Recent Development in Sludge Biochar-Based Catalysts for Advanced Oxidation Processes of Wastewater
Abstract
:1. Introduction
2. Preparation of Sludge Biochar-Based Catalysts
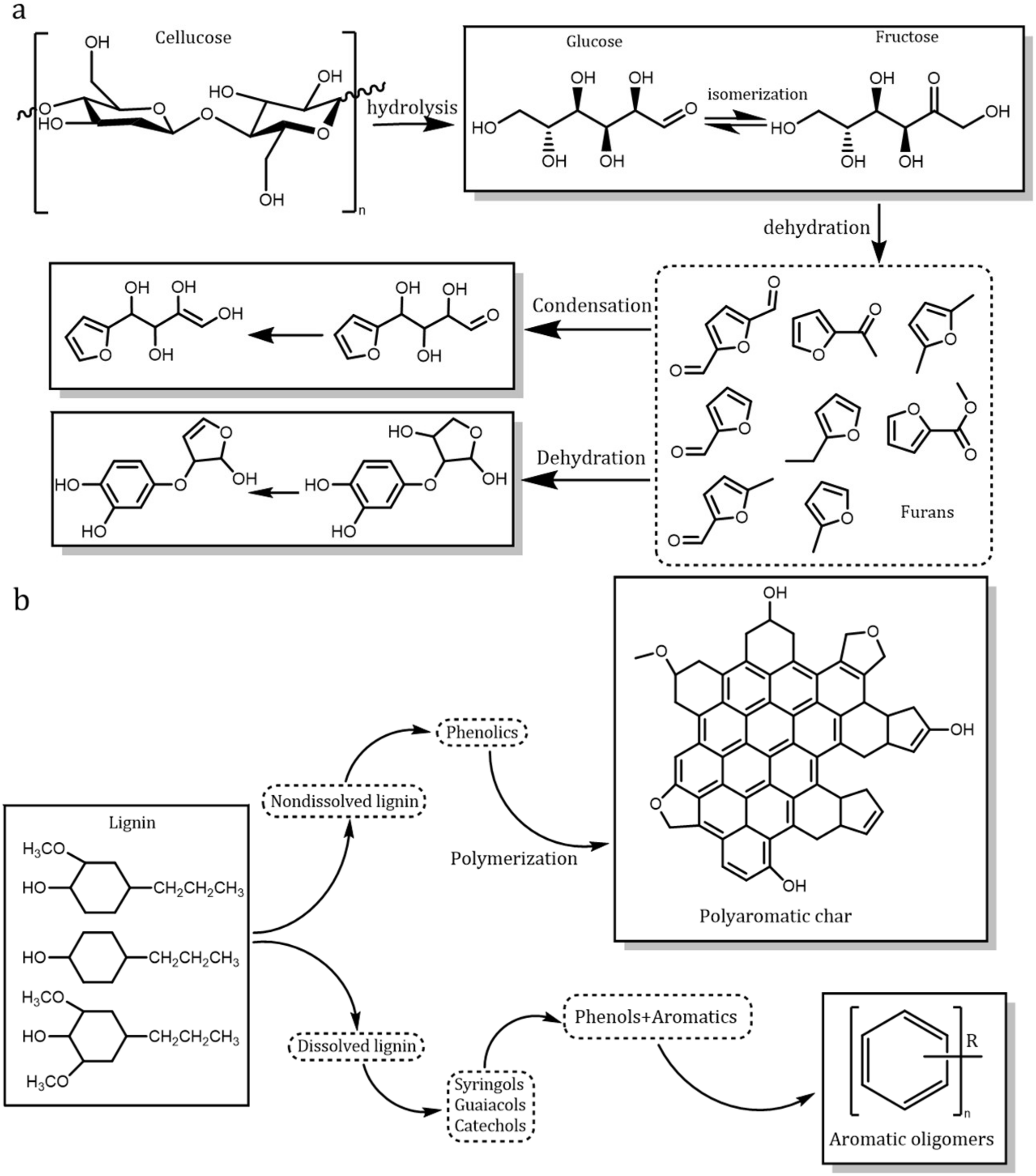
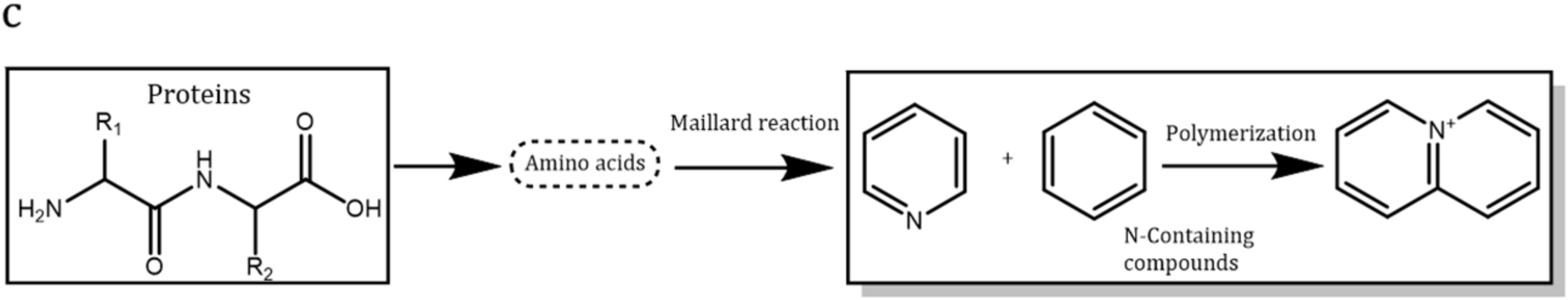
2.1. Sludge Components
2.2. Preparation Methods
2.2.1. Pyrolysis
2.2.2. Hydrothermal Carbonization
2.3. Dopants
3. Influence Parameters in AOPs
3.1. Initial Solution pH
3.2. Catalyst Dosage
3.3. Reaction Temperature
3.4. Coexisting Anions
4. Application in AOPs
4.1. Application in Sulfate-Based AOPs
4.1.1. Pure Sludge-Derived Biochar Catalysts
4.1.2. Fe-Based Catalysts
4.1.3. Mn-Based Catalysts
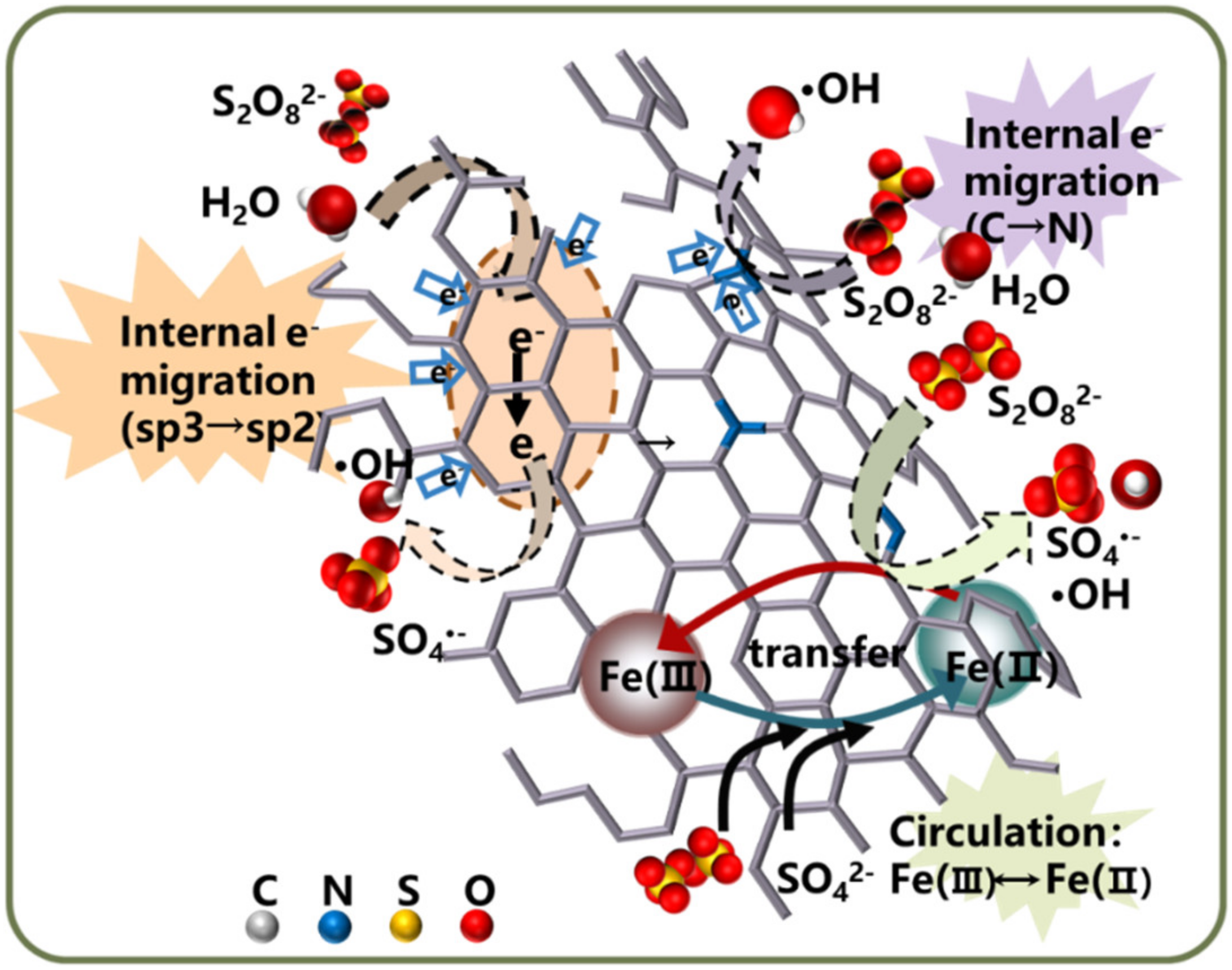
4.1.4. Heteroatom-Doped Hybrid Catalysts
4.1.5. Multimetallic Catalysts
4.2. Application in Fenton-like AOPs
4.2.1. Fenton Oxidation Process
4.2.2. Fe-Based Catalysts
4.2.3. Other Metals-Based Catalysts
4.2.4. Photo-Fenton Process
4.3. Application in Photocatalysis
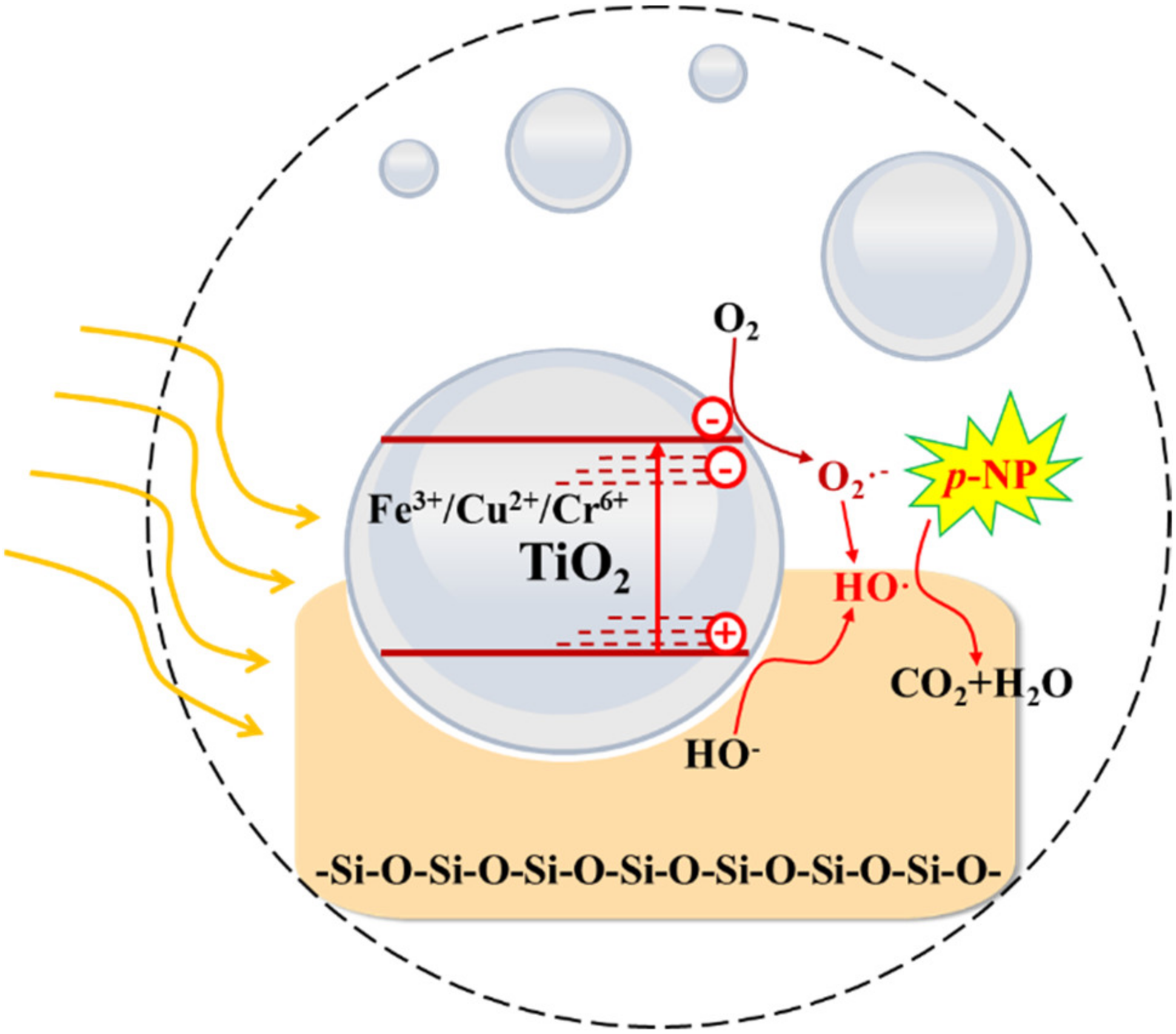
4.3.1. TiO2-Based Catalysts
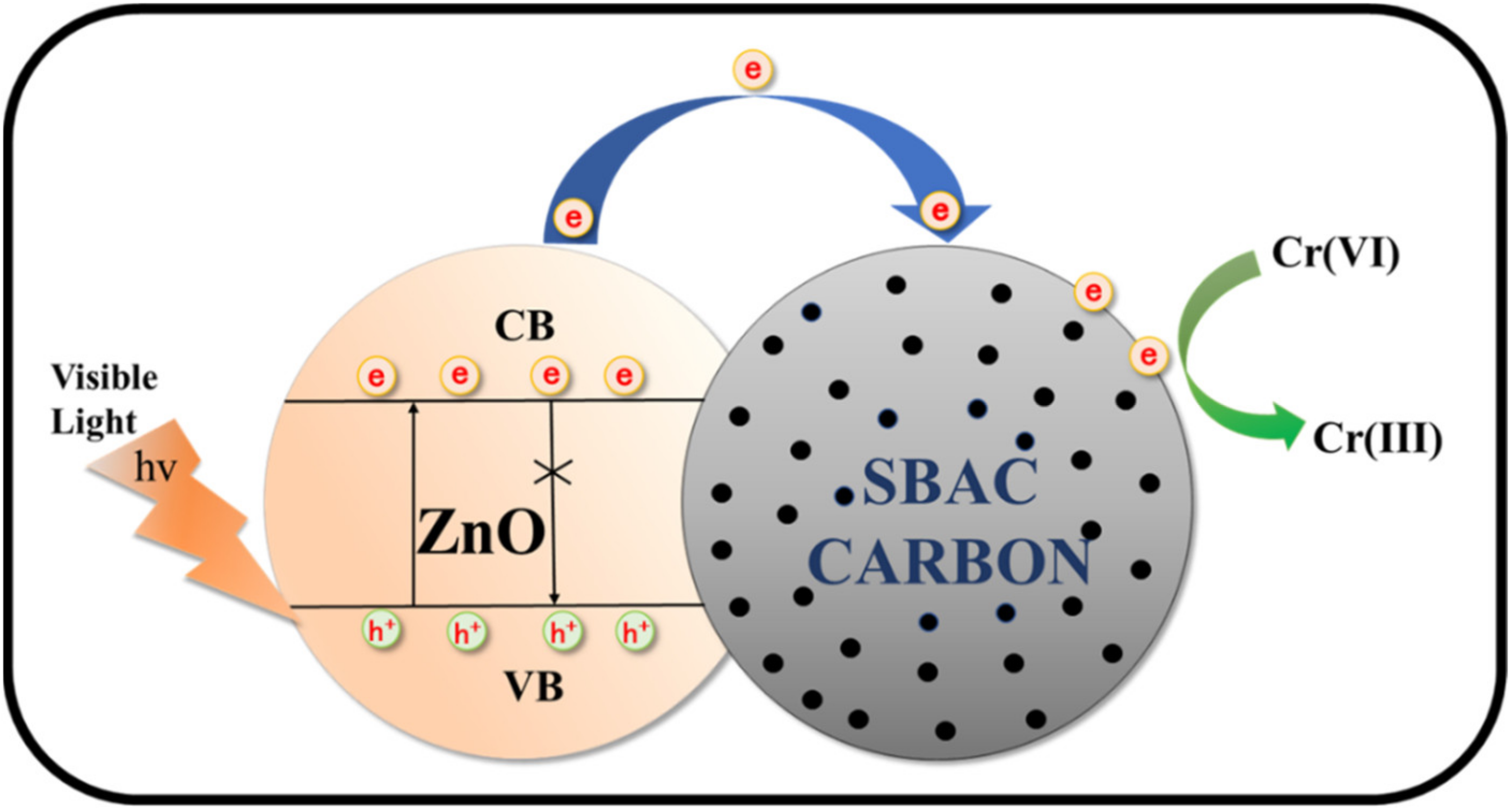
4.3.2. ZnO-Based Catalysts
4.3.3. Graphitic Carbon Nitride-Based Catalysts
4.4. Application in Ozonation
5. Costs and Viability
6. Conclusions and Prospects
6.1. Conclusions
6.2. Prospects
- When sludge biochar-based catalysts are repeated several times, catalytic activity may diminish, which is ascribed to the decrease of active sites and defect intensity as catalysts surface encounters the dilemma of being oxidized. Therefore, future sludge-based catalysts synthesis should focus on effectual modification methods and feasible dopants in order to strengthen the reusability and stability of sludge biochar-based catalysts;
- Few studies have concentrated on addressing the real wastewater treatment with sludge biochar-based catalysts in AOPs. Future investigations on catalytic efficiency in actual effluent are required;
- The mechanism involved is quite complicated. Understanding the correlation between sludge biochar-based catalysts, different parts and real wastewater components such as hydrophilic and hydrophobic organic matter will give direction for the synthesis or modification of sludge biochar-based catalysts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.M.; Gong, X.M.; Duan, A.; Zhang, Q.; et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Feng, L.Y.; Luo, J.Y.; Chen, Y.G. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.P.; Ma, X.Q.; Yao, Z.L.; Chen, X.F. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Kwon, E.E.; Kim, S.; Jeon, Y.J.; Yi, H. Biodiesel production from sewage sludge: New paradigm for mining energy from municipal hazardous material. Environ. Sci. Technol. 2012, 46, 10222–10228. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Wang, X.L.; Zhang, Q. Microwave-assisted pyrolysis of sewage sludge: A review. Fuel Process Technol. 2019, 187, 84–104. [Google Scholar] [CrossRef]
- Luo, J.Y.; Zhu, Y.; Zhang, Q.; Cao, M.; Guo, W.; Li, H.; Wu, Y.; Wang, H.; Su, Y.L.; Cao, J.S. Promotion of short-chain fatty acids production and fermented sludge properties via persulfate treatments with different activators: Performance and mechanisms. Bioresour. Technol. 2020, 295, 122278. [Google Scholar] [CrossRef]
- Zhang, H.X.; Song, Y.Y.; Nengzi, L.C.; Gou, J.F.; Li, B.; Cheng, X.W. Activation of persulfate by a novel magnetic CuFe2O4/Bi2O3 composite for lomefloxacin degradation. Chem. Eng. J. 2020, 379, 122362. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Zhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.S.; Zhang, Y.; Yu, J.Y.; Yuan, M.; Ma, Y.Q. Simultaneous photodegradation of multi-herbicides by oxidized carbon nitride: Performance and practical application. Appl. Catal. B 2017, 219, 194–199. [Google Scholar] [CrossRef]
- Guan, K.; Zhou, P.J.; Zhang, J.Y.; Zhu, L.L. Synthesis and characterization of ZnO@RSDBC composites and their Photo-Oxidative degradation of Acid Orange 7 in water. J. Mol. Struct. 2020, 1203, 127425. [Google Scholar] [CrossRef]
- Osegueda, O.; Dafinov, A.; Llorca, J.; Medina, F.; Sueiras, J. Heterogeneous catalytic oxidation of phenol by in situ generated hydrogen peroxide applying novel catalytic membrane reactors. Chem. Eng. J. 2015, 262, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Guan, K.; Zhou, P.J.; Zhang, J.Y.; Zhu, L.L. Catalytic degradation of Acid Orange 7 in water by persulfate activated with CuFe2O4@RSDBC. Mater. Res. Express 2020, 7, 016529. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Hu, P.D.; Long, M.C. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and application. Appl. Catal. B 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Singh, S.; Garg, A. Characterisation and utilization of steel industry waste sludge as heterogeneous catalyst for the abatement of chlorinated organics by advanced oxidation processes. Chemosphere 2020, 242, 125158. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.Q.; Yang, Y.; Han, Y.T.; Wang, T.S.; Chen, J.W.; Tsang, D.C.W. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, F.; Chen, H.Y.; Wang, H.J.; Li, G.G. Fe2O3/TiO2 functionalized biochar as a heterogeneous catalyst for dyes degradation in water under Fenton processes. J. Environ. Chem. Eng. 2020, 8, 103905. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Xiao, R.; Tafti, N.; DeLaune, R.D.; Seo, D.C. Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst. Bioresour. Technol. 2018, 249, 368–376. [Google Scholar] [CrossRef]
- Lyu, H.H.; Zhang, Q.R.; Shen, B.X. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2019, 391, 123499. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Wu, K.Y.; Zhou, H.T.; Hu, Y.; Sergei, P.; Wu, H.Z.; Wei, C.H. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater. J. Environ. Manage. 2018, 224, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yan, X.; Xu, Y.Y.; Yoza, B.A.; Wang, X.; Kou, Y.; Ye, H.F.; Wang, Q.H.; Li, Q.X. Activated petroleum waste sludge biochar for efficient catalytic ozonation of refinery wastewater. Sci. Total Environ. 2019, 651, 2631–2640. [Google Scholar] [CrossRef]
- Zhu, X.F.; Yuan, W.Y.; Lang, M.Q.; Zhen, G.Y.; Zhang, X.D.; Lu, X.Q. Novel methods of sewage sludge utilization for photocatalytic degradation of tetracycline-containing wastewater. Fuel 2019, 252, 148–156. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhu, Z.Y.; Shen, B.X.; Liu, L.N. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Zielinska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenerg. 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Ran, C.M.; Liu, Y.; Siddiqui, A.R.; Siyal, A.A.; Mao, X.; Kang, Q.H.; Fu, J.; Ao, W.Y.; Dai, J.J. Pyrolysis of textile dyeing sludge in fluidized bed: Analysis of products, and migration and distribution of heavy metals. J. Clean. Prod. 2019, 241, 118308. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.D.; Sun, L.Z.; Xie, X.P.; Yang, S.X.; Mei, N. Study on the fast pyrolysis of oil sludge by PY-GC/MS. Petrol. Sci. Technol. 2019, 37, 2108–2113. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.R.; Wang, Q.J.; Cui, Z.L.; Wang, L. Pyrolysis characteristics, kinetics, and evolved gas determination of chrome-tanned sludge by thermogravimetry–Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry. Waste Manage. 2019, 93, 130–137. [Google Scholar] [CrossRef]
- Yu, J.F.; Tang, L.; Pang, Y.; Zeng, G.M.; Wang, J.J.; Deng, Y.C.; Liu, Y.N.; Feng, H.P.; Chen, S.; Ren, X.Y. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Wang, X.P.; Gu, L.; Zhou, P.; Zhu, N.W.; Li, C.X.; Tao, H.; Wen, H.F.; Zhang, D.F. Pyrolytic temperature dependent conversion of sewage sludge to carbon catalyst and their performance in persulfate degradation of 2-Naphthol. Chem. Eng. J. 2017, 324, 203–215. [Google Scholar] [CrossRef]
- Huang, B.C.; Jiang, J.; Huang, G.X.; Yu, H.Q. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. A 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Wen, G.; Pan, Z.H.; Ma, J.; Liu, Z.Q.; Zhao, L.; Li, J.J. Reuse of sewage sludge as a catalyst in ozonation—Efficiency for the removal of oxalic acid and the control of bromate formation. J. Hazard. Mater. 2012, 239–240, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Sun, Y.R.; Xu, Z.H.; Luo, M.Y.; Zhu, C.L.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Wang, W.; Xu, Y.P.; Zhu, Z.G.; Liu, Z.Q.; Cu, F.Y. Iron sludge-derived magnetic Fe0/Fe3C catalyst for oxidation of ciprofloxacin via peroxymonosulfate activation. Chem. Eng. J. 2019, 365, 99–110. [Google Scholar] [CrossRef]
- Chen, Y.D.; Bai, S.W.; Li, R.X.; Su, G.Y.; Duan, X.G.; Ren, N.Q.; Ho, S.H. Magnetic biochar catalysts from anaerobic digested sludge: Production, application and environment impact. Environ. Int. 2019, 126, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Duan, X.G.; Zhang, C.F.; Wang, S.B.; Ren, N.Q.; Ho, S.H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar] [CrossRef]
- Huang, Y.F.; Huang, Y.Y.; Chiueh, P.T.; Lo, S.L. Heterogeneous Fenton oxidation of trichloroethylene catalyzed by sewage sludge biochar: Experimental study and life cycle assessment. Chemosphere 2020, 249, 126139. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.A.; Zhai, Y.B.; Hornung, A.; Wang, B.; Li, S.H.; Wang, T.F.; Li, C.T.; Zhu, Y. In-depth comparison of morphology, microstructure, and pathway of char derived from sewage sludge and relevant model compounds. Waste Manage. 2020, 102, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel. Bioprod. Bior. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Xu, Z.X.; Song, H.; Li, P.J.; He, Z.X.; Wang, Q.; Wang, K.; Duan, P.G. Hydrothermal carbonization of sewage sludge: Effect of aqueous phase recycling. Chem. Eng. J. 2019, 387, 123410. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, G.; Chen, H.; Li, X. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment. Chemosphere 2018, 191, 64–71. [Google Scholar] [CrossRef]
- Zhai, Y.B.; Peng, C.; Xu, B.B.; Wang, T.F.; Li, C.T.; Zeng, G.M.; Zhu, Y. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, G.; Chen, H.; Li, X. Hydrothermal synthesizing sludge-based magnetite catalyst from ferric sludge and biosolids: Formation mechanism and catalytic performance. Sci. Total Environ. 2019, 697, 133986. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.G.; Sun, H.Q.; Liu, S.M.; Tade, M.O.; Wang, S.B. N-doped graphene from metal–organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.J.; Fu, B.; Song, Y. Facile synthesis of sludge-derived MnOx-N-biochar as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B 2019, 255, 117765. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, S.B. Metal-free carbocatalysis in advanced oxidation reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.T.; Lee, J.H.; Kim, J.; Park, H.D.; Lee, J. Identifying the nonradical mechanism in the peroxymonosulfate activation process: Singlet oxygenation versus mediated eectron transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.J. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2019, 392, 123681. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.J. Sewage sludge-derived TiO2/Fe/Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chem. Eng. J. 2019, 215, 101–114. [Google Scholar] [CrossRef]
- Wang, J.; Kou, L.D.; Zhao, L.; Duan, W.J. One-pot fabrication of sludge-derived magnetic Fe,N-codoped carbon catalysts for peroxymonosulfate-induced elimination of phenolic contaminants. Chemosphere 2020, 248, 126076. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Wang, J.L. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Kong, L.J.; Zhu, Y.T.; Liu, M.X.; Chang, X.Y.; Xiong, Y.; Chen, D.Y. Conversion of Fe-rich waste sludge into nano-flake Fe-SC hybrid Fenton-like catalyst for degradation of AOII. Environ. Pollut. 2016, 216, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.F.; Han, H.J.; Jia, S.Y.; Hou, B.L.; Zhao, Q. Advanced treatment of biologically pretreated coal gasification wastewater by a novel integration of heterogeneous catalytic ozonation and biological process. Bioresour. Technol. 2014, 166, 592–595. [Google Scholar] [CrossRef]
- Zhu, S.J.; Xu, Y.P.; Zhu, Z.G.; Liu, Z.Q.; Wang, W. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation. Chem. Eng. J. 2020, 384, 123298. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.Q.; Zhang, H.G.; Tong, X.W.; Feng, J.N. Fabrication of sewage sludge-derived magnetic nanocomposites as heterogeneous catalyst for persulfate activation of Orange G degradation. Colloid. Surface. A 2017, 529, 856–863. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, F.; He, Y.; Liu, X.Y.; Song, C.J.; Xu, Y.H.; Zhang, Y.J. Heterogeneous fenton-like degradation of ofloxacin over sludge derived carbon as catalysts: Mechanism and performance. Sci. Total Environ. 2019, 654, 942–947. [Google Scholar] [CrossRef]
- Liu, J.J.; Diao, Z.H.; Liu, C.M.; Jiang, D.; Kong, L.J.; Xu, X.R. Synergistic reduction of copper (II) and oxidation of norfloxacin over a novel sewage sludge-derived char-based catalyst: Performance, fate and mechanism. J. Clean. Prod. 2018, 182, 794–804. [Google Scholar] [CrossRef]
- Zhuang, H.F.; Han, H.J.; Hou, B.L.; Jia, S.Y.; Zhao, Q. Heterogeneous catalytic ozonation of biologically pretreated Lurgi coal gasification wastewater using sewage sludge based activated carbon supported manganese and ferric oxides as catalysts. Bioresour. Technol. 2014, 166, 178–186. [Google Scholar] [CrossRef]
- Xiao, J.D.; Xie, Y.B.; Cao, H.B. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef]
- Mesquita, I.; Matos, L.C.; Duarte, F.; Maldonado-Hódar, F.J.; Mendes, A.; Madeira, L.M. Treatment of azo dye-containing wastewater by a Fenton-like process in a continuous packed-bed reactor filled with activated carbon. J. Hazard. Mater. 2012, 237–238, 30–37. [Google Scholar] [CrossRef]
- Meng, G.H.; Liu, B.H.; Sun, M.; Miao, Q.Q.; Ding, S.Y.; Zhang, J.L.; Liu, Z.L. Sludge-based activated carbon catalyzed H2O2 oxidation of reactive azo dyes. Environ. Technol. 2021, 42, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents-Application of mesoporous materials: A review. J. Environ. Manage. 2018, 211, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.F.; Liu, Y.; Yang, X.; Liang, Q.H. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yuan, R.X.; Guo, Y.G.; Xu, L.; Liu, J.S. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone reagent: Kinetic analysis. J. Hazard. Mater. 2011, 190, 1083–1087. [Google Scholar] [CrossRef]
- Kiwi, J.; Lopez, A.; Nadtochenko, V. Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger(Cl-). Environ. Sci. Technol. 2000, 34, 2162–2168. [Google Scholar] [CrossRef]
- Bacardit, J.; Stotzner, J.; Chamarro, E.; Esplugas, S. Effect of salinity on the photo-Fenton process. Ind. Eng. Chem. Res. 2007, 46, 7615–7619. [Google Scholar] [CrossRef]
- Hu, W.R.; Xie, Y.; Lu, S.; Li, P.Y.; Xie, T.H.; Zhang, Y.K.; Wang, Y.B. One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci. Total Environ. 2019, 680, 51–60. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.T.; Zhu, Y.H.; Li, J. Enhanced catalytic degradation of tetracycline antibiotic by persulfate activated with modified sludge bio-hydrochar. Chemosphere 2020, 247, 125854. [Google Scholar] [CrossRef]
- Li, D.Y.; Zhu, J.S.; Wu, J.H.; Yin, W.Z.; Liang, H.; Lin, G.H. Development of an activated carbon-supported zero-valent iron catalyst (AC-Fe0) for enhancing degradation of reactive brilliant orange and reducing iron sludge production. Environ. Prog. Sustain. 2016, 35, 949–956. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Gong, Q.; Wang, X.H.; Cai, J.Y.; Wang, S.L.; Chen, Z.Q. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation. Sci. Total Environ. 2020, 714, 136728. [Google Scholar] [CrossRef]
- Yun, E.T.; Yoo, H.Y.; Bae, H.; Kim, H.I.; Lee, J. Exploring the role of persulfate in the activation process: Radical precursor versus electron acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Wang, J.L. Kinetics of PMS activation by graphene oxide and biochar. Chemosphere 2020, 239, 124812. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.Y.; Lu, X.Q.; Su, L.H.; Kobayashi, T.; Kumar, G.; Zhou, T.; Xu, K.Q.; Li, Y.Y.; Zhu, X.F.; Zhao, Y.C. Unraveling the catalyzing behaviors of different iron species (Fe2+ vs. Fe0) in activating persulfate-based oxidation process with implications to waste activated sludge dewaterability. Water Res. 2018, 134, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Stathatos, E.; Dionysiou, D.D. Heterogeneous activation of oxone using Co3O4. J. Phys. Chem. B 2005, 109, 13052–13055. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Lyu, L.; Yu, G.F.; Zhang, L.L.; Hu, C.; Sun, Y. 4-phenoxyphenol-functionalized reduced graphene oxide nanosheets: A metal-free Fenton-like catalyst for pollutant destruction. Environ. Sci. Technol. 2018, 52, 747–756. [Google Scholar] [CrossRef]
- Voitko, K.; Tóth, A.; Demianenko, E.; Dobos, G.; Berke, B.; Bakalinska, O.; Grebenyuk, A.; Tombácz, E.; Kuts, V.; Tarasenko, Y.; et al. Catalytic performance of carbon nanotubes in H2O2 decomposition: Experimental and quantum chemical study. J. Colloid interf. Sci. 2015, 437, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.T.; Tian, S.H.; Kong, L.J.; Xiong, Y. Co-catalytic effect of sewage sludge-derived char as the support of Fenton-like catalyst. Chem. Eng. J. 2012, 185–186, 44–51. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.J.; Yu, G.W.; Xie, S.Y.; Li, C.X.; Lai, D.G.; Li, Z.W.; You, F.T.; Wang, Y. The synthesis of heterogeneous Fenton-like catalyst using sewage sludge biochar and its application for ciprofloxacin degradation. Sci. Total Environ. 2019, 654, 1284–1292. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, N.W.; Zhou, P. Preparation of sludge derived magnetic porous carbon and their application in Fenton-like degradation of 1-diazo-2-naphthol-4-sulfonic acid. Bioresour. Technol. 2012, 118, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.F.; Gu, L.; Yu, H.X.; Qiao, X.B.; Zhang, D.F.; Ye, J.F. Radical assisted iron impregnation on preparing sewage sludge derived Fe/carbon as highly stable catalyst for heterogeneous Fenton reaction. Chem. Eng. J. 2018, 352, 837–846. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.X.; Wen, Z.P.; Fida, H.; Zhang, G.K.; Chen, J.Y. Reutilization of iron sludge as heterogeneous Fenton catalyst for the degradation of rhodamine B: Role of sulfur and mesoporous structure. J. Colloid Interf. Sci. 2018, 532, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, W.J.; Liao, G.Y.; Chen, F.F.; Wang, D.S. A novel waste activated sludge multistage utilization strategy for preparing carbon-based Fenton-like catalysts: Catalytic performance assessment and micro-interfacial mechanisms. Water Res. 2019, 150, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.G.; Ou, C.J.; Faheem, M.; Shen, J.Y.; Yu, H.X.; Jiao, Z.H.; Han, W.Q.; Sun, X.Y.; Li, J.S.; et al. Reuse of Fenton sludge as an iron source for NiFe2O4 synthesis and its application in the Fenton-based process. J. Environ. Sci. 2017, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.J.; Liao, N.H.; Dong, B.; Dai, X.H. Optimization of a digested sludge-derived mesoporous material as an efficient and stable heterogeneous catalyst for the photo-Fenton reaction. Chinese J. Catal. 2016, 37, 735–742. [Google Scholar] [CrossRef]
- Laib, S.; Yazid, H.R.; Guendouz, N.; Belmedani, M.; Sadaoui, Z. Heterogeneous Fenton catalyst derived from hydroxide sludge as an efficient and reusable catalyst for anthraquinone dye degradation. Sep. Sci. Technol. 2019, 54, 1338–1352. [Google Scholar] [CrossRef]
- Zhou, G.Q.; Chen, Z.W.; Fang, F.; He, Y.F.; Sun, H.L.; Shi, H.X. Fenton-like degradation of Methylene Blue using paper mill sludge-derived magnetically separable heterogeneous catalyst: Characterization and mechanism. J. Environ. Sci. 2015, 35, 20–26. [Google Scholar] [CrossRef]
- Zhuang, H.F.; Han, H.J.; Ma, W.C.; Hou, B.L.; Jia, S.Y.; Zhao, Q. Advanced treatment of biologically pretreated coal gasification wastewater by a novel heterogeneous Fenton oxidation process. J. Environ. Sci. 2015, 33, 12–20. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, N.; Zhang, G.K.; Yu, J.C. Graphene modified iron sludge derived from homogeneous Fenton process as an efficient heterogeneous Fenton catalyst for degradation of organic pollutants. Micropor. Mesopor. Mat. 2017, 238, 62–68. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.M.; Huang, T.; Chong, S.; Liu, Y.C. Fe3O4 and Fe3O4/Fe2+/Fe0 catalyzed Fenton-like process for advanced treatment of pharmaceutical wastewater. Desalin. Water Treat. 2017, 93, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Vilardi, G.; Palma, L.D.; Verdone, N. On the critical use of zero valent iron nanoparticles and Fenton processes for the treatment of tannery wastewater. J. Water Process Eng. 2018, 22, 109–122. [Google Scholar] [CrossRef]
- Faheem, M.; Jiang, X.B.; Wang, L.J.; Shen, J.Y. Synthesis of Cu2O-CuFe2O4 microparticles from Fenton sludge and its application in the Fenton process: The key role of Cu2O in the catalytic degradation of phenol. RSC Adv. 2018, 8, 5740–5748. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.Y.; Liu, J.; Shan, N.; Zhang, H.; Dionysiou, D.D. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of Orange II in water. Appl. Catal. B 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Tu, Y.T.; Xiong, Y.; Descorme, C.; Kong, L.J.; Tian, S.H. Heterogeneous photo-Fenton oxidation of Acid Orange II over iron-sewage sludge derived carbon under visible irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 544–551. [Google Scholar] [CrossRef]
- Yuan, S.J.; Dai, X.H. Facile synthesis of sewage sludge-derived mesoporous material as an efficient and stable heterogeneous catalyst for photo-Fenton reaction. Appl. Catal. B 2014, 154, 252–258. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhang, Y.X.; Wang, H.T.; Chen, T.; Lu, W.J. Enhanced photodegradation of pentachlorophenol in a soil washing system under solar irradiation with TiO2 nanorods combined with municipal sewage sludge. Micropor. Mesopor. Mat. 2015, 201, 99–104. [Google Scholar] [CrossRef]
- Yuan, S.J.; Li, X.W.; Dai, X.H. Efficient degradation of organic pollutants with a sewage sludge support and in situ doped TiO2 under visible light irradiation conditions. RSC Adv. 2014, 4, 61036–61044. [Google Scholar] [CrossRef]
- Wang, X.P.; Huang, S.Q.; Zhu, N.W.; Lou, Z.Y.; Yuan, H.P. Facile synthesis of porous TiO2 photocatalysts using waste sludge as the template. Appl. Surf. Sci. 2015, 359, 917–922. [Google Scholar] [CrossRef]
- Rashed, M.N.; Eltaher, M.A.; Abdou, A.N.A. Adsorption and photocatalysis for methyl orange and Cd removal from wastewater using TiO2/sewage sludge-based activated carbon nanocomposites. Roy. Soc. Open Sci. 2017, 4, 170834. [Google Scholar] [CrossRef] [Green Version]
- Ramya, V.; Murugan, D.; Lajapathirai, C.; Sivasamy, A. Activated carbon (prepared from secondary sludge biomass) supported semiconductor zinc oxide nanocomposite photocatalyst for reduction of Cr (VI) under visible light irradiation. J. Environ. Chem. Eng. 2018, 6, 7327–7337. [Google Scholar]
- Zhu, X.D.; Wang, Y.J.; Sun, R.J.; Zhou, D.M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Botia, D.C.; Rodriguez, M.S.; Sarria, V.M. Evaluation of UV/TiO2 and UV/ZnO photocatalytic systems coupled to a biological process for the treatment of bleaching pulp mill effluent. Chemosphere 2012, 89, 732–736. [Google Scholar] [CrossRef]
- Matos, J.; Rosales, M.; Garcia, A.; Nieto-Delgado, C.; Rangel-Mendez, J.R. Hybrid photoactive materials from municipal sewage sludge for the photocatalytic degradation of methylene blue. Green Chem. 2011, 13, 3431. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.J.; Yousaf, B.; Fu, B.; Ahmed, R.; Abbas, Q.; Munir, M.A.M.; Ruijia, L. One-step synthesis of N-doped metal/biochar composite using NH3-ambiance pyrolysis for efficient degradation and mineralization of Methylene Blue. J. Environ. Sci. 2019, 78, 29–41. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; You, J.G.; Tseng, W.B.; Dwivedi, G.D.; Rajesh, N.; Jiang, S.J.; Tseng, W.L. Magnetically separable nanospherical g-C3N4@Fe3O4 as a recyclable material for chromium adsorption and visible-light-driven catalytic reduction of aromatic nitro compounds. ACS Sustain. Chem. Eng. 2019, 7, 6662–6671. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, H.B.; Yoon, G.S.; Kim, S.H.; Min, S.J.; Tsang, D.C.W.; Baek, K. Simultaneous oxidation and adsorption of arsenic by one-step fabrication of alum sludge and graphitic carbon nitride (g-C3N4). J. Hazard. Mater. 2020, 383, 121138. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Liu, Y.Z.; Feng, L.; Sun, Z.G.; Zhang, L.Q. Characterization of ferromagnetic sludge-based activated carbon and its application in catalytic ozonation of p-chlorobenzoic acid. Environ. Sci. Pollut. Res. 2018, 25, 5086–5094. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.J.; Diao, Z.H.; Chang, X.Y.; Xiong, Y.; Chen, D.Y. Synthesis of recoverable and reusable granular MgO-SCCA-Zn hybrid ozonation catalyst for degradation of methylene blue. J. Environ. Chem. Eng. 2016, 4, 4385–4391. [Google Scholar] [CrossRef]
- Xu, J.L.; Yu, Y.; Ding, K.; Liu, Z.Y.; Wang, L.; Xu, Y.H. Heterogeneous catalytic ozonation of hydroquinone using sewage sludge-derived carbonaceous catalysts. Water Sci. Technol. 2018, 77, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Zhang, L.Q.; Qi, F.; Wang, X.; Li, L.; Feng, L. Removal performance and mechanism of ibuprofen from water by catalytic ozonation using sludge-corncob activated carbon as catalyst. J. Nanosci. Nanotechno. 2014, 14, 7266–7271. [Google Scholar] [CrossRef]
- Sun, Z.G.; Feng, L.; Liu, Y.Z.; Lu, S.Y.; Zhang, L.Q. Magnetic sludge-based activated carbon: Preparation, characterization, and application for catalytic ozonation of ibuprofen. Sci. Adv. Mater. 2017, 9, 2066–2072. [Google Scholar] [CrossRef]
- Ma, J.; Sui, M.H.; Zhang, T.; Guan, C.Y. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, B.; Oyama, S.T. Gas phase ozone decomposition catalysts. Appl. Catal. B 1997, 11, 129–166. [Google Scholar] [CrossRef]

| Parameters | Catalysts | Removal Capacity (RC) | AOPs | References |
|---|---|---|---|---|
| pH = 3.2 pH = 7.2 pH = 10.2 | SBC | RC(triclosan)~70% RC(triclosan) = 99.2% RC(triclosan) = 46.7% | sulfate-based AOPs | [52] |
| pH = 4 | Fe-SC | RC(AOII) = 99% | Fenton-like | [53] |
| pH = 3.1 | BC | RC(trichloroethylene) = 83% | Fenton-like | [38] |
| pH = 7 pH = 11 | MnOx/SBAC | RC(COD)~65% RC(COD)~75% | ozonation | [54] |
| Catalyst dosage: 0.1 g/L 0.3 g/L 1 g/L | SBC | RC(triclosan) = 16% RC(triclosan) = 67% RC(triclosan)~100% | sulfate-based AOPs | [52] |
| Catalyst dosage: 0.5 g/L 0.9 g/L 1.1 g/L | ZnO@RSDBC | RC(AO7) = 64,7%; RC(AO7) = 93.7%; RC(AO7) = 95.9% | photocatalysis | [10] |
| T = 15–35 °C | SBC | The degradation rate of triclosan was increased | sulfate-based AOPs | [52] |
| T = 35 °C, 25 °C, 15 °C and 5 °C | Co-Fe/SiO2 LC | The degradation rate constants of ciprofloxacin for 35 °C, 25 °C, 15 °C and 5 °C were 1.615, 0.686, 0.398 and 0.173 min−1 | sulfate-based AOPs | [55] |
| T = 15 °C T = 55 °C | MnFe2O4-SAC | The degradation rate constants of Orange G for 15 °C and 15 °C were 0.07 and 0.247 min−1 | sulfate-based AOPs | [56] |
| T = 20 °C T = 40 °C T = 60 °C | SC-H2SO4 | RC(ofloxacin) = 23.3% RC(ofloxacin) = 80.4% RC(ofloxacin) = 91.5% | Fenton-like | [57] |
| HCO3− | Co-Fe/SiO2 LC | 0.5 mM, there was a slight decrease 1–10 mM, the removal of ciprofloxacin was accelerated | sulfate-based AOPs | [55] |
| HCO3− | CoFe2O4-SAC | 0–0.1 mg/L, the removal of norfloxacin was accelerated 0.1–0.4mg/L, it inhibited the performance of norfloxacin degradation | sulfate-based AOPs | [8] |
| CO32− | SC | 0 mM, RC(norfloxacin) = 99.0% 10 mM, RC(norfloxacin) = 65.8% | Fenton-like | [58] |
| HCO3− | SBC | 0 g/L, RC(TOC)~85% 0.6 g/L, RC(TOC)~70% 1 g/L, RC(TOC)~70% | ozonation | [22] |
| HCO3− | BC | 0 mM, RC(phenol)~89% 0.5 mM, RC(phenol)~91.5% 50 mM, RC(phenol)~99.9% | ozonation | [21] |
| HCO3− | MnOx/SBAC | 0 g/L, RC(TOC) = 64.4% 50 mg/L, RC(TOC) = 50.9% | ozonation | [59] |
| Cl− | Co-Fe/SiO2 LC | 2–50 mM, the degradation rate was decreased | sulfate-based AOPs | [55] |
| Cl− | CoFe2O4-SAC | 0–0.1 g/L, the degradation rate of norfloxacin was reduced 0.1–0.4 g/L, the degradation rate was increased | sulfate-based AOPs | [8] |
| Cl− | ADSBC 1000 | 0–20 mM, there was little effect on sulfathiazole degradation | sulfate-based AOPs | [37] |
| Cl− | SC | 10 mM, there was no significantly negative effect on the norfloxacin removal | Fenton-like | [58] |
| Cl− | BC | 50 mM, there was no effect on phenol removal | ozonation | [21] |
| PO43− | ADSBC 1000 | 0–20 mM, there was little effect on sulfathiazole degradation | sulfate-based AOPs | [37] |
| PO43− | SC | 0 mM, RC(norfloxacin) = 99.0% 10 mM, RC(norfloxacin) = 83.1% | Fenton-like | [58] |
| PO43− | BC | 0.5 mM, there was no effect on phenol removal | ozonation | [21] |
| NO3− | CoFe2O4-SAC | 0–0.4 g/L, there was weak influence on norfloxacin degradation | sulfate-based AOPs | [8] |
| NO3− | ADSBC 1000 | 0–20 mM, there was little effect on sulfathiazole degradation | sulfate-based AOPs | [37] |
| NO3− | SC | 10 mM, there was nearly no negative effect on norfloxacin removal | Fenton-like | [58] |
| NO3− | BC | 50 mM, there was little effect on phenol removal | ozonation | [21] |
| Synthesis Process | Product | Solution pH | Removal Capacity (RC) | Reusability and Chemical Stability | Mechanism | References |
|---|---|---|---|---|---|---|
| Iron sludge + ethylene glycol + CoCl2 + NaAc were vigorously stirred, then the suspension was solvothermal treated at 200 °C for 10 h. | Co-Fe/SiO2 LC | 7.0 | 0.2 g/L product; 10 mg/L ciprofloxacin; 0.5 g/L PMS. RC(ciprofloxacin) > 99.6% | RC(ciprofloxacin) decreased to 72.0% (4th run). | SO4•−, •OH | [55] |
| The dried sludge was calcined at 450 °C for 0.5 h under N2 and the obtained biochar was further activated by NaOH. | SBC | 7.2 | 0.5 g/L product; 0.034 mmol/L TCS; 0.8 mmol/L PMS. RC(TCS) = 99.2% | After 5 rounds, RC(TCS) decreased to 53.5%. | SO4•−, •OH, 1O2 | [52] |
| The dried sludge was carbonated at 600 °C for 6 h under NH3/Ar. | Biochar | 6 (phosphate buffer) | 0.2 g/L product; 10 ppm BPA; 0.1 g/L PMS. RC(TOC)~80% | - | 1O2 | [32] |
| ADS was annealed at 1000 °C for 90 min under N2. | ADSBC 1000 | 6 | 0.5 g/L product; 20 mg/L STZ; 10 mmol/L PDS. RC(STZ) = 90.31% | - | Nonradical process | [37] |
| The centrifuged sewage sludge + iron salt mixture was treated at 180 °C for 3 h in N2. | IBHC | 4 | 0.2 g/L product; 60 mg/L tetracycline; 5 mmol/L PDS. RC(tetracycline) = 99.72% | RC(tetracycline) was 94.7% in the fifth round reuse. | SO4•−, •OH | [69] |
| The preprocessed iron sludge was annealed at 900 °C for 2 h under Ar. | Fe0/Fe3C@C900 | 7.0 | 0.2 g/L product; 10 mg/L ciprofloxacin; 0.50 g/L PMS. RC(ciprofloxacin) = 98.2% | For the third run, 99% of ciprofloxacin removal was achieved and the concentration of leached Fe decreased from 0.684 to 0.227 mg/L. | SO4•−, •OH, O2•−, 1O2 | [35] |
| The dried sludge was treated by NaBH4 and then pyrolyzed at 400 °C for 2 h under N2. | ZVI-SDBC | 5.22 | 0.5 g/L product; 0.06 mmol/L AO7; 0.925 mmol/L PDS. RC(AO7) = 99.0% | The rate constants were 0.0718, 0.0655 and 0.0502 min−1 in the first-cycle, second-cycle and third-cycle reuse. | SO4•−, •OH, 1O2 | [70] |
| The sludge granule was pyrolyzed at 600 °C for 2 h (SDBC). Then, the SDBC + MnCl2 mixture was pyrolyzed at 600 °C for 30 min. | Mn-SDBC | 6 | 2 g/L product; 1500 mg/L OG; 3 mmol/L PDS. RC(OG) = 95.94% | - | SO4•−, •OH | [71] |
| The dried sludge + urea mixture was calcined at 700 °C under N2 for 2 h. | NC-700 | - | 0.3 g/L product; 50 mg/L MB; 0.4 g/L PMS. RC(MB) = 98.7% | >95% of MB could be removed over five cycles. | 1O2 | [68] |
| The urea + sludge mixture was calcined at 550 °C for 2 h. | UBC-0.5 | 6.84 | 0.5 g/L product; 0.1 mmol/L BPA; 1 mmol/L PMS. RC(BPA)~100% | A slight decrease was observed. | 1O2 | [51] |
| The dried sludge powders were pyrolyzed at 800 °C for 2 h under N2. | MS-800 | 2.17 | 0.2 g/L product; 10 mg/L tetracycline; 4.2 mmol/L PDS. RC(tetracycline) = 82.24% | The Fe highest leaching concentration was 0.8 mg/L. MS-800 exhibited a better reusability after four times application. | SO4•−, •OH | [30] |
| Sludge + agar powder + MnCl2 + NH4OH. Dried solid was thermal treated at 800 °C for 1 h under Ar. | ASMn-Nb | 6 (phosphate buffer) | 0.2 g/L product; 20 mg/L AO7; 1.6 mmol/L PMS. RC(AO7) = 100% | After 5 times recycling, the complete degradation of AO7 was achieved and there was negligible metal leaching. | Radical and Nonradical process | [46] |
| SAC + Co (NO3)3·9H2O + Fe (NO3)3·9H2O. Then NaOH solution was added until pH = 12. The suspension was treated at 180 °C for 12h. | CoFe2O4-SAC | - | 0.1 g/L product; 10 mg/L NOR; 0.15 g/L PMS. RC(TOC) = 81.0% | RC(NOR) maintained at 90% after five cycles. The leaching concentration of cobalt and iron was 0.57 and 0.25 mg/L, respectively. | SO4•−, •OH | [8] |
| SAC + FeCl3·6H2O + MnCl2·4H2O were dissolved in ethylene glycol under ultrasonication. Later, NaAc was added and stirred. Finally, the mixture was treated at 200 °C for 10 h. | MnFe2O4-SAC | - | 0.2 g/L product; 20 mg/L OG; 0.5 g/L PDS. RC(OG)>95% | RC(OG) was more than 94%, even after five cycles | SO4•−, •OH | [56] |
| Magnetic porous carbon was microwave digested and carbonized at 600 °C for 2 h under N2. | MS600 | 7.0 | 1 g/L product; 1 mmol/L 2-Napthol; 20 mmol/L PDS. RC(2-Napthol) = 88.7% | After the third time, RC(2-Napthol) was still above 80%. | SO4•−, •OH | [31] |
| Synthesis Process | Product | Solution pH | Removal Capacity (RC) | Reusability and Chemical Stability | Mechanism | References |
|---|---|---|---|---|---|---|
| Sodium lauryl sulfate + sludge biochar + Kaolin were sintered at 1100 °C for 30 min under N2. | SBC | 4.0 | 0.2 g/L product; 10 mg/L ciprofloxacin; 60 mmol/L H2O2. RC(ciprofloxacin) > 80% | - | •OH | [81] |
| The alkaline activated sludge was treated by microwave digestion and pyrolyzed at 600 °C for 2 h in N2. | PFC600 | 5.0 | 0.5 g/L product; 1.0 mmol/L 1,2,4-Acid; 15 mmol/L H2O2. RC(1,2,4-Acid) = 96.6% | RC(1,2,4-Acid) still reached 90.2% after three recycles. | •OH | [82] |
| Sludge was activated by H2O2 at acid pH and then mixed with FeSO4·7H2O. The resulting sample was carbonized at 600 °C under N2. | SC-F-0.2 | 3.0 | 1 g/L product; 1 mmol/L Black-T; 20 mmol/L/L H2O2. RC(TOC) = 71% | The catalyst presented 2.77% of the iron load loss. The dye removal reached 91% after three repeated reactions. | - | [83] |
| The dry sludge was carbonized at 600 °C for 4 h under N2 and then treated with sulfuric acid. | SC-H2SO4 | 6 | 1 g/L product; 30 mg/L ofloxacin; 138 mg/L H2O2. RC(ofloxacin) = 91.5% | - | •OH | [57] |
| The iron sludge was calcined at 600 °C for 3 h. | Fe-600 | 5.44 | 1 g/L product; 10 mg/L RhB; 10 mmol/Lol/L H2O2. RC(RhB) = 99% | - | •OH | [84] |
| The ferric sludge + biosolids mixture was stirred and sealed at 200 °C for 5 h. | SBMC | 3 | 1 g/L product; 0.21 mmol/L aniline; 60 mmol/L H2O2. RC(aniline) = 77.9%; RC(TOC) = 50.2% | In the 5th run, the catalytic ability of SBMC began to decrease and the leached iron was <0.75 mg/L. | •OH, •O2− | [44] |
| The sludge + starch mixture was heated at 600 °C for 3 h under N2 (SC). Then some SC was soaked with H2SO4 and the green tea extract was added. | SC-based catalyst | 4.0 | 1 g/L product; 20 mg/L NOR; 10 mg/L Cu2+; 1.5 mmol/L H2O2. RC(NOR) = 98.8%; RC(Cu2+) = 97.5% | After four runs, RC(Cu2+) decreased from 97.5 to 39.1%, RC(NOR) decreased from 98.8 to 76.4%. | •OH (NOR), •O2− (Cu2+) | [58] |
| The dry Fe-rich sludge was calcined at 800 °C for 2 h in N2. | Fe-SC-800 | 8 | 2 g/L product; AOII; 17 mmol/L H2O2. RC(AOII) = 98% | Fe-SC-800 performed similar RC after being recycled for three times. | •OH | [53] |
| WAS adsorbing the heavy metals was anaerobic pyrolyzed at 600 °C for 1 h. | Cu(II)-SBC | - | 0.1 g/L product; 800 μg/L E2; 600 mg/L H2O2. RC(E2) = 100% | - | •OH, •O2− | [85] |
| Ni(II)-SBC | 0.1 g/L product; 800 μg/L E2; 600 mg/L H2O2. RC(E2) = 79% | •O2- | ||||
| Obtained through a co-precipitation method followed by sintering at 800 °C | NiFe2O4 | 3.0 | 2.0 g/L product; 250 mg/L phenol; 120 mmol/Lol/L H2O2. RC(phenol) = 95% | The leached iron amounted to 6.3% ± 0.2% of total iron. | •OH | [86] |
| Steel sludge was acid washed with HCl. | SS_HCl | 4.4 | 1 g/L product; 200 mg/L 4-CP; 20.3 mmol/L H2O2. RC(TOC) = 64% | The catalyst can be reused without any regenerative treatment for up to 3 cycles. | •OH, •O2− | [15] |
| The sludge + (NH4)2Fe(SO4)2 mixture was calcined at 350 °C for 3 h in air. | FAS | 4.0 | 0.3 g/L product; 55.5 mg/L RhB; 3% H2O2. RC(TOC) = 69% | No obvious deactivation was observed over six repetitive trials. | •OH | [87] |
| Synthesis Process | Product | Solution pH | Removal Capacity (RC) | Reusability and Chemical Stability | Mechanism | References |
|---|---|---|---|---|---|---|
| The sludge + TiO2 mixture was carbonized at 200 °C for 20 h. Finally, the dried catalyst was heated at 800 °C for 2 h under N2. | TiO2 nanorods | 7 | 0.4 g/L product; 10 mg/L pentachlorophenol; RC(pentachlorophenol) = 97% | - | The photogenerated electrons | [99] |
| The sludge + HCl + TiOSO4·2H2O mixture was heated at 150 °C for 12 h. Finally, the dried solid was calcined at 700 °C for 5 h. | SS-Ti-700 | - | 35 mg/L p-NP; RC(p-NP) = 92.87% | SS-Ti-700 maintained excellent photoactivity in the six repeated experiments. | The photogenerated electrons, superoxide radical, and •OH | [100] |
| The pH of the sludge was adjusted to 1 and the titanium isopropoxide + sludge mixture was kept in a thermostat water bath for 6 h. The resulting precipitations were calcined in air at 500 °C for 4 h. | WSCT powder | 5 | 1 g/L product; 10 mg/L RhB; RC(RhB) = 82.4% | WSCT still exhibited high photo-activity after four times. | - | [101] |
| Titanium(IV) butoxide + ethanol + HNO3 + sludge were mixed. After gelation of the sol, the product was heated at 200 °C for 2 h. | TiO2/ASS nanocomposite | 7 | 4 g/L product; 25 mg/L MO; 30 mg/L Cd2+; RC(MO) = 94.28%. RC(Cd2+) > 90% | - | - | [102] |
| The sludge + TiO2 + NaOH mixture was heated at 200 °C for 20 h. The dried samples were heated at 600 °C for about 0.5 h under N2. | 0.01 g/L SS-TiO2 (ST2) | - | 0.01 g/L product; 5 mg/L tetracycline; RC(tetracycline) = 76.3% | - | - | [23] |
| The zinc acetate + SBAC + NaOH mixture was maintained at 180 °C for 12 h. | ZnO-SBAC (ZC) | 2.35 | 1 g/L product; 10 mg/L Cr(VI); RC(Cr) = 93.61% | RC(Cr) was similar after 3 cycles. | - | [103] |
| The RSDBC + zinc nitrate mixture was treated at 450 °C for 3 h under Ar. | ZnO@RSDBC | 7 | 0.9 g/L product; 20 mg/L AO7; RC(AO7)~95% | RC(AO7) remained at 78.6% after three cycling runs. | h+, O2•−, SO4•− and HO• | [10] |
| Synthesis Process | Product | Solution pH | Removal Capacity (RC) | Reusability and Chemical Stability | Mechanism | References |
|---|---|---|---|---|---|---|
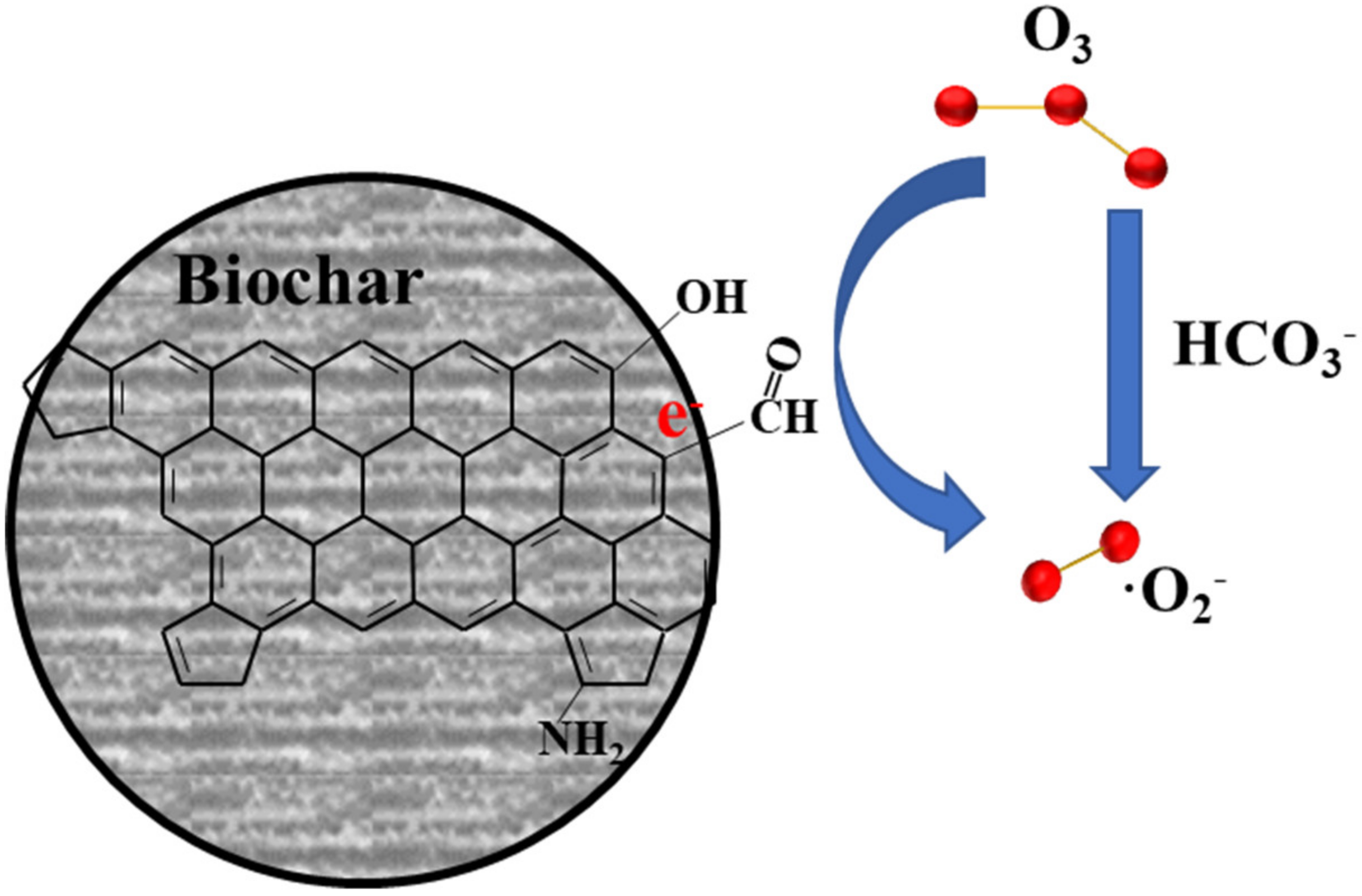
| Pyrolyzed at 700 °C for 2 h under N2. | Biochar | - | 1.0 g/L product; 0.2 g/L phenol; 14 ± 1 mg/L and 1.0 L/min O3. RC(phenol) = 95.4% | RC(phenol) dropped to 59.3% (fourth trial). | •O2− | [21] |
| Activated by ZnCl2/KOH/H2SO4 and then pyrolyzed at 700 °C for 1 h under N2. | SBC | 4.0 | 0.2 g/L product; 0.1 mmol/L oxalic acid; 0.7 mg/min O3. RC(oxalic acid) = 81.2% | - | Surface reaction | [33] |
| The SAC + FeCl3·6H2O + FeSO4·7H2O mixture was put into the thermostat water bath at 93 °C for 3 h. | FMSAC | 6.0 | 0.04 g/L product; 20 mg/L p-CBA; 1 mg/L O3. RC(p-CBA) = 80% | RC(p-CBA) only reduced by 13.2% after six repetitive runs. | •OH | [110] |
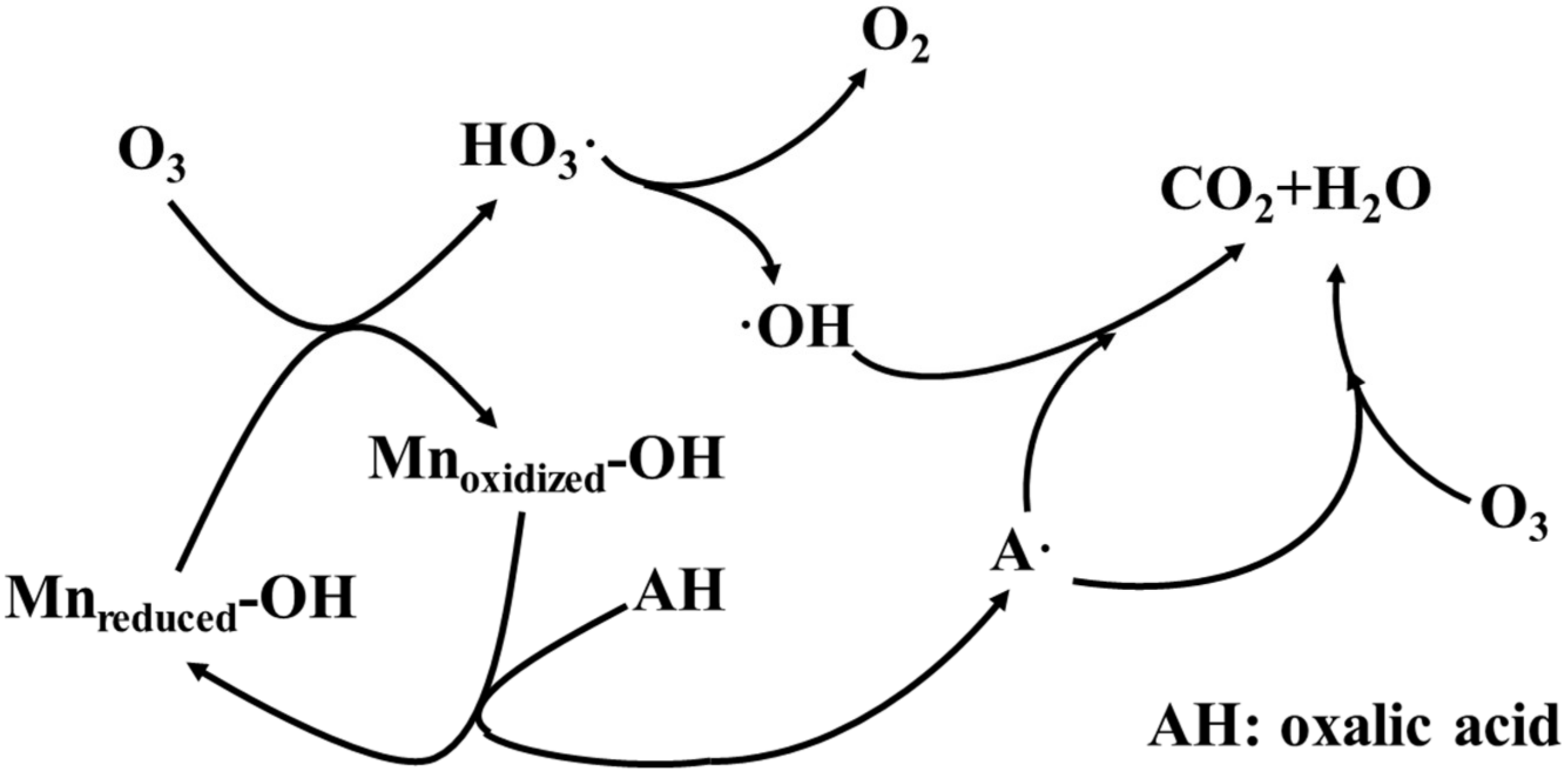
| SAC was obtained by ZnCl2+H2SO4 activation and then pyrolyzed at 550 °C for 1 h under N2. The SAC + manganese mixture was calcinated at 550 °C for 1 h under N2. | MnOx/SAC | 3.5 | 0.1 g/L product; 80 mg/L oxalic acid; 5.0 mg/L O3. RC(oxalic acid) = 72.1%; RC(TOC) = 92.2% | Manganese leaching was approximately 3.9% in 60 min. | Surface reaction was dominant | [34] |
| SCCA-Zn was obtained by ZnCl2 activation and then pyrolyzed under N2. SCCA-Zn + MgSO4 + NaOH were mixed. Then precipitation was squeezed into pellets and finally thermal treated at 500 °C for 2 h under N2. | Granular MgO-SCCA-Zn | 9.0 | 10 g/L product; 500 mg/L MB; 5 mg/L O3. RC(MB) = 98%; RC(COD) = 51.12% | Mg2+ was not detected in the aqueous solution. After the 3th reuse, RC(COD) was 49.68%. | - | [111] |
| SBC0: sludge was pyrolyzed at 850 °C for 1 h under N2. SBCa: SBC0 was treated with HCl solution. SBCb: SBCa was treated with NaOH solution. | Activated petroleum waste sludge biochar | 7.7 | 1.0 g/L product; petroleum refinery wastewater; 20 mg/min O3. The SBC0-COP (53.5%), SBCa-COP (49.3%) and SBCb-COP (51.8%) all exhibited higher TOC removal | After the 5th reuse, RC(TOC) was reduced to 48.5% (SBC0), 37.9% (SBCa) and 37.3% (SBCb). | •OH | [22] |
| SBAC: activated by ZnCl2 and then pyrolyzed at 700 °C for 1 h under N2. The SBAC + Mn nitrate mixture was calcinated at 600 °C for 3 h under N2. | MnOx/SBAC | 6.5–7.5 | 1 g/L product; coal gasification wastewater; 500 ml/min and 15 mg/L O3. RC(COD) = 78.1% | The maximum leaching of Mn was 0.41 mg/L. RC(COD) kept higher than 63.2% throughout ten successive runs. | •OH | [59] |
| SBAC: activated by ZnCl2 and then pyrolyzed at 700 °C for 1 h under N2. The SBAC + Fe nitrate mixture was calcinated at 600 °C for 3 h under N2. | FeOx/SBAC | 6.5-7.5 | 1 g/L product; coal gasification wastewater; 500 ml/min and 15 mg/L O3. RC(COD) = 73.7% | The maximum leaching of Fe was 1.45 mg/L. RC(COD) kept higher than 63.2% throughout ten successive runs. | •OH | [59] |
| Pyrolyzed at 700 °C for 4 h under N2. | SC-700 | 6 | 0.5 g/L product; 200 mg/L HQ; 50 ml/min and 17 mg/L O3. RC(HQ) = 97.86% | - | - | [112] |
| The sewage sludge + corncob + ZnCl2 mixture was pyrolyzed at 600°C for 1h. | SCAC | 6.0 | 0.025 g/L product; 0.5 mg/L Ibuprofen (IBP); 3.0 mg/L O3. RC(IBP) = 100% | - | •OH | [113] |
| MSAC: sludge was activated by ZnCl2 and then pyrolyzed under N2. The SAC + Fe(NO3)3·9H2O + HNO3 mixture was pyrolyzed at 600 °C for 1 h under N2. For the Mn loaded MSAC, the molar ratio of Fe to Mn was adjusted to 2. | MSAC-Mn | 7.0 (phosphate buffer) | 0.05 g/L product; 0.6 mg/L IBP; 1.0 mg/L O3. RC(IBP) = 86.2% | - | •OH | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Fu, L.; Yu, Y.; Wu, C.; Li, M.; Jin, X.; Yang, J.; Wang, P.; Chen, Y. Recent Development in Sludge Biochar-Based Catalysts for Advanced Oxidation Processes of Wastewater. Catalysts 2021, 11, 1275. https://doi.org/10.3390/catal11111275
Chen X, Fu L, Yu Y, Wu C, Li M, Jin X, Yang J, Wang P, Chen Y. Recent Development in Sludge Biochar-Based Catalysts for Advanced Oxidation Processes of Wastewater. Catalysts. 2021; 11(11):1275. https://doi.org/10.3390/catal11111275
Chicago/Turabian StyleChen, Xingxing, Liya Fu, Yin Yu, Changyong Wu, Min Li, Xiaoguang Jin, Jin Yang, Panxin Wang, and Ying Chen. 2021. "Recent Development in Sludge Biochar-Based Catalysts for Advanced Oxidation Processes of Wastewater" Catalysts 11, no. 11: 1275. https://doi.org/10.3390/catal11111275
APA StyleChen, X., Fu, L., Yu, Y., Wu, C., Li, M., Jin, X., Yang, J., Wang, P., & Chen, Y. (2021). Recent Development in Sludge Biochar-Based Catalysts for Advanced Oxidation Processes of Wastewater. Catalysts, 11(11), 1275. https://doi.org/10.3390/catal11111275





