Bimetallic PdCo Nanoparticles Loaded in Amine Modified Polyacrylonitrile Hollow Spheres as Efficient Catalysts for Formic Acid Dehydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Studies
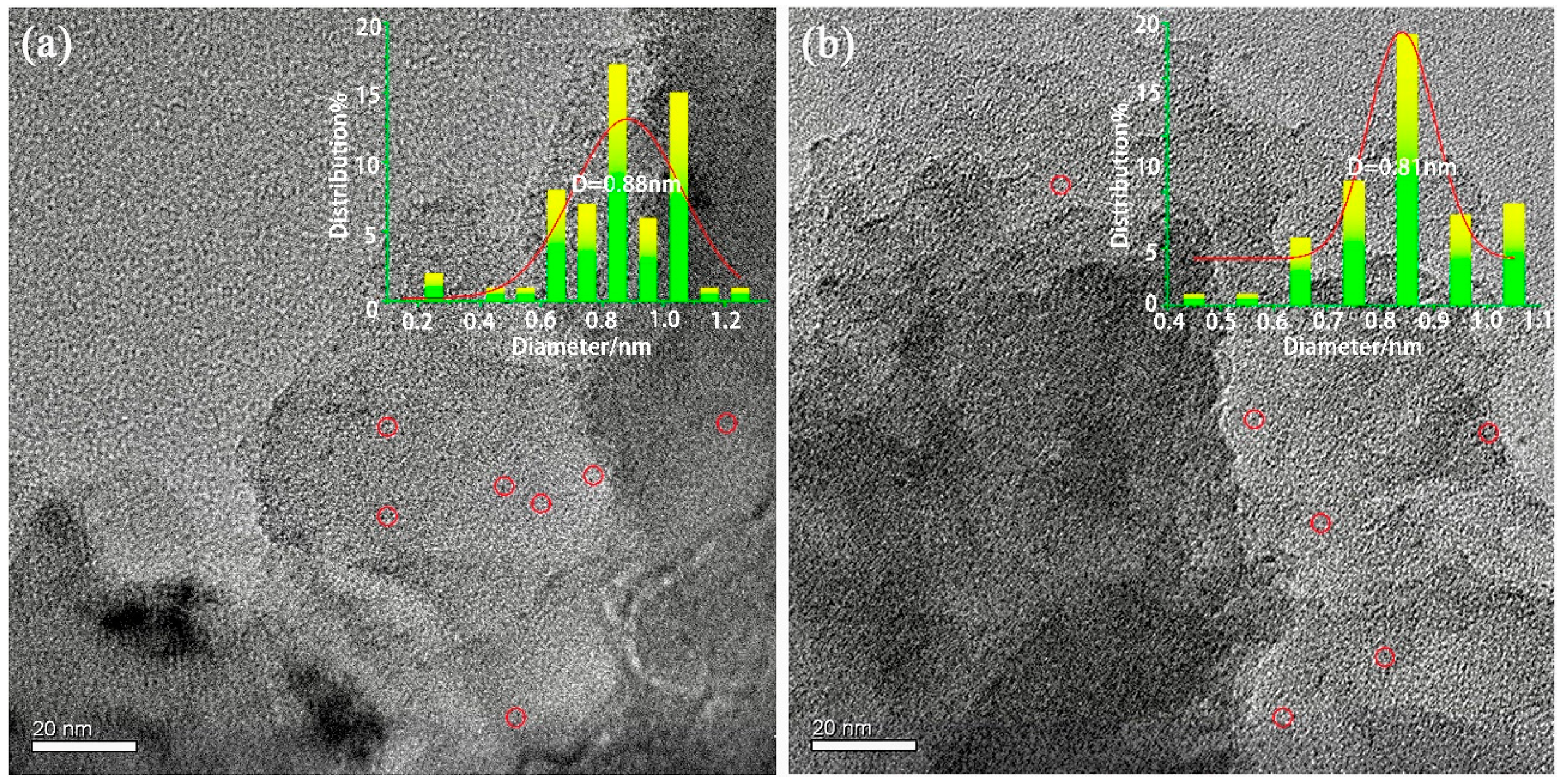
2.2. TEM Studies
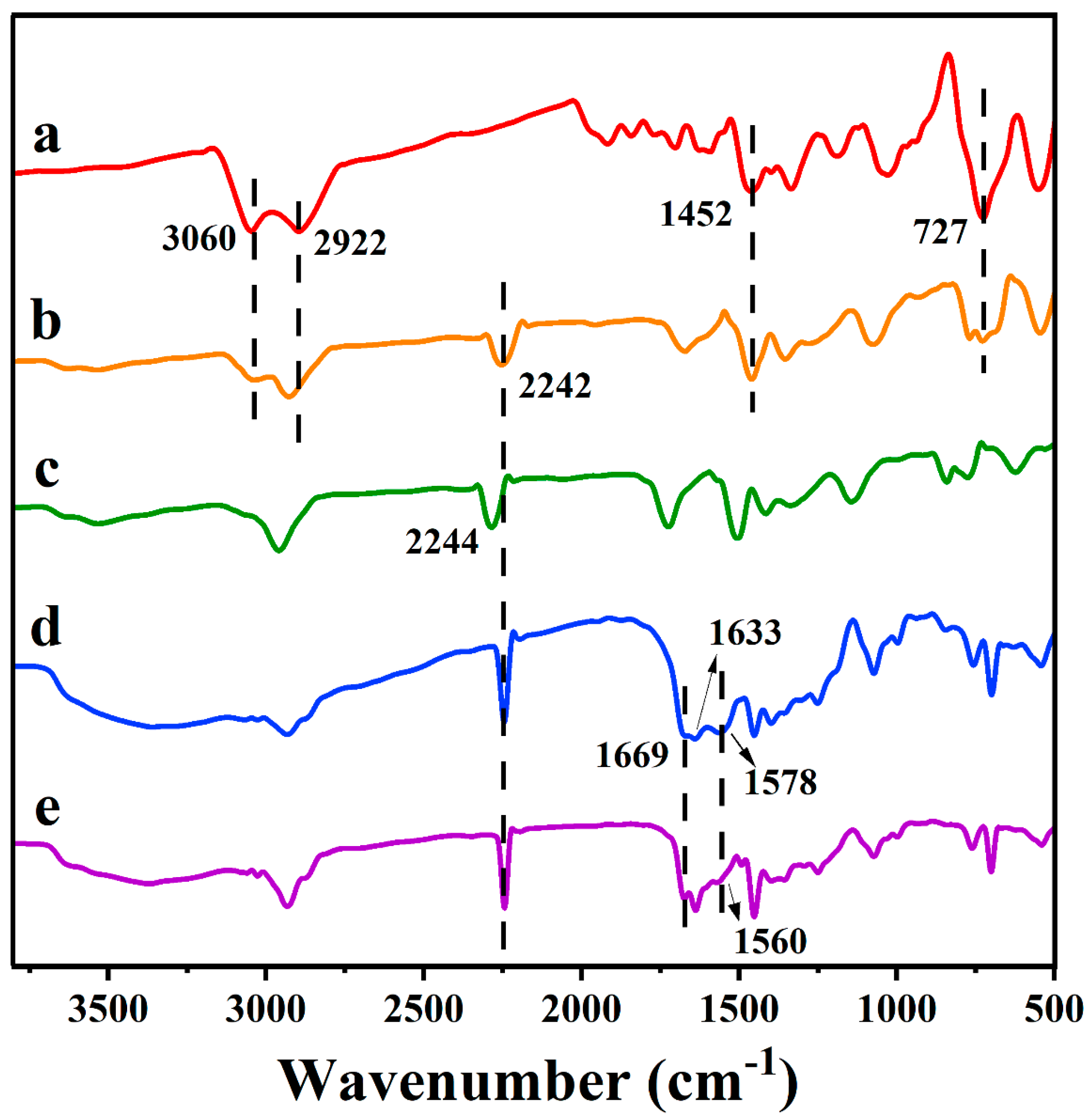
2.3. IR Spectra Analysis of Samples
2.4. Elemental Analyses and Structure Properties of Samples
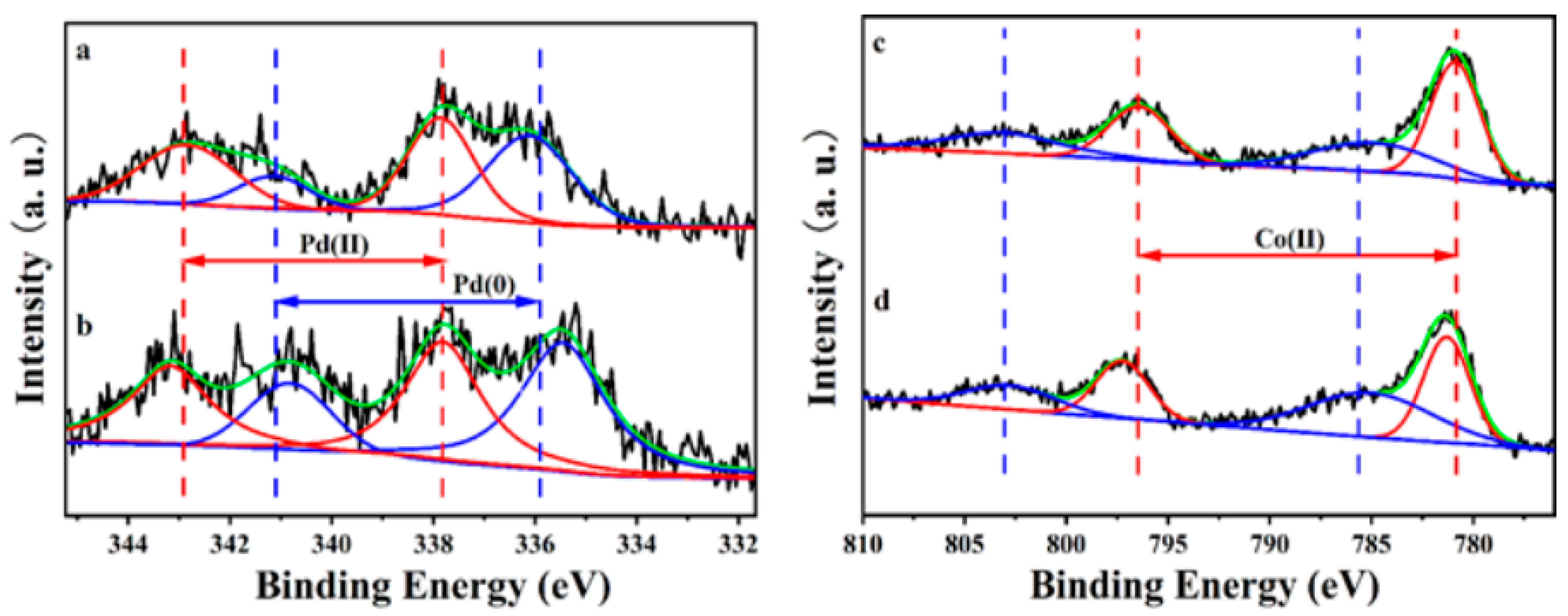
2.5. X-ray Photoelectron Spectroscopy Analyses
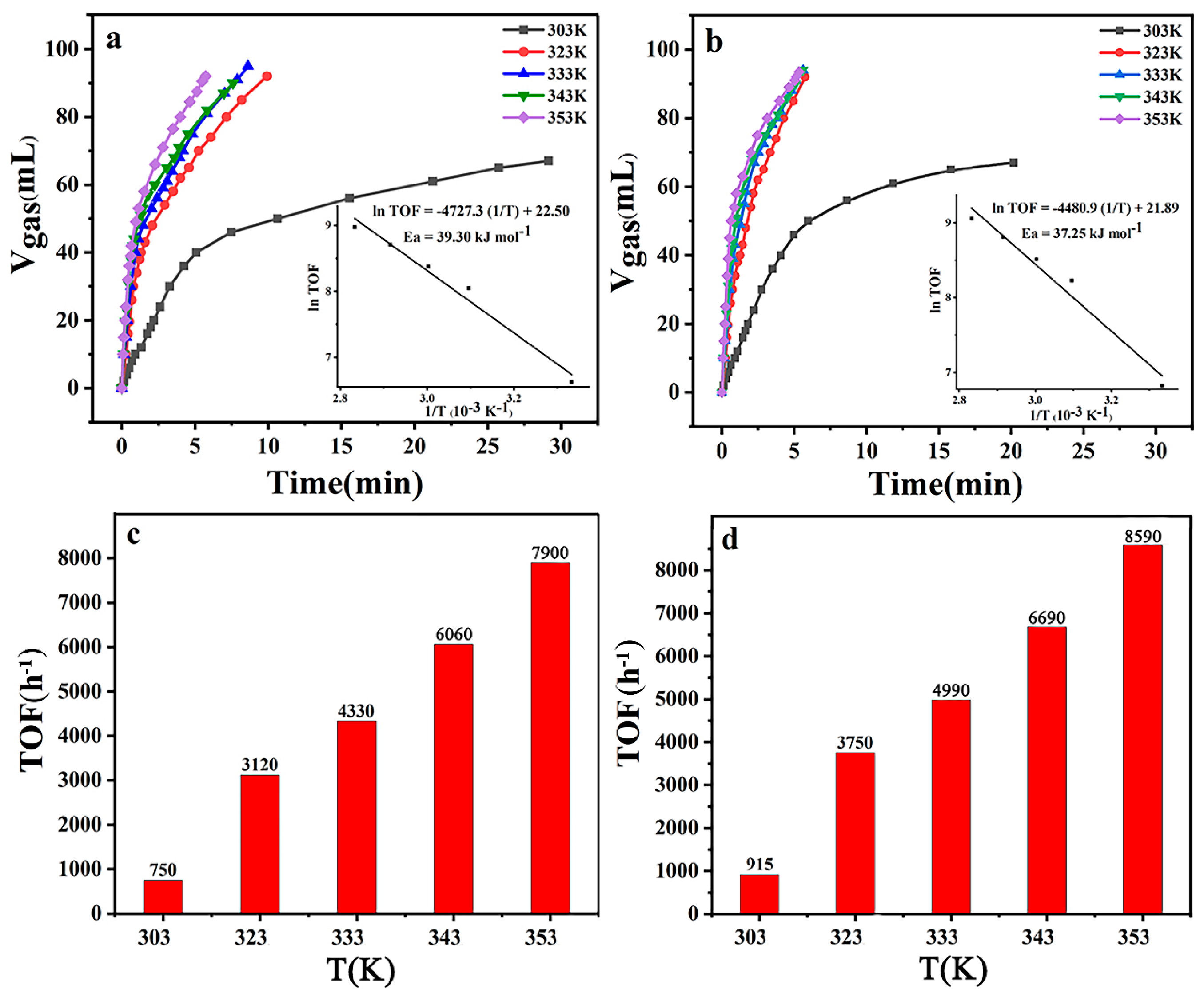
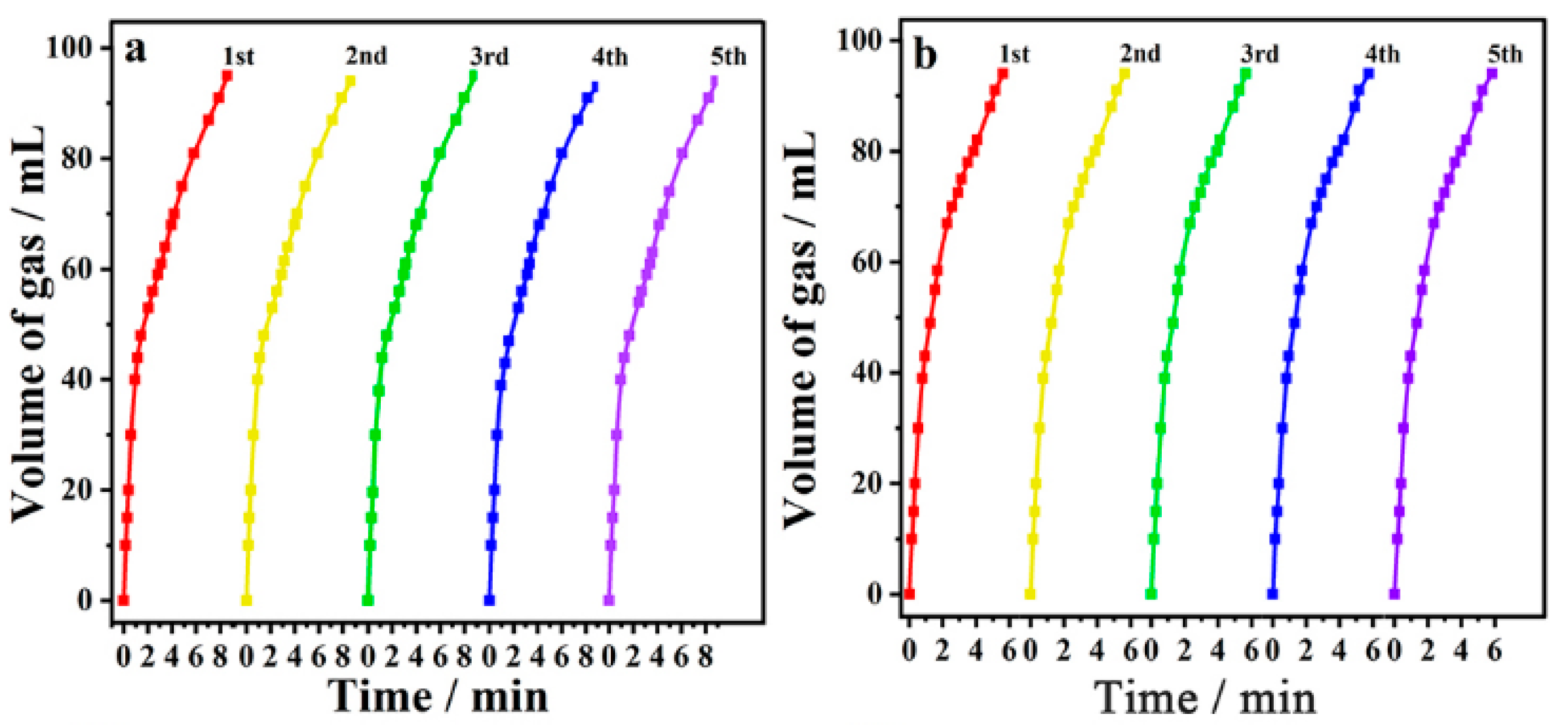
2.6. Evaluation of Catalytic Activity
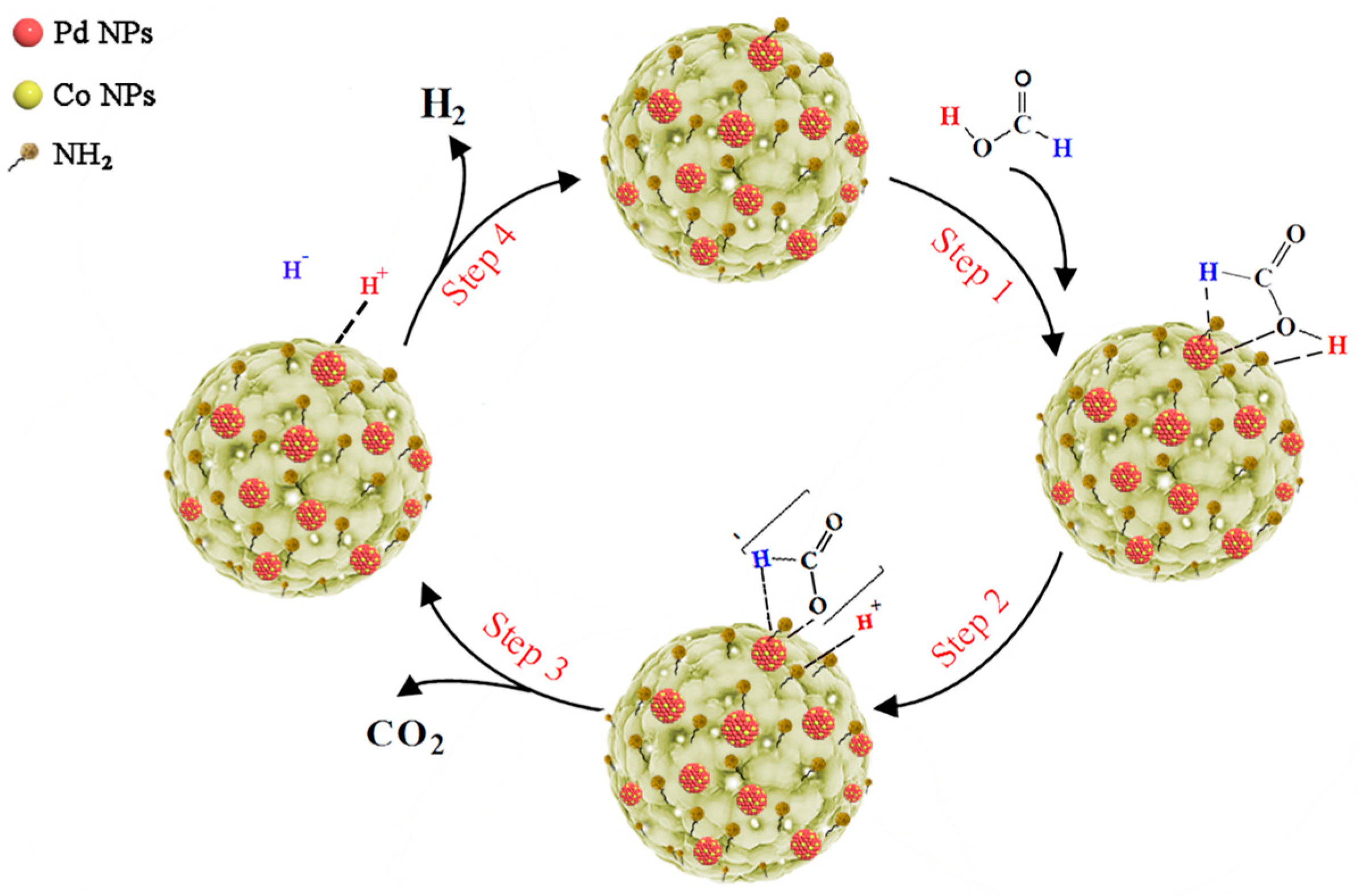
2.7. Catalytic Mechanism
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Polyacrylonitrile Hollow Nanospheres (HPAN)
3.3. Synthesis of Amine-Functionalized HPAN
3.4. Synthesis of Pd/EDA-HPAN and PdCox/EDA-HPAN
3.5. Catalyst Characterisation
3.6. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X. Green energy for sustainability and energy security. In Green Energy; British Library: London, UK, 2011; pp. 1–16. ISBN 978-1-84882-646-5. [Google Scholar]
- Oncel, S.S. Green energy engineering: Opening a green way for the future. J. Clean Prod. 2017, 142, 3095–3100. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Dörthe, M.; Peter, S.; Henrik, J.; Beller, M. Formic acid as a hydrogen storage material—Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar]
- Jiang, Y.Q.; Fan, X.L.; Xiao, X.Z.; Qin, T.; Zhang, L.T.; Jiang, F.L.; Li, M.; Li, S.Q.; Ge, H.W.; Chen, L.X. Novel AgPd hollow spheres anchored on graphene as an efficient catalyst for dehydrogenation of formic acid at room temperature. J. Mater. Chem. A 2016, 4, 657–666. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, P.; Xu, D.Y.; Tao, X.M.; Liu, X.E.; Ge, Q.J. Ultrafine PdAg alloy nanoparticles anchored on NH2-functionalized 2D/2D TiO2 nanosheet/rGO composite as efficient and reusable catalyst for hydrogen release from additive-free formic acid at room temperature. J. Energy Chem. 2021, 59, 455–464. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous catalysis for sustainable hydrogen storage in formic acid and alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef]
- Johnson, T.C.; Morris, D.J.; Wills, M. Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem. Soc. Rev. 2010, 39, 81–88. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.G.; Luca, L.D.; Giacomelli, G.; Porcheddu, A. Formic acid: A promising bio-renewable feedstock for fine chemicals. Adv. Synth. Catal. 2012, 354, 3180–3186. [Google Scholar] [CrossRef]
- Yao, M.Q.; Ye, Y.; Chen, H.L.; Zhang, X.M. Porous carbon supported Pd as catalysts for boosting formic acid dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 17398–17409. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Li, J.J.; Zhang, D.J.; Liu, C.B. Reexamination of formic acid decomposition on the Pt (111) surface both in the absence and in the presence of water, from periodic DFT calculations. Catal. Sci. Technol. 2015, 5, 3322–3332. [Google Scholar] [CrossRef]
- Ojeda, M.; Iglesia, E. Formic acid dehydrogenation on Au-based catalysts at near ambient temperatures. Angew. Chem. Int. Ed. 2009, 48, 4800–4803. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.J.; Clarkson, G.J.; Wills, M. Insights into hydrogen generation from formic acid using ruthenium complexe. Organometallics 2009, 28, 4133–4140. [Google Scholar] [CrossRef]
- Coffey, R.S. The decomposition of formic acid catalysed by soluble metal complexes. Chem. Commun. 1967, 923–924. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kobayashi, T.; Suenobu, T. Efficient catalytic decomposition of formic acid for the selective generation of H2 and H/D exchange with a water-soluble rhodium complex in aqueous solution. ChemSusChem 2008, 1, 827–834. [Google Scholar] [CrossRef]
- Shao, M.H.; Odell, J.; Humbert, M.; Yu, T.; Xia, Y.N. Electrocatalysis on shape-controlled palladium nanocrystals: Oxygen reduction reaction and formic acid oxidation. J. Phys. Chem. C 2013, 117, 4172–4180. [Google Scholar] [CrossRef]
- Sousa-Castillo, A.; Li, F.; Carbo’-Argibay, E.; Correa-Duarteb, M.A.; Klinkova, A. Pd-CNT-SiO2 nanoskein: Composite structure design for formic acid dehydrogenation. Chem. Commun. 2019, 55, 10733–10736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Yan, J.M.; Ping, Y.; Wang, H.L.; Zheng, W.T.; Jiang, Q. An efficient CoAuPd/C catalyst for hydrogen generation from formic acid at room temperature. Angew. Chem. Int. Ed. 2013, 52, 4406–4409. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.L.; Liu, Y.C.; Liang, F.; Wang, L.M. Preparation of Pd-Co-based nanocatalysts and their superior applications in formic acid decomposition and methanol oxidation. ChemSusChem 2015, 8, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Song, F.Z.; Zhu, Q.L.; Yang, X.C.; Zhan, W.W.; Pachfule, P.; Tsumori, N.; Xu, Q. Metal-organic framework templated porous carbon-metal oxide/reduced graphene oxide as superior support of bimetallic nanoparticles for efficient hydrogen generation from formic acid. Adv. Energy Mater. 2018, 8, 1701416. [Google Scholar] [CrossRef]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Yao, F.; Luo, J.L.; Wan, C.; Ye, M.F.; Cui, P.; An, Y. Facile synthesis of amine-functionalized SBA-15-supported bimetallic Au-Pd nanoparticles as an efficient catalyst for hydrogen generation from formic acid. RSC Adv. 2017, 7, 4746–4752. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, H.; Mahyari, M.; Bagheri, A.; Shaabani, A. Pd and PdCo alloy nanoparticles supported on polypropylenimine dendrimer-grafted graphene: A highly efficient anodic catalyst for direct formic acid fuel cells. J. Power Sources 2014, 247, 70–77. [Google Scholar] [CrossRef]
- Karatas, Y.; Bulut, A.; Yurderi, M.; Ertas, I.E.; Alal, O.; Gulcan, M.; Celebi, M.; Kivrak, H.; Kaya, M.; Zahmakiran, M. PdAu-MnOx nanoparticles supported on amine-functionalized SiO2 for the room temperature dehydrogenation of formic acid in the absence of additives. Appl. Catal. B-Environ. 2016, 180, 586–595. [Google Scholar] [CrossRef]
- Liu, Q.G.; Yang, X.F.; Huang, Y.Q.; Xu, S.T.; Su, X.; Pan, X.L.; Xu, J.M.; Wang, A.Q.; Liang, C.H.; Wang, X.K.; et al. A Schiff base modified gold catalyst for green and efficient H2 production from formic acid. Energy Environ. Sci. 2015, 8, 3204–3207. [Google Scholar] [CrossRef]
- Gao, S.T.; Liu, W.H.; Feng, C.; Shang, N.Z.; Wang, C. A Ag-Pd alloy supported on an amine functionalized UiO-66 as an efficient synergetic catalyst for the dehydrogenation of formic acid at room temperature. Catal. Sci. Technol. 2016, 6, 869–874. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Zahmakiran, M.; Kivrak, H.; Gulcan, M.; Kaya, M. Pd-MnOx nanoparticles dispersed on amine-grafted silica: Highly efficient nanocatalyst for hydrogen production from additive-free dehydrogenation of formic acid under mild conditions. Appl. Catal. B-Environ. 2015, 164, 324–333. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Tsumoriab, N.; Xu, Q. Sodium hydroxide-assisted growth of uniform Pd nanoparticles on nanoporous carbon MSC-30 for efficient and complete dehydrogenation of formic acid under ambient conditions. Chem. Sci. 2014, 5, 195–199. [Google Scholar] [CrossRef]
- Nie, W.D.; Luo, Y.X.; Yang, Q.F.; Feng, G.; Yao, Q.L.; Lu, Z.H. An amine-functionalized mesoporous silica-supported PdIr catalyst: Boosting room-temperature hydrogen generation from formic acid. Inorg. Chem. Front. 2020, 7, 709–717. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Caner, N.; Celebi, M.; Kayab, M.; Zahmakiran, M. Amine grafted silica supported CrAuPd alloy nanoparticles: Superb heterogeneous catalysts for the room temperature dehydrogenation of formic acid. Chem. Commun. 2015, 51, 11417–11420. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Jeon, M.; Yoon, C.W.; Asefa, T. Formic acid dehydrogenation over Pd NPs supported on amine-functionalized SBA-15 catalysts: Structure-activity relationships. J. Mater. Chem. A. 2017, 5, 16150–16161. [Google Scholar] [CrossRef]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd−Ag nanoparticles within a macroreticular basic resin: An efficient catalyst for hydrogen production from formic acid decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Yadav, M.; Akita, T.; Tsumoriab, N.; Xu, Q. Strong metal-molecular support interaction (SMMSI): Amine-functionalized gold nanoparticles encapsulated in silica nanospheres highly active for catalytic decomposition of formic acid. J. Mater. Chem. 2012, 22, 12582–12586. [Google Scholar] [CrossRef]
- Song, F.Z.; Zhu, Q.L.; Tsumori, N.; Xu, Q. Diamine-alkalized reduced graphene oxide: Immobilization of sub-2 nm palladium nanoparticles and optimization of catalytic activity for dehydrogenation of formic acid. ACS Catal. 2015, 5, 5141–5144. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, A.K.; Tsumoriab, N.; Xu, Q. Palladium silica nanosphere-catalyzed decomposition of formic acid for chemical hydrogen storage. J. Mater. Chem. 2012, 22, 19146–19150. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Wu, P.; Xu, L.; Tang, Y.W.; Lee, J.M. 3D graphene hollow nanospheres@palladium-networks as an efficient electrocatalyst for formic acid oxidation. Adv. Mater. Interfaces 2015, 2, 1500321. [Google Scholar] [CrossRef]
- Fang, B.Z.; Kim, M.S.; Yu, J.S. Hollow core/mesoporous shell carbon as a highly efficient catalyst support in direct formic acid fuel cell. Appl. Catal. B-Environ. 2008, 84, 100–105. [Google Scholar] [CrossRef]
- Li, Y.L.; Chen, L.L.; Jia, Y.H.; Li, D.; Hao, X.F.; Jia, M.J. The enhanced role of surface amination on the catalytic performance of polyacrylonitrile supported palladium nanoparticles in hydrogen generation from formic acid. J. Appl. Polym. Sci. 2021, 138, e50456. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhang, H.Y.; Li, L.; Miao, S.S.; Wu, S.J.; Hao, X.F.; Zhang, W.X.; Jia, M.J. Polyacrylinitrile beads supported Pd-based nanoparticles as superior catalysts for dehydrogenation of formic acid and reduction of organic dyes. Catal. Commun. 2018, 114, 51–53. [Google Scholar] [CrossRef]
- Chen, L.L.; Hao, D.F.; Wang, Z.Z.; Li, Y.L.; Gao, G.; Hao, X.F.; Zhang, W.X.; Jia, M.J. Use of amidoxime polyacrylonitrile bead-supported Pd-based nanoparticles as high efficiency catalysts for dehydrogenation of formic acid. J. Nanosci. Nanotechnol. 2020, 20, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.D.; Li, Y.L.; Leng, F.; Jia, M.J.; Yu, J.H.; Hao, X.F.; Xu, J.Q. PdAu nanoparticles supported by diamine-containing UiO-66 for formic acid dehydrogenation. ACS Appl. Nano Mater. 2021, 4, 9790–9798. [Google Scholar] [CrossRef]
- Chen, A.B.; Xia, K.C.; Yu, Y.F.; Sun, H.X.; Liu, L.; Ren, S.F.; Li, Y.T. “Dissolution-Capture” strategy to monodispersed nitrogen-doped hollow mesoporous carbon spheres. J. Electrochem. Soc. 2016, 163, A3063–A3068. [Google Scholar] [CrossRef]
- Monsores, K.G.D.C.; da Silva, A.O.; Oliveira, S.D.S.A.; Weber, R.P.; Filho, P.F.; Monteiro, S.N. Influence of ultraviolet radiation on polystyrene. J. Mater. Res. Technol. 2021, 13, 359–365. [Google Scholar] [CrossRef]
- Botan, R.; Nogueira, T.R.; Lona, L.M.F. Síntese e caracterização de nanocompósitos esfoliados de poliestireno-hidróxido duplo lamelar via polimerização in situ. Polímeros 2011, 21, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.G.; Choi, U.S. Observation of metal ions adsorption on novel polymeric chelating fiber and activated carbon fiber. Sep. Purif. Technol. 2007, 57, 338–347. [Google Scholar] [CrossRef]
- Kuo, H.H.; Chen, W.C.; Wen, T.C.; Gopalan, A. A novel composite gel polymer electrolyte for rechargeable lithium batteries. J. Power Sources 2002, 110, 27–33. [Google Scholar] [CrossRef]
- Park, K.O.; Lee, S.H.; Joh, H.I.; Kim, J.K.; Kang, P.H.; Lee, J.H.; Ku, B.C. Effect of functional groups of carbon nanotubes on the cyclization mechanism of polyacrylonitrile (PAN). Polymer 2012, 53, 2168–2174. [Google Scholar] [CrossRef]
- Wang, M.L.; Jiang, T.T.; Lu, Y.; Liu, H.J.; Chen, Y. Gold nanoparticles immobilized in hyperbranched polyethylenimine modified polyacrylonitrile fiber as highly efficient and recyclable heterogeneous catalysts for the reduction of 4-nitrophenol. J. Mater. Chem. A 2013, 1, 5923–5933. [Google Scholar] [CrossRef]



| Sample | N Content (wt %) | Pd Loading (wt %) | BET Surface Area (m2 g−1) |
|---|---|---|---|
| HPAN | 23.83 | - | 45 |
| EDA-HPAN | 25.34 | - | 49 |
| Pd/EDA-HPAN | 24.57 | 3.12 | - |
| PdCo0.2/EDA-HPAN | 24.43 | 3.09 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; She, P.; Ding, R.; Li, D.; Cai, H.; Hao, X.; Jia, M. Bimetallic PdCo Nanoparticles Loaded in Amine Modified Polyacrylonitrile Hollow Spheres as Efficient Catalysts for Formic Acid Dehydrogenation. Catalysts 2022, 12, 33. https://doi.org/10.3390/catal12010033
Li Y, She P, Ding R, Li D, Cai H, Hao X, Jia M. Bimetallic PdCo Nanoparticles Loaded in Amine Modified Polyacrylonitrile Hollow Spheres as Efficient Catalysts for Formic Acid Dehydrogenation. Catalysts. 2022; 12(1):33. https://doi.org/10.3390/catal12010033
Chicago/Turabian StyleLi, Yulin, Ping She, Rundong Ding, Da Li, Hongtan Cai, Xiufeng Hao, and Mingjun Jia. 2022. "Bimetallic PdCo Nanoparticles Loaded in Amine Modified Polyacrylonitrile Hollow Spheres as Efficient Catalysts for Formic Acid Dehydrogenation" Catalysts 12, no. 1: 33. https://doi.org/10.3390/catal12010033
APA StyleLi, Y., She, P., Ding, R., Li, D., Cai, H., Hao, X., & Jia, M. (2022). Bimetallic PdCo Nanoparticles Loaded in Amine Modified Polyacrylonitrile Hollow Spheres as Efficient Catalysts for Formic Acid Dehydrogenation. Catalysts, 12(1), 33. https://doi.org/10.3390/catal12010033








