Recent Advances in the Synthesis of Isothiocyanates Using Elemental Sulfur
Abstract
:1. Introduction
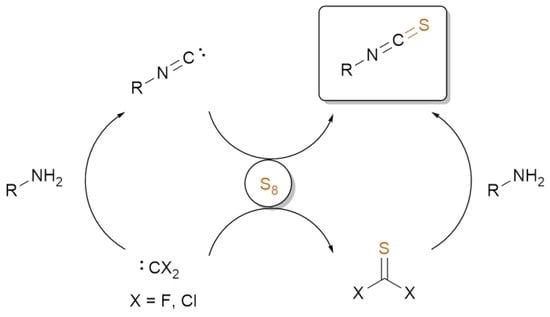
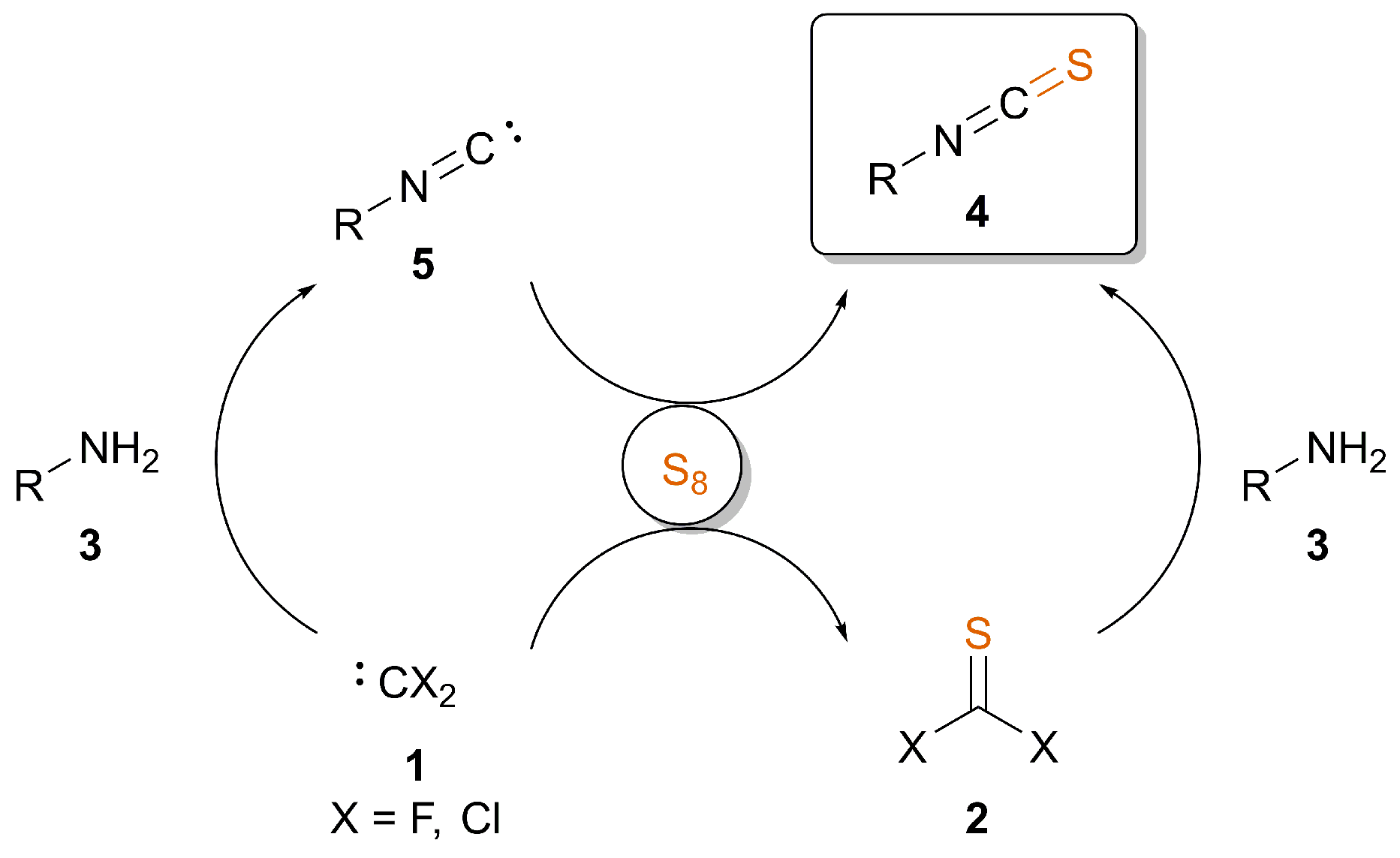
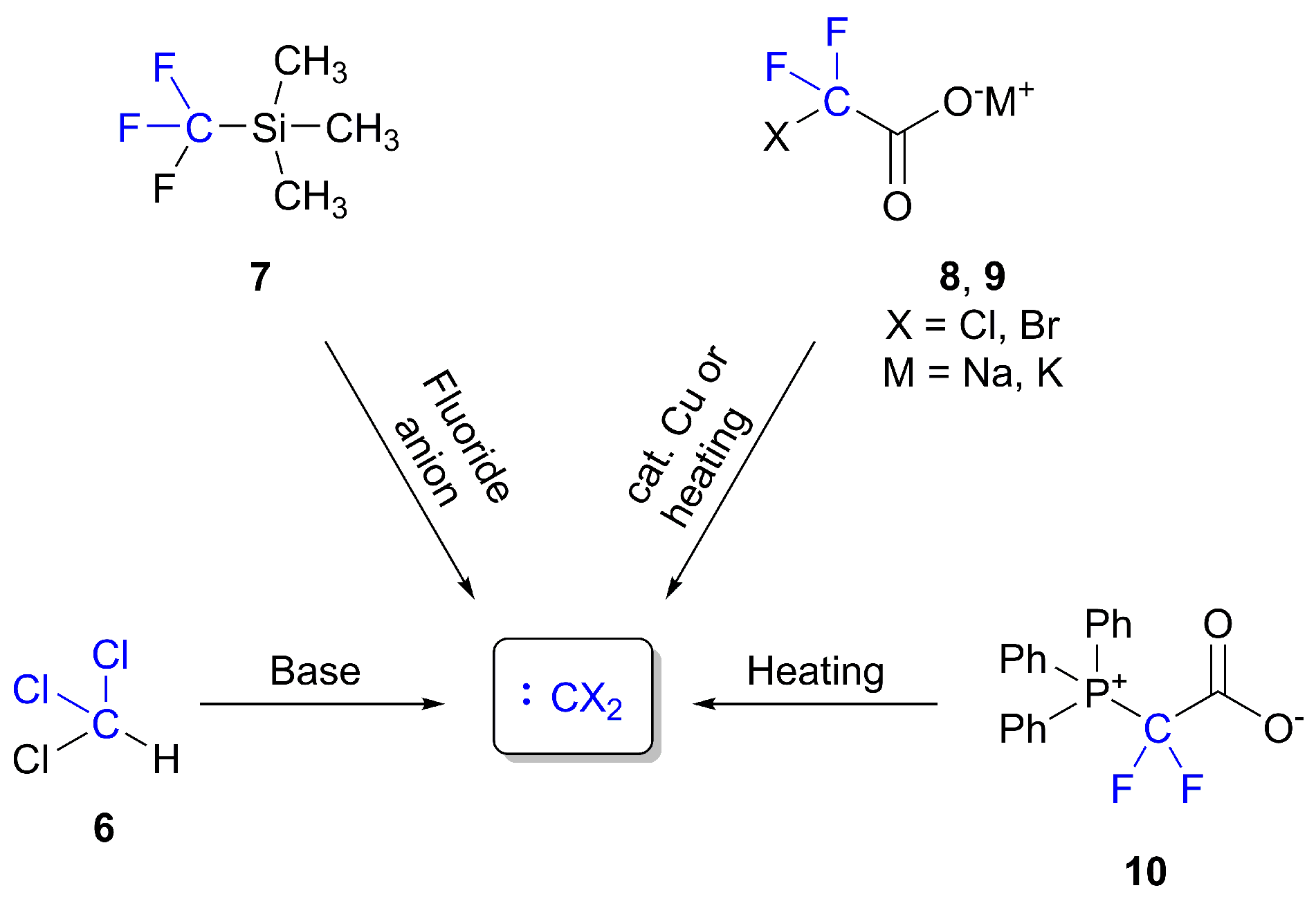
2. Synthesis of ITCs through Thiocarbonyl Surrogates
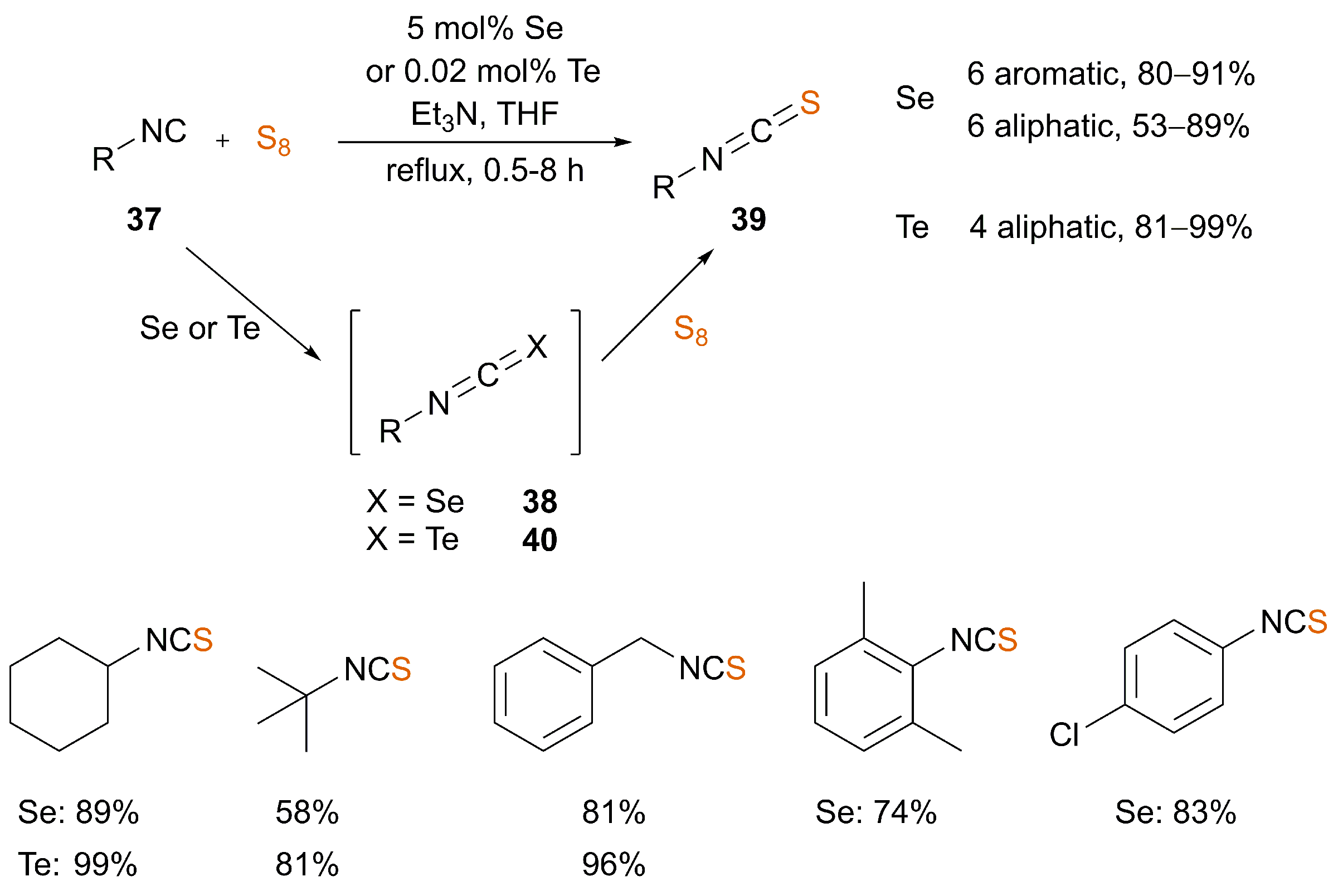
3. Synthesis of ITCs from Isocyanides
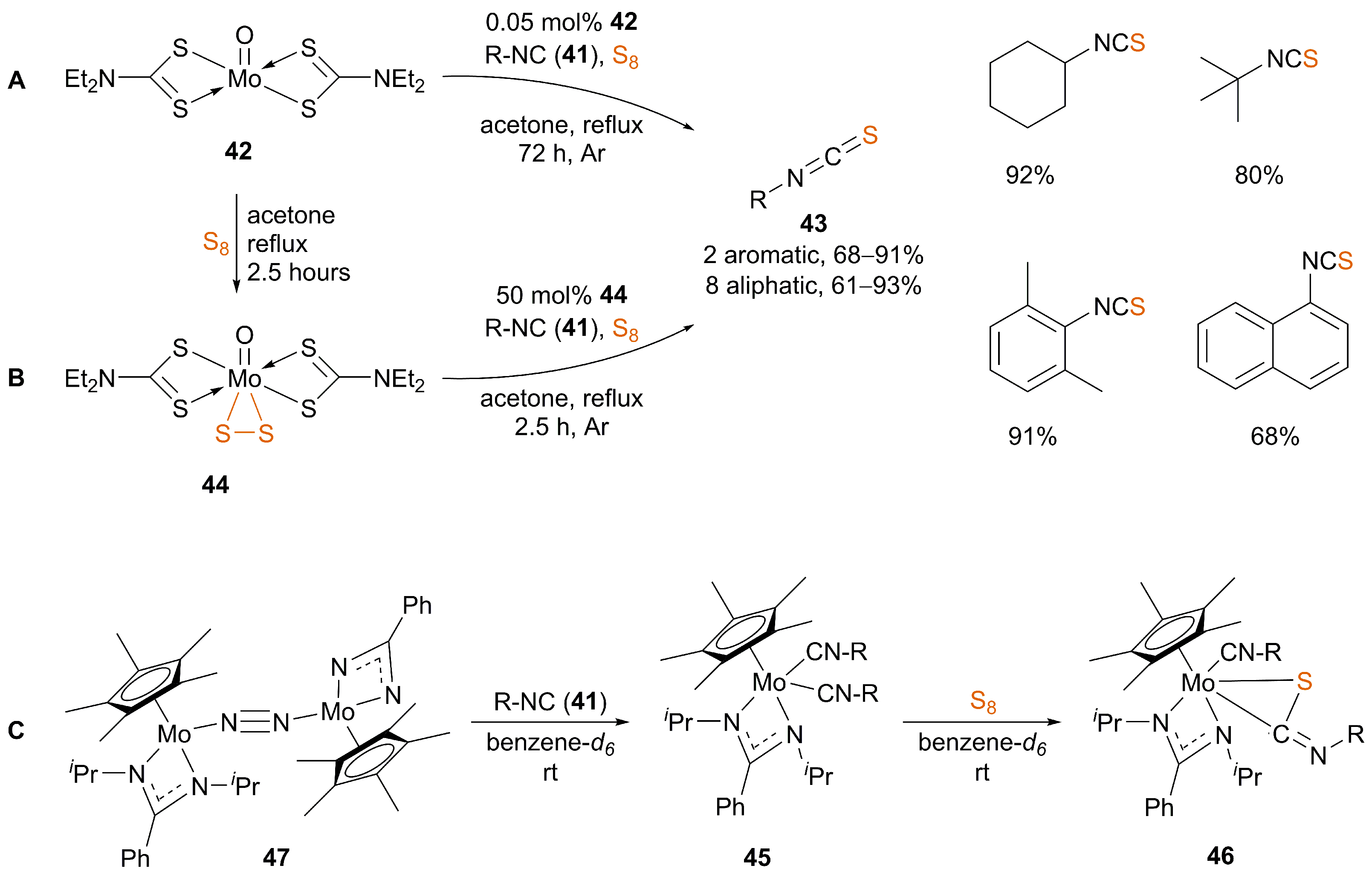
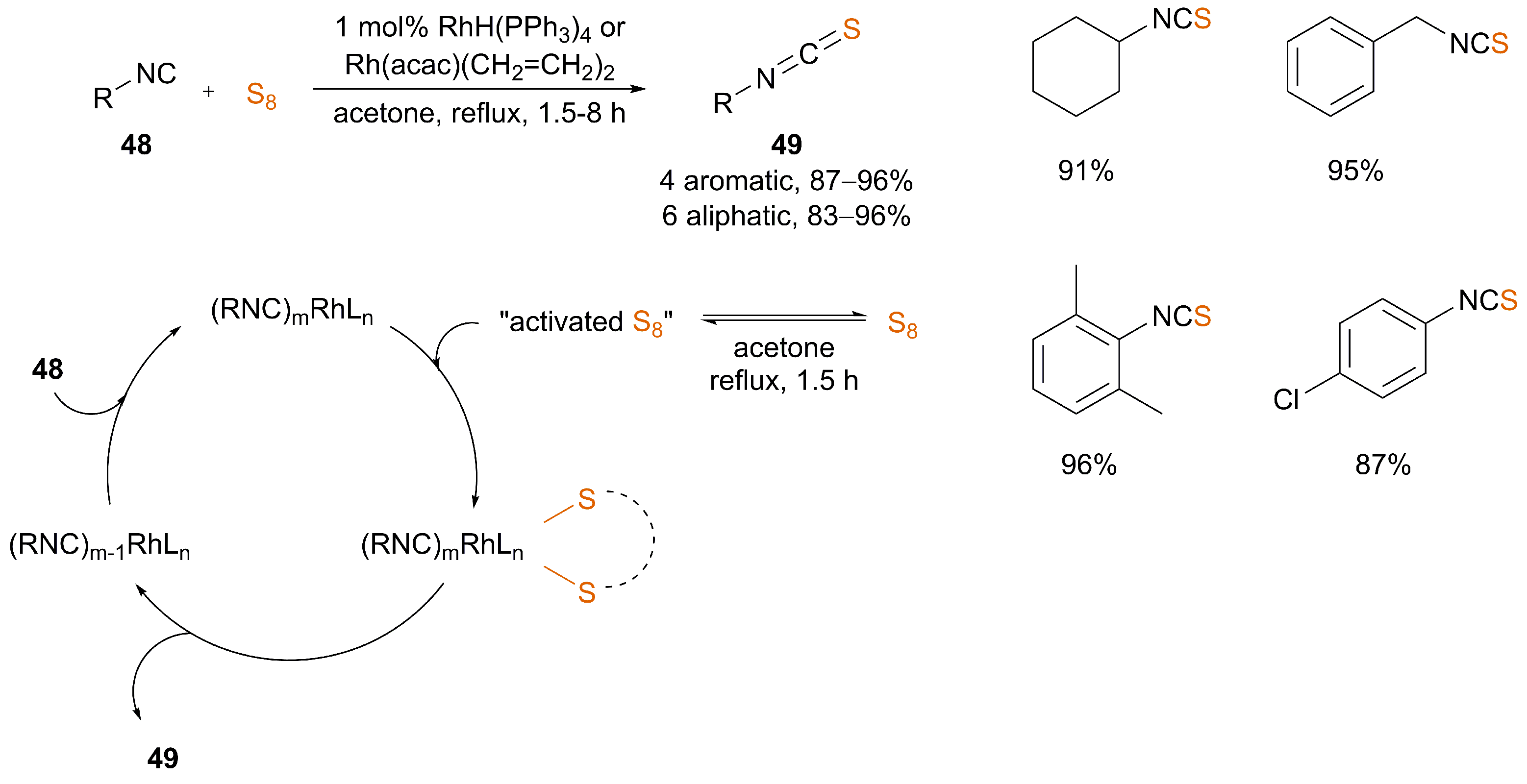
3.1. Catalysis
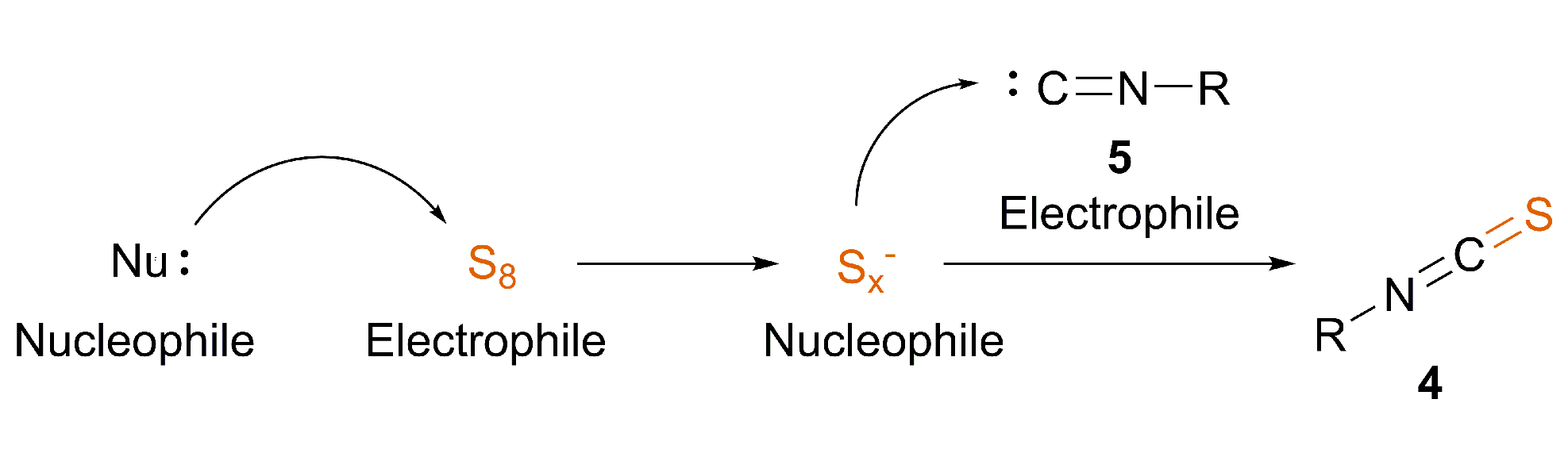
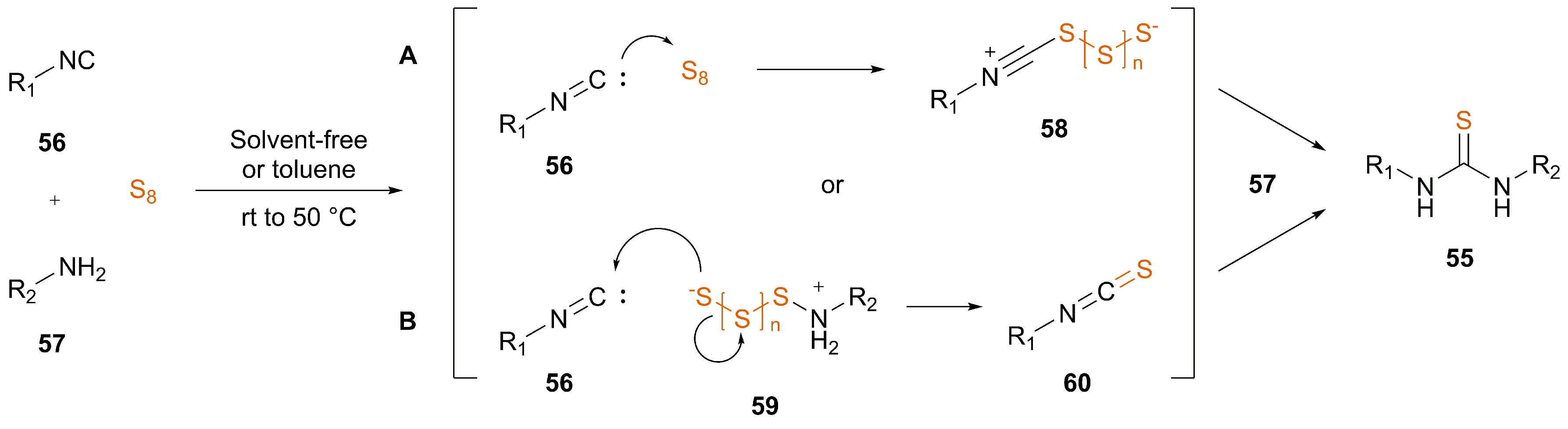
3.2. Nucleophile-Induced Transformation of Isocyanide to ITC
4. Overview and Practical Considerations of the Discussed Methods
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Kala, C.; Ali, S.S.; Ahmad, N.; Gilani, S.J.; Khan, N.A. Isothiocyanates: A Review Chandra. Res. J. Pharmacogn. 2018, 5, 71–89. [Google Scholar] [CrossRef]
- Sugiyama, R.; Li, R.; Kuwahara, A.; Nakabayashi, R.; Sotta, N.; Mori, T. Retrograde sulfur flow from glucosinolates to cysteine in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2017890118. [Google Scholar] [CrossRef]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.P.; Long, M.J.C.; Coffey, R.T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Naturally occurring isothiocyanates exert anticancer effects by inhibiting deubiquitinating enzymes. Cancer Res. 2015, 75, 5130–5142. [Google Scholar] [CrossRef] [Green Version]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petri, L.; Szijj, P.A.; Kelemen, Á.; Imre, T.; Gömöry, Á.; Lee, M.T.W.; Hegedus, K.; Ábrányi-Balogh, P.; Chudasama, V.; Keseru, G.M. Cysteine specific bioconjugation with benzyl isothiocyanates. RSC Adv. 2020, 10, 14928–14936. [Google Scholar] [CrossRef]
- Abdeldayem, A.; Raouf, Y.S.; Constantinescu, S.N.; Moriggl, R.; Gunning, P.T. Advances in covalent kinase inhibitors. Chem. Soc. Rev. 2020, 49, 2617–2687. [Google Scholar] [CrossRef]
- Kulkarni, P.M.; Kulkarni, A.R.; Korde, A.; Tichkule, R.B.; Laprairie, R.B.; Denovan-Wright, E.M.; Zhou, H.; Janero, D.R.; Zvonok, N.; Makriyannis, A.; et al. Novel Electrophilic and Photoaffinity Covalent Probes for Mapping the Cannabinoid 1 Receptor Allosteric Site(s). J. Med. Chem. 2016, 59, 44–60. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Hamachi, I. Chemistry for Covalent Modification of Endogenous/Native Proteins: From Test Tubes to Complex Biological Systems. J. Am. Chem. Soc. 2019, 141, 2782–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.D.; Tidwell, T.T. Ketenes and other cumulenes as reactive intermediates. Chem. Rev. 2013, 113, 7287–7342. [Google Scholar] [CrossRef]
- Mukerjee, A.K.; Ashare, R. Isothiocyanates in the Chemistry of Heterocycles. Chem. Rev. 1991, 91, 1–24. [Google Scholar] [CrossRef]
- Norris, B.C.; Bielawski, C.W. Structurally dynamic materials based on bis(N-heterocyclic carbene)s and bis(isothiocyanate)s: Toward reversible, conjugated polymers. Macromolecules 2010, 43, 3591–3593. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Gajda, A.; Gajda, T. Direct, Microwave-Assisted Synthesis of Isothiocyanates. Eur. J. Org. Chem. 2019, 2019, 2528–2532. [Google Scholar] [CrossRef]
- Munch, H.; Hansen, J.S.; Pittelkow, M.; Christensen, J.B.; Boas, U. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119. [Google Scholar] [CrossRef]
- Sun, N.; Li, B.; Shao, J.; Mo, W.; Hu, B.; Shen, Z.; Hu, X. A general and facile one-pot process of isothiocyanates from amines under aqueous conditions. Beilstein J. Org. Chem. 2012, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Yuan, W.; Chen, N.; Yang, Z.; Xu, J. Na2S2O8-mediated efficient synthesis of isothiocyanates from primary amines in water. Green Chem. 2018, 20, 4484–4491. [Google Scholar] [CrossRef]
- Bassetto, M.; Ferla, S.; Pertusati, F.; Kandil, S.; Westwell, A.D.; Brancale, A.; Mcguigan, C. Design and synthesis of novel bicalutamide and enzalutamide derivatives as antiproliferative agents for the treatment of prostate cancer. Eur. J. Med. Chem. 2016, 118, 230–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Yi, K.Y. Di-2-pyridyl thionocarbonate. A new reagent for the preparation of isothiocyanates and carbodiimides. Tetrahedron Lett. 1985, 26, 1661–1664. [Google Scholar] [CrossRef]
- Larsen, C.; Steliou, K.; Harpp, D.N. Thiocarbonyl Transfer Reagents. J. Org. Chem. 1978, 43, 337–339. [Google Scholar] [CrossRef]
- Wong, R.; Dolman, S.J. Isothiocyanates from Tosyl Chloride Mediated Decomposition of in Situ Generated Dithiocarbamic Acid Salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Ghosh, H.; Yella, R.; Patel, B.K. Molecular Iodine Mediated Preparation of Isothiocyanates from Dithiocarbamic Acid Salts. Eur. J. Org. Chem. 2009, 1849–1851. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, Y.K.; Kelley, J.A.; Burke, T.R. Preparation of Aryl Isothiocyanates via Protected Phenylthiocarbamates and Application to the Synthesis of Caffeic Acid (4-Isothiocyanato) phenyl Ester Isothiocyanates have been widely used in organic thetic isothiocyanates have been reported to exhibit. J. Org. Chem. 2000, 65, 6237–6240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, H.; Han, C.; Xi, H.; Meng, Q.; Chen, X.; Sun, X. Synthesis of Isothiocyanates by Reaction of Amines with Phenyl Chlorothiono- formate via One-Pot or Two-Step Process. Synthesis 2013, 45, 1667–1674. [Google Scholar] [CrossRef]
- Rong, H.J.; Chen, T.; Xu, Z.G.; Su, T.D.; Shang, Y.; Wang, Y.Q.; Yang, C.F. 4-Dimethylaminopyridine-catalyzed synthesis of isothiocyanates from amines and carbon disulfide. Tetrahedron Lett. 2021, 68, 152868. [Google Scholar] [CrossRef]
- Reagent, D.; Janczewski, Ł.; Kreigel, D. Synthesis of Isothiocyanates Using DMT/NMM/TsO—As a New Desulfurization Reagent. Molecules 2021, 26, 2740. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. The rapid generation of isothiocyanates in flow. Beilstein J. Org. Chem. 2013, 9, 1613–1619. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Uemura, S.; Okano, M. The Thallium(I) Salt-catalyzed Formation of Isothiocyanates form Isocyanides and Disulfides. Bull. Chem. Soc. Jpn. 1977, 50, 2785–2788. [Google Scholar] [CrossRef] [Green Version]
- Eschliman, K.; Bossmann, S.H. Synthesis of Isothiocyanates: An Update. Synthesis 2019, 51, 1746–1752. [Google Scholar] [CrossRef]
- Boyer, J.H.; Ramakrishnan, V.T. Sulfurization of Isocyanides. J. Org. Chem. 1972, 37, 1360–1364. [Google Scholar] [CrossRef]
- Reisfen, M. Zur Reaktion von Amidacetalen rnit Heterocumulenen. Chem. Ber. 1977, 110, 37–48. [Google Scholar] [CrossRef]
- Cunico, R.F.; Maity, B.C. Direct Carbamoylation of Alkenyl Halides. Org. Lett. 2003, 5, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Schanz, H.J.; Stevens, E.D.; Nolan, S.P.; Capps, K.B.; Bauer, A.; Hoff, C.D. Structural and solution calorimetric studies of sulfur binding to nucleophilic carbenes. Inorg. Chem. 2000, 39, 1042–1045. [Google Scholar] [CrossRef]
- Sharma, S. Isothiocyanates in Heterocyclic Synthesis. Sulf. Rep. 1989, 8, 327–454. [Google Scholar] [CrossRef]
- Kowaoka, Y. Studies of Rubber Vulcanization Accelerators, V. J. Soc. Chem. Ind. Jpn. Suppl. 1940, 43, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.E. Nucleophilic Displacement Reactions at the Sulfur-Sulfur Bond. In Survey of Progress in Chemistry: Volume 2; Scott, A.F., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1964; Volume 2, pp. 189–238. [Google Scholar]
- Nickisch, R.; Conen, P.; Gabrielsen, S.M.; Meier, M.A.R. A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv. 2021, 11, 3134–3142. [Google Scholar] [CrossRef]
- Németh, A.G.; Keserű, G.M.; Ábrányi-Balogh, P. A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: A mild, catalyst-free approach towards O -thiocarbamates and dithiocarbamates. Beilstein J. Org. Chem. 2019, 15, 1523–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Németh, A.G.; Szabó, R.; Domján, A.; Keserű, G.M.; Ábrányi-Balogh, P. Chromatography-free multicomponent synthesis of thioureas enabled by aqueous solution of elemental sulfur. ChemistryOpen 2020, 10, 16–27. [Google Scholar] [CrossRef]
- Németh, A.G.; Szabó, R.; Orsy, G.; Mándity, I.M.; Keserű, G.M.; Ábrányi-Balogh, P. Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution. Molecules 2021, 26, 303. [Google Scholar] [CrossRef]
- Németh, A.G.; Marlok, B.; Domján, A.; Gao, Q.; Han, X.; Keserű, G.M.; Ábrányi-Balogh, P. Convenient multicomponent one-pot synthesis of 2-iminothiazolines and 2-aminothiazoles using elemental sulfur under aqueous conditions. Eur. J. Org. Chem. 2021, 28–33. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. Three-component reaction between isocyanides, aliphatic amines and elemental sulfur: Preparation of thioureas under mild conditions with complete atom economy. Synthesis 2014, 46, 3172–3179. [Google Scholar] [CrossRef]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef]
- Srivastava, K.; Bhatt, A.; Singh, N.; Khare, R.; Shukla, R.; Chaturvedi, D.; Kant, R. Synthesis of Isothiocyanates: A Review. Chem. Biol. 2014, 4, 1–22. [Google Scholar]
- Nesterov, V.; Reiter, D.; Bag, P.; Frisch, P.; Holzner, R.; Porzelt, A.; Inoue, S. NHCs in Main Group Chemistry. Chem. Rev. 2018, 118, 9678–9842. [Google Scholar] [CrossRef]
- Wang, Y.; Hickox, H.P.; Xie, Y.; Wei, P.; Blair, S.A.; Johnson, M.K.; Schaefer, H.F.; Robinson, G.H. A Stable Anionic Dithiolene Radical. J. Am. Chem. Soc. 2017, 139, 6859–6862. [Google Scholar] [CrossRef] [Green Version]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable Cyclic Carbenes and Related Species beyond Diaminocarbenes. Angew. Chem. Int. Ed. Engl. 2010, 49, 8810–8849. [Google Scholar] [CrossRef] [Green Version]
- Ni, C.; Hu, J. Recent advances in the synthetic application of difluorocarbene. Synthesis 2014, 46, 842–863. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cai, J.; Lin, J.H.; Guo, Y.; Xiao, J.C. Synthesis and decarboxylative Wittig reaction of difluoromethylene phosphobetaine. Chem. Commun. 2013, 49, 7513–7515. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lin, J.H.; Cai, J.; Xiao, J.C. Conversion between difluorocarbene and difluoromethylene ylide. Chem. Eur. J. 2013, 19, 15261–15266. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, R.; Lin, J.-H.; Yu, D.-H.; Ma, L.; Jia, L.; Zhang, L.; Wang, L.; Xiao, J.-C.; Liang, S.H. An Unconventional Mechanistic Insight into SCF3 Formation from Difluorocarbene: Preparation of 18F-Labeled α-SCF3 Carbonyl Compounds. Angew. Chem. Int. Ed. 2017, 56, 3196–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Lin, J.H.; Xiao, J.C. Reaction of Thiocarbonyl Fluoride Generated from Difluorocarbene with Amines. Angew. Chem. Int. Ed. 2017, 56, 16669–16673. [Google Scholar] [CrossRef]
- Zhen, L.; Fan, H.; Wang, X.; Jiang, L. Synthesis of thiocarbamoyl fluorides and isothiocyanates using CF3SiMe3 and elemental sulfur or AgSCF3 and KBr with amines. Org. Lett. 2019, 21, 2106–2110. [Google Scholar] [CrossRef]
- Tavener, S.J.; Adams, D.J.; Clark, J.H. Trifluoromethylthiolation of aromatic substrates using thiophosgene—fluoride salt reagents, and formation of byproducts with multi-carbon chains. J. Fluor. Chem. 1999, 95, 171–176. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Yuan, Z.; Yang, D.-Y.; Weng, Z. One-Pot Synthesis of 3-Difluoromethyl Benzoxazole-2-thiones. Org. Lett. 2018, 20, 6407–6410. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Chu, L.; Wang, R.W.; Zhang, X.; Qing, F.L. Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids with TMSCF3 and elemental sulfur. Angew. Chem. Int. Ed. 2012, 51, 2492–2495. [Google Scholar] [CrossRef]
- Liu, J.B.; Xu, X.H.; Chen, Z.H.; Qing, F.L. Direct dehydroxytrifluoromethylthiolation of alcohols using silver(I) trifluoromethanethiolate and tetra-n-butylammonium iodide. Angew. Chem. Int. Ed. 2015, 54, 897–900. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, X.G. Organophosphine-free copper-catalyzed isothiocyanation of amines with sodium bromodifluoroacetate and sulfur. Chem. Commun. 2019, 55, 1144–1147. [Google Scholar] [CrossRef]
- Baars, H.; Engel, J.; Mertens, L.; Meister, D.; Bolm, C. The Reactivity of Difluorocarbene with Hydroxylamines: Synthesis of Carbamoyl Fluorides. Adv. Synth. Catal. 2016, 358, 2293–2299. [Google Scholar] [CrossRef]
- Fuchibe, K.; Aono, T.; Hu, J.; Ichikawa, J. Copper(I)-Catalyzed [4 + 1] Cycloaddition of Silyl Dienol Ethers with Sodium Bromodifluoroacetate: Access to β,β-Difluorocyclopentanone Derivatives. Org. Lett. 2016, 18, 4502–4505. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.P.; Greaney, M.F. S-, N-, and Se-difluoromethylation using sodium chlorodifluoroacetate. Org. Lett. 2013, 15, 5036–5039. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wei, J.; Jiang, X. Thiocarbonyl Surrogate via Combination of Sulfur and Chloroform for Thiocarbamide and Oxazolidinethione Construction. Org. Lett. 2017, 19, 2166–2169. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Shin-Ike, T.; Sonoda, N.; Aoki, M.; Okada, K.; Miyoshi, N.; Kambe, N. Novel selenium catalyzed synthesis of isothiocyanates from isocyanides and elemental sulfur. Tetrahedron Lett. 1991, 32, 3503–3506. [Google Scholar] [CrossRef]
- Fujiwara, S.; Shin-Ike, T.; Okada, K.; Aoki, M.; Kambe, N.; Sonoda, N. A marvelous catalysis of tellurium in the formation of isothiocyanates from isocyanides and sulfur. Tetrahedron Lett. 1992, 33, 7021–7024. [Google Scholar] [CrossRef]
- Adam, W.; Bargon, R.M.; Bosio, S.G.; Schenk, W.A.; Stalke, D. Direct Synthesis of Isothiocyanates from Isonitriles by Molybdenum-Catalyzed Sulfur Transfer with Elemental Sulfur. J. Org. Chem. 2002, 67, 7037–7041. [Google Scholar] [CrossRef]
- Farrell, W.S.; Zavalij, P.Y.; Sita, L.R. Catalytic Production of Isothiocyanates via a Mo(II)/Mo(IV) Cycle for the “Soft” Sulfur Oxidation of Isonitriles. Organometallics 2016, 35, 2361–2366. [Google Scholar] [CrossRef]
- Arisawa, M.; Ashikawa, M.; Suwa, A.; Yamaguchi, M. Rhodium-catalyzed synthesis of isothiocyanate from isonitrile and sulfur. Tetrahedron Lett. 2005, 46, 1727–1729. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Choudhary, S.; Doshi, A.; Liu, F.-Q.; Mohan, R.; Ravindra, M.P.; Shah, D.; Yang, X.; Fleming, F.F. Catalytic Isonitrile Insertions and Condensations Initiated by RNC-X Complexation. Adv. Synth. Catal. 2014, 356, 2135–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-mediated and metal-catalyzed reactions of isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef]
- Adam, W.; Bargon, R.M. Molybdenum-catalyzed episulfidation of (E)-cycloalkenes with elemental sulfur. Chem. Commun. 2001, 1, 1910–1911. [Google Scholar] [CrossRef]
- Adam, W.; Bargon, R.M.; Schenk, W.A. Direct episulfidation of alkenes and allenes with elemental sulfur and thiiranes as sulfur sources, catalyzed by molybdenum oxo complexes. J. Am. Chem. Soc. 2003, 125, 3871–3876. [Google Scholar] [CrossRef]
- Arisawa, M.; Ichikawa, T.; Yamaguchi, M. Rhodium-catalyzed synthesis of diaryl sulfides using aryl fluorides and sulfur/organopolysulfides. Org. Lett. 2012, 14, 5318–5321. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Ichikawa, T.; Yamaguchi, M. Synthesis of thiiranes by rhodium-catalyzed sulfur addition reaction to reactive alkenes. Chem. Commun. 2015, 51, 8821–8824. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Ichikawa, T.; Tanii, S.; Yamaguchi, M. Synthesis of Symmetrical and Unsymmetrical 1,4-Dithiins by Rhodium-Catalyzed Sulfur Addition Reaction to Alkynes. Synthesis 2016, 48, 3107–3119. [Google Scholar] [CrossRef] [Green Version]
- Arisawa, M.; Tanaka, K.; Yamaguchi, M. Rhodium-catalyzed sulfur atom exchange reaction between organic polysulfides and sulfur. Tetrahedron Lett. 2005, 46, 4797–4800. [Google Scholar] [CrossRef]
- Nguyen, T.B. Recent Advances in Organic Reactions Involving Elemental Sulfur. Adv. Synth. Catal. 2017, 359, 1066–1130. [Google Scholar] [CrossRef]
- Nguyen, T.B. Recent Advances in the Synthesis of Heterocycles via Reactions Involving Elemental Sulfur. Adv. Synth. Catal. 2020, 362, 3448–3484. [Google Scholar] [CrossRef]
- Liu, S.; Deng, G.J.; Huang, H. Recent Advances in Sulfur-Containing Heterocycle Formation via Direct C-H Sulfuration with Elemental Sulfur. Synlett 2021, 32, 142–158. [Google Scholar] [CrossRef]
- Davis, R.E.; Nakshbendi, H.F. Sulfur in Amine Solvents. J. Am. Chem. Soc. 1962, 84, 2085–2090. [Google Scholar] [CrossRef]
- Chivers, T.; Elder, P.J.W. Ubiquitous trisulfur radical anion: Fundamentals and applications in materials science, electrochemistry, analytical chemistry and geochemistry. Chem. Soc. Rev. 2013, 42, 5996–6005. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.W.; Nagashima, K.; Macdonald, P.M.; Ozin, G.A. From Sulfur−Amine Solutions to Metal Sulfide Nanocrystals: Peering into the Oleylamine−Sulfur Black Box. J. Am. Chem. Soc. 2011, 133, 5036–5041. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.D.; Cox, E.F.; Davis, R.E. Reactions of Elemental Sulfur. IV. Catalytic Effects in the Reaction of Sulfur with Triphenylphosphine. J. Am. Chem. Soc. 1961, 83, 103–109. [Google Scholar] [CrossRef]
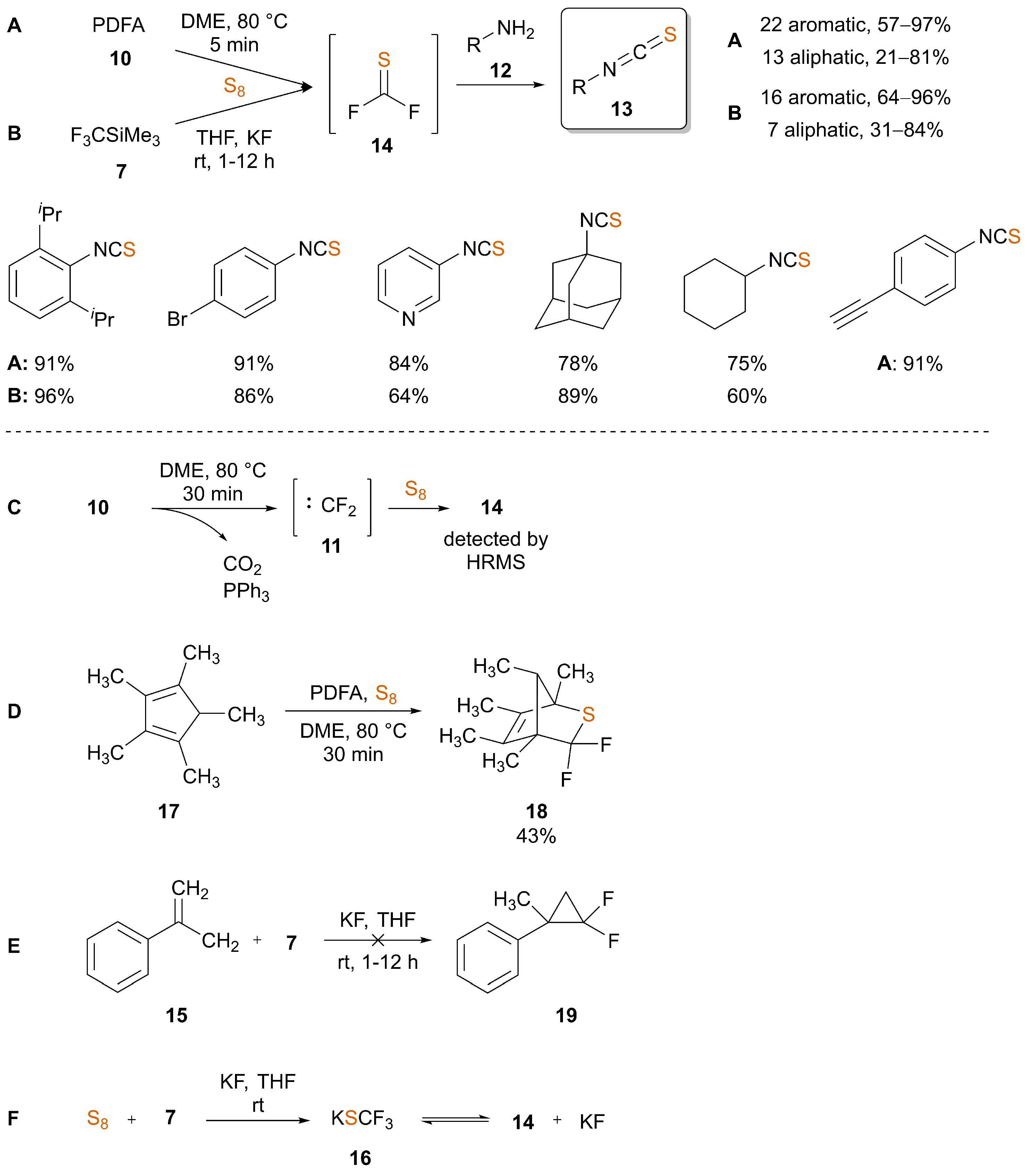
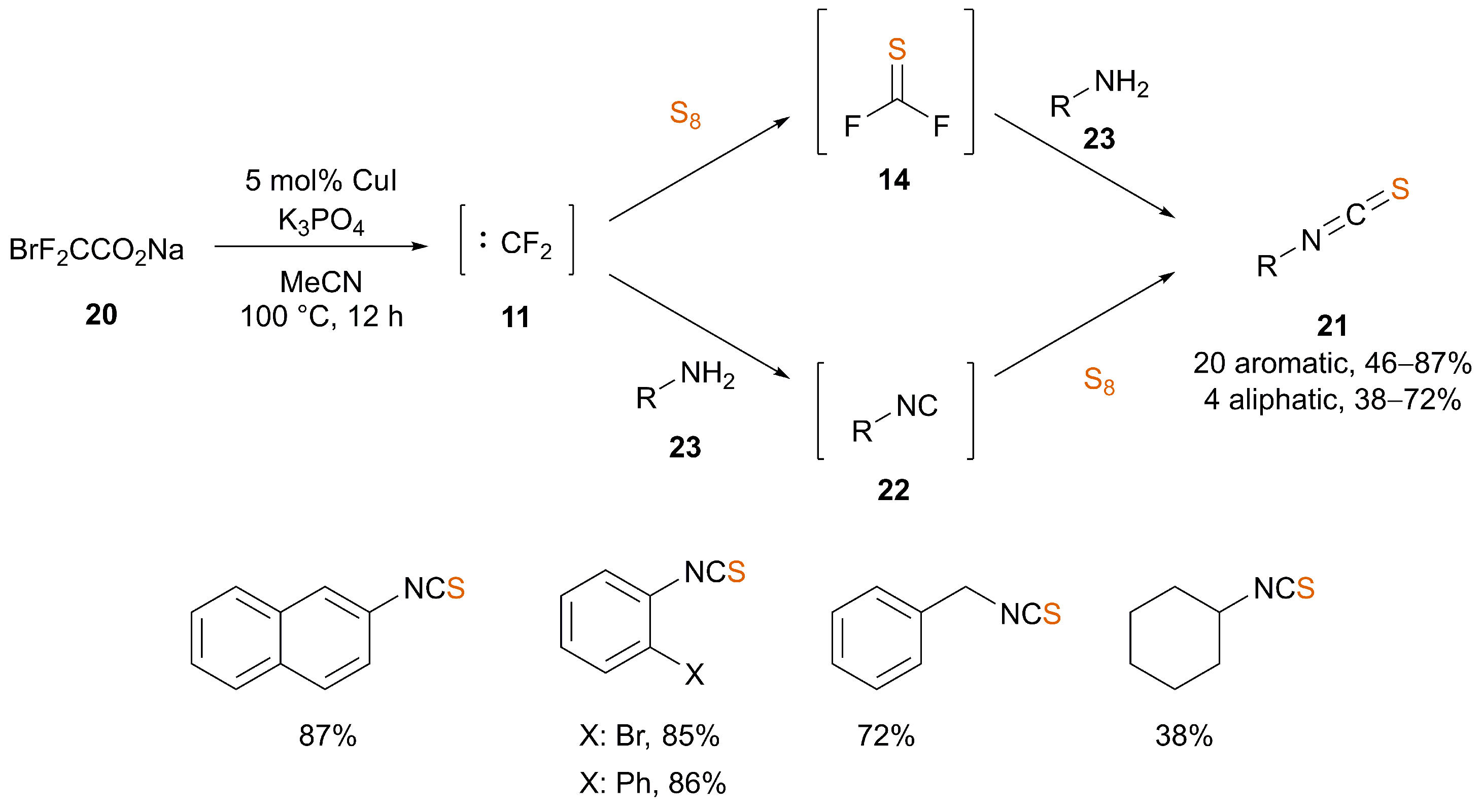
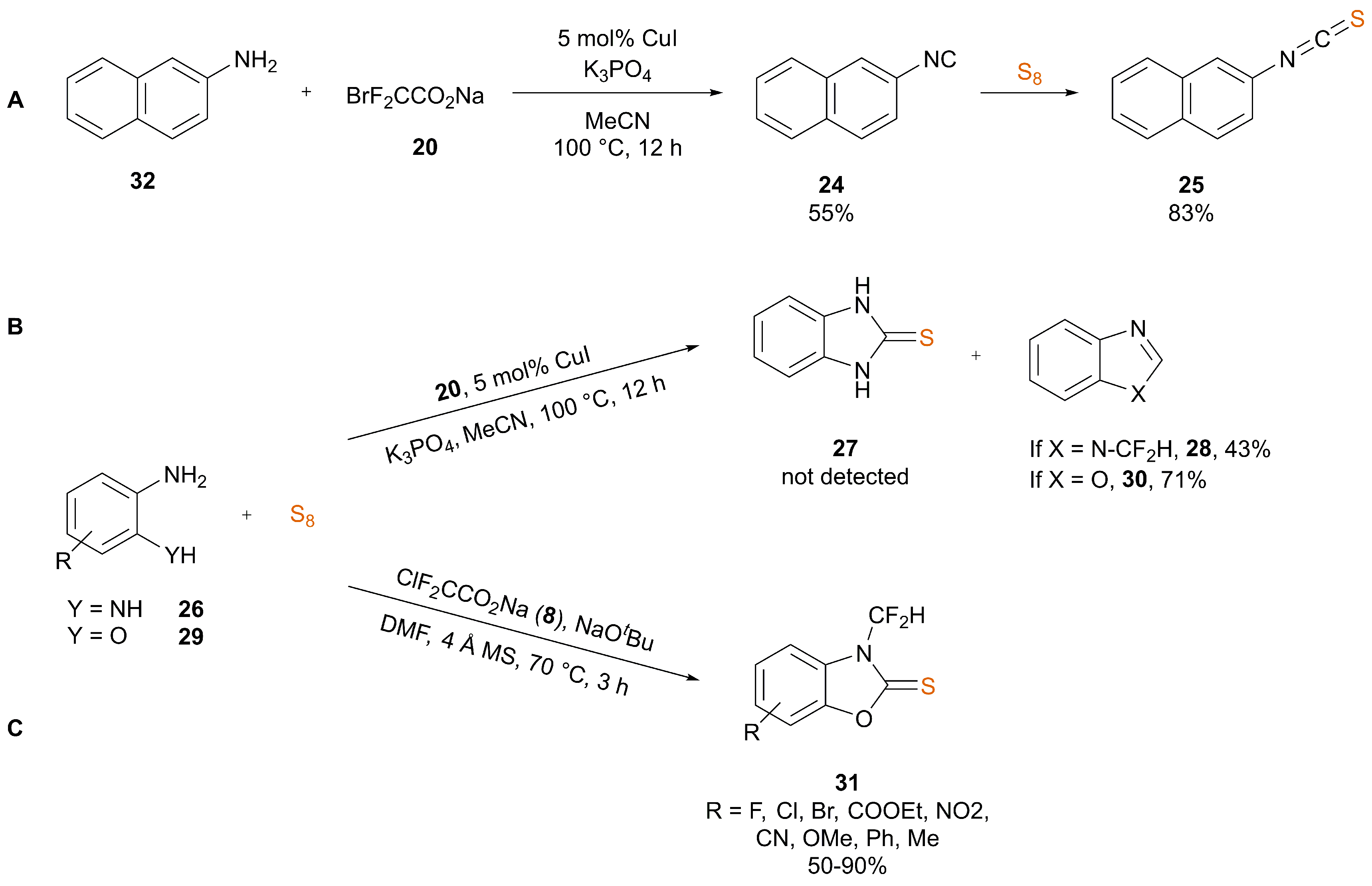
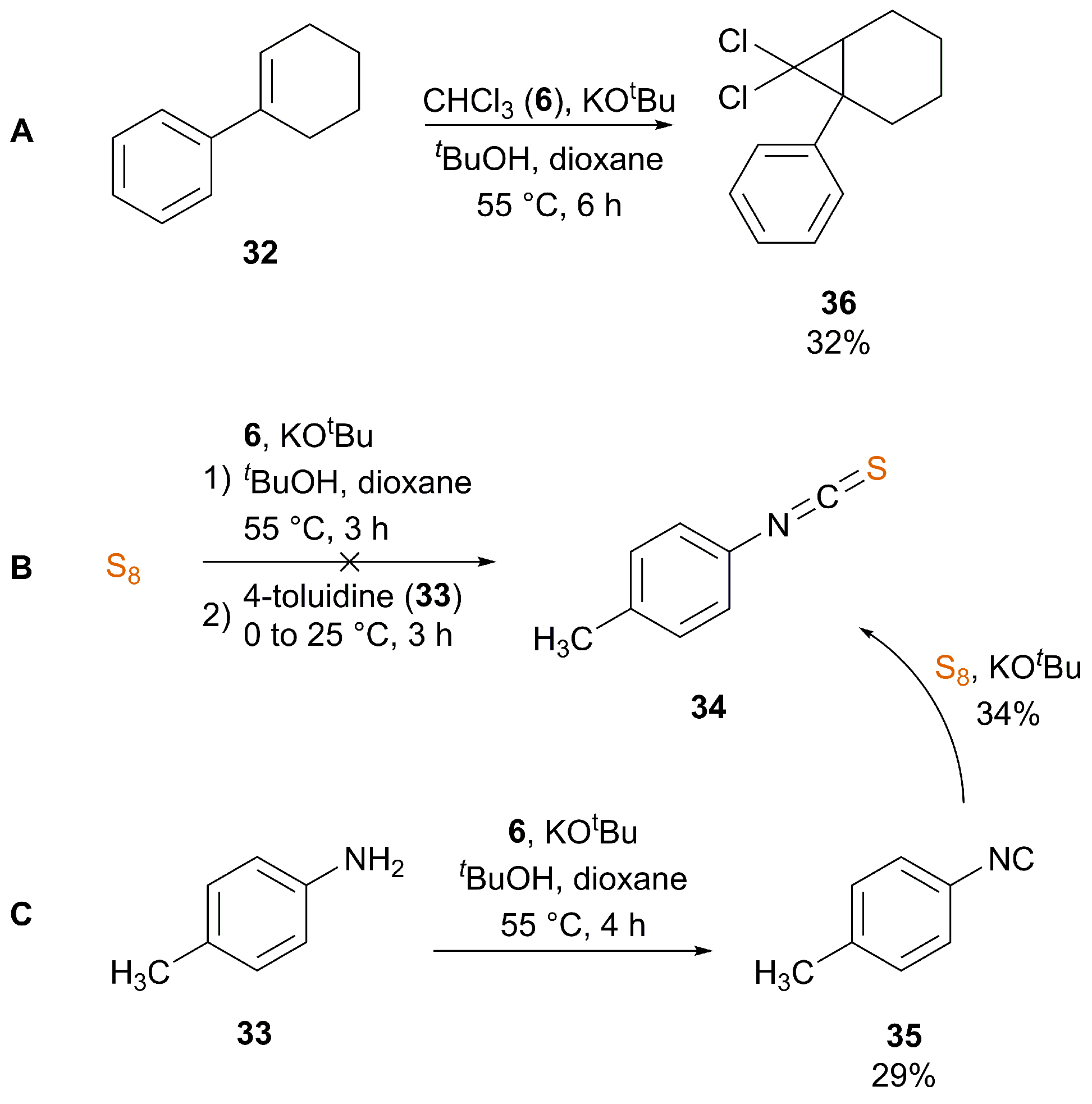
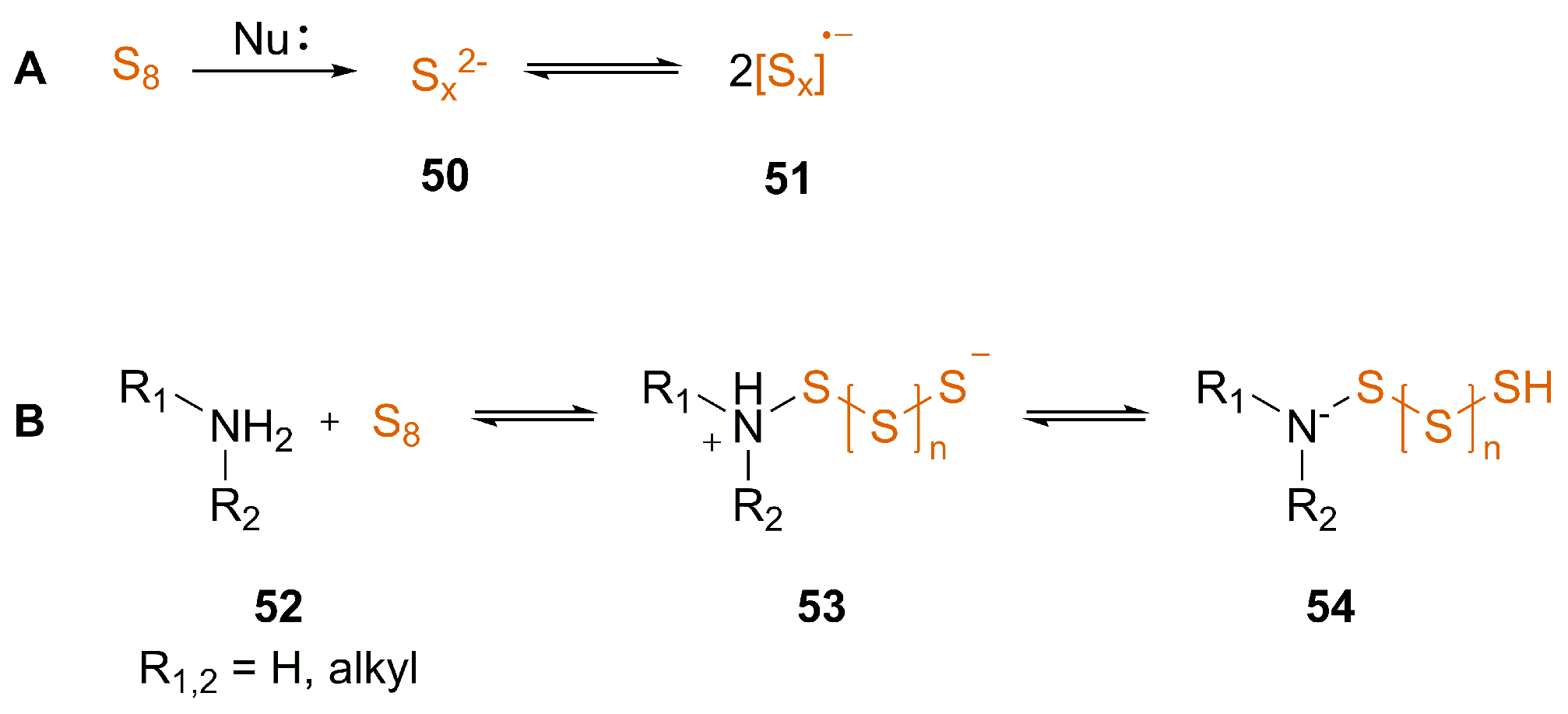
| Ref. | Starting Material | Additive | Inert Atmosphere | Solvent | T (°C) | T (h) | Yield (%) |
|---|---|---|---|---|---|---|---|
| [56] | Amine | PDFA | Yes | DME | 80 | 0.083 | 21–97 |
| [57] | F3CSiMe3 + KF | Yes | THF | rt | 1–12 | 31–96 | |
| [62] | BrF2CCOOK + 5 mol% CuI, K3PO4 | No | MeCN | 100 | 12 | 38–87 | |
| [67,68] | Isocyanide | 5 mol% Se or 0.02 mol% Te | No | THF | reflux | 0.5–8 | 53–99 |
| [69,70] | “Mo” (X) | No | acetone | reflux | 72 | 61–93 | |
| [71] | “Rh” (X) | Yes | acetone | reflux | 1.5–8 | 83–96 | |
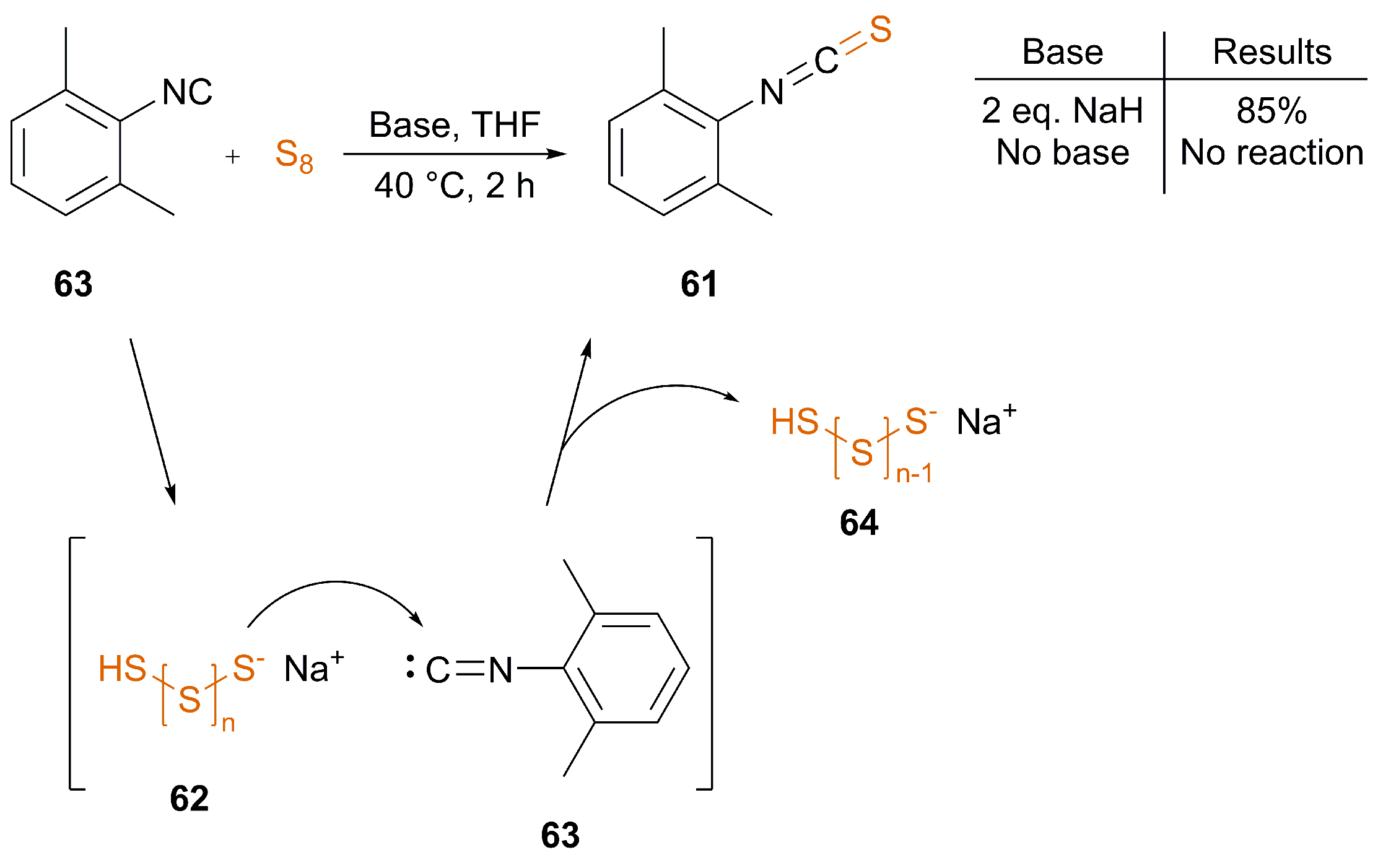
| [42] | 2 eq. NaH | Yes | THF | 40 | 2 | 85 | |
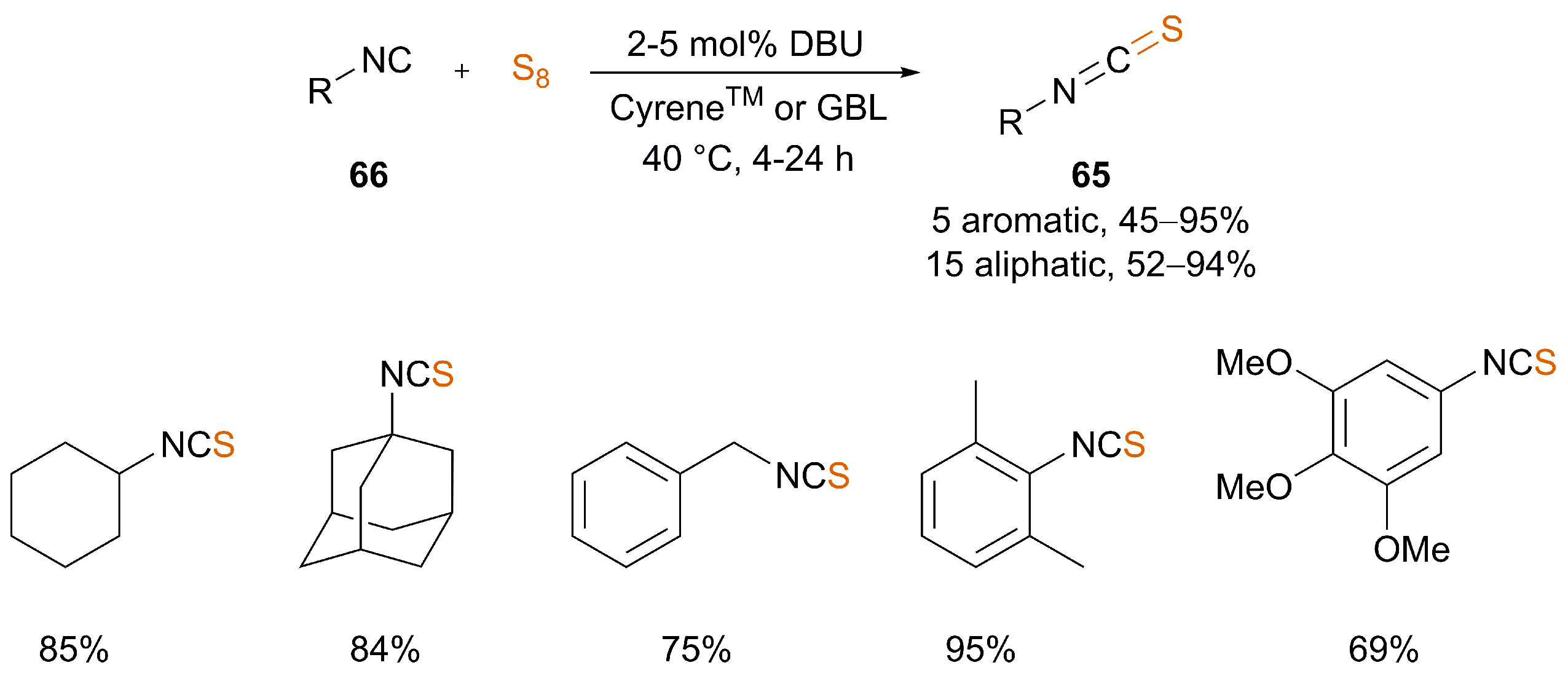
| [41] | 2–5 mol% DBU | No | CyreneTM or GBL | 40 | 4–24 | 34–95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, A.G.; Ábrányi-Balogh, P. Recent Advances in the Synthesis of Isothiocyanates Using Elemental Sulfur. Catalysts 2021, 11, 1081. https://doi.org/10.3390/catal11091081
Németh AG, Ábrányi-Balogh P. Recent Advances in the Synthesis of Isothiocyanates Using Elemental Sulfur. Catalysts. 2021; 11(9):1081. https://doi.org/10.3390/catal11091081
Chicago/Turabian StyleNémeth, András Gy., and Péter Ábrányi-Balogh. 2021. "Recent Advances in the Synthesis of Isothiocyanates Using Elemental Sulfur" Catalysts 11, no. 9: 1081. https://doi.org/10.3390/catal11091081
APA StyleNémeth, A. G., & Ábrányi-Balogh, P. (2021). Recent Advances in the Synthesis of Isothiocyanates Using Elemental Sulfur. Catalysts, 11(9), 1081. https://doi.org/10.3390/catal11091081