Conversion of Scenedesmus rubescens Lipid into Biodiesel by Biochar of Different Origin
Abstract
:1. Introduction
2. Results
2.1. Biochar Properties
2.1.1. SEM and EDX Analysis
2.1.2. XRD and FTIR Analysis
2.1.3. Specific Surface Area (SSA) Determination
2.1.4. Thermogravimetric Analysis (TGA)
2.1.5. Acid–Base Behavior of Biochars
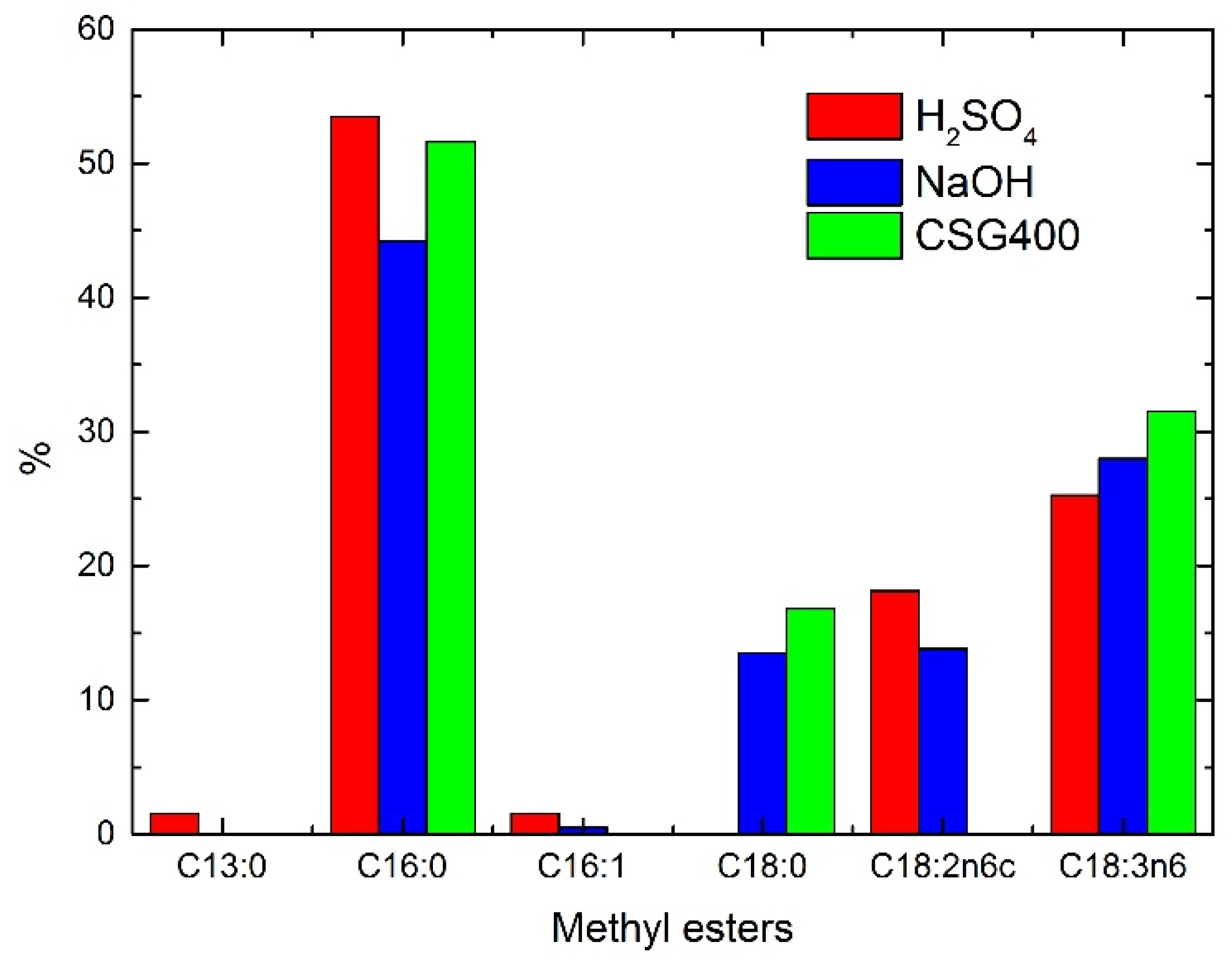
2.2. Transesterification Results
3. Discussion
4. Materials and Methods
4.1. Algal Cultures
4.2. Biochar Production
4.3. Physicochemical Characterization of Biochar
4.4. Lipid Extraction
4.5. Transesterification of Algal Lipid
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. 2050 Long-Term Strategy. Available online: https://ec.europa.eu/clima/policies/strategies/2050_en (accessed on 5 July 2021).
- Zhang, M.; Xu, Y.; Williams, B.L.; Xiao, M.; Wang, S.; Han, D.; Sun, L.; Meng, Y. Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2. J. Clean. Prod. 2021, 279, 123344. [Google Scholar] [CrossRef]
- Zhang, M.; Alferov, K.A.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. Continuous Dimethyl Carbonate Synthesis from CO2 and Methanol Using Cu-Ni@VSiO as Catalyst Synthesized by a Novel Sulfuration Method. Catalysts 2018, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Mašek, O.; Zhao, L.; Nan, H.; Yu, S.; Yin, J.; Li, Z.; Cao, X. Country-level potential of carbon sequestration and environmental benefits by utilizing crop residues for biochar implementation. Appl. Energy 2021, 282, 116275. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Manariotis, I.D. Effect of operating conditions on Chlorococcum sp. growth and lipid production. J. Environ. Chem. Eng. 2016, 4, 1217–1223. [Google Scholar] [CrossRef]
- Doussineau, M.; Gomez, J.; Holstein, F. Smart Specialisation and Blue Biotechnology in Europe; EU Publications: Luxembourg, 2020; ISBN 9789276277538. [Google Scholar]
- Tsavatopoulou, V.D.; Vakros, J.; Manariotis, I.D. Lipid conversion of Scenedesmus rubescens biomass into biodiesel using biochar catalysts from malt spent rootlets. J. Chem. Technol. Biotechnol. 2020, 95, 2421–2429. [Google Scholar] [CrossRef]
- Jo, S.W.; Do, J.M.; Na, H.; Hong, J.W.; Kim, I.S.; Yoon, H.S. Assessment of biomass potentials of microalgal communities in open pond raceways using mass cultivation. PeerJ 2020, 8, e9418. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Theodorakopoulos, M.A.; Manariotis, I.D. Selection of microalgae for wastewater treatment and potential lipids production. Bioresour. Technol. 2013, 147, 130–134. [Google Scholar] [CrossRef]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Tsavatopoulou, V.D.; Aravantinou, A.F.; Manariotis, I.D. Biofuel conversion of Chlorococcum sp. and Scenedesmus sp. biomass by one- and two-step transesterification. Biomass Convers. Biorefinery 2019, 11, 1301–1309. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Μ.M.; Fu, Z.; Xiao, M.; Yu, Y.; Wang, S.; Choi, M.J.; Meng, Y. Synthesis of Co1.5PW12O40 and its catalytic performance of completely converting methanol to ethylene. Chem. Commun. 2016, 52, 1151–1153. [Google Scholar] [CrossRef]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and Characterization of Biochar Sorbents Produced from Malt Spent Rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of sulfamethoxazole by rice husk biochar-activated persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Ntaflou, M.; Vakros, J. Transesterification activity of modified biochars from spent malt rootlets using triacetin. J. Clean. Prod. 2020, 259, 120931. [Google Scholar] [CrossRef]
- Vakros, J.; Manariotis, I.D.; Dracopoulos, V.; Mantzavinos, D.; Lianos, P. Biochar from spent malt rootlets and its application to an energy conversion and storage device. Chemosensors 2021, 9, 57. [Google Scholar] [CrossRef]
- Orfanos, A.G.; Manariotis, I.D.; Karapanagioti, H.K. Removal of methylene blue from water by food industry by-products and biochars. Desalin. Water Treat. 2018, 103, 113–121. [Google Scholar] [CrossRef]
- Ntzoufra, P.; Vakros, J.; Frontistis, Z.; Tsatsos, S.; Kyriakou, G.; Kennou, S.; Manariotis, I.D.; Mantzavinos, D. Effect of sodium persulfate treatment on the physicochemical properties and catalytic activity of biochar prepared from spent malt rootlets. J. Environ. Chem. Eng. 2021, 9, 105071. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Anto, S.; Rene, E.R.; Sekar, M.; Mathimani, T.; Chi, N.T.L.; Pugazhendhi, A. Effect of reaction temperature on the conversion of algal biomass to bio-oil and biochar through pyrolysis and hydrothermal liquefaction. Fuel 2021, 285, 119106. [Google Scholar] [CrossRef]
- Sotoudehniakarani, F.; Alayat, A.; McDonald, A.G. Characterization and comparison of pyrolysis products from fast pyrolysis of commercial Chlorella vulgaris and cultivated microalgae. J. Anal. Appl. Pyrolysis 2019, 139, 258–273. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochars from valorized olive stones for the degradation of sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Bekatorou, A.; Kopsahelis, N.; Mallouchos, A.; Plessas, S.; Droushiotis, N.; Kanellaki, M.; Komaitis, M.; Koutinas, A.A.; Nigam, P. Biotechnological exploitation of brewery solid wastes. In Current Topics on Bioprocesses in Food Industry; Rao, L.V., Pandey, A., Larroche, C., Soccol, C.R., Dussap, C.G., Eds.; Asiatech Publishers Inc.: New Delhi, India, 2010; pp. 193–209. [Google Scholar]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Gao, G.; Cheong, L.Z.; Wang, D.; Shen, C. Pyrolytic carbon derived from spent coffee grounds as anode for sodium-ion batteries. Carbon Resour. Convers. 2018, 1, 104–108. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Thoai, D.N.; Tongurai, C.; Prasertsit, K.; Kumar, A. Review on biodiesel production by two-step catalytic conversion. Biocatal. Agric. Biotechnol. 2019, 18, 101023. [Google Scholar] [CrossRef]
- Piloto-Rodríguez, R.; Sánchez-Borroto, Y.; Melo-Espinosa, E.A.; Verhelst, S. Assessment of diesel engine performance when fueled with biodiesel from algae and microalgae: An overview. Renew. Sustain. Energy Rev. 2017, 69, 833–842. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Prommuak, C.; Pavasant, P.; Quitain, A.T.; Goto, M.; Shotipruk, A. Microalgal lipid extraction and evaluation of single-step biodiesel production. Eng. J. 2012, 16, 157–166. [Google Scholar] [CrossRef]
- Shalaby, E.A. Algae as promising organisms for environment and health. Plant. Signal. Behav. 2011, 6, 1338–1350. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.R.; Yeh, Y.; Liu, H.S. A novel strategy of biodiesel production from wet microalgae by direct saponification–esterification conversion (DSEC). J. Taiwan Inst. Chem. Eng. 2018, 83, 23–31. [Google Scholar] [CrossRef]
- APHA/WEF/AWWA. Standard Methods for the Examination of Water and Wastewater, 25th ed.; American Public Health Association: Washington, DC, USA, 1989; pp. 1–101. ISBN 9780875532356. [Google Scholar]
- Salama, A.R.A.; El-Sahn, M.A.; Mesallam, A.S.; Shehata, A.M.E.T. The chemical composition, the nutritive value and the functional properties of malt sprout and its components (acrespires, rootlets and husks). J. Sci. Food Agric. 1997, 75, 50–56. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple Method for the Isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]

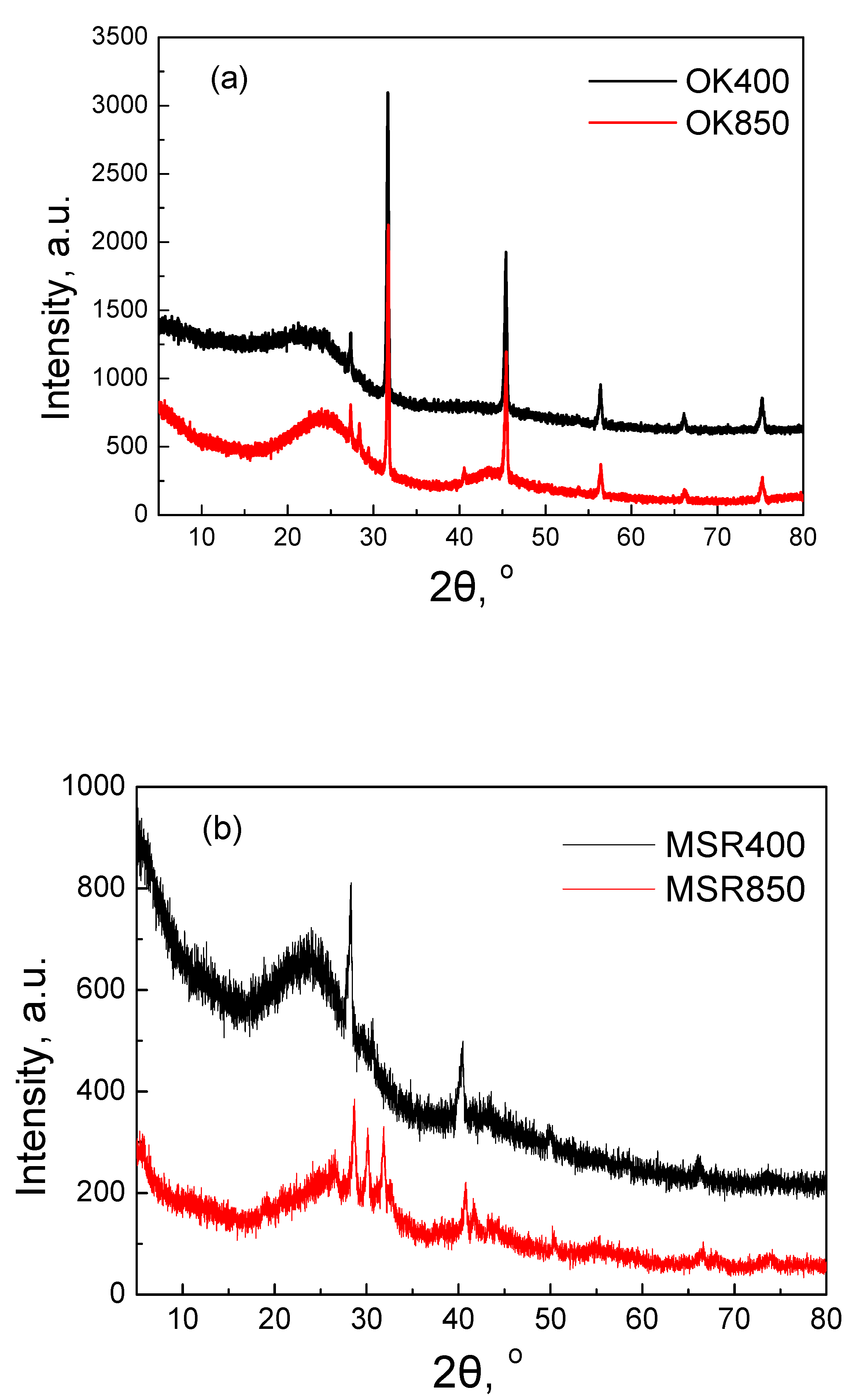
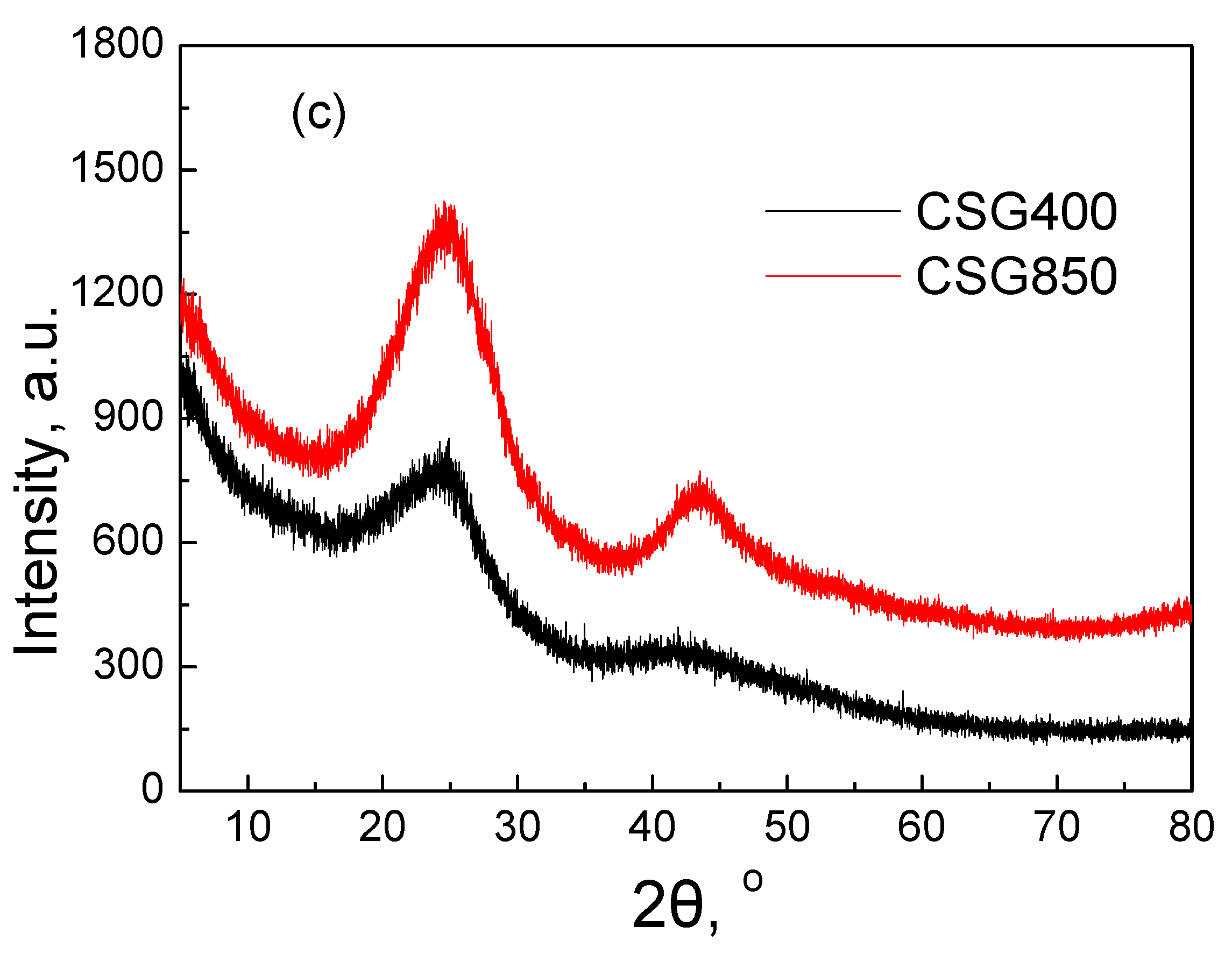


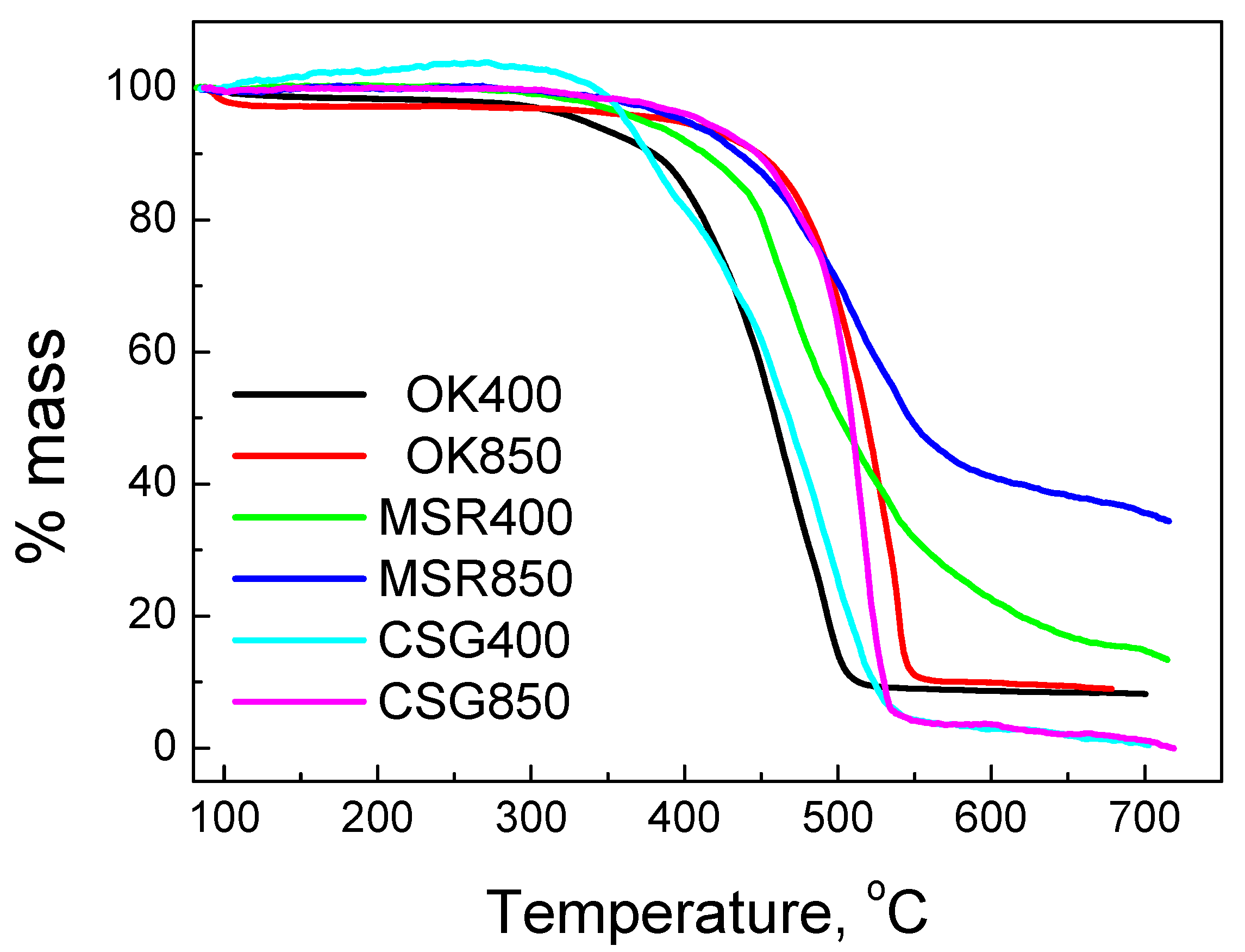
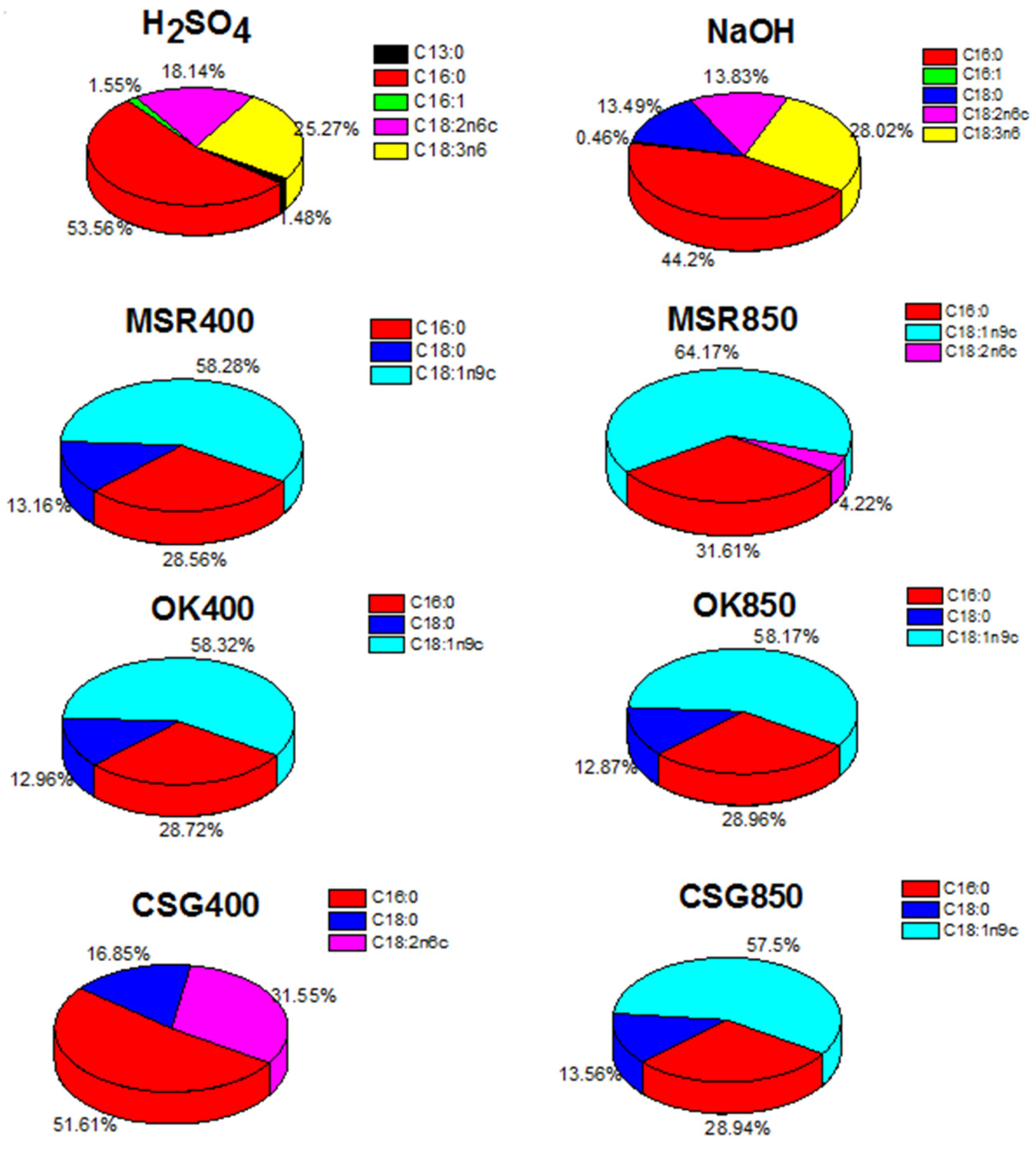
| Element | OK400 | OK850 | MSR400 | MSR850 | CSG400 | CSG850 |
|---|---|---|---|---|---|---|
| O | 15.75 | 17.38 | 47.06 | 42.23 | 31.8 | 34 |
| Mg | N.D. | N.D. | 2.83 | 15.27 | 11.47 | 12.46 |
| Si | N.D. | N.D. | 26.38 | N.D. | N.D. | N.D. |
| P | 2.15 | 2.08 | 8.67 | 21.61 | 9 | 11.53 |
| Cl | 45.33 | 36.76 | N.D. | N.D. | N.D. | N.D. |
| K | N.D. | 4.94 | 10.58 | 20.9 | 33.04 | 30.08 |
| Ca | 3.42 | 3.21 | 4.48 | N.D. | 14.69 | 11.93 |
| Na | 33.36 | 35.64 | N.D. | N.D. | N.D. | N.D. |
| Biochar | SSA | Micropores SSA | Pore Volume | TGA Minerals | pH (H2O) | pH (H2O-MeOH) |
|---|---|---|---|---|---|---|
| (m2g−1) | (m2g−1) | (mL g−1) | (%) | |||
| OK400 | 0.1 | - | - | 8 | 6.9 | 7.2 |
| OK850 | 107 | 69 | 0.01 | 11 | 10.5 | 10.0 |
| MSR400 | 0.04 | - | - | 14 | 7.7 | 7.8 |
| MSR850 | 125 | 74 | 0.1 | 35 | 11.4 | 11.2 |
| CSG400 | 0.4 | - | - | 2 | 8.1 | 9.5 |
| CSG850 | 307 | 217 | 0.15 | 3 | 10.7 | 10.5 |
| Catalysts | Total FAMEs (mg) |
|---|---|
| H2SO4 | 27.55 |
| NaOH | 29.04 |
| MSR850 | 21.34 |
| MSR400 | 16.78 |
| OK850 | 16.35 |
| OK400 | 19.12 |
| CSG850 | 18.96 |
| CSG400 | 25.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsavatopoulou, V.D.; Aravantinou, A.F.; Vakros, J.; Manariotis, I.D. Conversion of Scenedesmus rubescens Lipid into Biodiesel by Biochar of Different Origin. Catalysts 2021, 11, 1116. https://doi.org/10.3390/catal11091116
Tsavatopoulou VD, Aravantinou AF, Vakros J, Manariotis ID. Conversion of Scenedesmus rubescens Lipid into Biodiesel by Biochar of Different Origin. Catalysts. 2021; 11(9):1116. https://doi.org/10.3390/catal11091116
Chicago/Turabian StyleTsavatopoulou, Vasiliki D., Andriana F. Aravantinou, John Vakros, and Ioannis D. Manariotis. 2021. "Conversion of Scenedesmus rubescens Lipid into Biodiesel by Biochar of Different Origin" Catalysts 11, no. 9: 1116. https://doi.org/10.3390/catal11091116
APA StyleTsavatopoulou, V. D., Aravantinou, A. F., Vakros, J., & Manariotis, I. D. (2021). Conversion of Scenedesmus rubescens Lipid into Biodiesel by Biochar of Different Origin. Catalysts, 11(9), 1116. https://doi.org/10.3390/catal11091116







