Evaluation of Cr(VI) Reduction Using Indigenous Bacterial Consortium Isolated from a Municipal Wastewater Sludge: Batch and Kinetic Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bacteria Screening for Cr(VI) Reduction
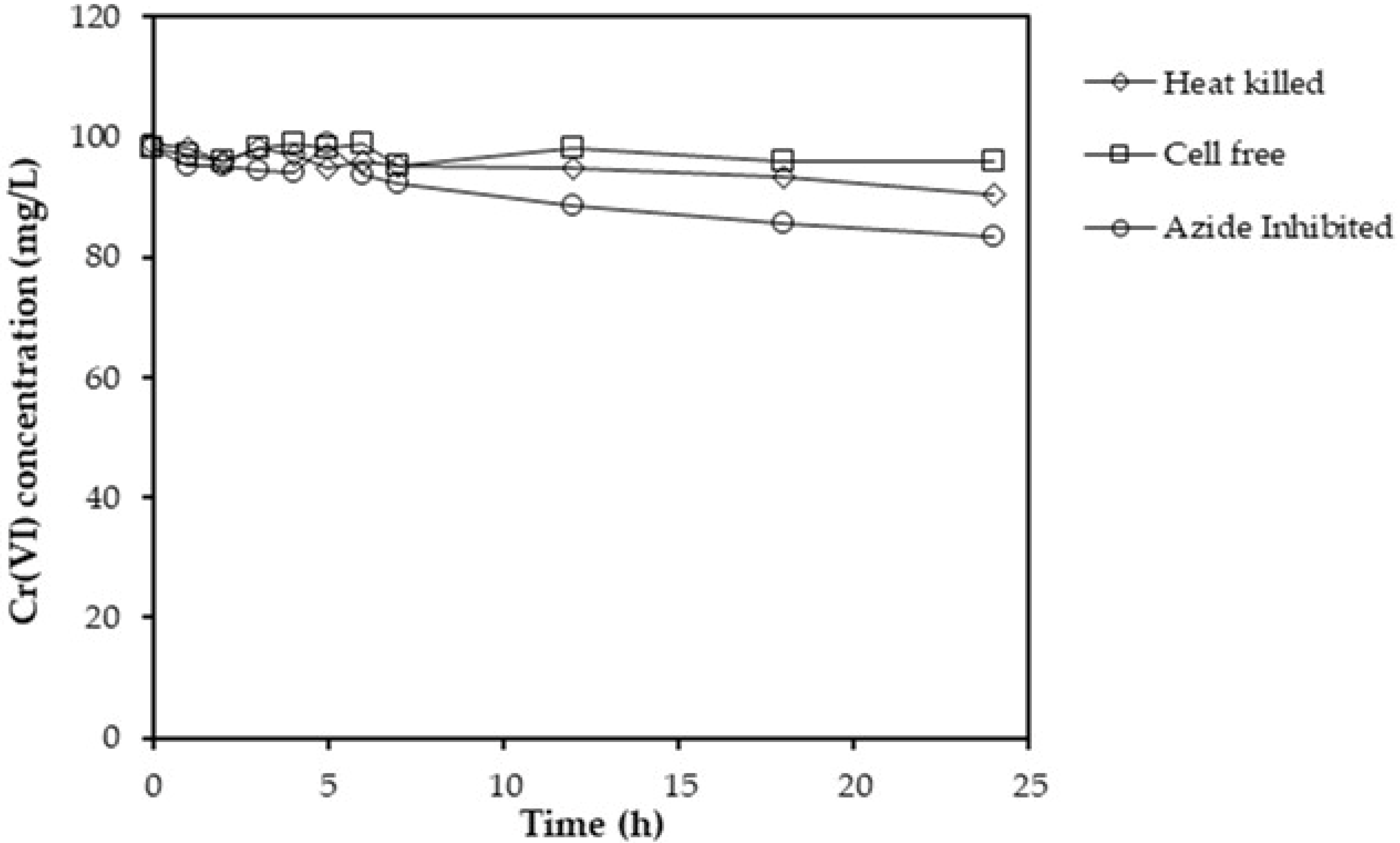
2.2. Abiotic Controls
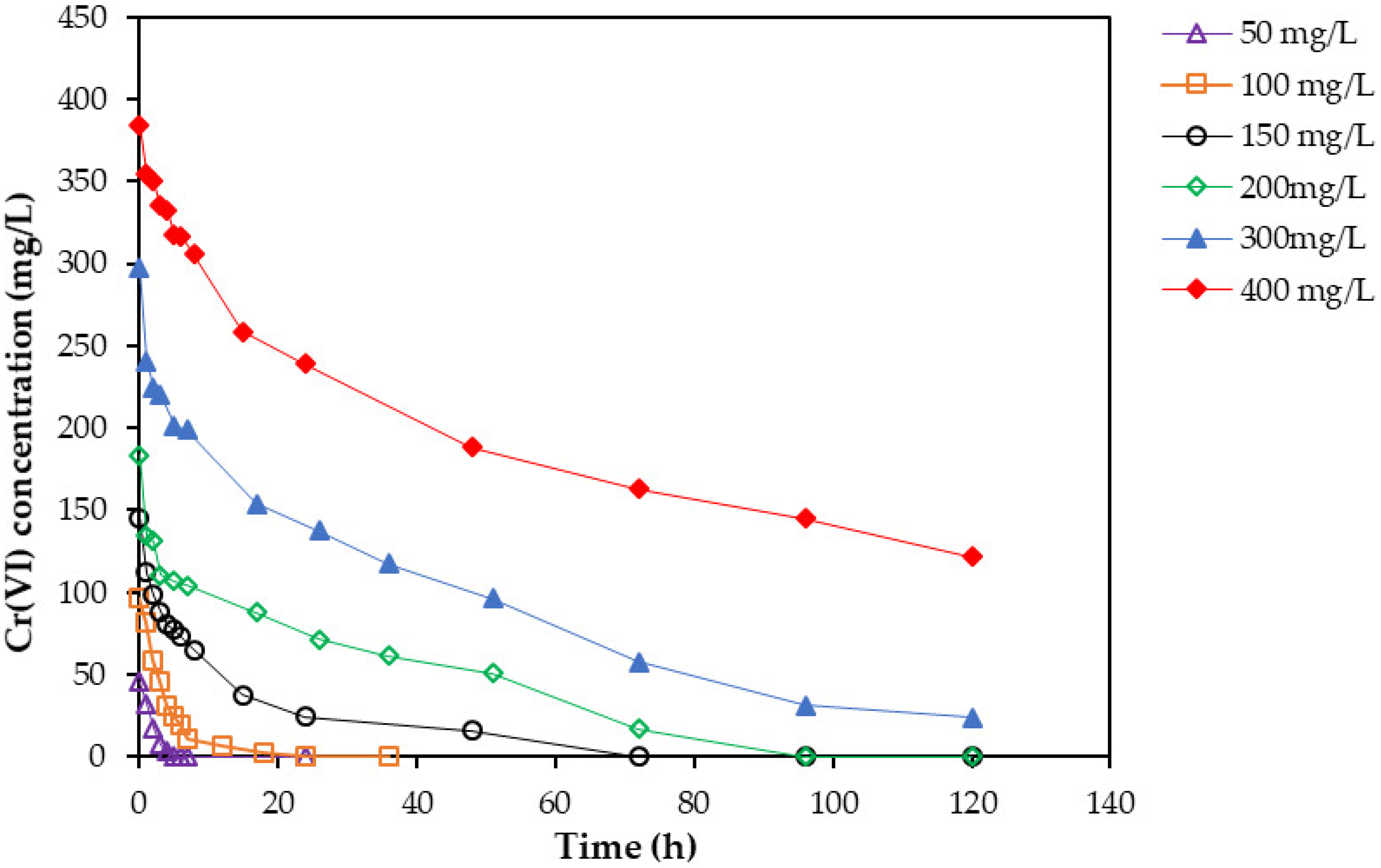
2.3. Effect of Cr(VI) Concentration
2.4. Bacteria Performance at Different Cr(VI) Concentrations
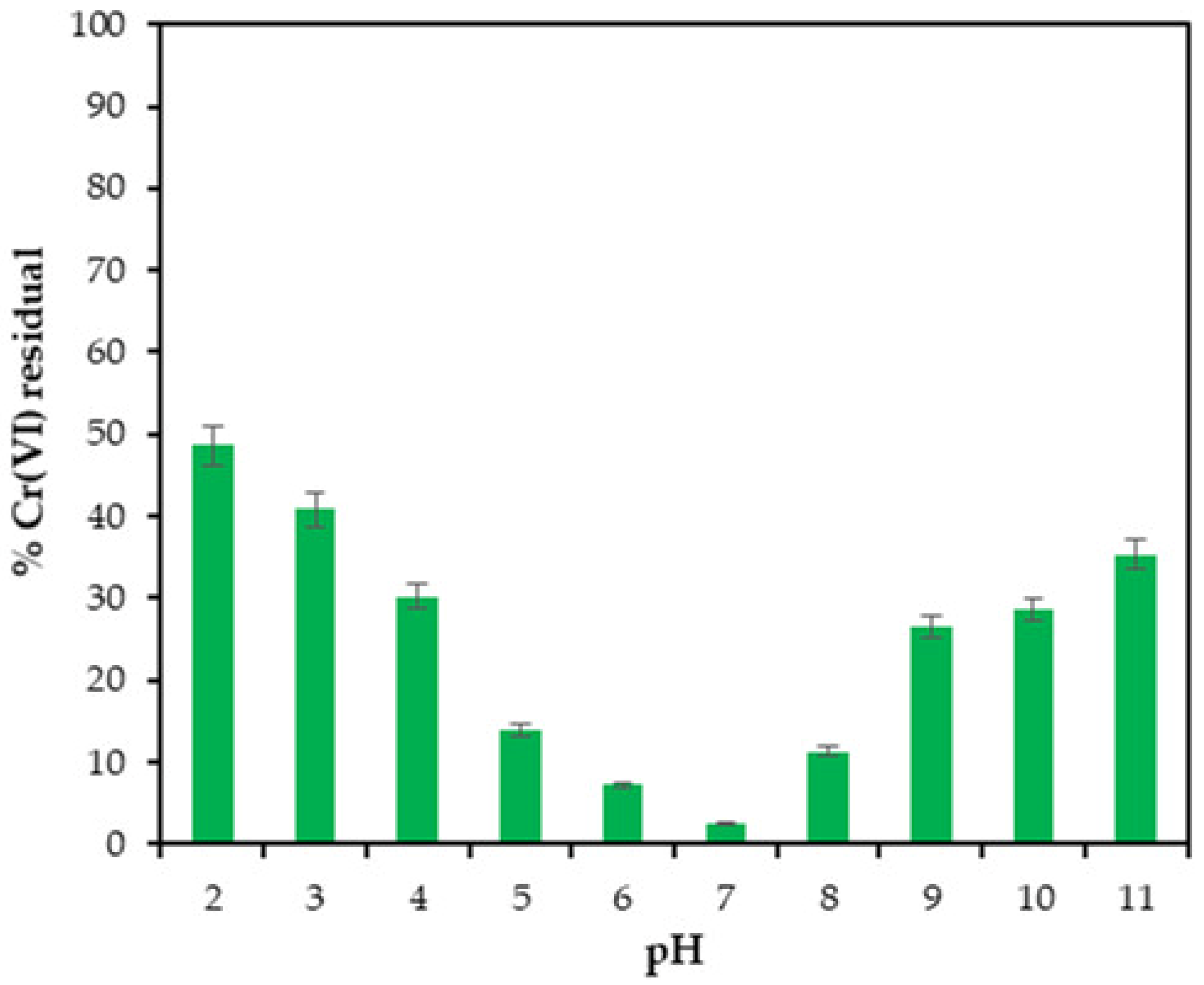
2.5. Effect of pH on Cr(VI) Reduction
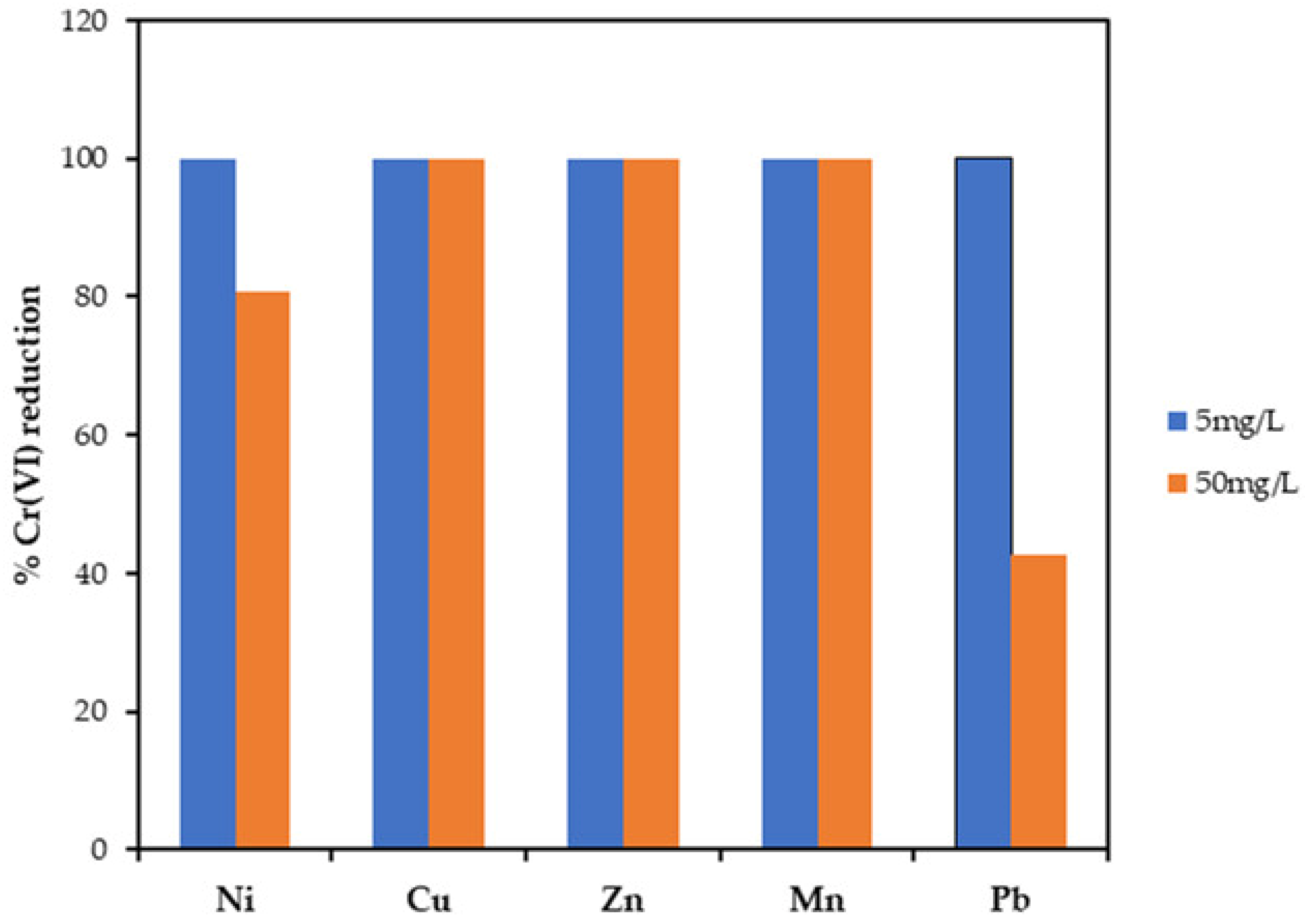
2.6. Effects of Coexisting Heavy Metals on Cr(VI) Reduction
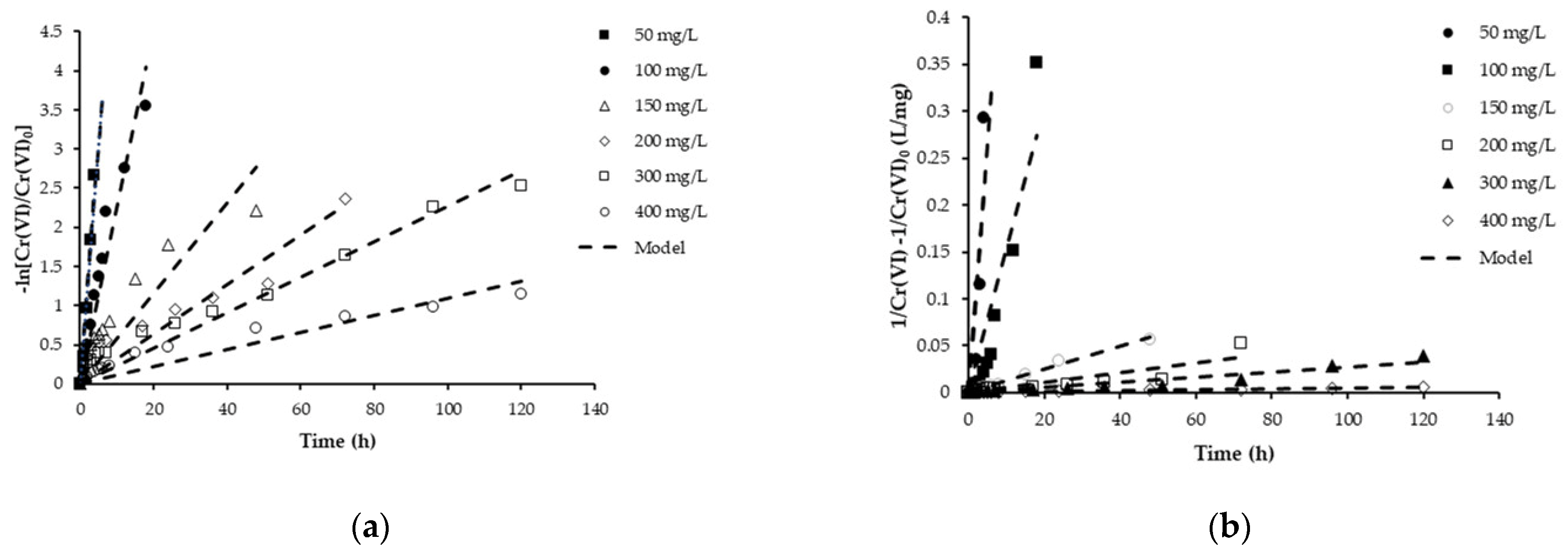
2.7. Kinetics of Cr(VI) Reduction by Bacteria Consortia
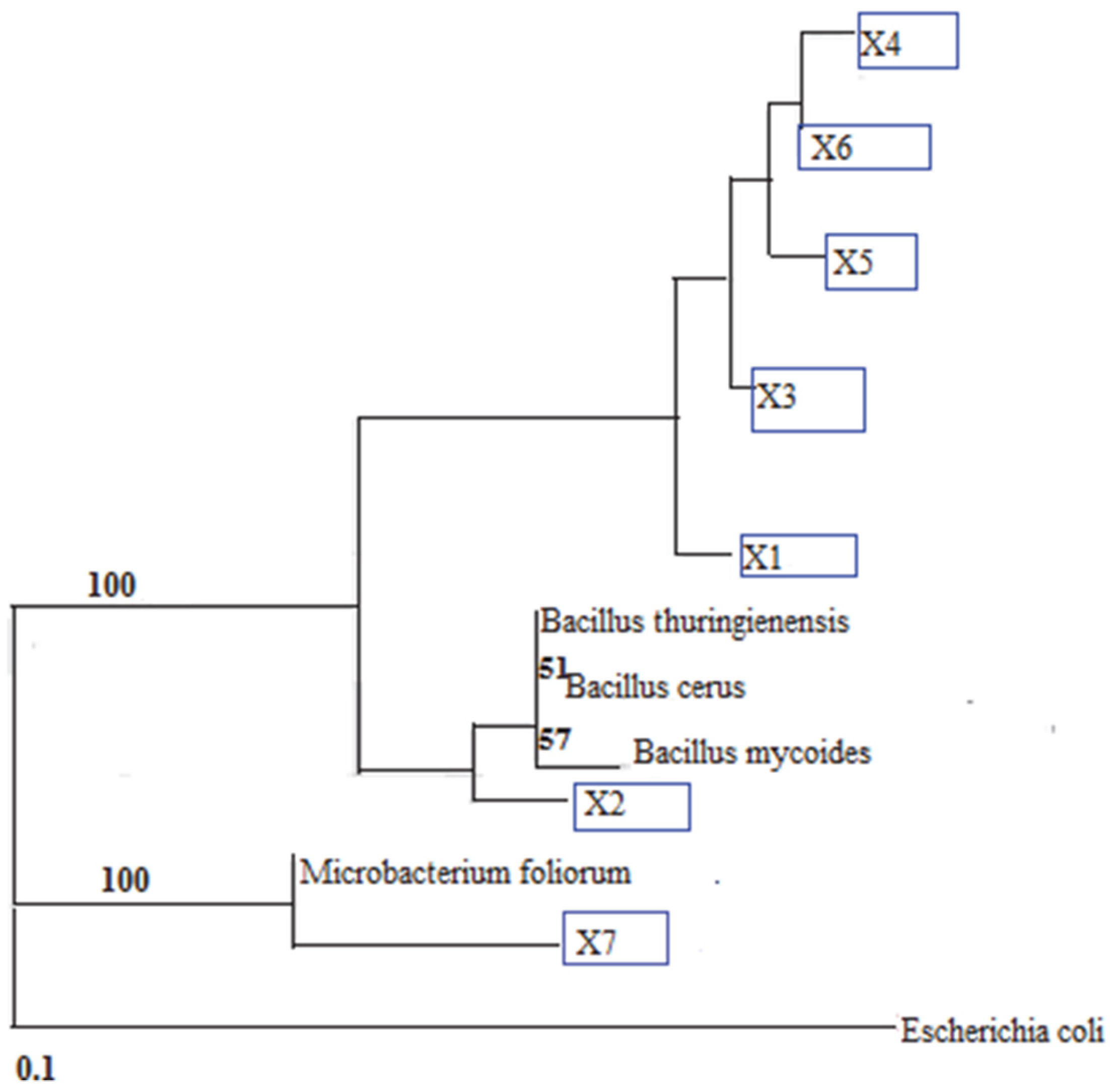
2.8. Microbial Characterization
3. Materials and Methods
3.1. Chemical Reagents
3.2. Bacteriul Culture
3.2.1. Source of Bacterial Culture
3.2.2. Culture Screening and Cr(VI) Tolerance
3.2.3. Microbial Characterization
3.3. Batch Studies
3.3.1. Abiotic Experiments
3.3.2. Aerobic Reduction Experiments
3.3.3. Effect of pH on Cr(VI) Reduction
3.3.4. Effect of Coexisting Heavy Metals on Cr(VI) Reduction
3.4. Kinetic Parameter Estimation for Cr(VI) Reduction by Bacteria Consortia
3.4.1. First-Order Kinetics
3.4.2. Second-Order Kinetics
3.5. Analytical Methods
3.6. Biomass Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and Microbial Remediation of Hexavalent Chromium from Contaminated Soil and Mining/Metallurgical Solid Waste: A Review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Fernandez, P.M.; Vinarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation Strategies for Chromium Removal: Current Research, Scale-up Approach and Future Perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Molokwane, P.E.; Meli, K.C.; Nkhalambayausi-chirwa, E.M. Chromium (VI) Reduction in Activated Sludge Bacteria Exposed to High Chromium Loading: Brits Culture (South Africa). Water Res. 2008, 42, 4538–4548. [Google Scholar] [CrossRef] [Green Version]
- Khakbaz, A.; De Nobili, M.; Mainardis, M.; Contin, M.; Aneggi, E.; Mattiussi, M.; Cabras, I.; Busut, M.; Goi, D. Monitoring of Heavy Metals, Eox and Las in Sewage Sludge for Agricultural Use: A Case Study. Detritus 2020, 12, 160–168. [Google Scholar] [CrossRef]
- Troiano, J.M.; Jordan, D.S.; Hull, C.J.; Geiger, F.M. Interaction of Cr(III) and Cr(VI) with Hematite Studied by Second Harmonic Generation. J. Phys. Chem. C 2013, 117, 5164–5171. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. Biosorption of Cr(VI) onto Marine Aspergillus Niger: Experimental Studies and Pseudo-Second Order Kinetics. World J. Microbiol. Biotechnol. 2009, 25, 1413–1421. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Hexavalent Chromium Reduction Ability and Bioremediation Potential of Aspergillus Flavus CR500 Isolated from Electroplating Wastewater. Chemosphere 2019, 237, 124567. [Google Scholar] [CrossRef] [PubMed]
- Murugavelh, S.; Mohanty, K. Bioreduction of Hexavalent Chromium by Free Cells and Cell Free Extracts of Halomonas Sp. Chem. Eng. J. 2012, 203, 415–422. [Google Scholar] [CrossRef]
- Villacís-García, M.; Villalobos, M.; Gutiérrez-Ruiz, M. Optimizing the Use of Natural and Synthetic Magnetites with Very Small Amounts of Coarse Fe(0) Particles for Reduction of Aqueous Cr(VI). J. Hazard. Mater. 2015, 281, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gai, L.; Tang, J.; Fu, J.; Wang, Q.; Zeng, E.Y. Reduction of Cr(VI) in Simulated Groundwater by FeS-Coated Iron Magnetic Nanoparticles. Sci. Total Environ. 2017, 595, 743–751. [Google Scholar] [CrossRef]
- Li, L.L.; Feng, X.Q.; Han, R.P.; Zang, S.Q.; Yang, G. Cr(VI) Removal via Anion Exchange on a Silver-Triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef]
- Ma, S.; Song, C.S.; Chen, Y.; Wang, F.; Chen, H.L. Hematite Enhances the Removal of Cr(VI) by Bacillus Subtilis BSn5 from Aquatic Environment. Chemosphere 2018, 208, 579–585. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, D.; Zhang, G.; Lan, H.; Liu, H.; Qu, J. Microfluidic Flow through Polyaniline Supported by Lamellar-Structured Graphene for Mass-Transfer-Enhanced Electrocatalytic Reduction of Hexavalent Chromium. Environ. Sci. Technol. 2015, 49, 13534–13541. [Google Scholar] [CrossRef]
- Cheng, S.F.; Huang, C.Y.; Tu, Y.T. Remediation of Soils Contaminated with Chromium Using Citric and Hydrochloric Acids: The Role of Chromium Fractionation in Chromium Leaching. Environ. Technol. 2011, 32, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Mtimunye, P.J.; Chirwa, E.M.N. Finite Difference Simulation of Biological Chromium (VI) Reduction in Aquifer Media Columns. Water SA 2014, 40, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Li, Y.; Zhang, X.; Liu, P.; Ren, J.; Wu, G.; Zhang, Y.; Chen, Y.; Li, X. A Bacillus Subtilis Strain Can Reduce Hexavalent Chromium to Trivalent and an NfrA Gene Is Involved. Int. Biodeterior. Biodegrad. 2015, 97, 90–96. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, J.; Xia, L.; Zhang, X.; Luo, L. Mechanisms of Cr(VI) Reduction by Bacillus Sp. CRB-1, a Novel Cr(VI)-Reducing Bacterium Isolated from Tannery Activated Sludge. Ecotoxicol. Environ. Saf. 2019, 186, 109792. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wang, C.; Zeng, G.; Luo, Y.; Li, H.; Xu, H. Bioreduction and Biosorption of Cr(VI) by a Novel Bacillus Sp. CRB-B1 Strain. J. Hazard. Mater. 2020, 386, 121628. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Murugan, K.; Benelli, G.; Higuchi, A.; Rajasekar, A. Bioreduction of Hexavalent Chromium by Pseudomonas Stutzeri L1 and Acinetobacter Baumannii L2. Ann. Microbiol. 2017, 67, 91–98. [Google Scholar] [CrossRef]
- Wani, P.A.; Wahid, S.; Khan, M.S.A.; Rafi, N.; Wahid, N. Investigation of the Role of Chromium Reductase for Cr (VI) Reduction by Pseudomonas Species Isolated from Cr (VI) Contaminated Effluent. Biotechnol. Res. Innov. 2019, 3, 38–46. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Luo, X.; Zhu, Y.; Xia, L.; Luo, L. Simultaneous Ammonia and Cr (VI) Removal by Pseudomonas Aeruginosa LX in Wastewater. Biochem. Eng. J. 2020, 157, 107551. [Google Scholar] [CrossRef]
- Kumar, M.; Saini, H.S. Reduction of Hexavalent Chromium (VI) by Indigenous Alkaliphilic and Halotolerant Microbacterium Sp. M5: Comparative Studies under Growth and Nongrowth Conditions. J. Appl. Microbiol. 2019, 127, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Elahi, A.; Ajaz, M.; Rehman, A.; Vuilleumier, S.; Khan, Z.; Hussain, S.Z. Isolation, Characterization, and Multiple Heavy Metal-Resistant and Hexavalent Chromium-Reducing Microbacterium Testaceum B-HS2 from Tannery Effluent. J. King Saud Univ.-Sci. 2019, 31, 1437–1444. [Google Scholar] [CrossRef]
- Mbonambi, N.C.; Chirwa, E.M.N. Biological Remediation of Chromium (VI) in Aquifer Media Columns. Chem. Eng. Trans. 2019, 76, 1333–1338. [Google Scholar] [CrossRef]
- Sun, Y.; Lan, J.; Du, Y.; Guo, L.; Du, D.; Chen, S.; Ye, H.; Zhang, T.C. Chromium(VI) Bioreduction and Removal by Enterobacter Sp. SL Grown with Waste Molasses as Carbon Source: Impact of Operational Conditions. Bioresour. Technol. 2020, 302, 121974. [Google Scholar] [CrossRef]
- Murugavelh, S.; Mohanty, K. Performance of Halomonas Sp. to Reduce Hexavalent Chromium in Batch and Continuous Fixed Film Reactor. J. Environ. Chem. Eng. 2018, 6, 2561–2567. [Google Scholar] [CrossRef]
- Mohamed, M.S.M.; El-Arabi, N.I.; El-Hussein, A.; El-Maaty, S.A.; Abdelhadi, A.A. Reduction of Chromium-VI by Chromium-Resistant Escherichia Coli FACU: A Prospective Bacterium for Bioremediation. Folia Microbiol. 2020, 65, 687–696. [Google Scholar] [CrossRef]
- Liu, Y.G.; Xu, W.H.; Zeng, G.M.; Li, X.; Gao, H. Cr(VI) Reduction by Bacillus Sp. Isolated from Chromium Landfill. Process. Biochem. 2006, 41, 1981–1986. [Google Scholar] [CrossRef]
- Banerjee, S.; Misra, A.; Chaudhury, S.; Dam, B. A Bacillus Strain TCL Isolated from Jharia Coalmine with Remarkable Stress Responses, Chromium Reduction Capability and Bioremediation Potential. J. Hazard. Mater. 2019, 367, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, M.; Liu, L.; Chang, J.; Zhang, J. Treatment of High-Concentration Chromium-Containing Wastewater by Sulfate-Reducing Bacteria Acclimated with Ethanol. Water Sci. Technol. 2019, 80, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lingen, E.; Paton, A. Market Implications for Technology Acquisition Modes in the South African Ferrochrome Context. J. South. Afr. Inst. Min. Metall. 2018, 118, 1087–1094. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.T.; Shen, H. Modelling Cr(VI) Reduction by Pure Bacterial Cultures. Water Res. 1997, 31, 727–732. [Google Scholar] [CrossRef]
- Elangovan, R.; Abhipsa, S.; Rohit, B.; Ligy, P.; Chandraraj, K. Reduction of Cr(VI) by a Bacillus Sp. Biotechnol. Lett. 2006, 28, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Morales-Barrera, L.; Cristiani-Urbina, E. Hexavalent Chromium Removal by a Trichoderma Inhamatum Fungal Strain Isolated from Tannery Effluent. Water Air Soil Pollut. 2008, 187, 327–336. [Google Scholar] [CrossRef]
- Middleton, S.S.; Latmani, R.B.; Mackey, M.R.; Ellisman, M.H.; Tebo, B.M.; Criddle, C.S. Cometabolism of Cr(VI) by Shewanella Oneidensis MR-1 Produces Cell-Associated Reduced Chromium and Inhibits Growth. Biotechnol. Bioeng. 2003, 83, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.A.; Zakaria, Z.; Surif, S.; Ahmad, W.A. Hexavalent Chromium Reduction by Acinetobacter Haemolyticus Isolated from Heavy-Metal Contaminated Wastewater. J. Hazard. Mater. 2007, 146, 30–38. [Google Scholar] [CrossRef]
- Jeyasingh, J.; Philip, L. Bioremediation of Chromium Contaminated Soil: Optimization of Operating Parameters under Laboratory Conditions. J. Hazard. Mater. 2005, 118, 113–120. [Google Scholar] [CrossRef]
- Karthika, N.; Jananee, K.; Murugaiyan, V. Remediation of Contaminated Soil Using Soil Washing—A Review. J. Eng. Res. Appl. 2016, 6, 13–18. [Google Scholar]
- Mangaiyarkarasi, M.S.M.; Rao, T.S.; Tata, B.V.R. Bioreduction of Cr ( VI ) by Alkaliphilic Bacillus Subtilis and Interaction of the Membrane Groups. Saudi J. Biol. Sci. 2011, 18, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zeng, Q.; Hu, L.; Zhong, H.; He, Z. Bioreduction Performances and Mechanisms of Cr(VI) by Sporosarcina Saromensis W5, a Novel Cr(VI)-Reducing Facultative Anaerobic Bacteria. J. Hazard. Mater. 2021, 413, 125411. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jian, H.; Liu, Y. Bioreduction of Chromate by an Isolated Bacillus Anthracis Cr-4 with Soluble Cr (III) Product. Water Air Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gupta, A. Evaluation of Acinetobacter Sp. B9 for Cr (VI) Resistance and Detoxification with Potential Application in Bioremediation of Heavy-Metals-Rich Industrial Wastewater. Environ. Sci. Pollut. Res. 2013, 20, 6628–6637. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.N.; Karthick, R.; Muthukumar, K. Ex Situ Bioremediation of Cr(VI) Contaminated Soil by Bacillus Sp.: Batch and Continuous Studies. Chem. Eng. J. 2011, 169, 107–115. [Google Scholar] [CrossRef]
- Soni, S.K.; Singh, R.; Awasthi, A. In Vitro Cr(VI) Reduction by Cell-Free Extracts of Chromate-Reducing Bacteria Isolated from Tannery Effluent Irrigated Soil. Environ. Sci. Pollut. Res. 2013, 20, 1661–1674. [Google Scholar] [CrossRef]
- Upadhyay, N.; Vishwakarma, K.; Singh, J.; Mishra, M.; Tripathi, D.K.; Sharma, S. Tolerance and Reduction of Chromium (VI) by Bacillus Sp. MNU16 Isolated from Contaminated Coal Mining Soil. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, N.; Coetzee, J.J.; Chirwa, E.M.N. In Situ Bioremediation of Hexavalent Chromium in Presence of Iron by Dried Sludge Bacteria Exposed to High Chromium Concentration. Ecotoxicol. Environ. Saf. 2019, 172, 281–289. [Google Scholar] [CrossRef]

| Cr(VI) Concentration (mg/L) | Percentage Reduction | ||
|---|---|---|---|
| Sludge A | Sludge B | Sludge C | |
| 100 | 100 | 100 | 100 |
| 200 | 60.7 | 65.4 | 57.2 |
| 300 | 25.9 | 29.9 | 20.1 |
| 400 | 4.7 | 4.5 | 11.4 |
| 500 | 0.29 | 5.0 | 6.2 |
| Cr(VI) Concentration | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|
| k1 | R2 | k2 | R2 | |
| 50 | 0.615 | 0.96 | 0.0532 | 0.74 |
| 100 | 0.225 | 0.93 | 0.0152 | 0.85 |
| 150 | 0.056 | 0.69 | 0.0012 | 0.99 |
| 200 | 0.032 | 0.82 | 0.0005 | 0.79 |
| 300 | 0.023 | 0.94 | 0.0003 | 0.91 |
| 400 | 0.011 | 0.86 | 0.00005 | 0.97 |
| Blast Results | Pure Isolates | ID Index | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | ||
| B. cereus ATCC 10987 | - | √ | √ | - | - | - | - | 0.99 |
| B. thuringiensis serovar finitimus strain BGSC 4B2 16S | - | - | - | √ | √ | √ | - | 0.99 |
| B. thuringiensis str. Al Hakam | - | √ | √ | - | - | - | - | 0.99 |
| Bacillus cereus strain 213 16S | √ | - | - | - | - | - | - | 0.99 |
| Bacillus mycoides strain BGSC 6A13 16S | - | - | - | √ | √ | √ | - | 0.99 |
| Bacillus sp. ZZ2 16S | - | √ | √ | - | - | - | - | 0.99 |
| Bacillus thuringiensis 16S | √ | - | - | - | - | - | - | 0.99 |
| Microbacterium foliorum | - | - | - | - | - | - | √ | 0.99 |
| Microbacterium sp. S15-M4 | - | - | - | - | - | - | √ | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholisa, B.; Matsena, M.; Chirwa, E.M.N. Evaluation of Cr(VI) Reduction Using Indigenous Bacterial Consortium Isolated from a Municipal Wastewater Sludge: Batch and Kinetic Studies. Catalysts 2021, 11, 1100. https://doi.org/10.3390/catal11091100
Kholisa B, Matsena M, Chirwa EMN. Evaluation of Cr(VI) Reduction Using Indigenous Bacterial Consortium Isolated from a Municipal Wastewater Sludge: Batch and Kinetic Studies. Catalysts. 2021; 11(9):1100. https://doi.org/10.3390/catal11091100
Chicago/Turabian StyleKholisa, Buyisile, Mpumelelo Matsena, and Evans M. N. Chirwa. 2021. "Evaluation of Cr(VI) Reduction Using Indigenous Bacterial Consortium Isolated from a Municipal Wastewater Sludge: Batch and Kinetic Studies" Catalysts 11, no. 9: 1100. https://doi.org/10.3390/catal11091100
APA StyleKholisa, B., Matsena, M., & Chirwa, E. M. N. (2021). Evaluation of Cr(VI) Reduction Using Indigenous Bacterial Consortium Isolated from a Municipal Wastewater Sludge: Batch and Kinetic Studies. Catalysts, 11(9), 1100. https://doi.org/10.3390/catal11091100







