Abstract
Metalloenzymes such as the carbonic anhydrases (CAs, EC 4.2.1.1) possess highly specialized active sites that promote fast reaction rates and high substrate selectivity for the physiologic reaction that they catalyze, hydration of CO2 to bicarbonate and a proton. Among the eight genetic CA macrofamilies, α-CAs possess rather spacious active sites and show catalytic promiscuity, being esterases with many types of esters, but also acting on diverse small molecules such as cyanamide, carbonyl sulfide (COS), CS2, etc. Although artificial CAs have been developed with the intent to efficiently catalyse non-biologically related chemical transformations with high control of stereoselectivity, the activities of these enzymes were much lower when compared to natural CAs. Here, we report an overview on the catalytic activities of α-CAs as well as of enzymes which were mutated or artificially designed by incorporation of transition metal ions. In particular, the distinct catalytic mechanisms of the reductase, oxidase and metatheses-ase such as de novo designed CAs are discussed.
1. Introduction
Enzymes play a pivotal role in the life of every living organism by efficiently catalyzing a large number of biological reactions and thus assuring that metabolic needs are met. In some cases, the rate of biotransformation is increased up to 1017-fold [1]. Often the elevated catalytic turnover of enzymes requires minimal energy to happen and in comparison to the uncatalyzed chemical transformation, no influences on the equilibrium constants are encountered, whereas a residual amount of side products is formed, and the stereospecific information within starting materials and/or products are highly conserved. These features have attracted researchers in all chemistry fields due to the possibility of developing enzymes capable of catalyzing transformations difficult or impossible to perform using classical synthetic organic tools. The appealing properties of the enzymes prompt researchers to both look for new applications of the naturally occurring ones and to re-engineer them in order to generate new bio-inspired catalysts, possibly endowed with novel and features not previously reported [2,3,4,5]. Among all known proteins, Nature offers a well-studied superfamily of metalloenzymes, the carbonic anhydrases (CAs, EC 4.2.1.1), which have genetically evolved independently into at least eight families (i.e., the α-, β-, γ-, δ-, ζ- η-, θ- and ι-CAs) according to a convergent evolution process [6,7,8,9,10,11,12,13]. The α-CAs are present in vertebrates, protozoa, algae, cytoplasm of green plants and in many Gram negative bacteria [6,7,14]; the β-CAs are found in both Gram negative and positive bacteria, algae and chloroplasts of mono- as well as di-cotyledons, and also in many fungi and some Archaea [6,7,8,9,10,15]. The γ-CAs are found in Archaea, cyanobacteria and most types of bacteria [8,11,16], the δ-, ζ- and θ-CAs seem to be present only in marine diatoms [9,10,13], whereas the η-Cas are present in protozoa [12]. Finally, ι-CAs were discovered in marine phytoplankton but are also present in bacteria [17]. The present review outlines the different catalytic mechanisms of natural and engineered CAs and their application within different chemistry fields.
2. Catalytic Mechanism of Carbonic Anhydrases
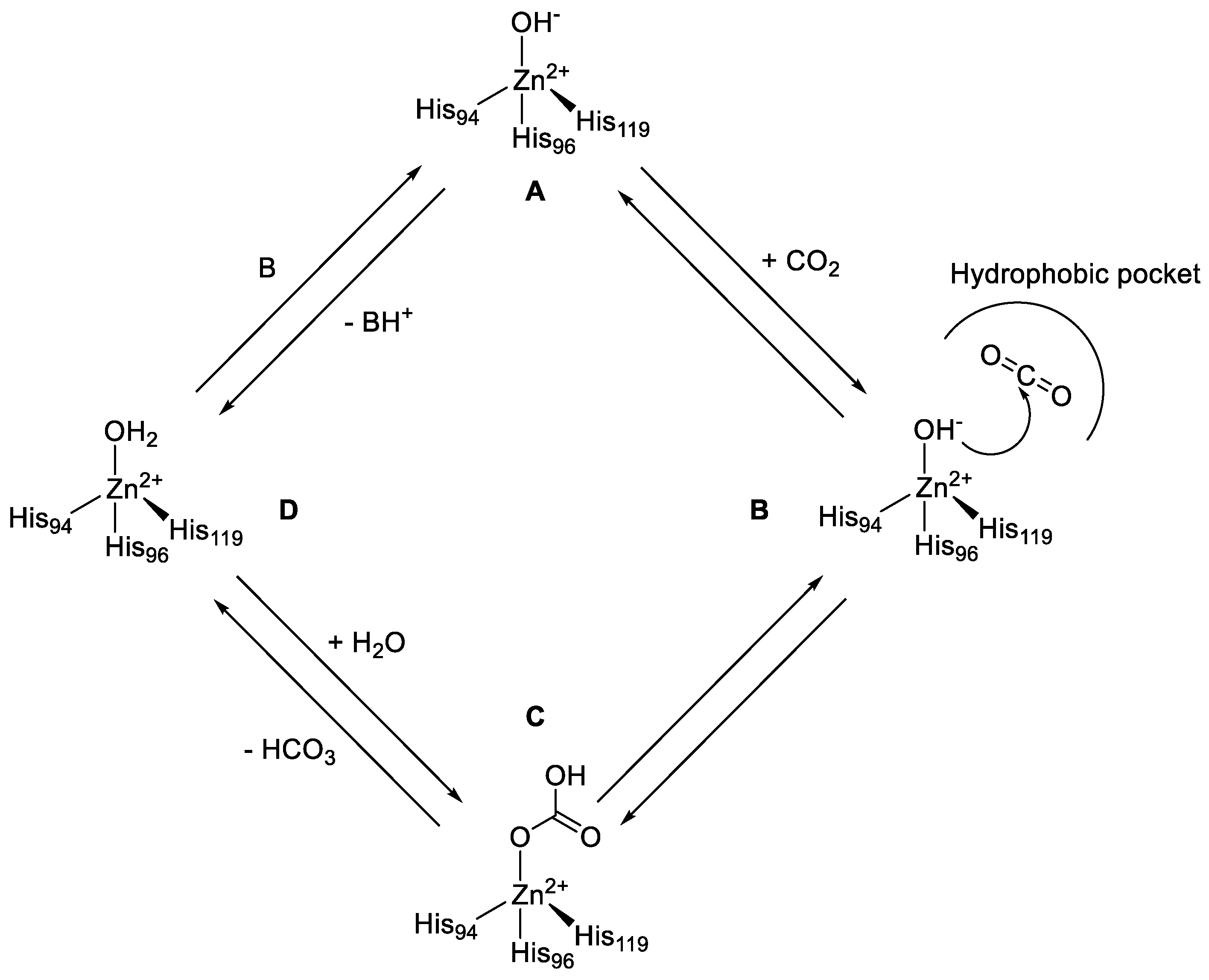
CAs were first discovered in 1933, when the erythrocytes were observed to contain stoichiometric amounts of zinc and an abundant protein (later denominated carbonic anhydrase), which was also proven essential for the enzymatic activity of CO2 hydration [18]. CAs are ubiquitously expressed within all life kingdoms and, as mentioned above, are encoded by eight distinct gene families. All of them differ for their catalytic activities, subcellular localizations, and tissue distributions. All the catalytically active CAs reversibly hydrate carbon dioxide to the bicarbonate ion and a proton. Although this reaction may also occur spontaneously, at physiological pH values it is too slow to meet the metabolic needs of most organisms/cells. The α-CAs catalytic mechanism occurs in two main steps according to Figure 1 [19].
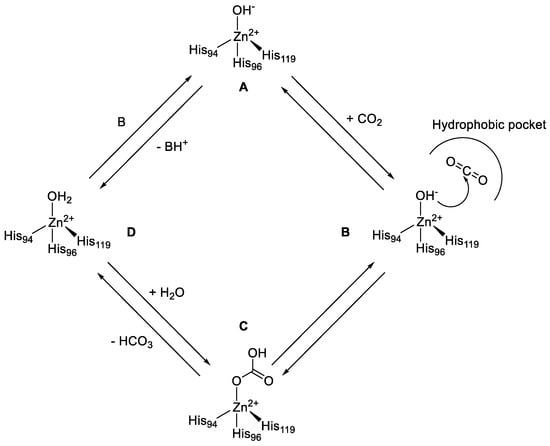
Figure 1.
Catalytic mechanism of reversible hydration of CO2 to HCO3− and a proton in the presence of α-CAs.
The first step is the nucleophilic attack of the Zn2+-bound hydroxide ion to a CO2 with the consequent formation of the enzyme-HCO3 adduct (B to C), which is thereafter displaced from the active site by a water molecule (C to D). The last step (D to A), which is the kinetically rate limiting one, regenerates the catalytically active Zn2+-bound hydroxide ion through a proton transfer reaction from the Zn2+-bound water molecule to an exogenous proton acceptor or to an active site residue (Figure 1). CO2 is not only an essential molecule in physiological processes, but also one of the principal products in combustion reactions, being produced in a large quantity by anthropogenic emissions, becoming thus one of the most significant and long-lived greenhouse gases in the earth’s atmosphere. As a result, CO2 capture and sequestration is becoming a critical point and has started to be highly investigated in the last decade. Recently, CAs have been reported to have the potential to accelerate CO2 capture from large combustion emitters [20]. However, the poor stability and activity of mammalian CAs under the harsh conditions of these processes such as temperatures ranging from 50 °C to over 125 °C, the high concentrations of organic amines, trace contaminants such as heavy metals, sulfur and nitrogen oxides, have drastically limited their use [21]. Approaches to overcome these limitations have included sourcing CAs from thermophilic organisms, or the use of protein engineering techniques to create thermo-tolerant enzymes [22,23,24,25]. However, CAs are not only highly effective catalysts for the interconversion between carbon dioxide and bicarbonates, but show catalytic versatility, participating in several other hydrolytic processes, which are reported below in this review. To date, only α-CAs and a few β-CAs were reported to have other catalytic functions than the CO2 hydration one.
3. Hydrolysis Reactions Catalyzed by CAs
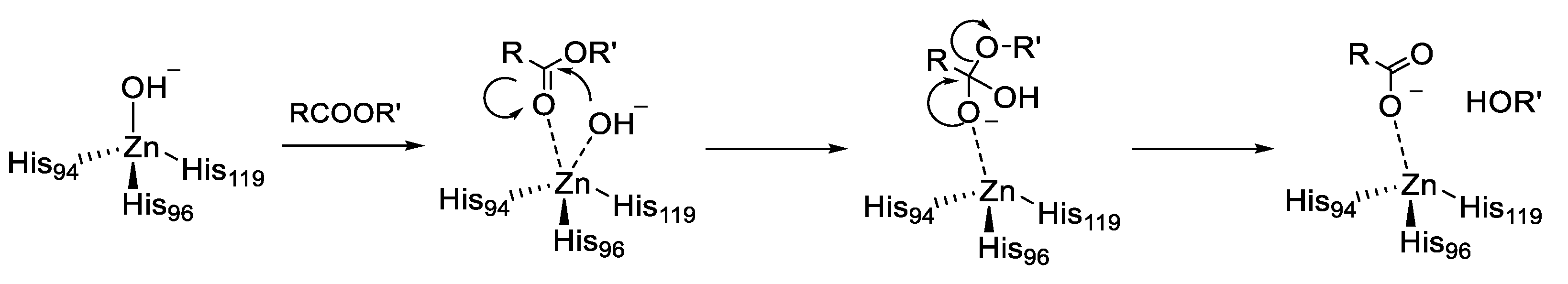
In addition to the physiologically relevant reaction, α-CAs were reported to catalyze other reactions involving carbonyl systems such as the hydrolysis of esters [26,27]. Catalysis takes place in a 15-Å wide by 15-Å deep hydrophobic pocket, close to the zinc ion, that can accommodate ligands much larger than CO2. This esterase activity probably stems from the mechanistic similarity between hydration of CO2 and hydrolysis of an ester, i.e., nucleophilic attack by a zinc-coordinated OH- ion to the CO and the stabilization of the resulting oxyanionic intermediate, as shown in Figure 2.
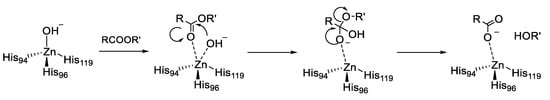
Figure 2.
Esterase Activity of carbonic anhydrases (CAs).
In several studies, the increase in the esterase activity through different mutations in the CA active site was investigated, which led to a 40-fold improvement in the activity towards several carboxylic acid esters [28,29]. These mutations enhanced the activity principally through steric adaptation of the engineered CA active site, which enhanced the ability to accommodate bulkier ligands. In addition, in the last 10 years this activity was used to generate novel and highly selective CA inhibitors targeting the tumor associate isoforms (CA IX and XII). Indeed, the coumarins and their isosters were shown to act as prodrug inhibitors. Such compounds undergo the hydrolysis of the lactone ring due to the esterase activity of these enzymes [30,31,32,33]. Recently, Supuran’s group also showed CAs to possess thioesterase activity [34], whereas in the last year, CAs were found to hydrolyze selenoesters, acting in the same manner with regard to coumarin scaffolds, and generate CA inhibitors of the selenol type [35].
The hydration of other carbonyl systems was not limited to esters, but was extended to aldehyde moieties [36,37]. The reaction follows pseudo-first-order kinetics and is enhanced when the enzyme is in imidazole buffers, which probably act as CA activators [38].
The availability within the CAs catalytic site of a strong nucleophile, the Zn(II)-bound hydroxide ion, gives the opportunity to hydrolyze other molecules such as cyanamide. This is a linear molecule isoelectronic with CO2, and the CA-catalyzed hydration leads to the formation of urea, bound as a ureate anion to the Zn(II) from the enzyme active site. The conversion of cyanamide to urea is a very slow reaction and it proceeded until a stoichiometric amount of enzyme-urea adduct was formed [39,40].
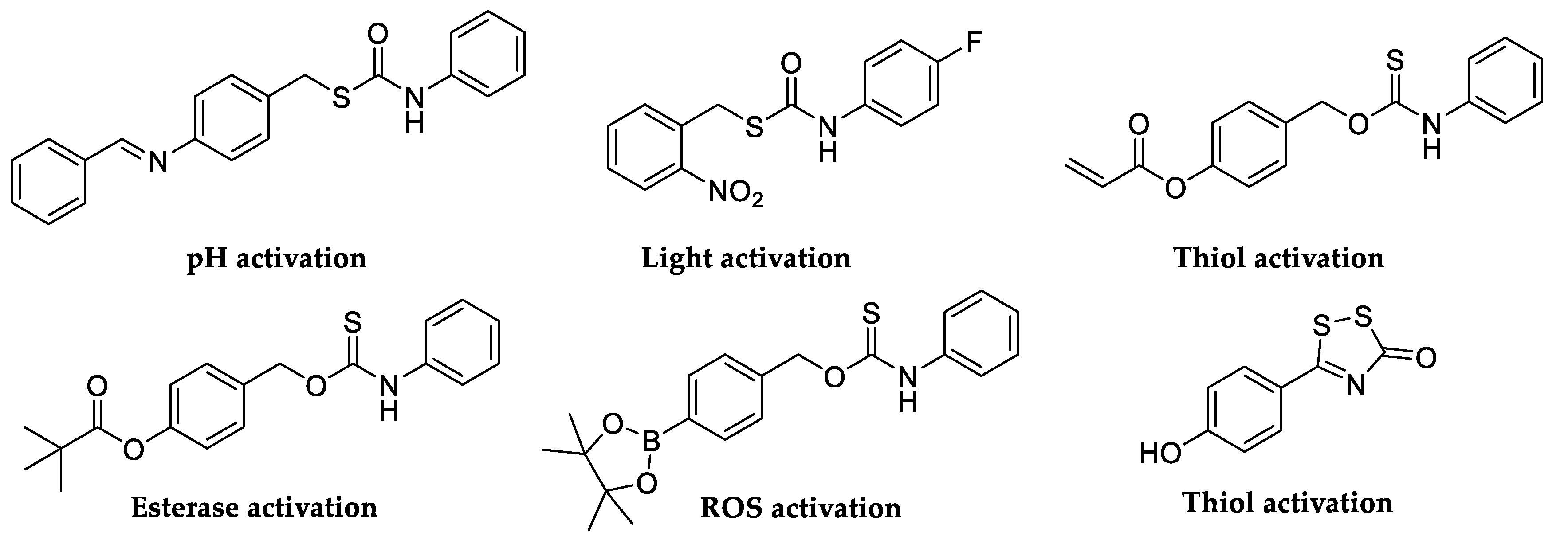
In recent years, the hydrolysis reaction of CAs has been employed to generate hydrogen sulfide (H2S). A small gaseous biomolecules such as H2S has attracted significant attention due to its important physiological role as a signaling molecule. Both endogenous H2S synthesis and exogenous H2S administration exhibited promising protections in different stages of diverse diseases [41]. In this scenario, researchers modulated biological levels of H2S developing novel class of H2S-donor molecules by engineering the release of carbonyl sulfide (COS) from different scaffolds (Figure 3), as an intermediate which can be quickly converted to H2S by CAs [42,43].
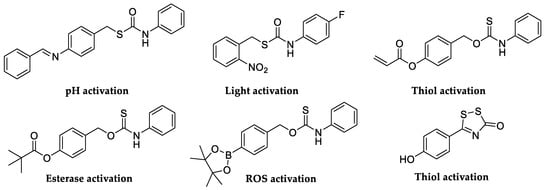
Figure 3.
Selected small molecule incorporating H2S donors and their chemical motifs.
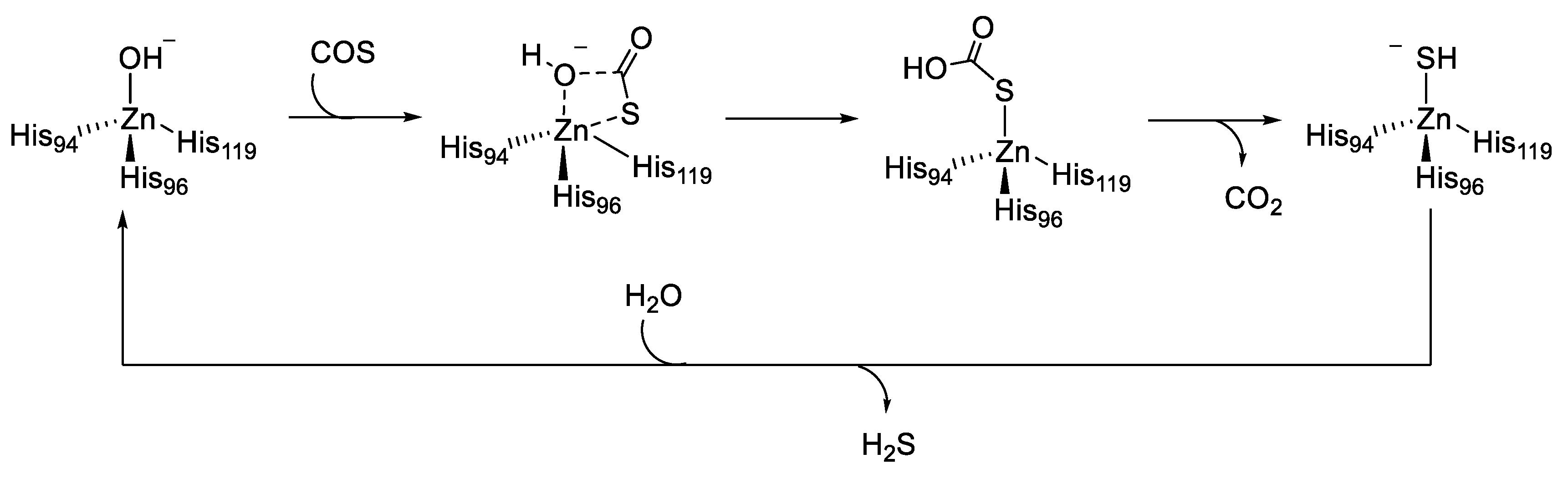
Although there was no single universal ‘‘ideal donor,’’ certain donor classes provided distinct advantages and useful properties. First, the donors should be stable until the activating group is cleaved or modified. Subsequently, they should have readily accessible control compounds that can be used to clearly delineate observed biological activities and outcomes associated with H2S from those of donor by-products. Similarly, donors that respond to specific stimuli enable experiments in which H2S delivery can be controlled or triggered by specific activators such as light, various pH ranges, other enzymes, biological thiols, and hydrogen peroxide (H2O2) [44,45,46,47]. COS shares an interconnection with H2S generation through the action of CAs, which convert it to H2S. Growing evidence shows that COS may play roles in sulfide transport in several disease pathologies. Indeed, the consumption of COS by many organisms was found to be CA-dependent. Furthermore, CA played an important role in explaining the toxicity of COS. During COS consumption, H2S is formed, which was recognized as being the actual toxin responsible for the noxious effect of some heterocumulene derivatives [48]. The mechanism by which CAs catalyze the irreversible hydration of COS, even though it is not their natural substrate, has been investigated by computational model by Anders et al. (Figure 4) [48].
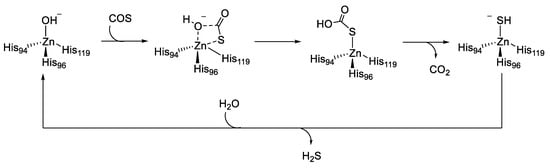
Figure 4.
Catalytic hydration mechanism of carbonyl sulfide (COS).
The reaction follows the same principle as the CO2 hydration reaction. COS is attacked by the zinc-bound hydroxide, which occurs exclusively at the C=S bond. The result is a four-center transition structure, whereby a zinc bound thiocarbonate is formed (Figure 4). CO2 is formed by a water-assisted proton transfer, then expelled, and the zinc hydrosulfide complex is obtained. This species is stabilized by a strong Zn-S bond. Finally, the mode of reactivation of the CA catalytic domain required a water molecule [48].
4. CA as a New Reductase Enzyme
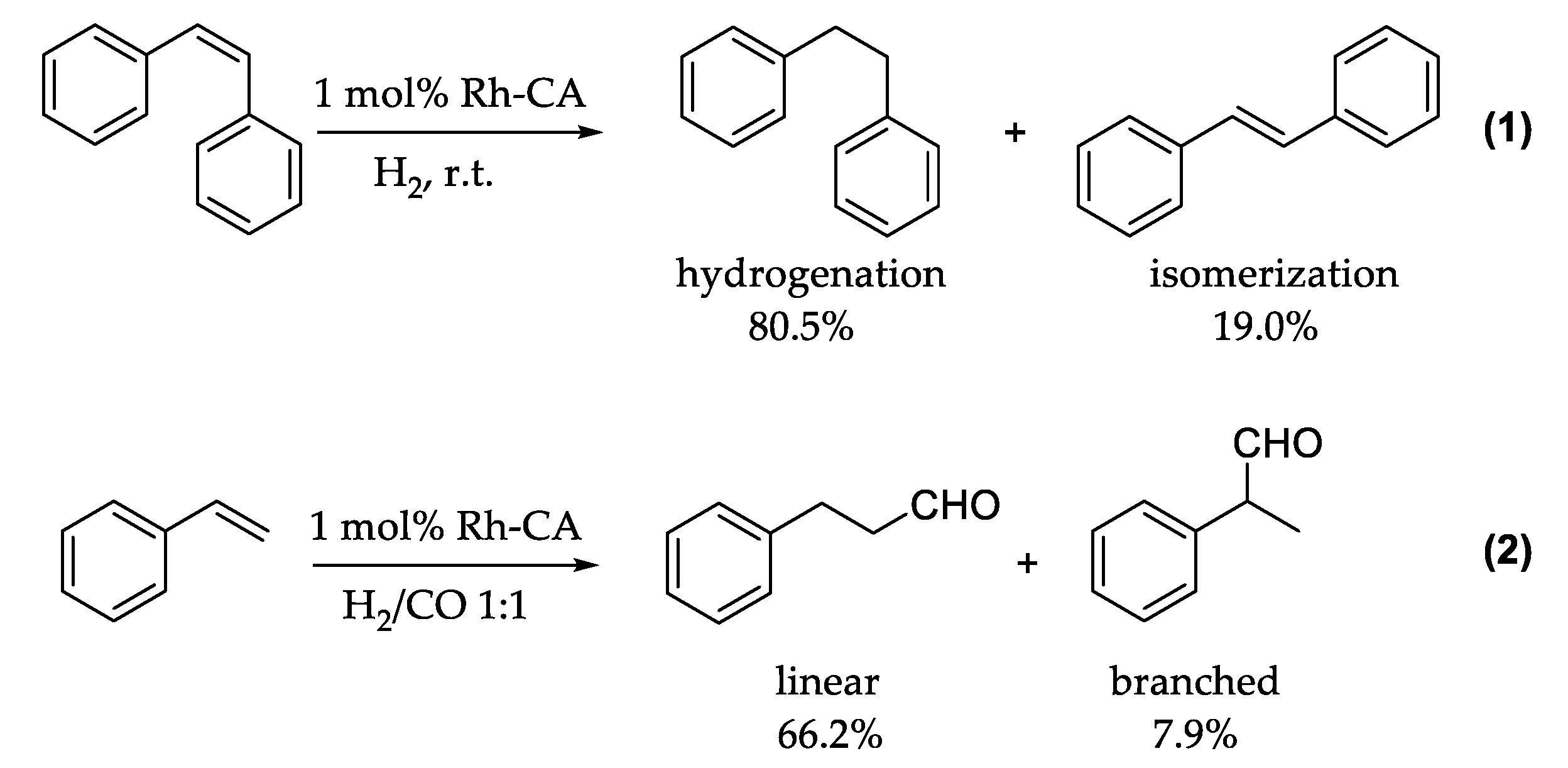
A common and useful reaction in organic chemistry is the direct hydrogenation of reagents using hydrogen gas. Nature, on the other hand, does not utilize this technique, and reduces organic molecules during metabolic pathways involving cofactors such as NADPH or FADH2, which provide evolutionary advantages over the direct use of hydrogen [49]. This likely makes the enzymes more efficient than they would be if H2 was used. In 2009, Kazlauskas and coworkers replaced the zinc ion in the CA active site with a rhodium ion, introducing new catalytic activity to the enzyme. [50] This choice was made on the basis of different considerations: first, ionic radii, similar in both zinc and rhodium conserve the native structure of the active site [51]. Second, histidine residues in the active site may also act as good ligands for rhodium [52]. Finally, CA II lacks cysteine residues within its active site and consequently may have less anchoring points for additional rhodium ions. The generated Rh-CA has been studied for its reductase ability. In the first work, the authors reported stereoselective hydrogenation of cis-stilbene over trans-stilbene. Human Rh-CA II with two mutated histidines, in order to minimize the side anchored points for rhodium ions, was the best engineered enzyme with the new reductase function. An interesting point was the lower amount of isomerization of cis-stilbene to trans-stilbene during the hydrogenation reaction, as outlined in Figure 5(1) [53].
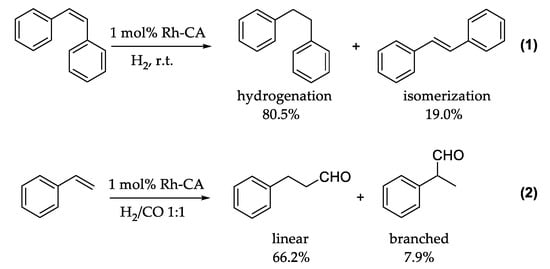
Figure 5.
Hydrogenation and isomerization of cis-stilbene (1) and hydroformylation of styrene (2).
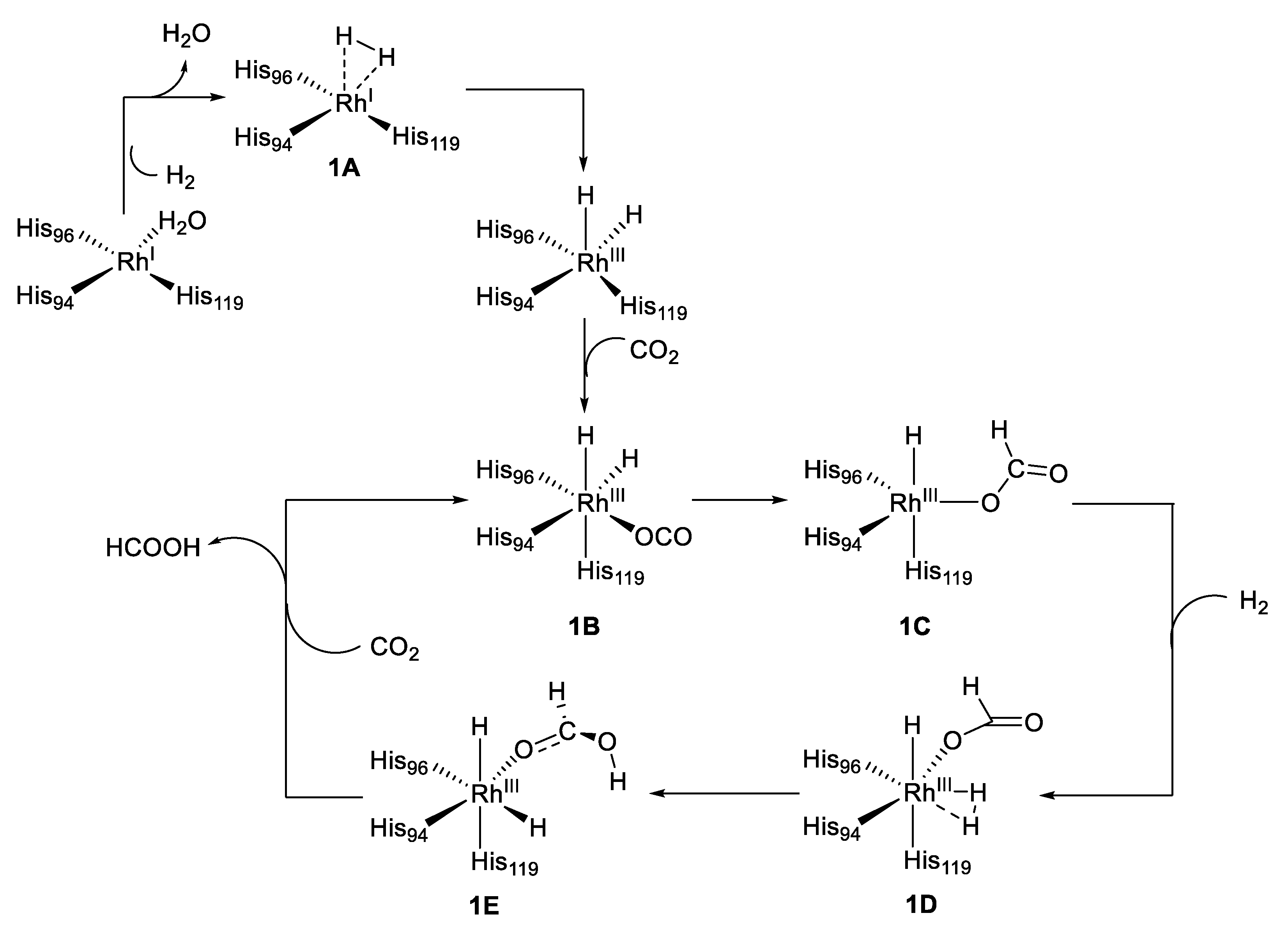
Subsequently, the same authors reported the Rh-CA catalyzed hydroformylation of an unfunctionalized olefin with a regioselectivity of approximately 8.4 for a linear versus branched aldehyde product, as shown in Figure 5(2) [53]. Moreover, in this case the rhodium outside the active site generated most of the side products (branched aldehyde), whereas the rhodium within the active site produced mostly linear product. Consistent experiments with different variants of Rh-CA were conducted, which confirmed this hypothesis. Due to the novelty of these results and the lack of mechanistic information, Piazzetta et al., explored by computational methods the reaction mechanisms beside the reductase catalytic process of CO2 [54,55]. CO2 is thermodynamically stable, and its transformation into formic acid can be considered as an innovative means for hydrogen storage [56]. The reduction of CO2 to HCOOH can be done by a hydrogenation reaction by using efficient and specific catalyst such as Rh-CA. This process involved the roles of H2 and H2O. The carbon dioxide reduction process started with the activation of the enzyme through the substitution of a coordinated water molecule by the insertion of H2 (1A) as outlined in Figure 6.
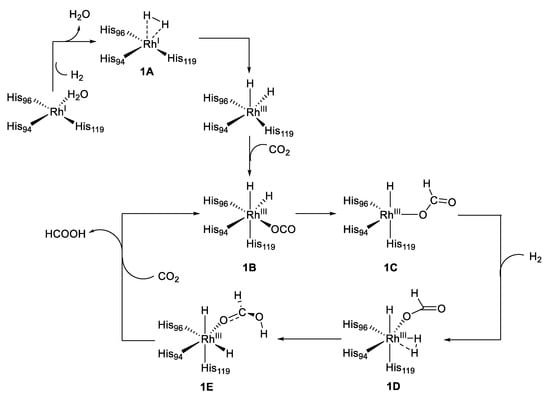
Figure 6.
Proposed mechanism for the reduction of CO2 with Rh-CA.
The subsequent insertion of a CO2 molecule allowed the formation of the rhodium complex 1B followed by the formation of the penta-coordinated complex 1C. A concerted transition state, δ-bond metathesis, and rotation of the formate anion were triggered by a hydrogen molecule, which restored the octahedral geometry 1E. The 1E species, with formic acid coordinated to the Rh, was then released with concomitant insertion of a CO2 molecule, which restored the Rh octahedral geometry. This was the rate-determining step of the process and governed the regeneration of the catalyst [55].
A second type of CA with reductase function is Cu-CA [57]. This enzyme showed nitrite reductase reducing nitrite (NO2−) to nitric oxide (NO), and thus, may play a role in vasodilation and regulation of blood pressure [58,59,60]. Interestingly, this enzyme has reductase function only if two sites are occupied by copper, one within the active site and, and the second in the T-2 site, which is situated at the entrance of the cavity. The mechanism is poorly understood at the moment and the oxidation state of the two copper ions (if Cu(I) or Cu(II), or mixed) has also not been elucidated [57].
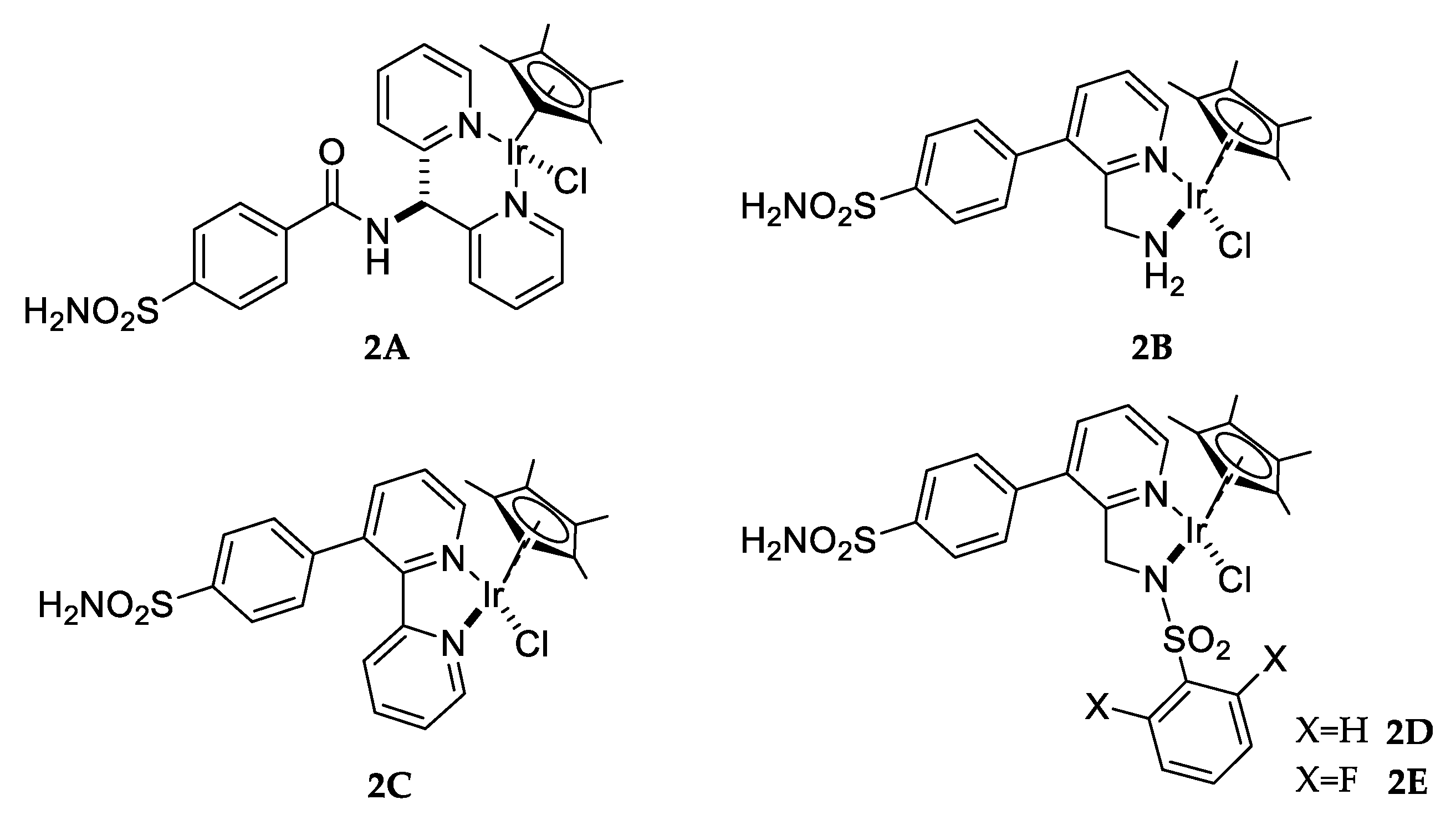
Alternatively, CA reductase activity developed by Ward and co-workers was obtained as a result of incorporation of an organometallic moiety within a native CA protein. The adduct was obtained by anchoring different para-substituted arylsulfonamides to produce an incorporate active iridium complex (Figure 7) and showed transfer hydrogenase activity [61].
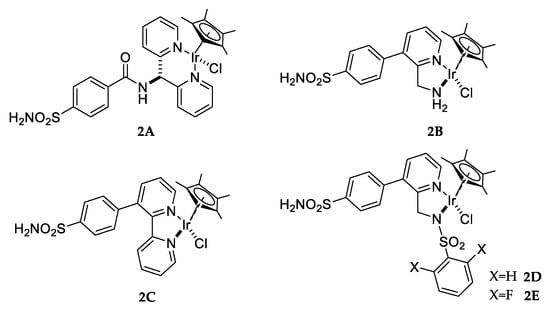
Figure 7.
Pianostool complexes 2A–E.
Iridium complexes were chosen because they have been proven to exhibit superior reduction of imines in water than congeners with rhodium or ruthenium [62]. In addition, protein host CA II was mutated in different critical residues in order to determine the best conformation for obtaining more space within the substrate binding pocket. The complexes showed only moderate activity upon incorporation in hCA II. However, complex 2D displayed significantly improved catalytic performance both in terms of activity and selectivity at 4 °C compared to the other complexes reported by the authors [61].
5. CA as a New Oxidase Enzyme
The well-characterized structure of the CA protein permitted the synthesis of several artificial isoforms with varied and novel activities. Recent studies have focused on Iridium complexes with exclusively high water-oxidizing activity [63]. The Ir-CA was prepared by binding Iridium to the apo-protein of CA and the new enzyme showed catalytic water-oxidizing activity comparable to those of other molecular catalysts [64,65]. Moreover, the oxygen-evolving catalytic activity could be displayed only when Iridium was bound to the active site of CA and this activity was sensitive to physiological conditions such as pH and temperature. Indeed, the activity was promoted by increasing the pH, which indicated that the water-oxidation activity of Ir-CA was higher under alkaline conditions [63].
Enantioselective oxidation activity was carried out by replacement of the zinc ion with manganese(II) by Soumillion et al. [66], thus discovering a Mn-CA with Epoxide Synthase activity. Several alkene substrates and variants of CAs were tested to evaluate the specificity of the epoxidation, and the best ones reached a yield of 59% and an ee of 50%. More interestingly, the replacement of Thr199 with a different residue produced amounts of styrene oxide similar to the wild type, but the enantioselectivity was severely affected. This feature showed this side chain essential for asymmetric induction [66].
6. CA as Host Protein for Metatheses-Ases Activity
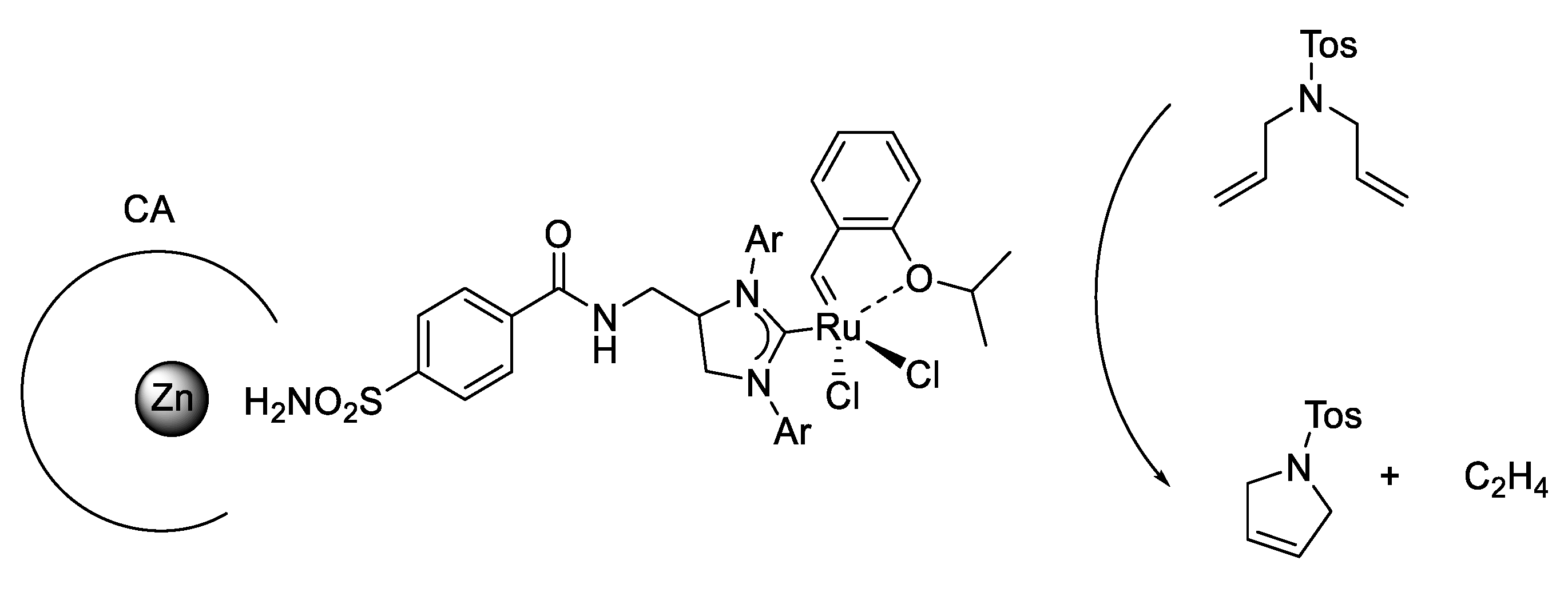
Artificial metathesases activity was reported by exploiting human CA II through the creation of an adduct with benzenesulfonamide derivatives. The sulfonamide group is a well-known inhibitor of CAs. For obtaining this activity an arylsulfonamide anchored on a Hoveyda-Grubbs 2nd generation-type catalyst was synthetized (Figure 8) [67].
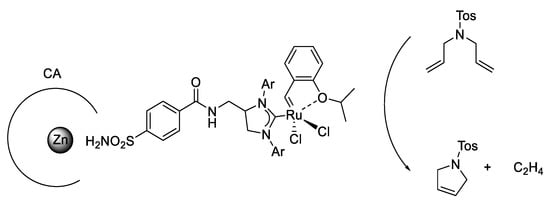
Figure 8.
Artificial metalloenzyme for ring-closing metathesis.
This approach had the advantage of ensuring its localization within the CA active site so it did not require an inert atmosphere; the substrate concentration was the lowest of all reported systems to date and it was operating under physiological conditions. In addition, metathesases activity was evaluated with site-directed mutagenesis in order to modulate lipophilic, polar and coordinating amino acid residues to improve the catalytic performance [67].
7. Summary and Outlook
The CAs represents a unique example of highly investigated enzymes both for basic science studies, which allowed the understanding of intricate structure–function relationships in proteins at the atomic level, but also from the biochemistry viewpoint, with several different mutations the researchers could use for a variety of reactions not catalyzed normally by these enzymes, such as the hydratase, reductase and other catalytic propriety. All CA families use a metal hydroxide nucleophilic species of the enzyme, and possess a unique active site architecture, with half of it hydrophilic and the opposing part hydrophobic, allowing these enzymes to act as some of the most effective catalysts known in Nature. In addition, the introduction of different metal ions, mutation of key protein residues and different molecules bound to the CA can create beneficial mutations for different activities. In this scenario, compounds containing chalcogenide atoms can be use as novel tools to catalyze different activity [68,69,70,71,72].
Author Contributions
Writing—original draft preparation, A.A.; writing—review and editing, F.C., C.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
Fabrizio Carta (F.C.) is grateful to Fondazione Cassa di Risparmio di Firenze (Grant Number ECR2018.1001) for partially supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benkovic, S.; Hammes-Schiffer, S. A perspective on enzyme catalysis. Science 2003, 301, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.; Abdallah, W.; Banta, S.; Wheeldon, I. Engineering enzyme microenvironments for enhanced biocatalysis. Chem. Soc. Rev. 2018, 47, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ding, Y. Recent advances in biocatalyst discovery, development and applications. Bioorg. Med. Chem. 2014, 22, 5604–5612. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Sinha, P.; Jaiswal, P.; Mahendru, S.; Roy, K.; Kukreti, S. Protein engineering and de novo designing of a biocatalyst. J. Mol. Recognit. 2016, 29, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, L.; Jeffrey, P.D.; Shi, Y.; Morel, F.M. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008, 452, 56–61. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef]
- Supuran, C.T.; Capasso, C. The eta-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin. Ther. Targets 2015, 19, 551–563. [Google Scholar] [CrossRef]
- Del Prete, S.; Vullo, D.; De Luca, V.; Supuran, C.T.; Capasso, C. Biochemical characterization of the δ- carbonic anhydrase from the marine diatom Thalassiosira weissflogii, TweCA. J. Enzym. Inhib. Med. Chem. 2014, 29, 906–911. [Google Scholar] [CrossRef]
- Stefanucci, A.; Angeli, A.; Dimmito, M.P.; Luisi, G.; Del Prete, S.; Capasso, C.; Donald, W.A.; Mollica, A.; Supuran, C.T. Activation of β- and γ-carbonic anhydrases from pathogenic bacteria with tripeptides. J. Enzym. Inhib. Med. Chem. 2018, 33, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Del Prete, S.; Alasmary, F.A.S.; Alqahtani, L.S.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. The first activation studies of the η-carbonic anhydrase from the malaria parasite Plasmodium falciparum with amines and amino acids. Bioorg. Chem. 2018, 80, 94–98. [Google Scholar] [CrossRef]
- Angeli, A.; Buonanno, M.; Donald, W.A.; Monti, S.M.; Supuran, C.T. The zinc—But not cadmium—Containing ζ-carbonic from the diatom Thalassiosira weissflogii is potently activated by amines and amino acids. Bioorg. Chem. 2018, 80, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Kuuslahti, M.; Parkkila, S.; Supuran, C.T. Activation studies with amines and amino acids of the α-carbonic anhydrase from the pathogenic protozoan Trypanosoma cruzi. Bioorg. Med. Chem. 2018, 26, 4187–4190. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Del Prete, S.; Osman, S.M.; Alasmary, F.A.S.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation studies with amines and amino acids of the β-carbonic anhydrase encoded by the Rv3273 gene from the pathogenic bacterium Mycobacterium tuberculosis. J. Enzym. Inhib. Med. Chem. 2018, 33, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Del Prete, S.; Donald, W.A.; Capasso, C.; Supuran, C.T. The γ-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae is potently activated by amines and amino acids. Bioorg. Chem. 2018, 77, 1–5. [Google Scholar] [CrossRef]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B. A new widespread subclass of carbonic anhydrase in marine phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef]
- Keilin, D.; Mann, T. Carbonic anhydrase. Nature 1939, 144, 442–443. [Google Scholar] [CrossRef]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef]
- Chu, S. Carbon capture and sequestration. Science 2009, 325, 1599. [Google Scholar] [CrossRef]
- Savile, C.K.; Lalonde, J.J. Biotechnology for the acceleration of carbon dioxide capture and sequestration. Curr. Opin. Biotechnol. 2011, 22, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic anhydrase Enzyme-MOFs composite with a superior catalytic performance to promote CO2 absorption into tertiary amine solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Vinoba, M.; Bhagiyalakshmi, M.; Jeong, S.K.; Nam, S.C.; Yoon, Y. Carbonic anhydrase immobilized on encapsulated magnetic nanoparticles for CO2 sequestration. Chemistry 2012, 18, 12028–12034. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, H.; Lu, Y. Enhanced stability and chemical resistance of a new nanoscale biocatalyst for accelerating CO2 absorption into a carbonate solution. Environ. Sci. Technol. 2013, 47, 13882–13888. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, X.; Kaczmarek, M.B.; Chen, P.; Li, K.; Jin, P.; Liang, Y.; Daroch, M. Accelerated CO2 hydration with thermostable sulfurihydrogenibium azorense carbonic anhydrase-chitin binding domain fusion protein immobilised on chitin support. Int. J. Mol. Sci. 2019, 20, 1494. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Scozzafava, A.; Parkkila, S.; Puccetti, L.; De Simone, G.; Supuran, C.T. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg. Med. Chem. Lett. 2008, 18, 2267–2271. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Gould, S.M.; Tawfik, D.S. Directed evolution of the promiscuous esterase activity of carbonic anhydrase II. Biochemistry 2005, 44, 5444–5452. [Google Scholar] [CrossRef]
- Höst, G.; Mårtensson, L.G.; Jonsson, B.H. Redesign of human carbonic anhydrase II for increased esterase activity and specificity towards esters with long acyl chains. Biochim. Biophys. Acta 2006, 1764, 1601–1606. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef]
- Zengin Kurt, B.; Sonmez, F.; Ozturk, D.; Akdemir, A.; Angeli, A.; Supuran, C.T. Synthesis of coumarin-sulfonamide derivatives and determination of their cytotoxicity, carbonic anhydrase inhibitory and molecular docking studies. Eur. J. Med. Chem. 2019, 183, 111702. [Google Scholar] [CrossRef] [PubMed]
- Angapelly, S.; Sri Ramya, P.V.; Angeli, A.; Supuran, C.T.; Arifuddin, M. Sulfocoumarin-, coumarin-, 4-sulfamoylphenyl-bearing indazole-3-carboxamide hybrids: Synthesis and selective inhibition of tumor-associated carbonic anhydrase isozymes IX and XII. ChemMedChem 2017, 12, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.S.; Angeli, A.; Argulwar, O.S.; Tiwari, P.L.; Arifuddin, M.; Supuran, C.T. Design, synthesis and biological evaluation of coumarin linked 1,2,4-oxadiazoles as selective carbonic anhydrase IX and XII inhibitors. Bioorg. Chem. 2020, 98, 103739. [Google Scholar] [CrossRef] [PubMed]
- Tanc, M.; Carta, F.; Scozzafava, A.; Supuran, C.T. α-Carbonic anhydrases possess thioesterase activity. ACS Med. Chem. Lett. 2015, 6, 292–295. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Donnini, S.; Capperucci, A.; Ferraroni, M.; Tanini, D.; Supuran, C.T. Selenolesterase enzyme activity of carbonic anhydrases. Chem. Commun. 2020, 56, 4444–4447. [Google Scholar] [CrossRef]
- Pocker, Y.; Meany, J.E. The catalytic versatility of erythrocyte carbonic anhydrase. II. Kinetic studies of the enzyme-catalyzed hydration of pyridine aldehydes. Biochemistry 1967, 6, 239–246. [Google Scholar] [CrossRef]
- Cheshnovsky, D.; Navon, G. Nuclear magnetic resonance studies of carbonic anhydrase catalyzed reversible hydration of acetaldehyde by the saturation transfer method. Biochemistry 1980, 19, 1866–1873. [Google Scholar] [CrossRef]
- Pocker, Y.; Meany, J.E. photoregeneration of faded alkali metal solutions. The catalytic versatility of carbonic anhydrase from erythrocytes. The enzyme-catalyzed hydration of acetaldehyde. J. Am. Chem. Soc. 1965, 87, 1809–1811. [Google Scholar] [CrossRef]
- Briganti, F.; Mangani, S.; Scozzafava, A.; Vernaglione, G.; Supuran, C.T. Carbonic anhydrase catalyzes cyanamide hydration to urea: Is it mimicking the physiological reaction? J. Biol. Inorg. Chem. 1999, 4, 528–536. [Google Scholar] [CrossRef]
- Guerri, A.; Briganti, F.; Scozzafava, A.; Supuran, C.T.; Mangani, S. Mechanism of cyanamide hydration catalyzed by carbonic anhydrase II suggested by cryogenic X-ray diffraction. Biochemistry 2000, 39, 12391–12397. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Dis. 2015, 14, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 2015, 439, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 2018, 149, 101–109. [Google Scholar] [CrossRef]
- Gilbert, A.K.; Zhao, Y.; Otteson, C.E.; Pluth, M.D. Development of acid-mediated H2S/COS donors that respond to a specific pH window. J. Org. Chem. 2019, 84, 14469–14475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bolton, S.G.; Pluth, M.D. Light-activated COS/H2S donation from photocaged thiocarbamates. Org. Lett. 2017, 19, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Activatable small-molecule hydrogen sulfide donors. Antioxid. Redox Signal. 2020, 32, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Henthorn, H.A.; Pluth, M.D. Kinetic insights into hydrogen sulfide delivery from caged-carbonyl sulfide isomeric donor platforms. J. Am. Chem. Soc. 2017, 139, 16365–16376. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Schenk, S.; Protoschill-Krebs, G.; Kesselmeier, J.; Anders, E. The missing link in COS metabolism: A model study on the reactivation of carbonic anhydrase from its hydrosulfide analogue. Chembiochem 2007, 8, 530–536. [Google Scholar] [CrossRef]
- Esmieu, C.; Raleiras, P.; Berggren, G. From protein engineering to artificial enzymes—Biological and biomimetic approaches towards sustainable hydrogen production. Sustain. Energy Fuels 2018, 2, 724–750. [Google Scholar] [CrossRef]
- Jing, Q.; Okrasa, K.; Kazlauskas, R.J. Stereoselective hydrogenation of olefins using rhodium-substituted carbonic anhydrase—A new reductase. Chemistry 2009, 15, 1370–1376. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics, 59th ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1978–1979. [Google Scholar]
- Christianson, D.W.; Cox, J.D. Catalysis by metal-activated hydroxide in zinc and manganese metalloenzymes. Annu. Rev. Biochem. 1999, 68, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Kazlauskas, R.J. Regioselective hydroformylation of styrene using Rhodium-Substituted carbonic anhydrase. ChemCatChem 2010, 2, 953–957. [Google Scholar] [CrossRef]
- Piazzetta, P.; Marino, T.; Russo, N.; Salahub, D.R. Direct hydrogenation of carbon dioxide by an artificial reductase obtained by substituting rhodium for zinc in the carbonic anhydrase catalytic center. A mechanistic study. ACS Catal. 2015, 5, 5397–5409. [Google Scholar] [CrossRef]
- Piazzetta, P.; Marino, T.; Russo, N.; Salahub, D.R. Explicit water molecules play a key role in the mechanism of rhodium-substituted human carbonic anhydrase. ChemCatChem 2017, 9, 1047–1053. [Google Scholar] [CrossRef]
- Boddien, A.; Mellmann, D.; Gartner, F.; Jackstell, R.; Junge, H.; Dyson, P.J.; Laurenczy, G.; Ludwig, R.; Beller, M. Efficient dehydrogenation of formic acid using an iron catalyst. Science 2011, 333, 1733–1736. [Google Scholar] [CrossRef]
- Andring, J.T.; Kim, C.U.; McKenna, R. Structure and mechanism of copper-carbonic anhydrase II: A nitrite reductase. IUCrJ 2020, 7, 287–293. [Google Scholar] [CrossRef]
- Andring, J.T.; Lomelino, C.L.; Tu, C.; Silverman, D.N.; McKenna, R.; Swenson, E.R. Carbonic anhydrase II does not exhibit Nitrite reductase or Nitrous Anhydrase Activity. Free Radic. Biol. Med. 2018, 117, 1–5. [Google Scholar] [CrossRef]
- Aamand, R.; Dalsgaard, T.; Jensen, F.B.; Simonsen, U.; Roepstorff, A.; Fago, A. Generation of nitric oxide from nitrite by carbonic anhydrase: A possible link between metabolic activity and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2068–H2074. [Google Scholar] [CrossRef]
- Hanff, E.; Zinke, M.; Böhmer, A.; Niebuhr, J.; Maassen, M.; Endeward, V.; Maassen, N.; Tsikas, D. GC-MS determination of nitrous anhydrase activity of bovine and human carbonic anhydrase II and IV. Anal. Biochem. 2018, 550, 132–136. [Google Scholar] [CrossRef]
- Monnard, F.W.; Nogueira, E.S.; Heinisch, T.; Schirmerb, T.; Ward, T.R. Human carbonic anhydrase II as host protein for the creation of artificial metalloenzymes: The asymmetric transfer hydrogenation of imines. Chem. Sci. 2013, 4, 3269–3274. [Google Scholar] [CrossRef]
- Dürrenberger, M.; Heinisch, T.; Wilson, Y.M.; Rossel, T.; Nogueira, E.; Knörr, L.; Mutschler, A.; Kersten, K.; Zimbron, M.J.; Pierron, J.; et al. Artificial transfer hydrogenases for the enantioselective reduction of cyclic imines. Angew. Chem. Int. Ed. Engl. 2011, 50, 3026–3029. [Google Scholar] [CrossRef]
- Kim, M.C.; Lee, S.Y. Catalytic water oxidation by iridium-modified carbonic anhydrase. Chem. Asian, J. 2018, 13, 334–341. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Crabtree, R.H.; Brudvig, G.W. Molecular catalysts for water oxidation. Chem. Rev. 2015, 115, 12974–13005. [Google Scholar] [CrossRef] [PubMed]
- Rüttinger, W.; Dismukes, G.C. Synthetic water-oxidation catalysts for artificial photosynthetic water oxidation. Chem. Rev. 1997, 97, 1–24. [Google Scholar]
- Fernández-Gacio, A.; Codina, A.; Fastrez, J.; Riant, O.; Soumillion, P. Transforming carbonic anhydrase into epoxide synthase by metal exchange. Chembiochem 2006, 7, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kajetanowicz, A.; Ward, T.R. Carbonic anhydrase II as host protein for the creation of a biocompatible artificial metathesase. Org. Biomol. Chem. 2015, 13, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Pinteala, M.; Maier, S.S.; Simionescu, B.C.; Milaneschi, A.; Abbas, G.; Del Prete, S.; Capasso, C.; Capperucci, A.; Tanini, D.; et al. Evaluation of thio- and seleno-acetamides bearing benzenesulfonamide as inhibitor of carbonic anhydrases from different pathogenic bacteria. Int. J. Mol. Sci. 2020, 21, 598. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A.; Ferraroni, M.; Carta, F.; Angeli, A.; Supuran, C.T. Direct and straightforward access to substituted alkyl selenols as novel carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2020, 185, 111811. [Google Scholar] [CrossRef]
- Tanini, D.; Ricci, L.; Capperucci, A.; Di Cesare Mannelli, L.; Ghelardini, C.; Peat, T.S.; Carta, F.; Angeli, A.; Supuran, C.T. Synthesis of novel tellurides bearing benzensulfonamide moiety as carbonic anhydrase inhibitors with antitumor activity. Eur. J. Med. Chem. 2019, 181, 111586. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A.; Supuran, C.T.; Angeli, A. Sulfur, selenium and tellurium containing amines act as effective carbonic anhydrase activators. Bioorg. Chem. 2019, 87, 516–522. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C.T. Synthesis of Novel Selenides Bearing Benzenesulfonamide Moieties as Carbonic Anhydrase I, II, IV, VII, and IX Inhibitors. ACS Med. Chem. Lett. 2017, 8, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).