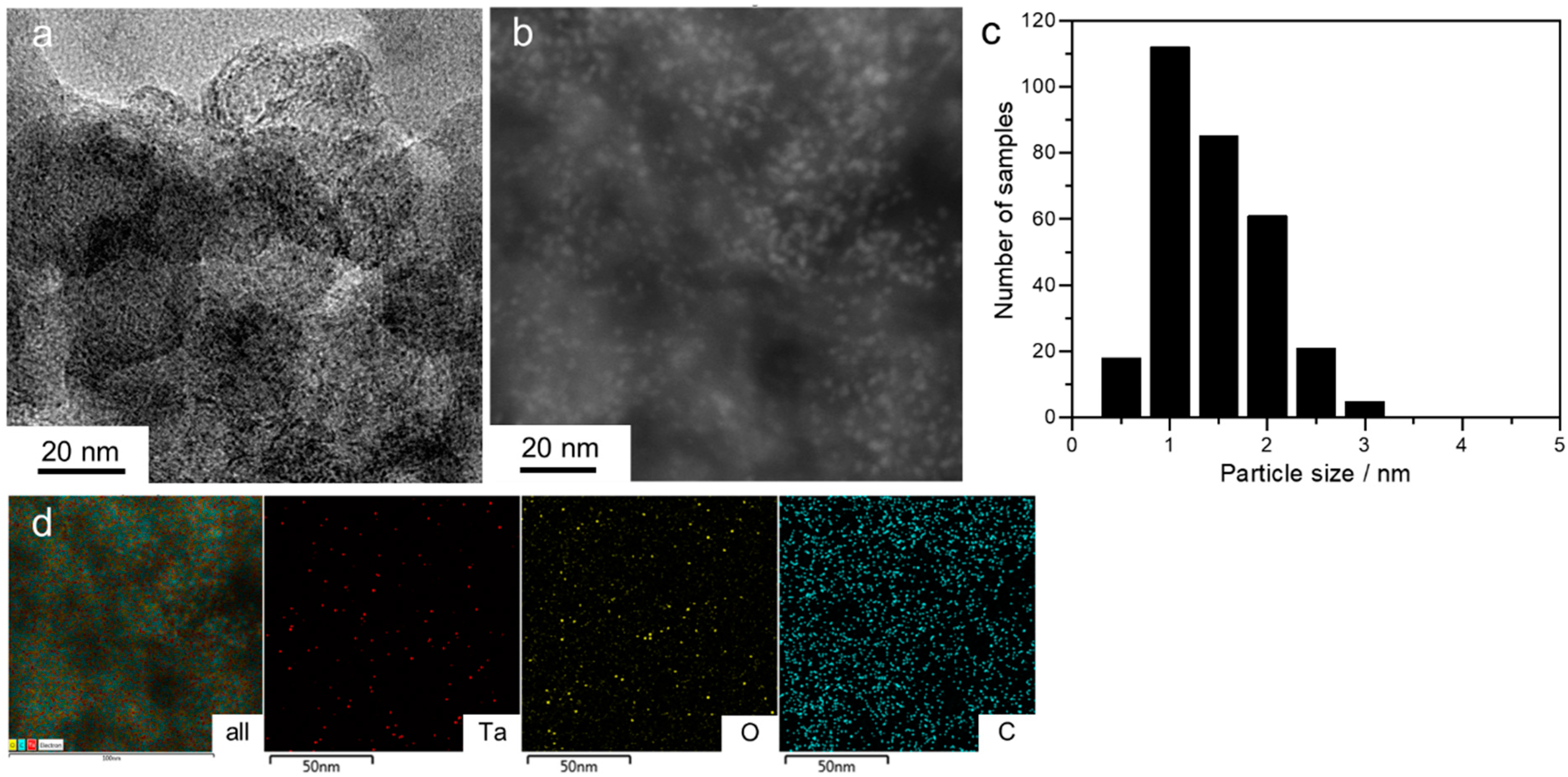
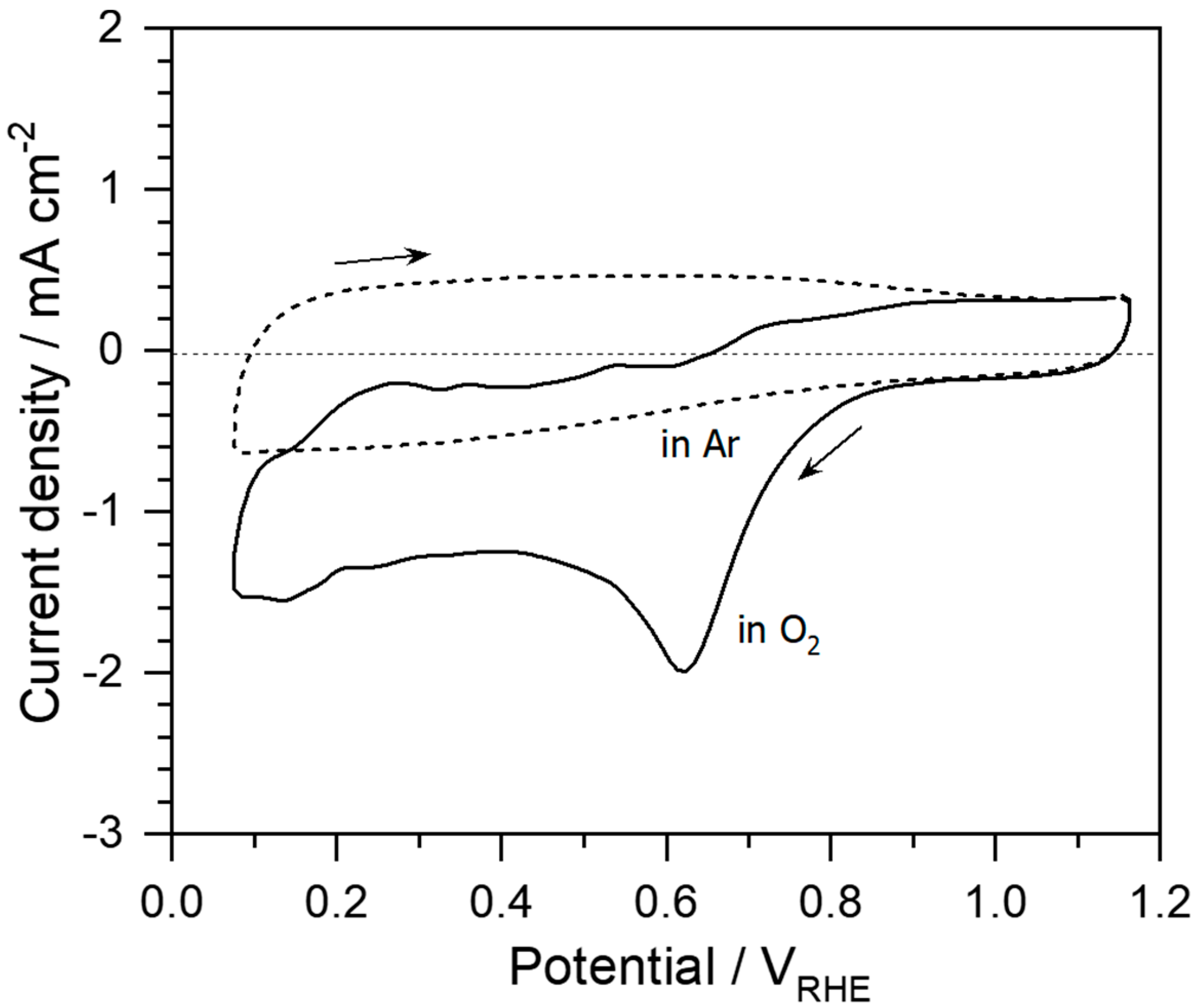
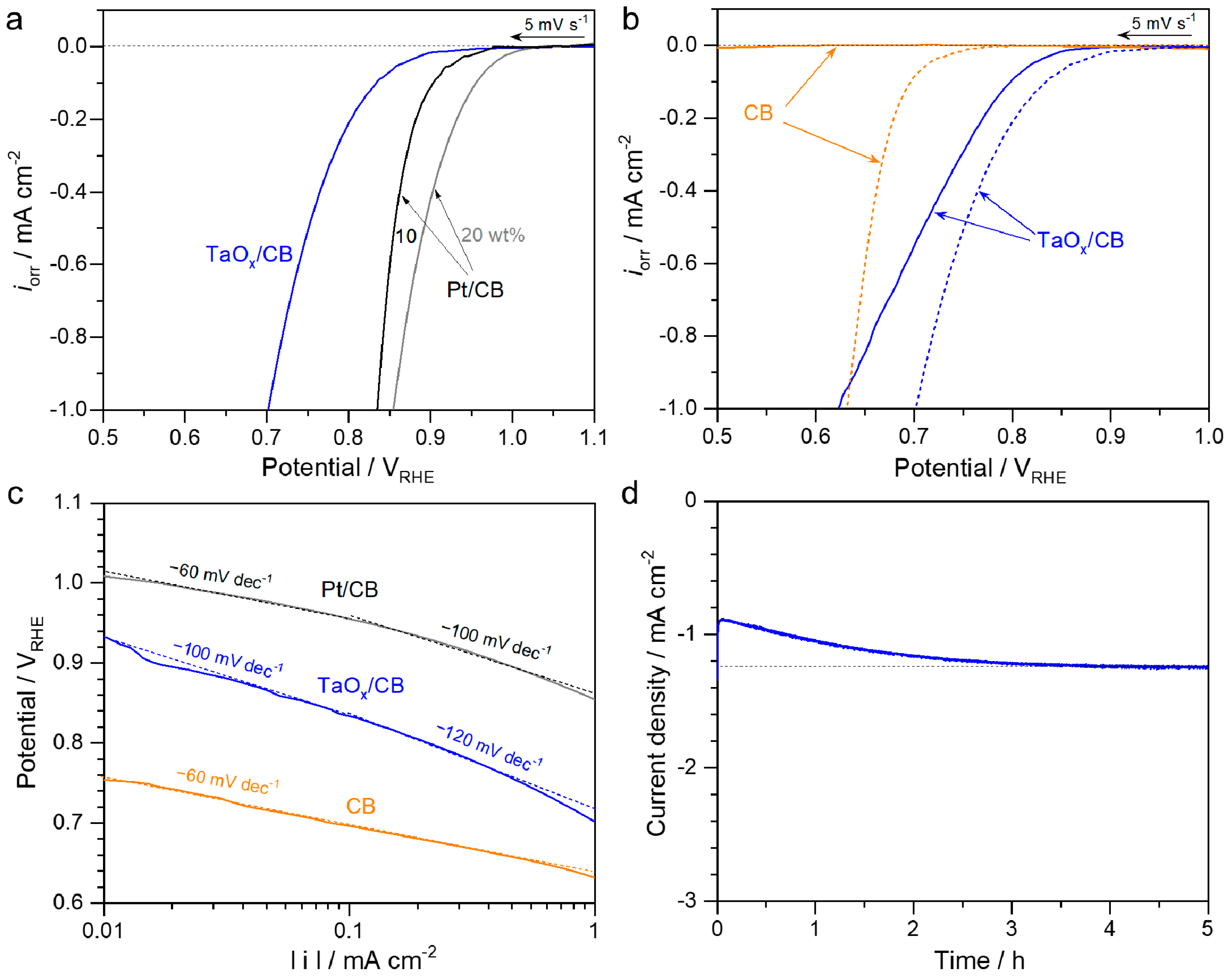
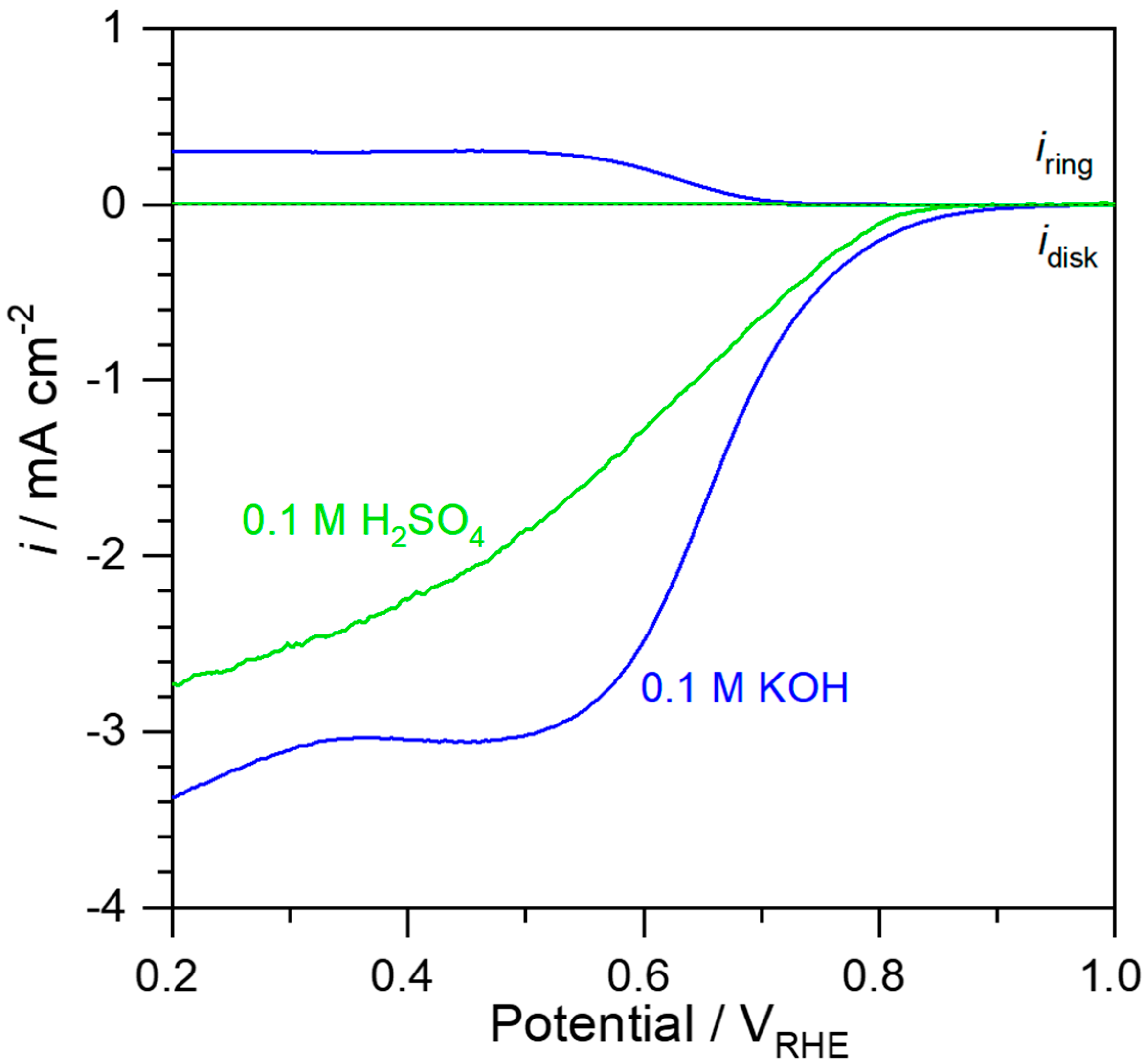
2. Results and Discussion
3. Experimental
3.1. Preparation of TaOx/CB Electrocatalyst
3.2. Electrochemical Measurements
3.3. Surface and Physical Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Geniès, L.; Faure, R.; Durand, R. Electrochemical reduction of oxygen on platinum nanoparticles in alkaline media. Electrochim. Acta 1998, 44, 1317–1327. [Google Scholar] [CrossRef]
- Perez, J.; Gonzalez, E.R.; Ticianelli, E.A. Oxygen electrocatalysis on thin porous coating rotating platinum electrodes. Electrochim. Acta 1998, 44, 1329–1339. [Google Scholar] [CrossRef]
- Awaludin, Z.; Suzuki, M.; Masud, J.; Okajima, T.; Ohsaka, T. Enhanced Electrocatalysis of Oxygen Reduction on Pt/TaOx/GC. J. Phys. Chem. C 2011, 115, 25557–25567. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst a thin-film rotating raing-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Esswein, A.J.; McMurdo, M.J.; Ross, P.N.; Bell, A.T.; Tilley, T.D. Size-Dependent Activity of Co3O4 Nanoparticle Anodes for Alkaline Water Electrolysis. J. Phys. Chem. C 2009, 113, 15068–15072. [Google Scholar] [CrossRef]
- Lee, J.-S.; Park, G.-S.; Lee, H.-I.; Kim, S.-T.; Cao, R.; Liu, M.; Cho, J. Ketjenblack carbon supported amorphous manganese oxides nanowires as highly efficient electrocatalyst for oxygen reduction reaction in alkaline solutions. Nano Lett. 2011, 11, 5362–5366. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous graphitic-C3N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef]
- Li, J.-C.; Xiao, F.; Zhong, H.; Li, T.; Xu, M.; Ma, L.; Cheng, M.; Liu, D.; Feng, S.; Shi, Q.; et al. Secondary-Atom-Assisted Synthesis of Single Iron Atoms Anchored on N-Doped Carbon Nanowires for Oxygen Reduction Reaction. ACS Catal. 2019, 9, 5929–5934. [Google Scholar] [CrossRef]
- Li, J.C.; Meng, Y.; Zhang, L.; Li, G.; Shi, Z.; Hou, P.X.; Liu, C.; Cheng, H.M.; Shao, M. Dual-Phasic Carbon with Co Single Atoms and Nanoparticles as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. Adv. Funct. Mater. 2021, 31, 2103360. [Google Scholar] [CrossRef]
- Ishihara, A.; Lee, K.; Doi, S.; Mitsushima, S.; Kamiya, N.; Hara, M.; Domen, K.; Fukuda, K.; Ota, K.-I. Tantalum Oxynitride for a Novel Cathode of PEFC. Electrochem. Solid-State Lett. 2005, 8, A201. [Google Scholar] [CrossRef]
- Ohgi, Y.; Ishihara, A.; Matsuzawa, K.; Mitsushima, S.; Ota, K. Zirconium Oxide-Based Compound as New Cathode without Platinum Group Metals for PEFC. J. Electrochem. Soc. 2010, 157, B885. [Google Scholar] [CrossRef]
- Ohnishi, R.; Takanabe, K.; Katayama, M.; Kubota, J.; Domen, K. Nano-nitride Cathode Catalysts of Ti, Ta, and Nb for Polymer Electrolyte Fuel Cells: Temperature-Programmed Desorption Investigation of Molecularly Adsorbed Oxygen at Low Temperature. J. Phys. Chem. C 2012, 117, 496–502. [Google Scholar] [CrossRef]
- Ohnishi, R.; Katayama, M.; Cha, D.; Takanabe, K.; Kubota, J.; Domen, K. Titanium Nitride Nanoparticle Electrocatalysts for Oxygen Reduction reaction in Alkaline solution. J. Electrochem. Soc. 2013, 160, F501–F506. [Google Scholar] [CrossRef]
- Seo, J.; Cha, D.; Takanabe, K.; Kubota, J.; Domen, K. Electrodeposited Ultrafine NbOx, ZrOx, and TaOx Nanoparticles on Carbon Black Supports for Oxygen Reduction Electrocatalysts in Acidic Media. ACS Catal. 2013, 3, 2181–2189. [Google Scholar] [CrossRef]
- Mancheri, N.A.; Sprecher, B.; Deetman, S.; Young, S.B.; Bleischwitz, R.; Dong, L.; Kleijn, R.; Tukker, A. Resilience in the tantalum supply chain. Resour. Conserv. Recycl. 2018, 129, 56–69. [Google Scholar] [CrossRef]
- Lee, J.; Seo, J.; Han, K.; Kim, H. Preparation of low Pt loading electrodes on Nafion (Na+)-bonded carbon layer with galvanostatic pulses for PEMFC application. J. Power Sources 2006, 163, 349–356. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Hsu, I.J.; Esposito, D.V.; Mahoney, E.G.; Black, A.; Chen, J.G. Particle shape control using pulse electrodeposition: Methanol oxidation as a probe reaction on Pt dendrites and cubes. J. Power Sources 2011, 196, 8307–8312. [Google Scholar] [CrossRef]
- Khan, M.S.; Khattak, R.; Khan, A.; Chen, Q.; Nisar, J.; Iqbal, Z.; Rashid, A.; Kamran, A.W.; Zekker, I.; Zahoor, M.; et al. Synthesis and Characterizations of PdNi Carbon Supported Nanomaterials: Studies of Electrocatalytic Activity for Oxygen Reduction in Alkaline Medium. Molecules 2021, 26, 3440. [Google Scholar] [CrossRef]
- Seo, J.; Zhao, L.; Cha, D.; Takanabe, K.; Katayama, M.; Kubota, J.; Domen, K. Highly Dispersed TaOx Nanoparticles Prepared by Electrodeposition as Oxygen Reduction Electrocatalysts for Polymer Electrolyte Fuel Cells. J. Phys. Chem. C 2013, 117, 11635–11646. [Google Scholar] [CrossRef]
- Seo, J.; Anjum, D.H.; Takanabe, K.; Kubota, J.; Domen, K. Electrodeposited Ultrafine TaOx/CB Catalysts for PEFC Cathode Application: Their Oxygen Reduction Reaction Kinetics. Electrochim. Acta 2014, 149, 76–85. [Google Scholar] [CrossRef]
- Seo, J.; Cha, D.; Takanabe, K.; Kubota, J.; Domen, K. Particle size dependence on oxygen reduction reaction activity of electrodeposited TaO(x) catalysts in acidic media. Phys. Chem. Chem. Phys. 2014, 16, 895–898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Z.-F.; Wang, J.; Peng, Y.; Jung, C.-Y.; Fisher, A.; Wang, X. Design of Efficient Bifunctional Oxygen Reduction/Evolution Electrocatalyst: Recent Advances and Perspectives. Adv. Energy Mater. 2017, 7, 1700544. [Google Scholar] [CrossRef]
- Atanassova, E.; Spassov, D. X-ray photoelectron spectroscopy of thermal thin Ta2O5 films on Si. Appl. Surf. Sci. 1998, 135, 71–82. [Google Scholar] [CrossRef]
- Kerrec, O.; Devilliers, D.; Groult, H.; Marcus, P. Study of dry and electrogenerated Ta2O5 and Ta/Ta2O5/Pt structures by XPS. Mater. Sci. Eng. 1998, B55, 134–142. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Ji, S.; Goh, J.; Feng, H.; Wang, R. Highly active Vulcan carbon composite for oxygen reduction reaction in alkaline medium. Electrochim. Acta 2014, 133, 391–398. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Allen, R.J.; Mukerjee, S. Electrochemical Kinetics and X-ray Absorption Spectroscopic Investigations of Oxygen Reduction on Chalcogen-Modified Ruthenium Catalysts in Alkaline Media. J. Phys. Chem. C 2011, 115, 12650–12664. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Influence of Inner- and Outer-Sphere Electron Transfer Mechanisms during Electrocatalysis of Oxygen Reduction in Alkaline Media. J. Phys. Chem. C 2011, 115, 18015–18026. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen Reduction on Silver Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskAg(hkl) Studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Buttry, D.A. Comparison of Oxygen Reduction Reaction at Silver Nanoparticles and Polycrystalline Silver Electrodes in Alkaline Solution. J. Phys. Chem. C 2012, 116, 10656–10663. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen electroreduction on Ag(111): The pH effect. Electrochim. Acta 2007, 52, 2264–2271. [Google Scholar] [CrossRef]


| Potential/VRHE | ik/mA cm−2 | Number of Electrons Transferred |
|---|---|---|
| 0.6 | 7.08 | 3.57 |
| 0.4 | 31.84 | 3.91 |
| 0.2 | 28.24 | 3.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Seo, J. Ultrafine TaOx/CB Oxygen Reduction Electrocatalyst Operating in Both Acidic and Alkaline Media. Catalysts 2022, 12, 35. https://doi.org/10.3390/catal12010035
Park J-W, Seo J. Ultrafine TaOx/CB Oxygen Reduction Electrocatalyst Operating in Both Acidic and Alkaline Media. Catalysts. 2022; 12(1):35. https://doi.org/10.3390/catal12010035
Chicago/Turabian StylePark, Jun-Woo, and Jeongsuk Seo. 2022. "Ultrafine TaOx/CB Oxygen Reduction Electrocatalyst Operating in Both Acidic and Alkaline Media" Catalysts 12, no. 1: 35. https://doi.org/10.3390/catal12010035
APA StylePark, J.-W., & Seo, J. (2022). Ultrafine TaOx/CB Oxygen Reduction Electrocatalyst Operating in Both Acidic and Alkaline Media. Catalysts, 12(1), 35. https://doi.org/10.3390/catal12010035





