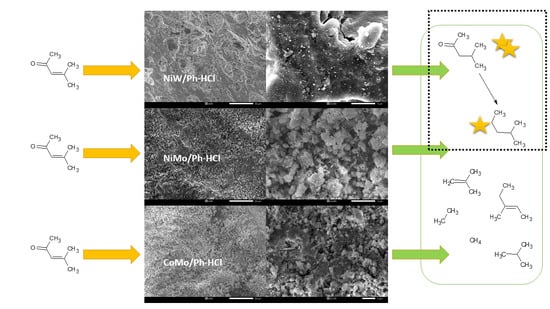
Mesityl Oxide Reduction by Using Acid-Modified Phonolite Supported NiW, NiMo, and CoMo Catalysts
Abstract
:1. Introduction
2. Results and Discussion
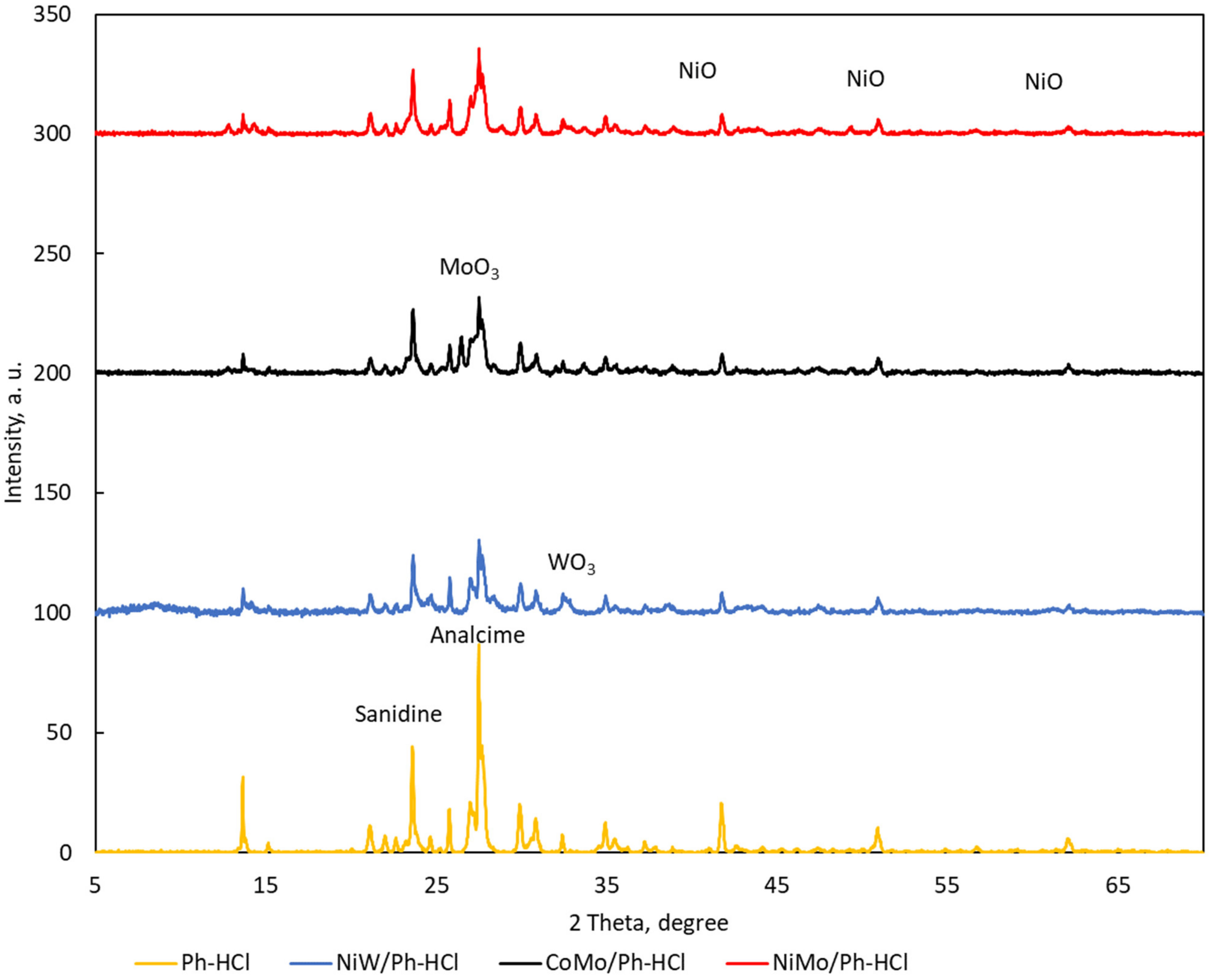
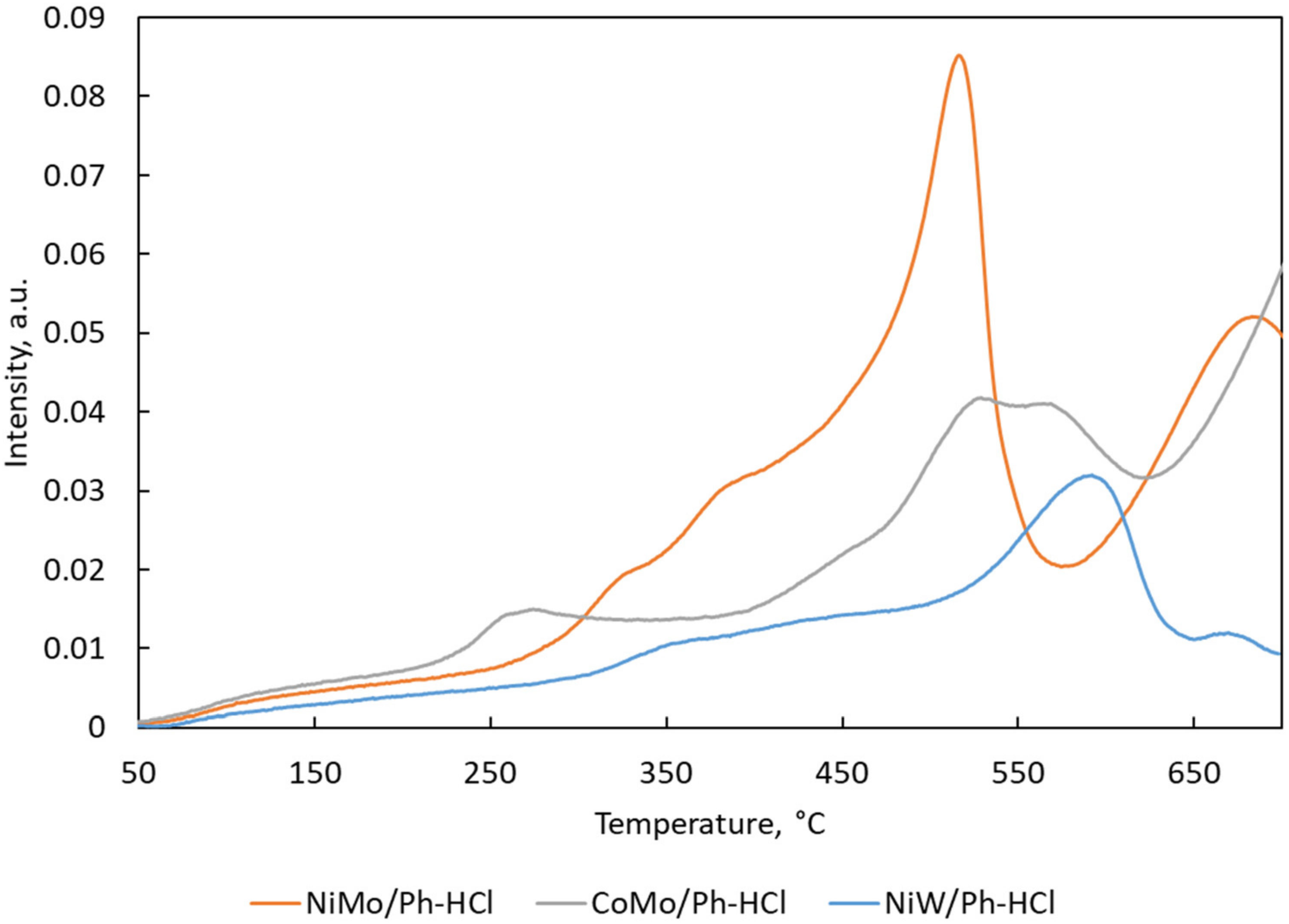
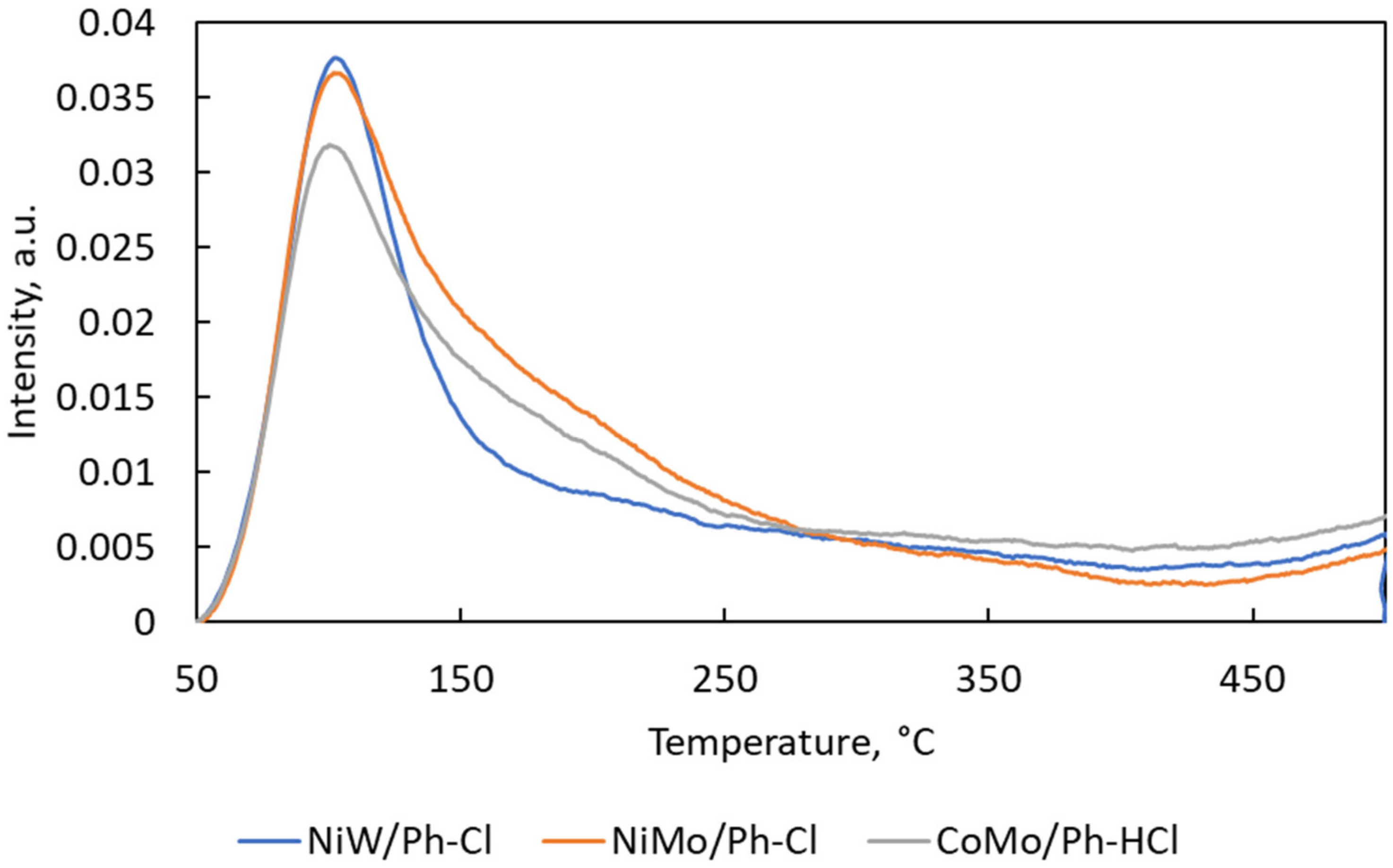
2.1. Catalysts Characterization
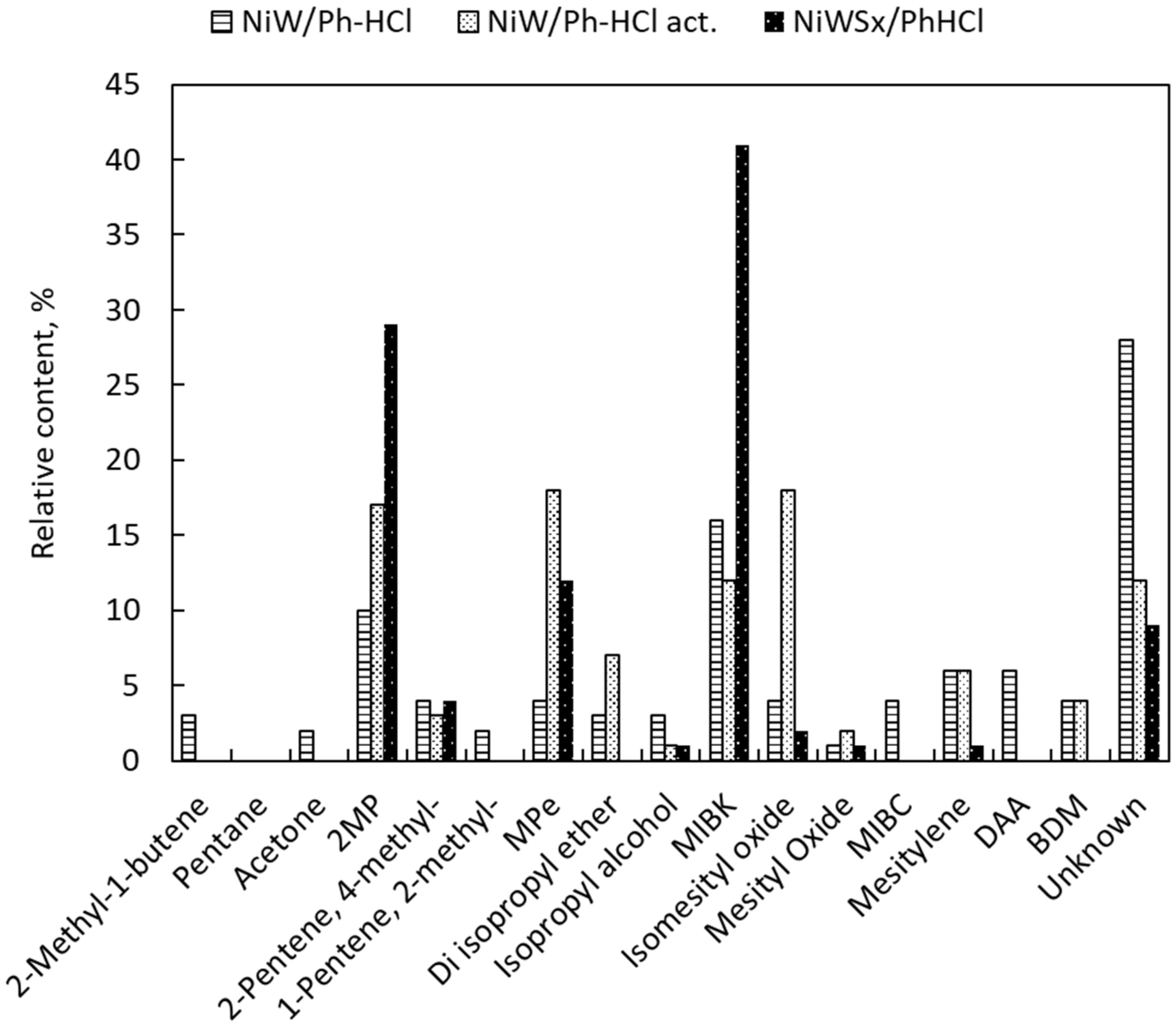
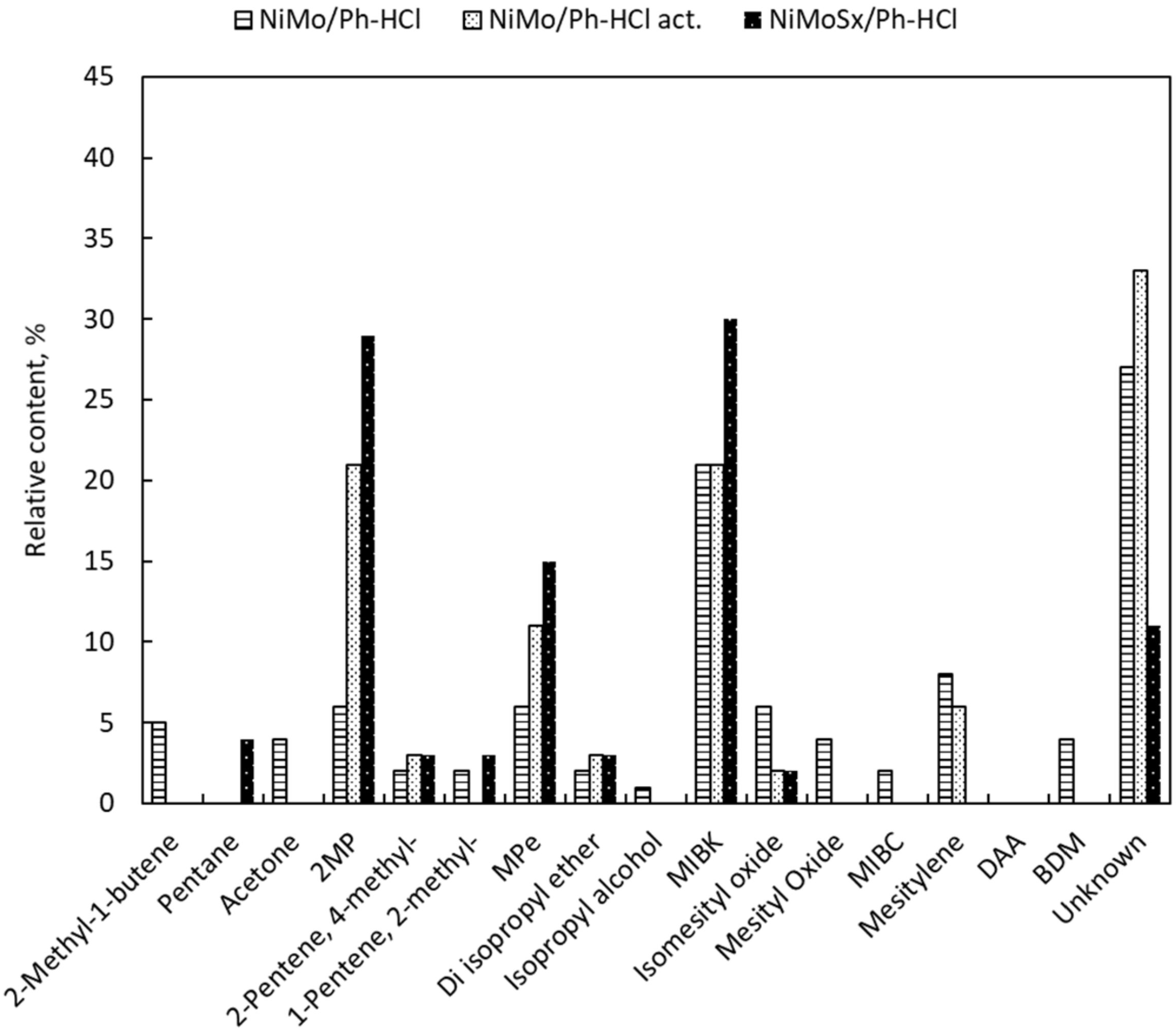
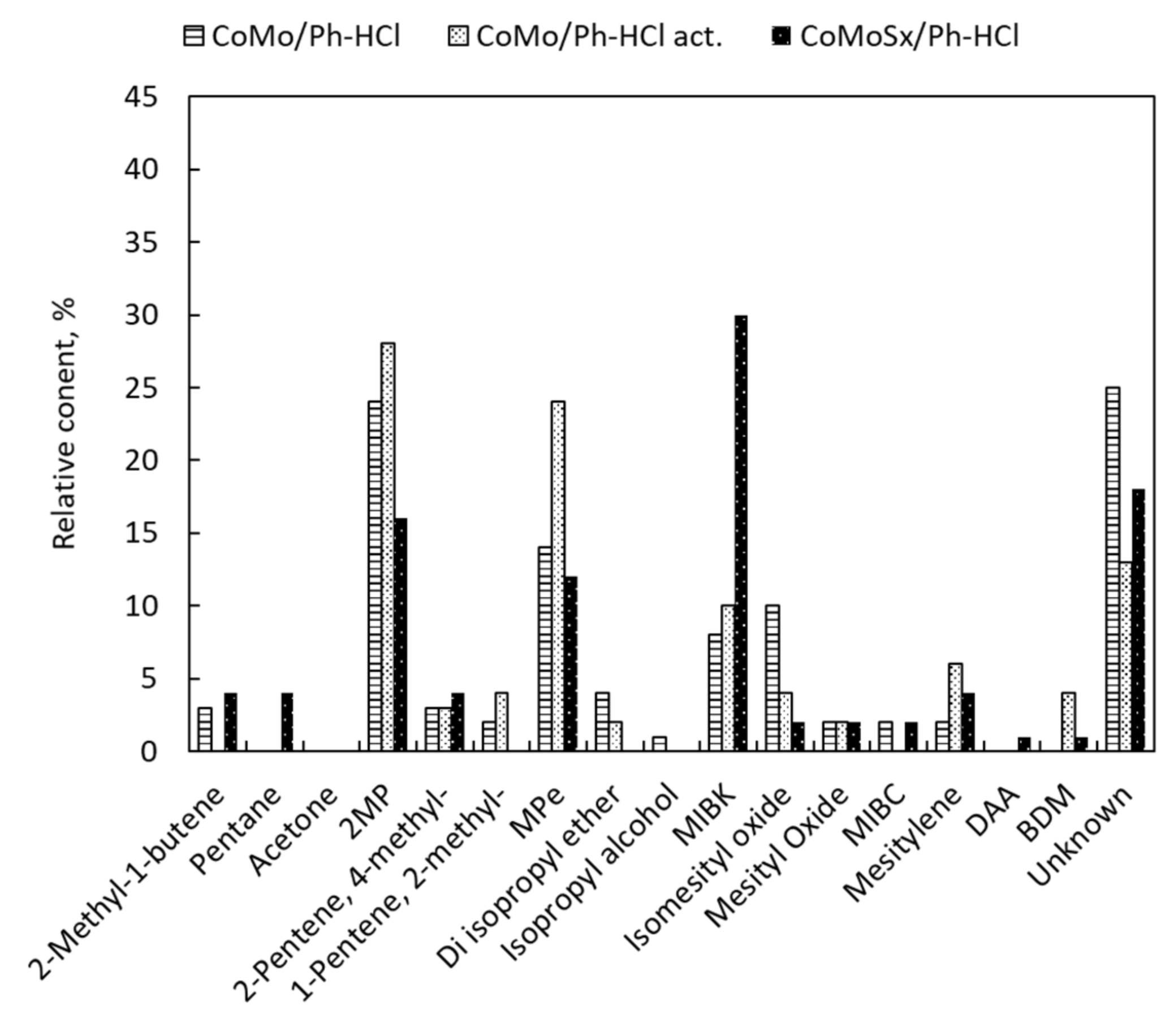
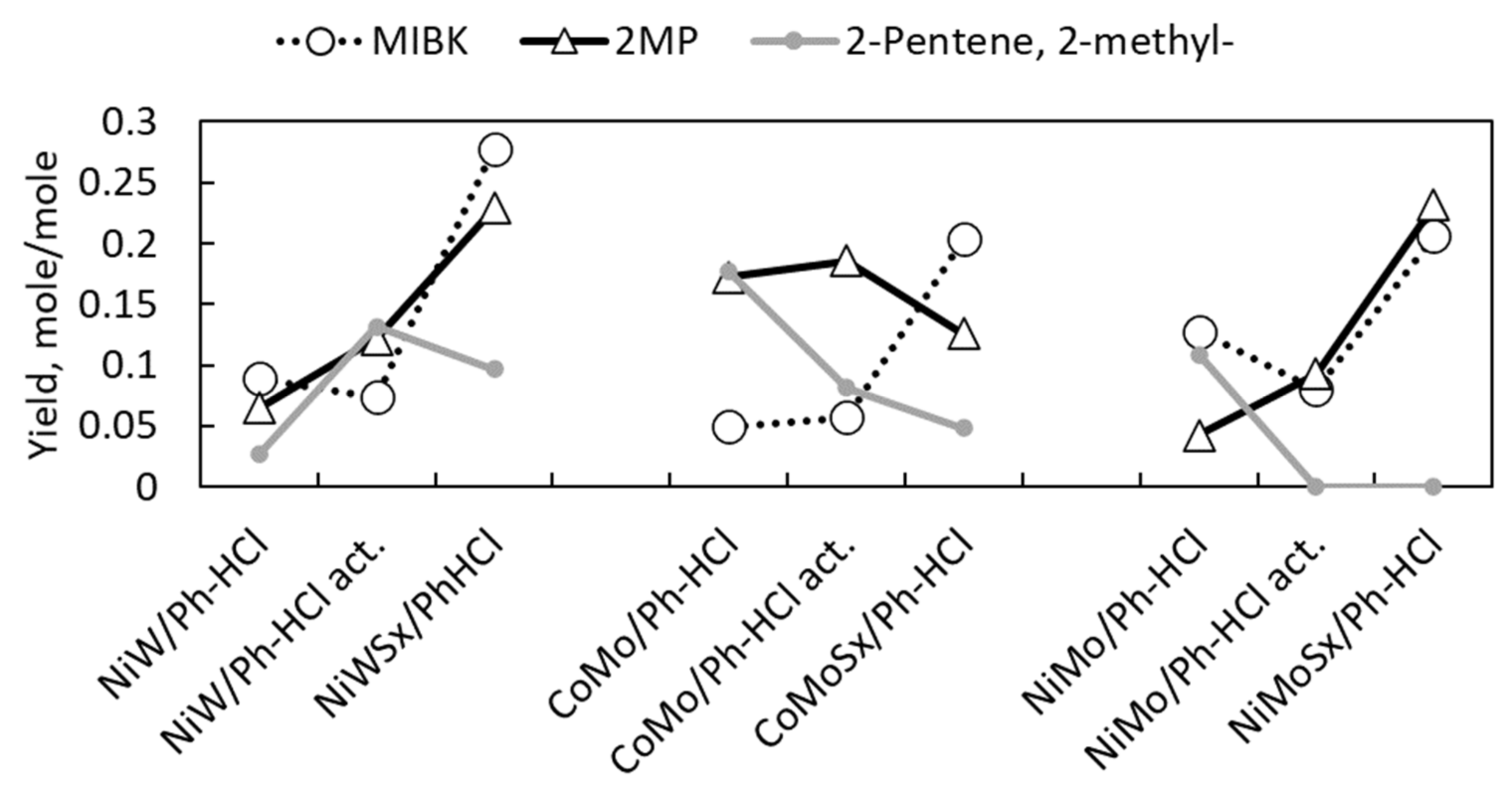
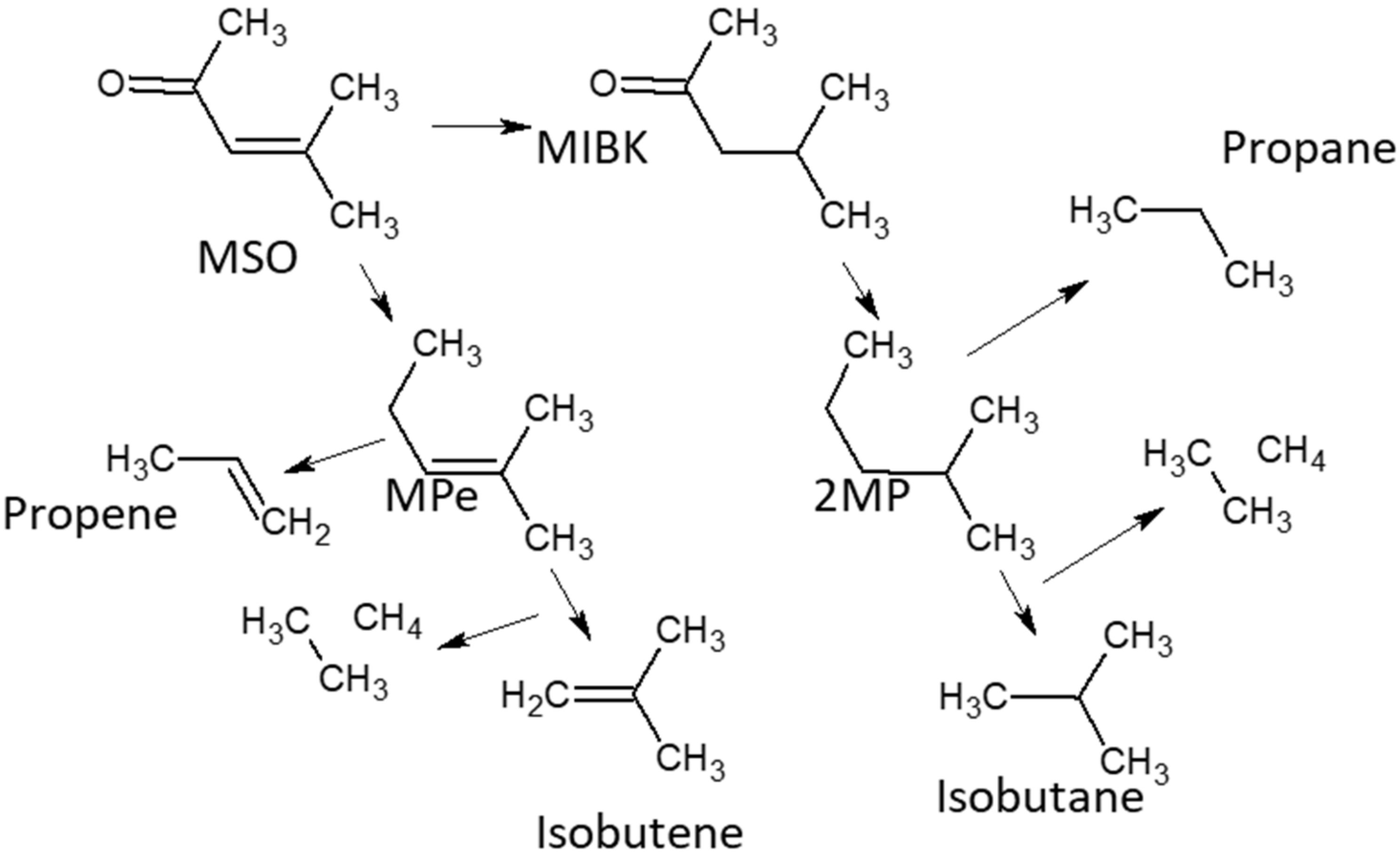
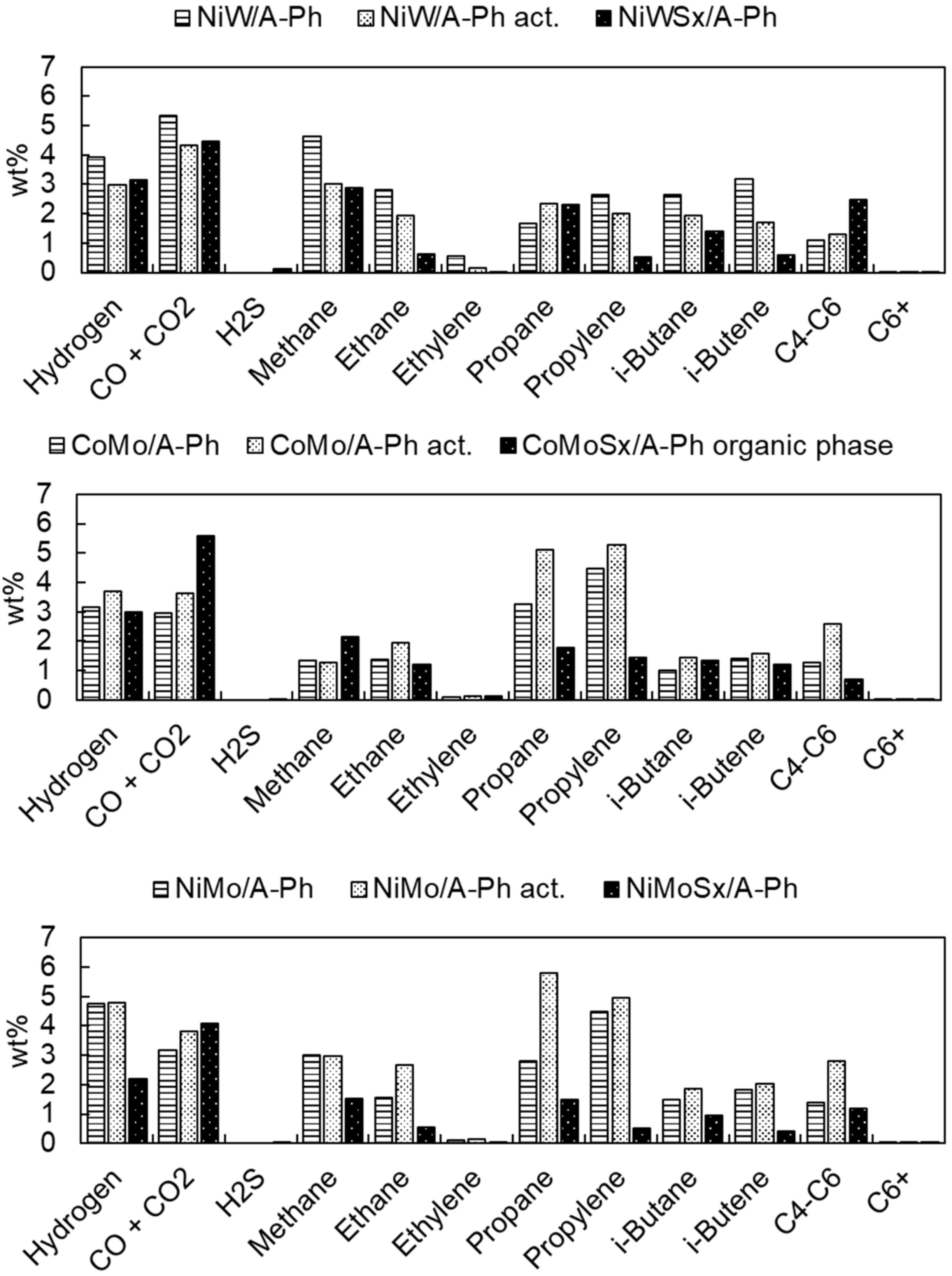
2.2. Catalyst Activity
3. Materials and Methods
3.1. Raw Materials
3.2. Catalysts Synthesis and Characterization
3.3. Experimental Set up and Catalytic Tests
- i.
- For the activation by the active metal sulfidation, the metal oxide-supported catalysts were activated—sulfided using di-t-butyl polysulfide (Lubrizol Company, Wicklie, OH, USA). In total, 2.1 g of the catalyst and 10 g of di-t-butyl polysulfide were introduced into the reactor. The reactor was pressurized to 50 bar and heated from room temperature to 375 °C (8.3 °C min−1) while maintaining the temperature for 1 h. The reactor was then cooled to room temperature and depressurized.
- ii.
- For the activation by the metal reduction only under H2 atmosphere, the autoclave was pressurized to 50 bar and heated from room temperature to 400 °C (8.3 °C min−1). The temperature of 400 °C was maintained for 2 h. Then, the reactor was cooled to room temperature and depressurized. About 2.1 g of catalyst (metal oxide) was used for this case.
- iii.
- For the direct use of the catalyst, 2.1 g of fresh catalyst was directly introduced in the batch reactor to start the reaction.
3.4. Product Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salvapati, G.S.; Ramanamurty, K.V.; Janardanarao, M. Selective catalytic self-condensation of acetone. J. Mol. Catal. 1989, 54, 9–30. [Google Scholar] [CrossRef]
- Mesityl Oxide. Hazardous Substance Fact Sheet. New Jersey Department of Health and Senior Services. Occupational Health Service. Trenton, NJ 08625-0360, June 1999. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1195.pdf (accessed on 6 September 2021).
- Kuśtrowski, P.; Sułkowska, D.; Chmielarz, L.; Rafalska-Łasocha, A.; Dudek, B.; Dziembaj, R. Influence of thermal treatment conditions on the activity of hydrotalcite-derived Mg–Al oxides in the aldol condensation of acetone. Microporous Mesoporous Mater. 2005, 78, 11–22. [Google Scholar] [CrossRef]
- Thotla, S.; Agarwal, V.; Mahajani, S.M. Simultaneous production of diacetone alcohol and mesityl oxide from acetone using reactive distillation. Chem. Eng. Sci. 2007, 62, 18–20. [Google Scholar] [CrossRef]
- Tan, S.T.; Ali Umar, A.A.; Salleh, M.M. Synthesis of defect-rich, (001) faceted-ZnO nanorod on a FTO substrate as efficient photocatalysts for dehydrogenation of isopropanol to acetone. J. Phys. Chem. Solids 2016, 93, 73–78. [Google Scholar] [CrossRef]
- Hauser, M. The reactions of mesityl oxide. Chem. Rev. 1963, 63, 311–324. [Google Scholar] [CrossRef]
- Wisniak, J.; Herskowltz, M.; Roffe, D.; Smllovltz, S. Reduction of Mesityl Oxide. Ind. Eng. Chem. Prod. Res. Dev. 1976, 15, 163–168. [Google Scholar] [CrossRef]
- O’Keefe, W.K.; Jiang, M.; Ng, F.T.T.; Rempel, G.L. Liquid phase kinetics for the selective hydrogenation of mesityl oxide to methyl isobutyl ketone in acetone over a Pd/Al2O3 catalyst. Chem. Eng. Sci. 2005, 60, 4131–4140. [Google Scholar] [CrossRef]
- Nicol, W.; du Toit, E.L. One-step methyl isobutyl ketone synthesis from acetone and hydrogen using Amberlyst® CH28. Chem. Eng. Process. Process. Intensif. 2004, 43, 1539–1545. [Google Scholar] [CrossRef]
- Ho, C.R.; Zheng, S.; Shylesh, S.; Bell, A.T. The mechanism and kinetics of methyl isobutyl ketone synthesis from acetone over ion-exchanged hydroxyapatite. J. Catal. 2018, 365, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, M.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Hydrogenation of methyl isobutyl ketone over bifunctional Pt–zeolite catalyst. J. Catal. 2012, 293, 141–144. [Google Scholar] [CrossRef]
- Weisser, O.; Landa, S. Sulphide Catalysts, Their Properties and Applications. Institute of Chemical Technology, Prague. ACADEMIA Publ. House Czechoslov. Acad. Sci. 1972, 6.6.1., 168–171. [Google Scholar]
- Hidalgo-Herrador, J.M.; Frątczak, J.; Velvarská, R.; de Paz Carmona, H. Oxalic acid-mediated catalytic transfer hydrodeoxygenation of waste cooking oil. Mol. Catal. 2020, 491, 110973. [Google Scholar] [CrossRef]
- Hidalgo Herrador, J.M.; Fratczak, J.; Tišler, Z.; de Paz Carmona, H.; Velvarská, R. Oxalic Acid as a Hydrogen Donor for the Hydrodesulfurization of Gas Oil and Deoxygenation of Rapeseed Oil Using Phonolite-Based Catalysts. Molecules 2020, 25, 3732. [Google Scholar] [CrossRef]
- Hidalgo, J.M.; Tišler, Z.; Vráblík, A.; Velvarská, R.; Lederer, J. Acid-modified phonolite and foamed zeolite as supports for NiW catalysts for deoxygenation of waste rendering fat. Reac. Kinet. Mech. Cat. 2019, 126, 773–793. [Google Scholar] [CrossRef]
- Hidalgo Herrador, J.M.; Fratzak, J.; Tišler, Z.; Velvarská, R.; Kocík, J. Nickel-Tungsten Catalyst Based on Oxalic Acid Modified Phonolite. UTILITY MODEL. Group UM App. No. 2021-38717. Document No. CZ 35055 U1. Industrial Property Office of the Czech Republic. Available online: https://isdv.upv.cz/webapp/resdb.print_detail.det?pspis=PUV/38717&plang=EN (accessed on 10 September 2021).
- Hidalgo-Herrador, J.M.; Tišler, Z.; Hajková, P.; Soukupová, L.; Zárybnická, L.; Černá, K. Cold Plasma and Acid Treatment Modification Effects on Phonolite. Acta Chim. Slov. 2017, 64, 598–602. [Google Scholar] [CrossRef] [Green Version]
- de Paz Carmona, H.; Hidalgo Herrador, J.M.; Fratzak, J.; Tišler, Z. Nickel-Molybdenum Catalyst on an Acid-Modified Unit. UTILITY MODEL. Group UM App. No. 2020-38225. Document No. CZ CZ 34683 U1. Industrial Property Office of the Czech Republic. Available online: https://isdv.upv.cz/webapp/resdb.print_detail.det?pspis=PUV/38225&plang=CS (accessed on 10 September 2021).
- Henderson, C.M.B. Composition, Thermal Expansion and Phase Transitions in Framework Silicates: Revisitation and Review of Natural and Synthetic Analogues of Nepheline-, Feldspar- and Leucite-Mineral Groups. Solids 2021, 2, 1. [Google Scholar] [CrossRef]
- Long, H.; Shi, T.; Hu, H.; Jiang, S.; Xi, S.; Tang, Z. Growth of Hierarchal Mesoporous NiO Nanosheets on Carbon Cloth as Binder-free Anodes for High-performance Flexible Lithium-ion Batteries. Sci. Rep. 2014, 4, 7413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.G.; Yang, N.H.; Yuan, L.; Pang, S.J. High-resolution electron microscopy analysis of microstructural changes in WOx electrochromic films. Surf. Coat. Technol. 1998, 110, 184–187. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Cherepanova, S.V.; Malakhov, V.V.; Dovlitova, L.S.; Ishchenko, A.V.; Tsybulya, S.V. In situ XRD study of nanocrystalline cobalt oxide reduction. Kinet. Catal. 2009, 50, 192–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Chen, H.; Zhang, X. Hydroconversion of Waste Cooking Oil into Green Biofuel over Hierarchical USY-Supported NiMo Catalyst: A Comparative Study of Desilication and Dealumination. Catalysts 2017, 7, 281. [Google Scholar] [CrossRef] [Green Version]
- Gnanamani, M.K.; Jacobs, G.; Graham, U.M.; Pendyala, V.R.R.; Martinelli, M.; MacLennan, A.; Hu, Y.; Davis, B.H. Effect of sequence of P and Co addition over silica for Fischer-Tropsch synthesis. Appl. Catal. A-Gen. 2017, 538, 190–198. [Google Scholar] [CrossRef]


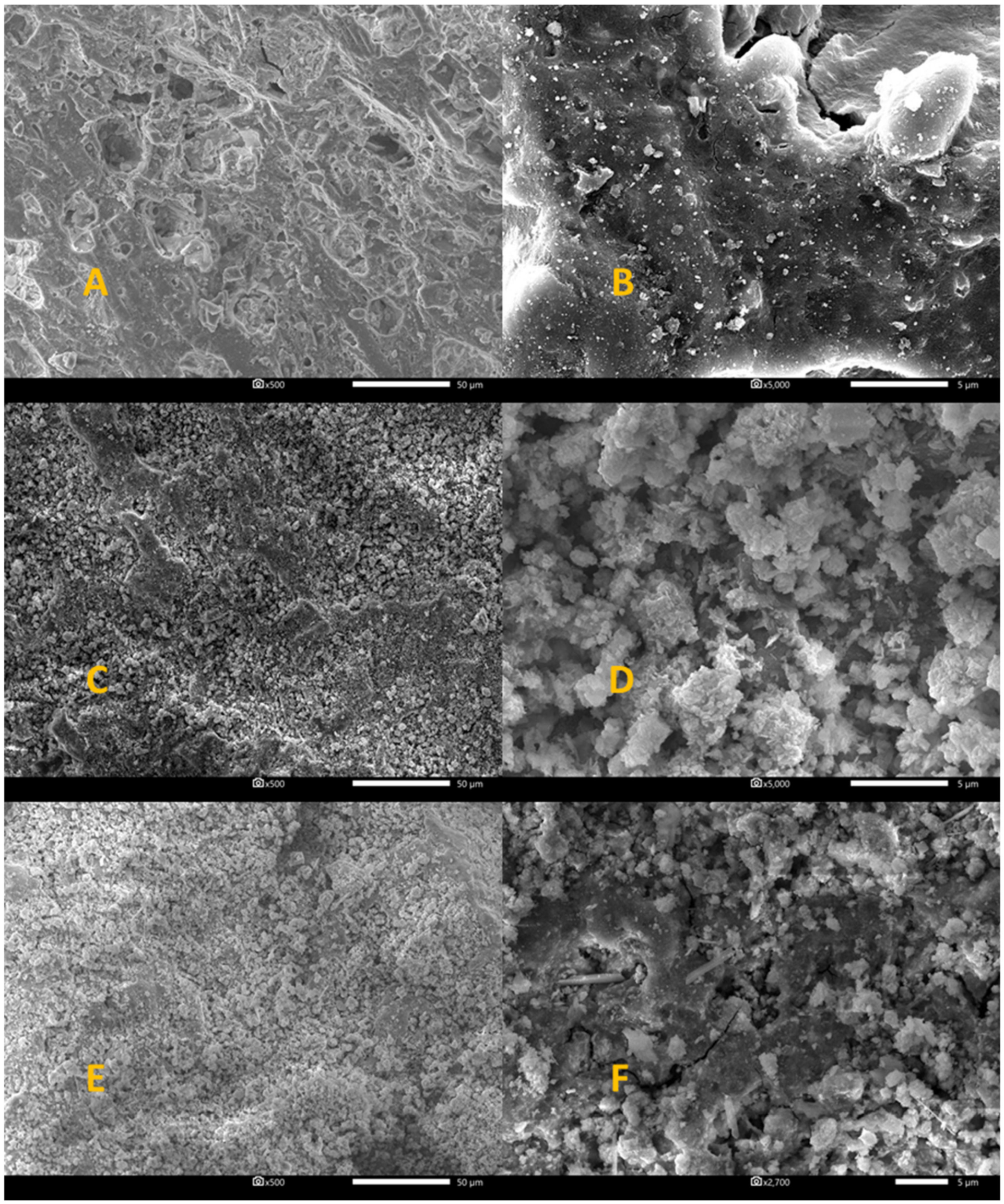

| Ph 1 | Ph-HCl 1 | NiW/Ph-HCl 1 | NiMo/Ph-HCl 2 | CoMo/Ph-HCl | |
|---|---|---|---|---|---|
| Si, wt% | 26.5 | 34.8 | 28.5 | 28.9 | 29.6 |
| Al, wt% | 11.8 | 6.7 | 5.3 | 5.5 | 5.7 |
| Ni, wt% | 0.0 | 0.0 | 5.2 | 5.4 | 0.0 |
| W, wt% | 0.0 | 0.0 | 9.8 | 0.0 | 0.0 |
| Mo, wt% | 0.0 | 0.0 | 0.0 | 10.0 | 9.0 |
| Co, wt% | 0.0 | 0.0 | 0.0 | 0.0 | 4.6 |
| Na, wt% | 7.9 | 2.8 | 1.7 | 1.2 | 1.4 |
| K, wt% | 5.1 | 6.6 | 5.2 | 2.8 | 2.7 |
| Fe, wt% | 1.4 | 0.8 | 0.5 | 0.6 | 0.6 |
| Ca, wt% | 0.7 | 0.0 | 0.0 | 0.1 | 0.1 |
| Ti, wt% | 0.2 | 0.0 | 0.0 | 0.1 | 0.1 |
| Cl, wt% | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Others, wt% | 0.0 | <0.1 | 0.5 | 0.0 | 0.0 |
| O 3, wt% | 46.1 | 48.3 | 43.3 | 45.4 | 46.2 |
| * Total Intrusion Volume, cm−3 g−1 | 0.008 | 0.179 | 0.118 | 0.200 | 0.209 |
| * Pore volume 3–50 nm (cm3 g−1) | 0.003 | 0.030 | 0.016 | 0 | 0 |
| ** BET surface Area, m2 g−1 | 7.6 | 120.1 | 53.9 | 41.8 | 51.3 |
| Phonolite (×5000)—Figure 5B | |||
| Element | Line | Mass% | Atom% |
| O | K | 45.91 ± 0.03 | 59.79 ± 0.04 |
| Na | K | 6.15 ± 0.01 | 5.57 ± 0.01 |
| Al | K | 11.97 ± 0.01 | 9.24 ± 0.01 |
| Si | K | 29.76 ± 0.02 | 22.08 ± 0.02 |
| K | K | 6.21 ± 0.01 | 3.31 ± 0.01 |
| Acid treated phonolite (×5000)—Figure 5D | |||
| O | K | 43.74 ± 0.03 | 58.97 ± 0.05 |
| Na | K | 3.67 ± 0.01 | 3.44 ± 0.01 |
| Al | K | 7.13 ± 0.01 | 5.70 ± 0.01 |
| Si | K | 34.74 ± 0.03 | 26.68 ± 0.02 |
| K | K | 6.05 ± 0.01 | 3.34 ± 0.01 |
| Ca | K | 0.2 ± 0.01 | 0.11 ± 0.00 |
| Ti | K | 0.65 ± 0.01 | 0.29 ± 0.00 |
| Fe | K | 3.83 ± 0.02 | 1.48 ± 0.01 |
| NiW/Ph-HCl (×5000)—Figure 6B | |||
| Element | Line | Mass% | Atom% |
| O | K | 39.93 ± 0.03 | 60.51 ± 0.05 |
| Na | K | 2.12 ± 0.01 | 2.24 ± 0.01 |
| Al | K | 6.65 ± 0.01 | 5.97 ± 0.01 |
| Si | K | 26.86 ± 0.02 | 23.19 ± 0.02 |
| K | K | 6.34 ± 0.01 | 3.93 ± 0.01 |
| Fe | K | 0.38 ± 0.01 | 0.16 ± 0.00 |
| Ni | K | 4.87 ± 0.03 | 2.01 ± 0.01 |
| Sr | L | 2.01 ± 0.05 | 0.56 ± 0.01 |
| W | M | 10.84 ± 0.07 | 1.43 ± 0.01 |
| NiMo/Ph-HCl (×5000)—Figure 6D | |||
| O | K | 31.88 ± 0.04 | 65.58 ± 0.08 |
| Na | K | 0.32 ± 0.01 | 0.46 ± 0.01 |
| Al | K | 1.57 ± 0.01 | 1.91 ± 0.01 |
| Si | K | 7.28 ± 0.01 | 8.53 ± 0.02 |
| K | K | 2.11 ± 0.01 | 1.78 ± 0.01 |
| Ni | K | 10.34 ± 0.04 | 5.80 ± 0.02 |
| Mo | L | 46.49 ± 0.05 | 15.95 ± 0.02 |
| CoMo/Ph-HCl (×5000)—Figure 6F | |||
| O | K | 36.44 ± 0.07 | 70.68 ± 0.14 |
| Na | K | 0.38 ± 0.01 | 0.51 ± 0.01 |
| Al | K | 1.37 ± 0.01 | 1.58 ± 0.01 |
| Si | K | 5.04 ± 0.02 | 5.56 ± 0.02 |
| K | K | 1.02 ± 0.01 | 0.81 ± 0.01 |
| Co | K | 13.96 ± 0.08 | 7.35 ± 0.04 |
| Mo | L | 41.80 ± 0.08 | 13.52 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo Herrador, J.M.; Tišler, Z.; Kocík, J.; Frątczak, J.; Hradecká, I.; Velvarská, R.; de Paz Carmona, H. Mesityl Oxide Reduction by Using Acid-Modified Phonolite Supported NiW, NiMo, and CoMo Catalysts. Catalysts 2021, 11, 1101. https://doi.org/10.3390/catal11091101
Hidalgo Herrador JM, Tišler Z, Kocík J, Frątczak J, Hradecká I, Velvarská R, de Paz Carmona H. Mesityl Oxide Reduction by Using Acid-Modified Phonolite Supported NiW, NiMo, and CoMo Catalysts. Catalysts. 2021; 11(9):1101. https://doi.org/10.3390/catal11091101
Chicago/Turabian StyleHidalgo Herrador, José Miguel, Zdeněk Tišler, Jaroslav Kocík, Jakub Frątczak, Ivana Hradecká, Romana Velvarská, and Héctor de Paz Carmona. 2021. "Mesityl Oxide Reduction by Using Acid-Modified Phonolite Supported NiW, NiMo, and CoMo Catalysts" Catalysts 11, no. 9: 1101. https://doi.org/10.3390/catal11091101
APA StyleHidalgo Herrador, J. M., Tišler, Z., Kocík, J., Frątczak, J., Hradecká, I., Velvarská, R., & de Paz Carmona, H. (2021). Mesityl Oxide Reduction by Using Acid-Modified Phonolite Supported NiW, NiMo, and CoMo Catalysts. Catalysts, 11(9), 1101. https://doi.org/10.3390/catal11091101