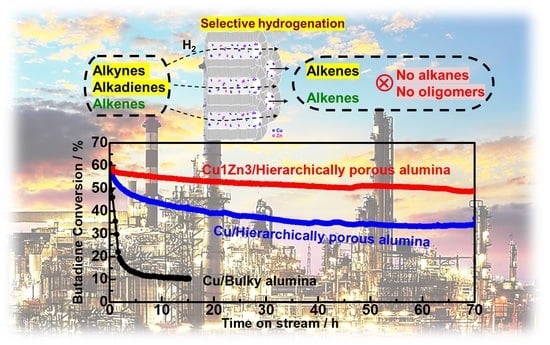
Highly Enhanced Catalytic Stability of Copper by the Synergistic Effect of Porous Hierarchy and Alloying for Selective Hydrogenation Reaction
Abstract
:1. Introduction
2. Results and Discussion
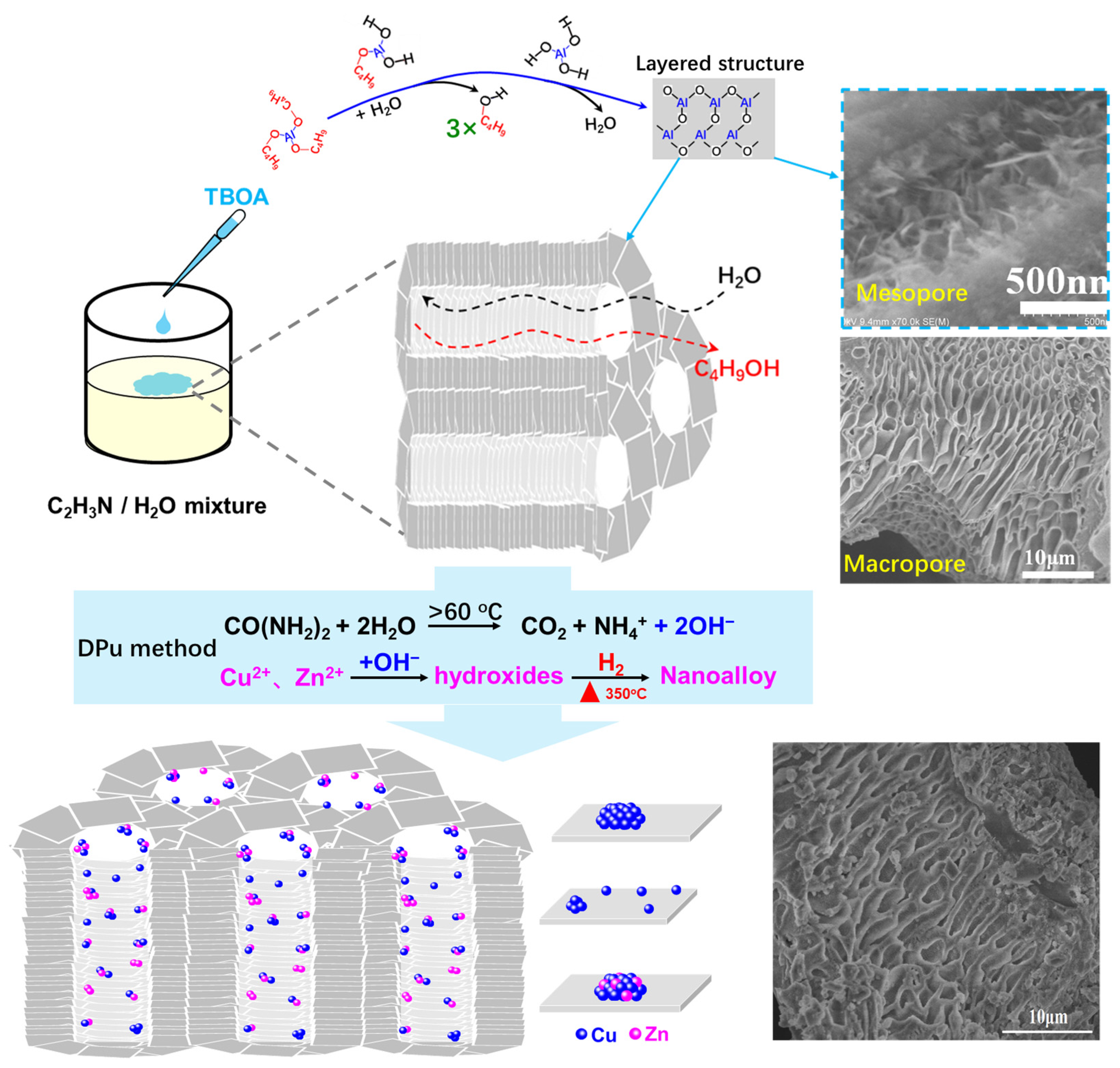
2.1. Preparation of the Hierarchically Porous Alumina Supported Bimetallic Cu–Zn Catalysts
2.2. The Porous Hierarchy in Cu–Zn Supported on Hierarchically Porous γ-Al2O3
2.3. The Property of Bimetallic Cu–Zn Supported on Hierarchically Porous γ-Al2O3
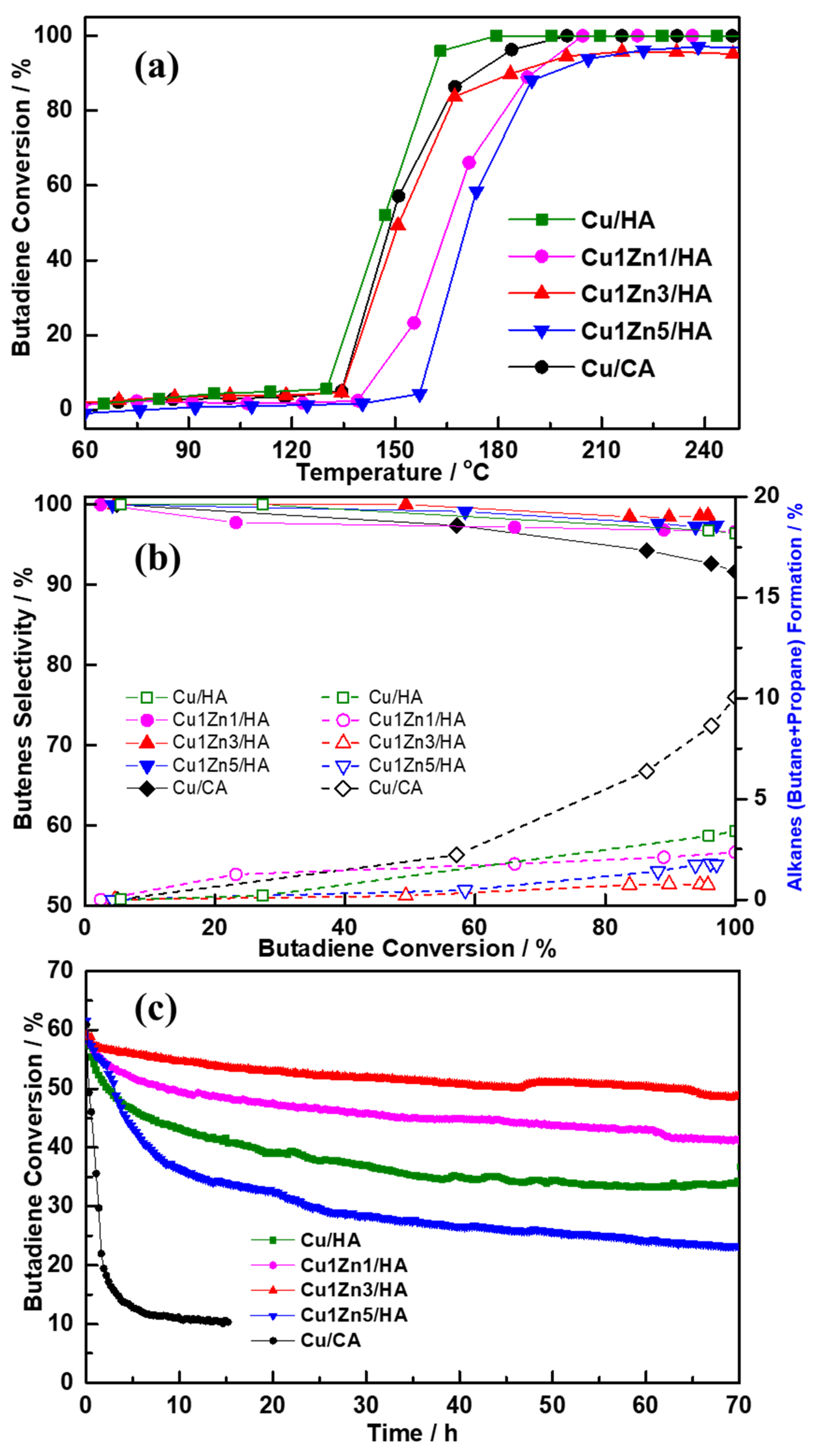
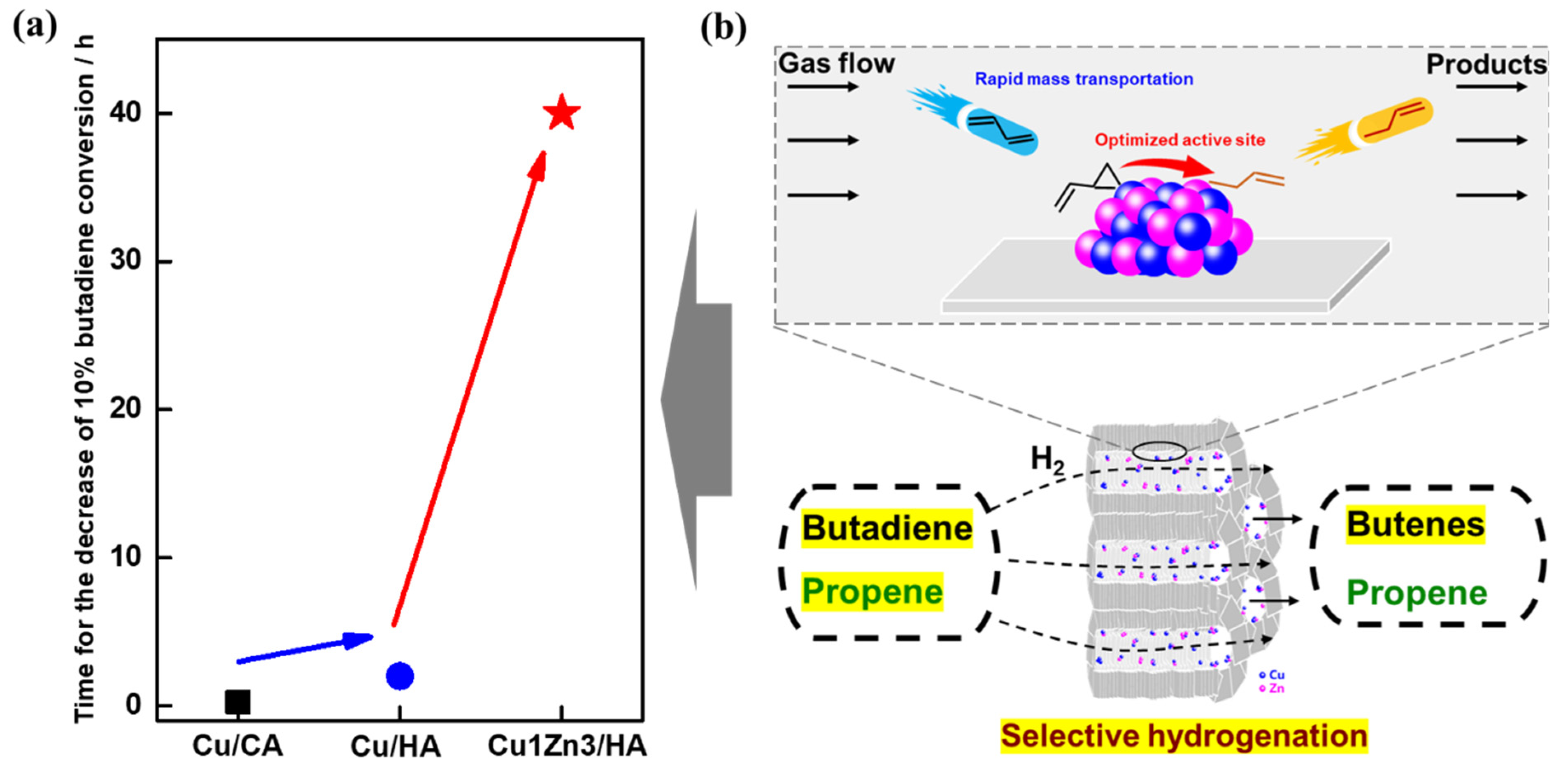
2.4. Catalytic Performance for Selective Butadiene Hydrogenation in an Excess of Propene
3. Experiment
3.1. Sample Preparation
3.1.1. Materials and Chemicals
3.1.2. Preparation of Hierarchically Porous Alumina
3.1.3. Preparation of Cu–Zn/HA Catalysts
3.2. Catalysts Characterization
3.3. Butadiene Selective Hydrogenation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Corresa, E.; Mathieu, Y.; Sauvanaud, L.; Al-Bogami, S.; Al-Ghrami, M.S.; Bourane, A. Crude oil to chemicals: Light olefins from crude oil. Catal. Sci. Technol. 2017, 7, 12–46. [Google Scholar] [CrossRef] [Green Version]
- Marcilly, C. Evolution of refining and petrochemicals: What is the place of zeolites. Oil Gas Sci. Technol. 2001, 56, 499–514. [Google Scholar] [CrossRef]
- Derrien, M.L. Chapter 18 Selective Hydrogenation Applied to the Refining of Petrochemical Raw Materials Produced by Steam Cracking. In Studies in Surface Science and Catalysis; Cerveny, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 27, pp. 613–666. [Google Scholar]
- Puls, F.H.; Ruhnke, K.D. Butene-1 Containing Feed Purification Process (CS-165). U.S. Patent 4260840A, 7 April 1981. [Google Scholar]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Albers, P.; Pietsch, J.; Parker, S.F. Poisoning and deactivation of palladium catalysts. J. Mol. Catal. A Chem. 2001, 173, 275–286. [Google Scholar] [CrossRef]
- Burger, B.J.; Thompson, M.E.; Cotter, W.D.; Bercaw, J.E. Ethylene insertion and. beta.-hydrogen elimination for permethylscandocene alkyl complexes. A study of the chain propagation and termination steps in Ziegler-Natta polymerization of ethylene. J. Am. Chem. Soc. 1990, 112, 1566–1577. [Google Scholar] [CrossRef]
- Campos, K.R.; Cai, D.; Journet, M.; Kowal, J.J.; Larsen, R.D.; Reider, P.J. Controlled Semihydrogenation of Aminoalkynes Using Ethylenediamine as a Poison of Lindlar’s Catalyst. J. Org. Chem. 2001, 66, 3634–3635. [Google Scholar] [CrossRef]
- Lu, F.; Xu, Y.; Jiang, X.; Liu, Y.; Huang, J.; Sun, D. Biosynthesized Pd/γ-Al2O3 catalysts for low-temperature 1,3-butadiene hydrogenation: The effect of calcination atmosphere. New J. Chem. 2017, 41, 13036–13042. [Google Scholar] [CrossRef]
- Lu, F.; Sun, D.; Jiang, X. Plant-mediated synthesis of AgPd/γ-Al2O3 catalysts for selective hydrogenation of 1,3-butadiene at low temperature. New J. Chem. 2019, 43, 13891–13898. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Krotova, I.N. Partial hydrogenation of phenylacetylene over gold- and palladium-containing catalysts. Pet. Chem. 2013, 53, 394–400. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Liu, X.; Zhu, Q. Synergetic effect of Pd and Ag dispersed on Al2O3 in the selective hydrogenation of acetylene. Appl. Catal. A Gen. 2000, 197, 221–228. [Google Scholar] [CrossRef]
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 2008, 320, 1320–1322. [Google Scholar] [CrossRef]
- Totarella, G.; Beerthuis, R.; Masoud, N.; Louis, C.; Delannoy, L.; de Jongh, P.E. Supported Cu Nanoparticles as Selective and Stable Catalysts for the Gas Phase Hydrogenation of 1,3-Butadiene in Alkene-Rich Feeds. J. Phys. Chem. C 2021, 125, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Brouri, D.; Casale, S.; Delannoy, L.; Louis, C. Exploration of the preparation of Cu/TiO2 catalysts by deposition–precipitation with urea for selective hydrogenation of unsaturated hydrocarbons. J. Catal. 2016, 340, 95–106. [Google Scholar]
- Shi, X.; Lin, Y.; Huang, L.; Sun, Z.; Yang, Y.; Zhou, X.; Vovk, E.; Liu, X.; Huang, X.; Sun, M.; et al. Copper Catalysts in Semihydrogenation of Acetylene: From Single Atoms to Nanoparticles. ACS Catal. 2020, 10, 3495–3504. [Google Scholar] [CrossRef]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Xie, J.; Xiao, D.; Wen, X.; Wang, N.; et al. Anchoring Cu1 species over nanodiamond-graphene for semi-hydrogenation of acetylene. Nat. Commun. 2019, 10, 4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, C.; Delannoy, L. Chapter One—Selective hydrogenation of polyunsaturated hydrocarbons and unsaturated aldehydes over bimetallic catalysts. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 64, pp. 1–88. [Google Scholar]
- Xiaoke, H.; Xiaoyun, L.; Zhao, W.; Nian, H.; Zhao, D.; Lihua, C.; Baolian, S. Self-reduction for the Synthesis of Co Supported on Hierarchically Porous Carbon for Selective Hydrogenation Reaction. Chem. J. Chin. Univ. 2020, 41, 639–945. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Louis, C.; Delannoy, L. Bimetallic Ni–Zn/TiO2 catalysts for selective hydrogenation of alkyne and alkadiene impurities from alkenes stream. Res. Chem. Intermed. 2021, 47, 91–116. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Ji, S.; Sui, Z.; Jiang, Z.; Wang, D.; Zaera, F.; Zhou, X.; Duan, X.; Li, Y. Adsorption Site Regulation to Guide Atomic Design of Ni–Ga Catalysts for Acetylene Semi-Hydrogenation. Angew. Chem. Int. Ed. 2020, 59, 11647–11652. [Google Scholar] [CrossRef] [PubMed]
- Putro, W.S.; Kojima, T.; Hara, T.; Ichikuni, N.; Shimazu, S. Selective hydrogenation of unsaturated carbonyls by Ni–Fe-based alloy catalysts. Catal. Sci. Technol. 2017, 7, 3637–3646. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, S.; Gao, Z.; Li, Y.; Zhang, Q.; Xiang, Q.; Qin, Y. The precise decoration of Pt nanoparticles with Fe oxide by atomic layer deposition for the selective hydrogenation of cinnamaldehyde. Appl. Catal. B Environ. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Wehrli, J.T.; Thomas, D.J.; Wainwright, M.S.; Trimm, D.L.; Cant, N.W. Selective hydrogenation of propyne over supported copper catalysts: Influence of support. Appl. Catal. 1991, 70, 253–262. [Google Scholar] [CrossRef]
- Stammbach, M.; Thomas, D.; Trimm, D.; Wainwright, M. Hydrogenation of ethyne over an ion-exchanged copper on silica catalyst. Appl. Catal. 1990, 58, 209–217. [Google Scholar] [CrossRef]
- Ossipoff, N.J.; Cant, N. The hydrogenation and oligomerization of propyne over an ion-exchanged copper on silica catalyst. J. Catal. 1994, 148, 125–133. [Google Scholar] [CrossRef]
- Sárkány, A.; Guczi, L.; Weiss, A.H. On the aging phenomenon in palladium catalysed acetylene hydrogenation. Appl. Catal. 1984, 10, 369–388. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, L.; Thrimurthulu, G.; Reddy, P.S.; Méthivier, C.; Nelayah, J.; Reddy, B.M.; Ricolleau, C.; Louis, C. Selective hydrogenation of butadiene over TiO2 supported copper, gold and gold–copper catalysts prepared by deposition–precipitation. Phys. Chem. Chem. Phys. 2014, 16, 26514–26527. [Google Scholar] [CrossRef] [PubMed]
- McCue, A.J.; McRitchie, C.J.; Shepherd, A.M.; Anderson, J.A. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation. J. Catal. 2014, 319, 127–135. [Google Scholar] [CrossRef]
- Boucher, M.B.; Zugic, B.; Cladaras, G.; Kammert, J.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.H.; Flytzani-Stephanopoulos, M. Single atom alloy surface analogs in Pd 0.18 Cu 15 nanoparticles for selective hydrogenation reactions. Phys. Chem. Chem. Phys. 2013, 15, 12187–12196. [Google Scholar] [CrossRef] [PubMed]
- McCue, A.J.; Shepherd, A.M.; Anderson, J.A. Optimisation of preparation method for Pd doped Cu/Al2O3 catalysts for selective acetylene hydrogenation. Catal. Sci. Technol. 2015, 5, 2880–2890. [Google Scholar] [CrossRef]
- Lucci, F.R.; Liu, J.; Marcinkowski, M.D.; Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective hydrogenation of 1, 3-butadiene on platinum-copper alloys at the single-atom limit. Nat. Commun. 2015, 6, 8550. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Wang, G.; Louis, C.; Delannoy, L. Novel non-noble bimetallic Cu-Zn/TiO2 catalysts for selective hydrogenation of butadiene. J. Catal. 2017, 347, 185–196. [Google Scholar]
- Zhao, Y.; Guo, Z.; Zhang, H.; Peng, B.; Xu, Y.; Wang, Y.; Zhang, J.; Xu, Y.; Wang, S.; Ma, X. Hydrogenation of diesters on copper catalyst anchored on ordered hierarchical porous silica: Pore size effect. J. Catal. 2018, 357, 223–237. [Google Scholar] [CrossRef]
- Hu, N.; Li, X.-Y.; Liu, S.-M.; Wang, Z.; He, X.-K.; Hou, Y.-X.; Wang, Y.-X.; Deng, Z.; Chen, L.-H.; Su, B.-L. Enhanced stability of highly-dispersed copper catalyst supported by hierarchically porous carbon for long term selective hydrogenation. Chin. J. Catal. 2020, 41, 1081–1090. [Google Scholar] [CrossRef]
- Su, B.L.; Vantomme, A.; Surahy, L.; Pirard, R.; Pirard, J.P. Hierarchical Multimodal Mesoporous Carbon Materials with Parallel Macrochannels. Chem. Mater. 2007, 19, 3325–3333. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Ren, T.Z.; Azioune, A.; Pireaux, J.J.; Su, B.L. Self-Assembly of Hierarchically Mesoporous−Macroporous Phosphated Nanocrystalline Aluminum (Oxyhydr)oxide Materials. Chem. Mater. 2006, 18, 1753–1767. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Hakim, S.H.; Su, B.-L.; Shanks, B.H. Direct observation of macropore self-formation in hierarchically structured metal oxides. Chem. Commun. 2010, 46, 8980–8982. [Google Scholar] [CrossRef]
- Zanella, R.; Delannoy, L.; Louis, C. Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition–precipitation with NaOH and urea. Appl. Catal. A Gen. 2005, 291, 62–72. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Jiang, B.; Yang, Y. Diffusion measurements of isopentane, 1-hexene, cyclohexane in polyethylene particles by the intelligent gravimetric analyzer. J. Appl. Polym. Sci. 2013, 127, 1098–1104. [Google Scholar] [CrossRef]
- Huang, H.H.; Yan, F.Q.; Kek, Y.M.; Chew, C.H.; Xu, G.Q.; Ji, W.; Oh, P.S.; Tang, S.H. Synthesis, Characterization, and Nonlinear Optical Properties of Copper Nanoparticles. Langmuir 1997, 13, 172–175. [Google Scholar] [CrossRef]
- Condorelli, G.G.; Costanzo, L.L.; Fragalà, I.L.; Giuffrida, S.; Ventimiglia, G. A single photochemical route for the formation of both copper nanoparticles and patterned nanostructured films. J. Mater. Chem. 2003, 13, 2409–2411. [Google Scholar] [CrossRef]
- Savinova, E.R.; Chuvilin, A.L.; Parmon, V.N. Copper colloids stabilized by water-soluble polymers: Part I. Preparation and properties. J. Mol. Catal. 1988, 48, 217–229. [Google Scholar] [CrossRef]
- Derrouiche, S.; La Fontaine, C.; Thrimurtulu, G.; Casale, S.; Delannoy, L.; Lauron-Pernot, H.; Louis, C. Unusual behaviour of Au/ZnO catalysts in selective hydrogenation of butadiene due to the formation of a AuZn nanoalloy. Catal. Sci. Technol. 2016, 6, 6794–6805. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, M.; Furukawa, S.; Takayama, T.; Yamazoe, S.; Komatsu, T. Surface Modification of PdZn Nanoparticles via Galvanic Replacement for the Selective Hydrogenation of Terminal Alkynes. ACS Appl. Nano Mater. 2019, 2, 3307–3314. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef]
- Wang, Z. Selective Hydrogenation of Butadiene over Non-Noble Bimetallic Catalysts. (Catalyseurs Bimétalliques à Base de Métaux Non Nobles Pour L’hydrogénation Sélective du Butadiène—Paris VI). Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2017. [Google Scholar]
- Wiame, F.; Islam, M.M.; Salgın, B.; Światowska, J.; Costa, D.; Diawara, B.; Maurice, V.; Marcus, P. Zn effect on STM imaging of brass surfaces. Surf. Sci. 2016, 644, 148–152. [Google Scholar] [CrossRef]
- Krajčí, M.; Hafner, J. Intermetallic Compounds as Selective Heterogeneous Catalysts: Insights from DFT. ChemCatChem 2016, 8, 34–48. [Google Scholar] [CrossRef]
- Bridier, B.; López, N.; Pérez-Ramírez, J. Molecular understanding of alkyne hydrogenation for the design of selective catalysts. Dalton Trans. 2010, 39, 8412–8419. [Google Scholar] [CrossRef] [PubMed]
- Berteau, P.; Ceckiewicz, S.; Delmon, B. Role of the acid-base properties of aluminas, modified γ-alumina, and silica-alumina in 1-butanol dehydration. Appl. Catal. 1987, 31, 361–383. [Google Scholar] [CrossRef]
- Hugon, A.; Delannoy, L.; Louis, C. Supported gold catalysts for selective hydrogenation of 1,3-butadiene in the presence of an excess of alkenes. Gold Bull. 2008, 41, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Catillon-Mucherie, S.; Ammari, F.; Krafft, J.-M.; Lauron-Pernot, H.; Touroude, R.; Louis, C. Preparation of Coimpregnated Cu−Zn/SiO2 Catalysts: Influence of the Drying Step on Metallic Particle Size and on Cu0−ZnII Interactions. J. Phys. Chem. C 2007, 111, 11619–11626. [Google Scholar] [CrossRef]
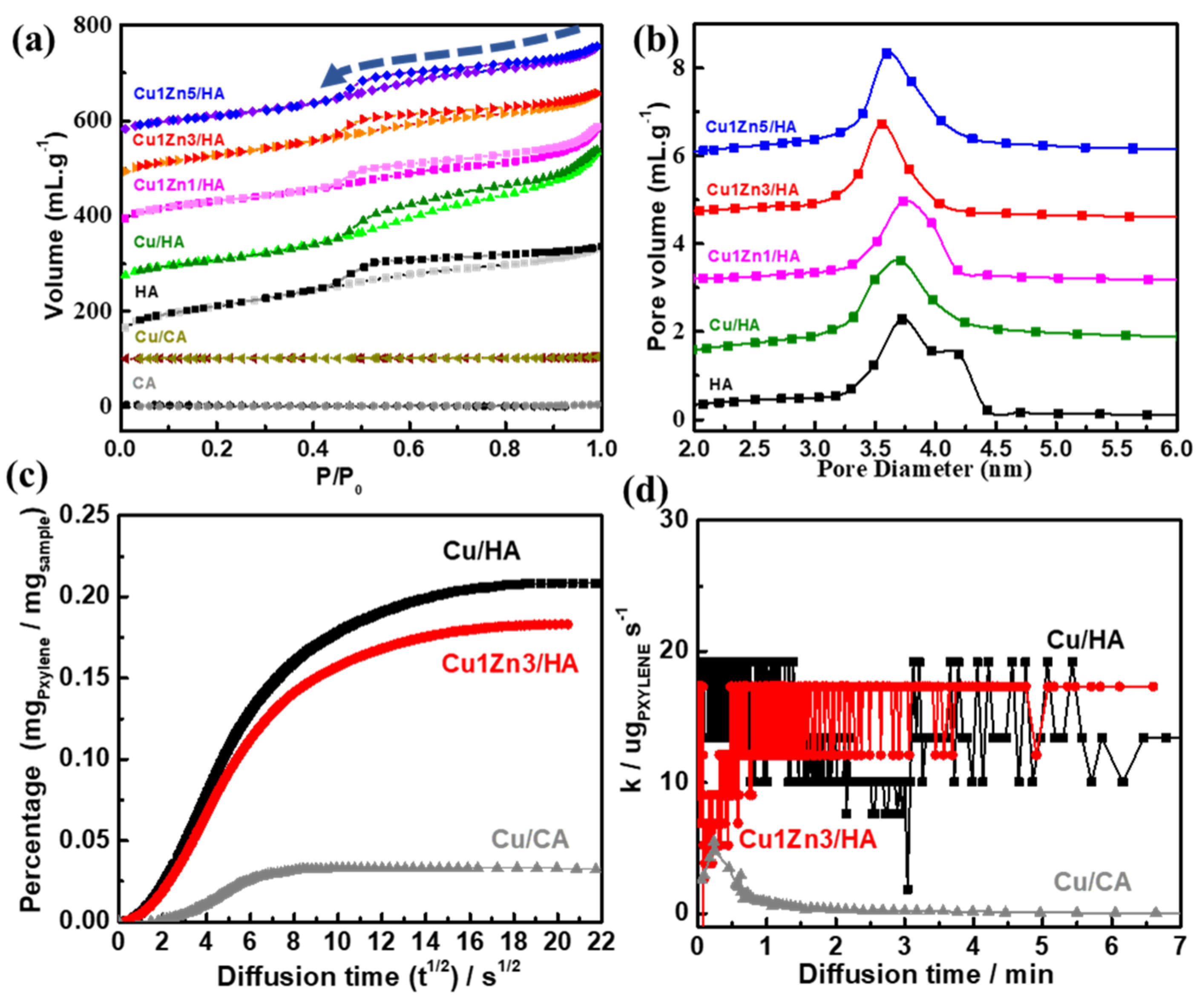


| Sample | Theoretical Values | Experimental Values | Total BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Meso Pore Diameter (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Loading (wt%) | Cu:Zn | Loading (wt%) | Cu:Zn | ||||||
| Cu | Zn | Cu | Zn | ||||||
| HA | - | - | - | - | - | - | 394 | 0.57 | 3.8, 4.2 |
| Cu/HA | 2.5 | 0 | 1:0 | 2.3 | 0 | 1:0 | 355 | 0.48 | 3.7 |
| Cu1Zn1/HA | 2.5 | 2.5 | 1:1 | 2.4 | 2.2 | 1:0.9 | 315 | 0.38 | 3.7 |
| Cu1Zn3/HA | 2.5 | 7.5 | 1:3 | 2.3 | 5.3 | 1:2.3 | 291 | 0.33 | 3.5 |
| Cu1Zn5/HA | 2.5 | 12.5 | 1:5 | 2.3 | 8.2 | 1:3.6 | 259 | 0.33 | 3.5 |
| Cu/CA | 2.5 | 0 | 1:0 | 2.3 | 0 | 1:0 | 4 | - | - |
| CA | - | - | - | - | - | - | 3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Wang, Z.; Jin, S.; Xiao, S.; Liu, S.; Hu, Z.; Chen, L.; Su, B. Highly Enhanced Catalytic Stability of Copper by the Synergistic Effect of Porous Hierarchy and Alloying for Selective Hydrogenation Reaction. Catalysts 2022, 12, 12. https://doi.org/10.3390/catal12010012
Yuan H, Wang Z, Jin S, Xiao S, Liu S, Hu Z, Chen L, Su B. Highly Enhanced Catalytic Stability of Copper by the Synergistic Effect of Porous Hierarchy and Alloying for Selective Hydrogenation Reaction. Catalysts. 2022; 12(1):12. https://doi.org/10.3390/catal12010012
Chicago/Turabian StyleYuan, Hao, Zhao Wang, Shunjing Jin, Shanshan Xiao, Siming Liu, Zhiyi Hu, Lihua Chen, and Baolian Su. 2022. "Highly Enhanced Catalytic Stability of Copper by the Synergistic Effect of Porous Hierarchy and Alloying for Selective Hydrogenation Reaction" Catalysts 12, no. 1: 12. https://doi.org/10.3390/catal12010012
APA StyleYuan, H., Wang, Z., Jin, S., Xiao, S., Liu, S., Hu, Z., Chen, L., & Su, B. (2022). Highly Enhanced Catalytic Stability of Copper by the Synergistic Effect of Porous Hierarchy and Alloying for Selective Hydrogenation Reaction. Catalysts, 12(1), 12. https://doi.org/10.3390/catal12010012