Valence Regulation of Ultrathin Cerium Vanadate Nanosheets for Enhanced Photocatalytic CO2 Reduction to CO
Abstract
:1. Introduction
2. Results and Discussion
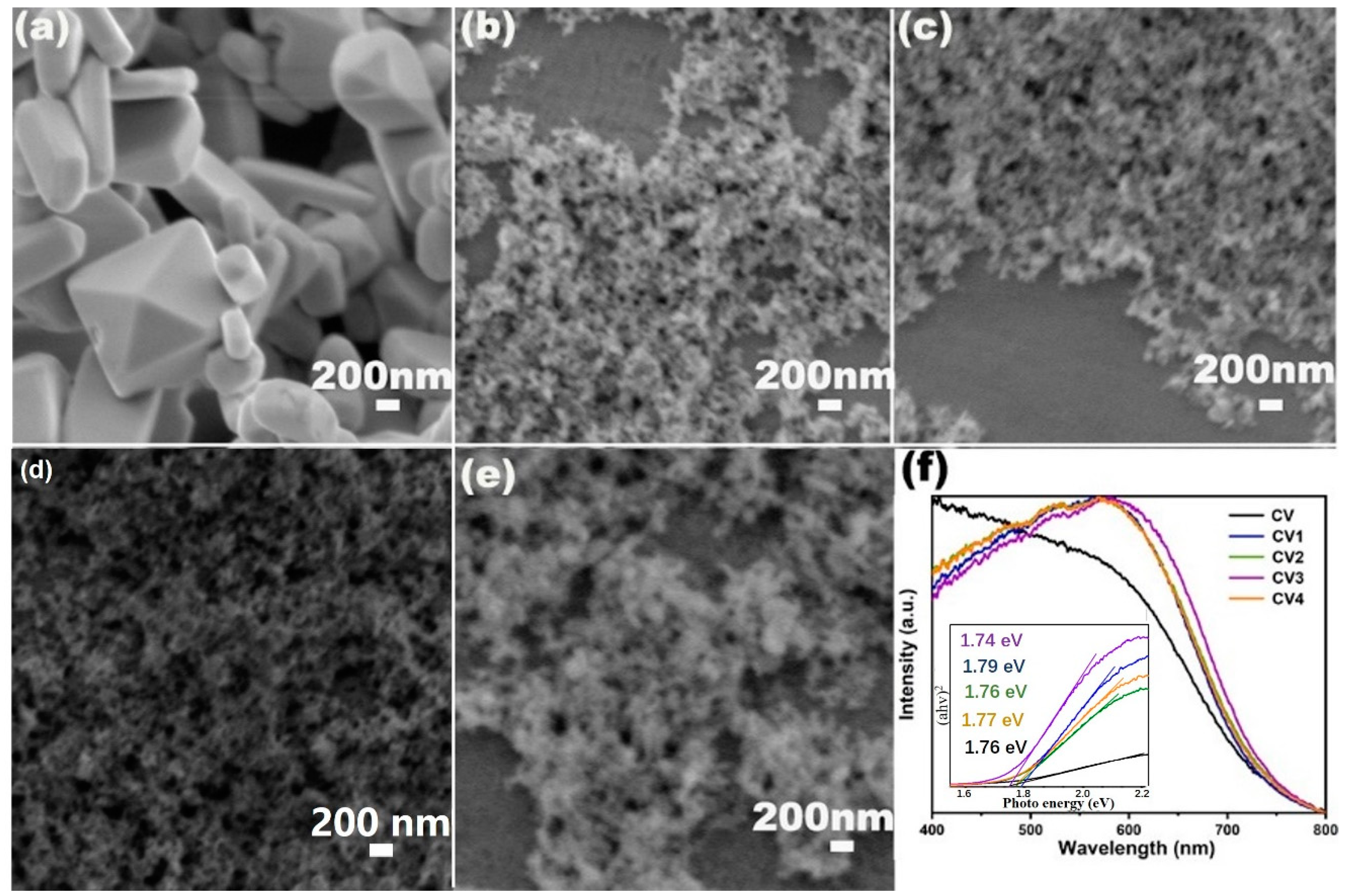
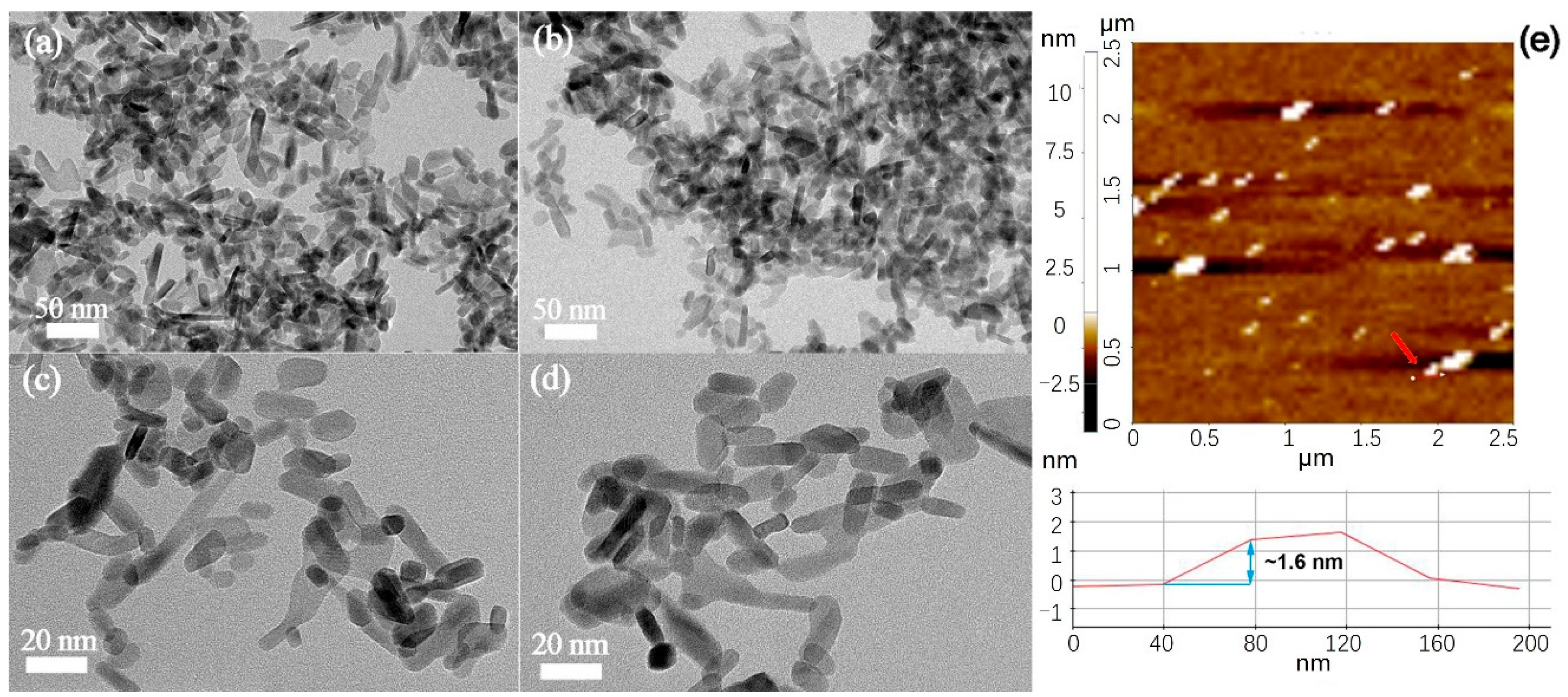
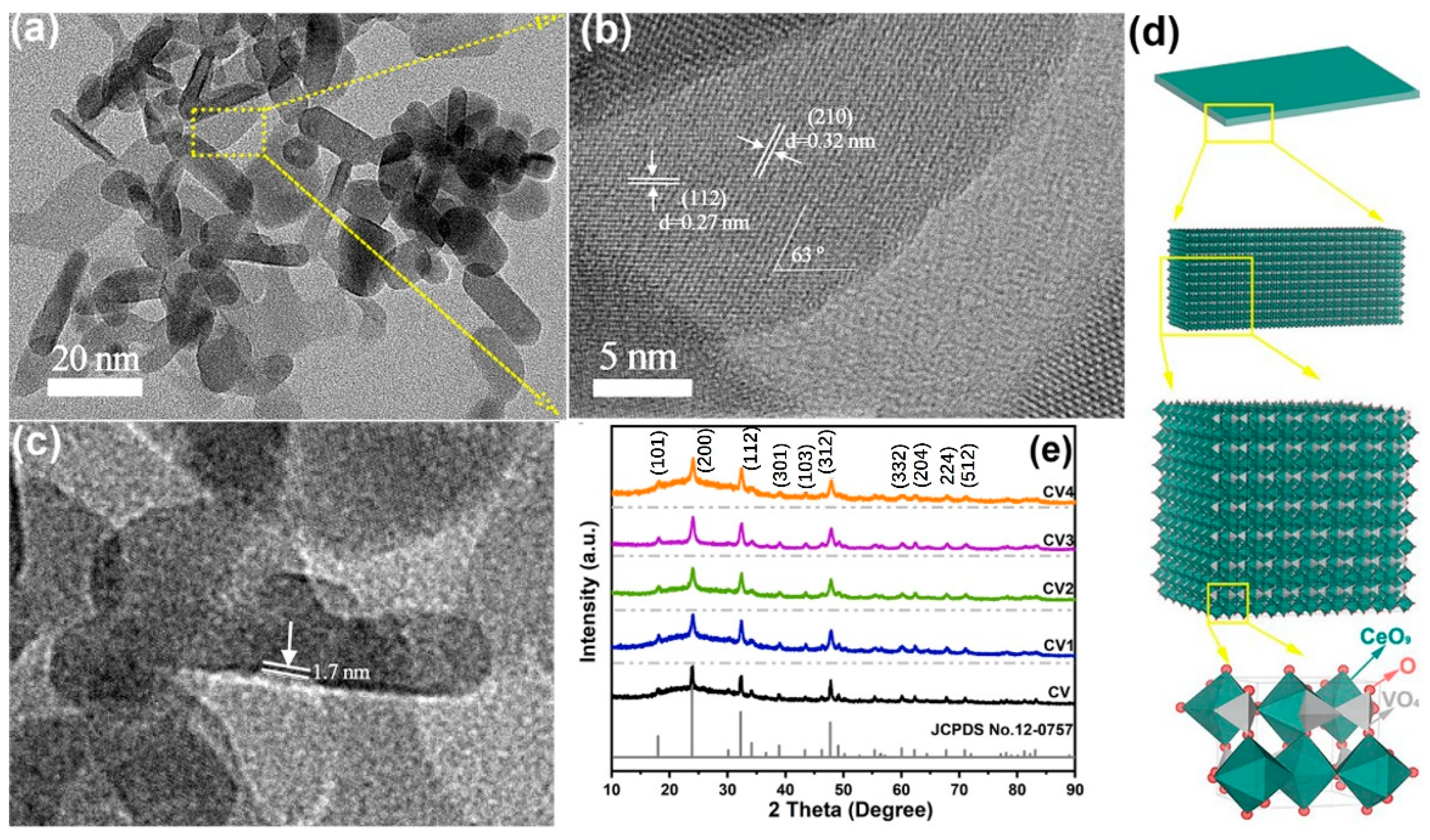
2.1. Morphology and Phase Characterization
2.2. Photocatalytic CO2 Reduction Activity
2.3. Spectroscopy Analysis
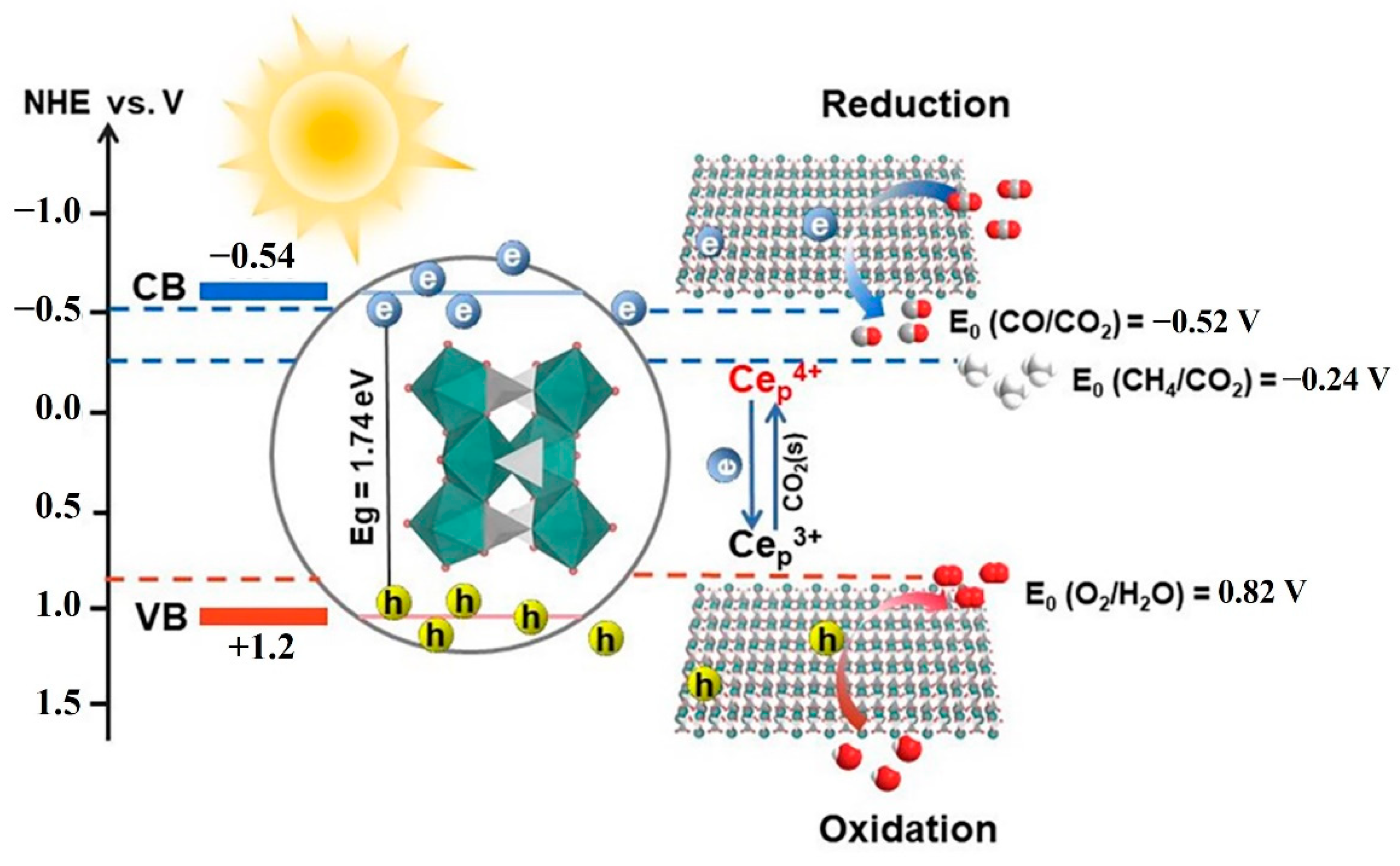
2.4. Photocatalytic Mechanism
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stolarczyk, J.K.; Bhattacharyya, S.; Polavarapu, L.; Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Amal, R.; Qiao, S.; Wu, J.; Yin, Z. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5305–5426. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Ayyub, M.; Rao, C.N.R. Recent progress in the photocatalytic reduction of carbon dioxide. ACS Omega 2017, 2, 2740–2748. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Bai, X.; Man, Z.; He, H.; Li, L.; Hu, J.; Alsaedi, A.; Hayat, T.; Yu, Z.; Zhang, W.; et al. Convincing synthesis of atomically thin, single-crystalline InVO4 sheets toward promoting highly selective and efficient solar conversion of CO2 into CO. J. Am. Chem. Soc. 2019, 141, 4209–4213. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, L.; Su, Y.; Sun, S.; Wang, Q.; Wang, H.; Wang, W. Boosted CO2 photoreduction to methane via Co doping in bismuth vanadate atomic layers. Catal. Sci. Technol. 2018, 8, 3115–3122. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, C.; Wang, X.; Liao, A.; Li, L.; Liu, Q.; Tang, Y.; Wu, C.; Shen, Q.; Yu, Z.; et al. Two-Step Synthesis of Laminar Vanadate via a Facile Hydrothermal Route and Enhancing the Photocatalytic Reduction of CO2 into Solar Fuel through Tuning of the Oxygen Vacancies by in Situ Vacuum Illumination Treatment. ACS Appl. Energy Mater. 2018, 1, 6857–6864. [Google Scholar] [CrossRef]
- Watanabe, A. Highly conductive oxides, CeVO4, Ce12xMxVO420.5x(M Ca, Sr, Pb) and Ce12yBiyVO4, with zircon-type structure prepared by solid-state reaction in air. J. Solid State Chem. 2000, 153, 174–179. [Google Scholar] [CrossRef]
- Mahapatra, S.; Madras, G.; Row, T.N.G. Synthesis, characterization and photocatalytic activity of lanthanide (Ce, Pr and Nd) orthovanadates. Ind. Eng. Chem. Res. 2007, 46, 1013–1017. [Google Scholar] [CrossRef]
- Dolgos, M.R.; Paraskos, A.M.; Stoltzfus, M.W.; Yarnell, S.C.; Woodward, P.M. The electronic structures of vanadate salts: Cation substitution as a tool for band gap manipulation. J. Solid State Chem. 2009, 182, 1964–1971. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Kuntalue, B.; Thongtem, S.; Thongtem, T. Effect of PEG on phase, morphology and photocatalytic activity of CeVO4 nanostructures. Mater. Lett. 2016, 174, 138–141. [Google Scholar] [CrossRef]
- Lu, G.; Zou, X.; Wang, F.; Wang, H.; Li, W. Facile fabrication of CeVO4 microspheres with efficient visible-light photocatalytic activity. Mater. Lett. 2017, 195, 168–171. [Google Scholar] [CrossRef]
- Allen, J.P.; Galea, N.M.; Watson, G.W. Valence states in CeVO4 and Ce0.5Bi0.5VO4 probed by density functional theory calculations and X-ray photoemission spectroscopy. J. Phys. Chem. C 2014, 118, 25330–25339. [Google Scholar] [CrossRef]
- Luo, F.; Jia, C.; Liu, R.; Sun, L.; Yan, C. Nanorods-assembled CeVO4 hollow spheres as active catalyst for oxidative dehydrogenation of propane. Mater. Res. Bull. 2013, 48, 1122–1127. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, Y.; Zheng, S.; Guo, X.; Chen, Z.; Peng, L.; Ding, W. Nanocrystals of CeVO4 doped by metallic heteroions. Inorg. Chem. 2011, 50, 6189–6194. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mugesh, G. CeVO4 nanozymes catalyze the reduction of dioxygen to water without releasing partially reduced oxygen species. Angew. Chem. Int. Ed. 2019, 58, 7797–7801. [Google Scholar] [CrossRef] [PubMed]
- Ao, B.; Qiu, R.; Hu, S. First-principles Insights into the oxidation states and electronic structures of ceria-based binary, ternary and quaternary oxides. J. Phys. Chem. C 2019, 123, 175–184. [Google Scholar] [CrossRef]
- Chang, M.; Wang, M.; Chen, Y.; Shu, M.; Zhao, Y.; Ding, B.; Hou, Z.; Lin, J. Self-assembled CeVO4/Ag nanohybrid as photo conversion agents with enhanced solar-driven photocatalysis and NIR-responsive photothermal/photodynamic synergistic therapy performance. Nanoscale 2019, 11, 10129–10136. [Google Scholar] [CrossRef]
- Juez, A.I.; Huerta, M.V.M.; García, E.R.; Jehng, J.M.; Bañares, M.A. On the nature of the unusual redox cycle at the vanadia ceria interface. J. Phys. Chem. C 2018, 122, 1197–1205. [Google Scholar] [CrossRef]
- Bandiello, E.; Errandonea, D.; Platas, J.G.; Hernandez, P.R.; Munoz, A.; Bettinelli, M.; Popescu, C. Phase behavior of TmVO4 under hydrostatic compression: An experimental and theoretical study. Inorg. Chem. 2020, 59, 4882–4894. [Google Scholar] [CrossRef]
- Guan, J.; Li, J.; Ye, Z.; Wu, D.; Liu, C.; Wang, H.; Ma, C.; Huo, P.; Yan, Y. La2O3 media enhanced electrons transfer for improved CeVO4@halloysite nanotubes photocatalytic activity for removing tetracycline. J. Taiwan Inst. Chem. E 2019, 96, 281–298. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Priya, T.S.; Wang, T.J. Surface engineering three-dimensional flowerlike cerium vanadate nanostructures used as electrocatalysts: Real time monitoring of clioquinol in biological samples. ACS Sustain. Chem. Eng. 2019, 7, 16121–16130. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, F.; Zhao, D. One-step synthesis and assembly of copper sulfide nanoparticles to nanowires, nanotubes, and nanovesicles by a simple organic amine-assisted hydrothermal process. Nano Lett. 2002, 2, 725–728. [Google Scholar] [CrossRef]
- Jin, M.H.; Shin, E.; Jin, S.; Jo, H.; Min, K.; Hong, J. Solvothermal synthesis of ferroelectric BaTiO3 nanoparticles and their application to dye-sensitized solar cells. J. Korean Phys. Soc. 2018, 73, 627–631. [Google Scholar] [CrossRef]
- Xing, G.; Huang, M.; Hao, S.; He, C.; Li, X.; Fan, L.; Li, Y. One-pot and high-yield preparation of ultrathin β-PbO nanowires and nanosheets for high-capacity positive electrodes in lead-acid batteries. J. Alloys Compd. 2020, 831, 154845–154855. [Google Scholar] [CrossRef]
- Gillot, S.; Dacquin, J.P.; Dujardin, C.; Tricot, G.; Vezin, H.; Granger, P. Impact of thermal aging on the SCR performance of tungsten doped CeVO4 mixed oxides. Top. Catal. 2018, 62, 49–55. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, H.; Sharafudeen, K.; Dai, W.; Liu, Y.; Dong, G.; Qiu, J. Up-conversion luminescence from single vanadate through blackbody radiation harvesting broadband near-infrared photons for photovoltaic cells. J. Alloys Compd. 2016, 663, 204–210. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Namuangruk, S.; Hu, H.; Hu, X.; Shia, L.; Zhang, D. Morphology-dependent performance of Zr-CeVO4/TiO2 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 5543–5553. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the roadmap for photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Huang, B.; Lia, Y.; Zhang, S.; Zhang, Y.; Hua, X.; Liu, G.; Li, B.; Zhou, J.; Xie, E.; et al. Methanol gas detection of electrospun CeO2 nanofibers by regulating Ce3+/ Ce4+ mole ratio via Pd doping. Sens. Actuat. B Chem. 2020, 307, 127638. [Google Scholar] [CrossRef]
- Meltsner, M.; Wohlberg, C.; Kleiner, M.J. Reduction of organic compounds by ethanolamines. J. Am. Chem. Soc. 1935, 57, 2554. [Google Scholar] [CrossRef]
- Pratt, H.D., III; Rose, A.J.; Staiger, C.L.; Ingersoll, D.; Anderson, T.M. Synthesis and characterization of ionic liquids containing copper, manganese, or zinc coordination cations. Dalton Trans. 2011, 40, 11396–11401. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; He, Y.; Li, X.; Duan, M.; Tang, J.; Wang, Y.; Chamas, M.; Wang, H. Adjustable band position of strontium titanate by doping-free solvents effect and its correlation with photodegradation performance. J. Mater. Sci. Mater. Electron. 2017, 28, 14981–14987. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Kadirov, Z.C.; Makinose, Y.; Zhu, G.; Emin, S.; Matsushit, N.; Hasegawa, M.; Okada, K. Involving CeVO4 in improving the photocatalytic activity of a Bi2WO6/allophane composite for the degradation of gaseous acetaldehyde under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 600–612. [Google Scholar] [CrossRef]
- Ye, T.; Huang, W.; Zeng, L.; Li, M.; Shi, J. CeO2-x platelet from monometallic cerium layered double hydroxides and its photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 210, 141–148. [Google Scholar] [CrossRef]
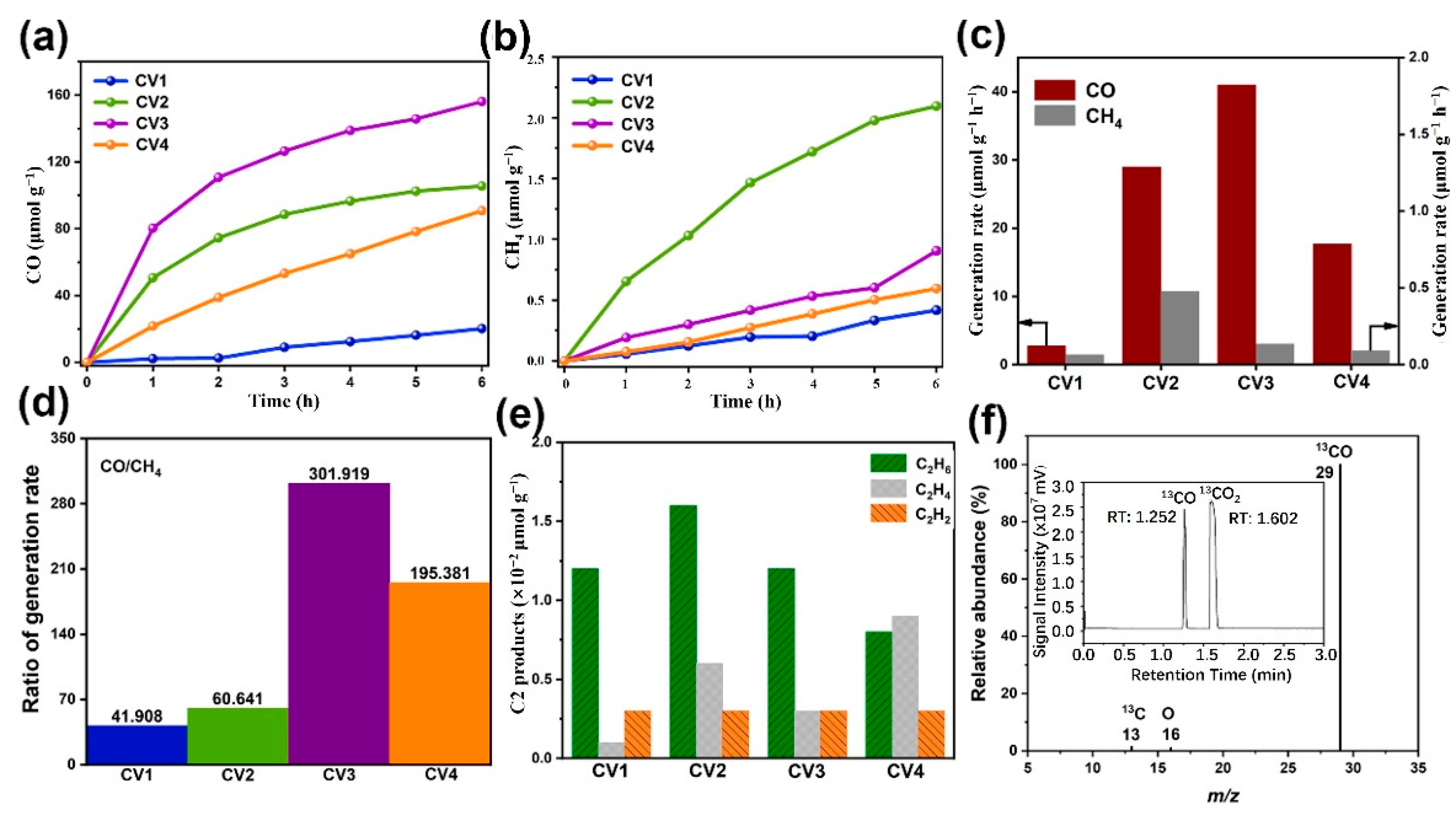
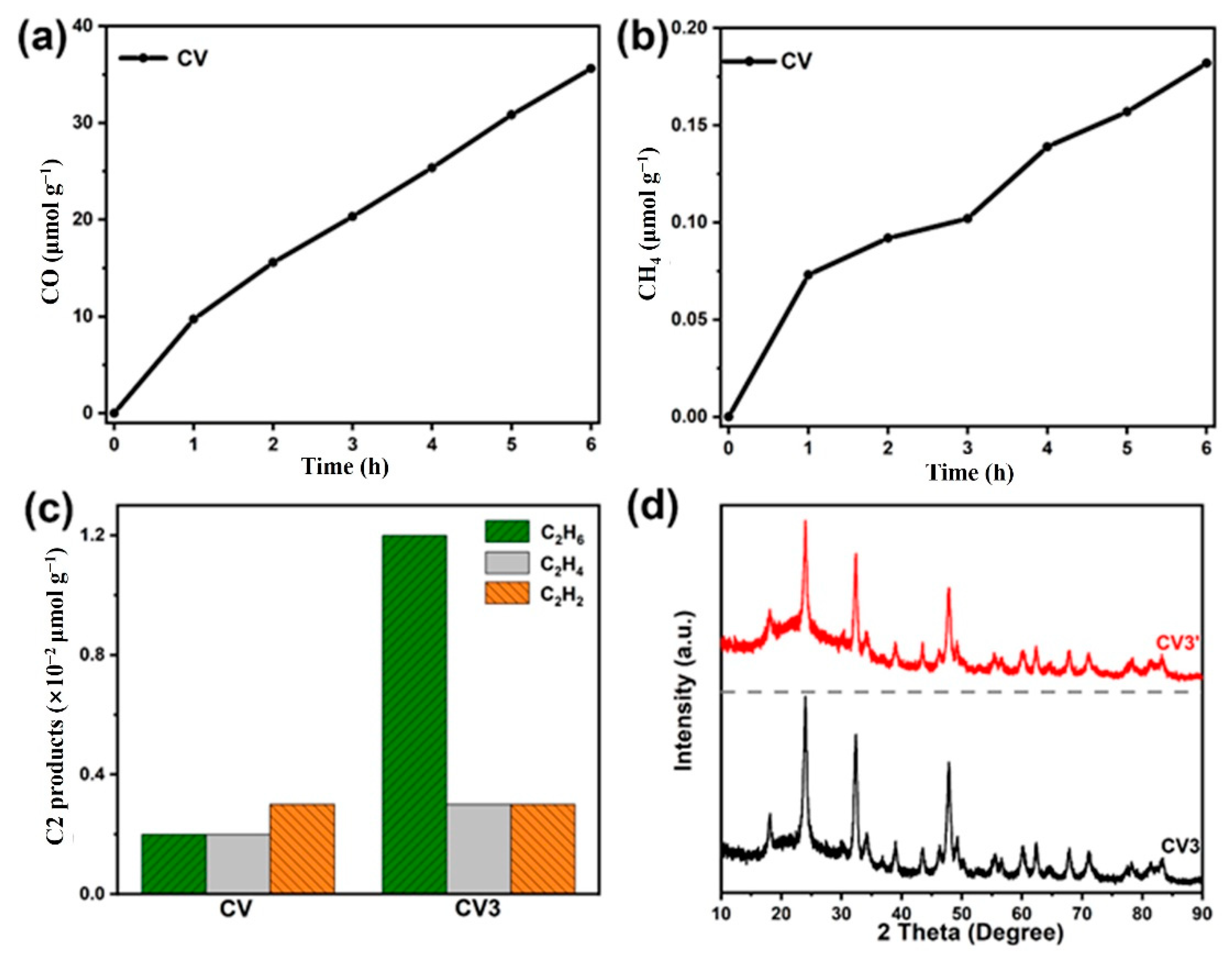
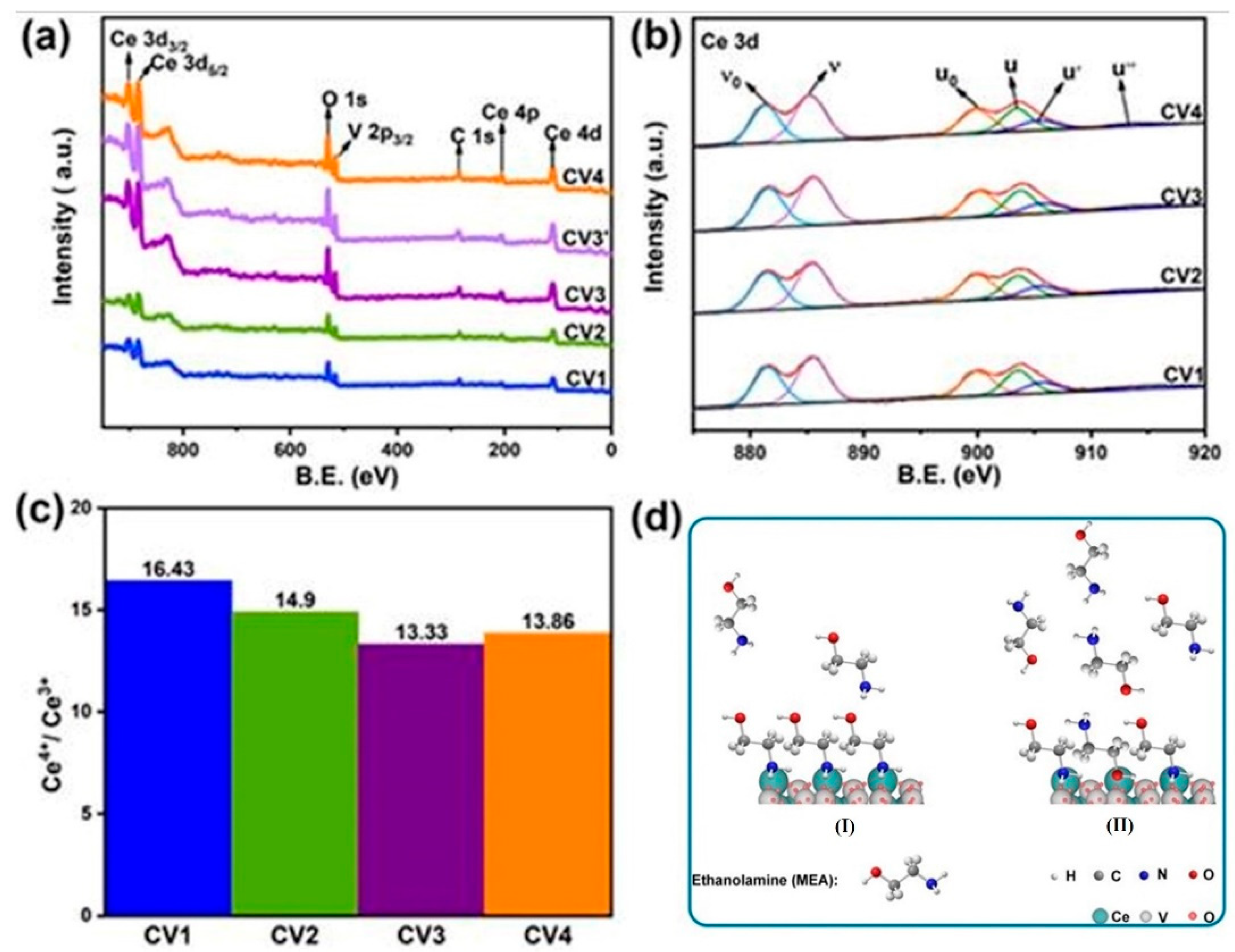

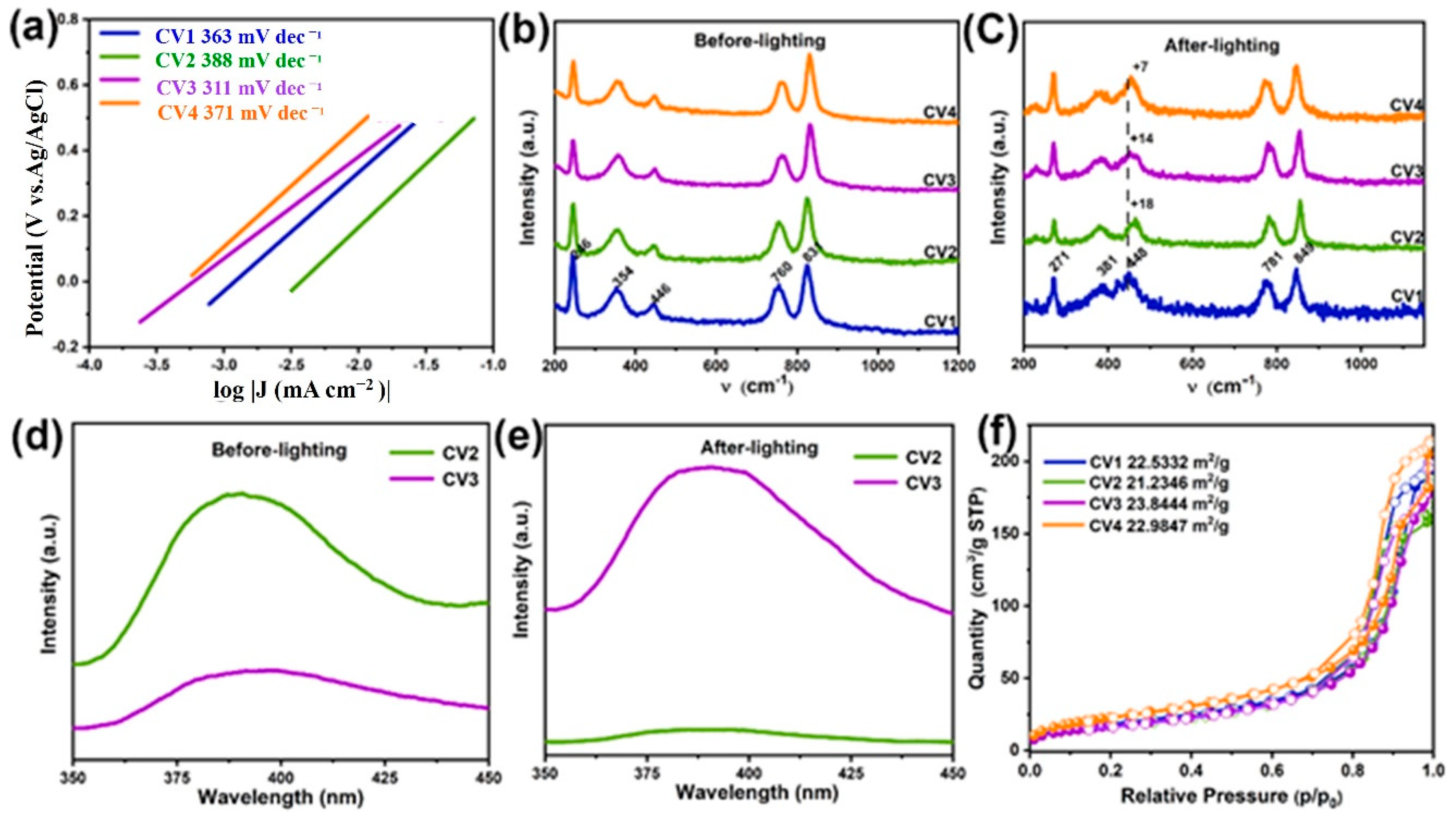
| Samples | CO (μmol g−1h−1) | CH4 (μmol g−1h−1) | CO/CH4 |
|---|---|---|---|
| CV | 6.68 | 0.0325 | 205.61 |
| CV1 | 2.75 | 0.0655 | 41.91 |
| CV2 | 28.94 | 0.477 | 60.64 |
| CV3 | 40.97 | 0.136 | 301.92 |
| CV4 | 17.68 | 0.0905 | 195.38 |
| Raman Peaks | Group | Raman Vibrations Modes |
|---|---|---|
| 831 cm−1 | -VO43− | symmetric stretching (A1g) |
| 760 cm−1 | -VO43− | anti-symmetric stretching (B1g) |
| 446 cm−1 | -VO43− | bending mode (B1g) |
| 354 cm−1 | -VO43− | bending mode (A1g) |
| below 250 cm−1 | Ce-VO4 | external mode (Eg) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Han, Q.; Lu, H.; Yang, Y.; Lu, G.; Zhang, H.; Ran, X.; Xia, Y.; Li, P.; Chen, Y.; et al. Valence Regulation of Ultrathin Cerium Vanadate Nanosheets for Enhanced Photocatalytic CO2 Reduction to CO. Catalysts 2021, 11, 1115. https://doi.org/10.3390/catal11091115
Ding L, Han Q, Lu H, Yang Y, Lu G, Zhang H, Ran X, Xia Y, Li P, Chen Y, et al. Valence Regulation of Ultrathin Cerium Vanadate Nanosheets for Enhanced Photocatalytic CO2 Reduction to CO. Catalysts. 2021; 11(9):1115. https://doi.org/10.3390/catal11091115
Chicago/Turabian StyleDing, Lujia, Qiutong Han, Hong Lu, Yong Yang, Gang Lu, Hui Zhang, Xueqin Ran, Yingdong Xia, Ping Li, Yonghua Chen, and et al. 2021. "Valence Regulation of Ultrathin Cerium Vanadate Nanosheets for Enhanced Photocatalytic CO2 Reduction to CO" Catalysts 11, no. 9: 1115. https://doi.org/10.3390/catal11091115
APA StyleDing, L., Han, Q., Lu, H., Yang, Y., Lu, G., Zhang, H., Ran, X., Xia, Y., Li, P., Chen, Y., & Zhou, Y. (2021). Valence Regulation of Ultrathin Cerium Vanadate Nanosheets for Enhanced Photocatalytic CO2 Reduction to CO. Catalysts, 11(9), 1115. https://doi.org/10.3390/catal11091115







