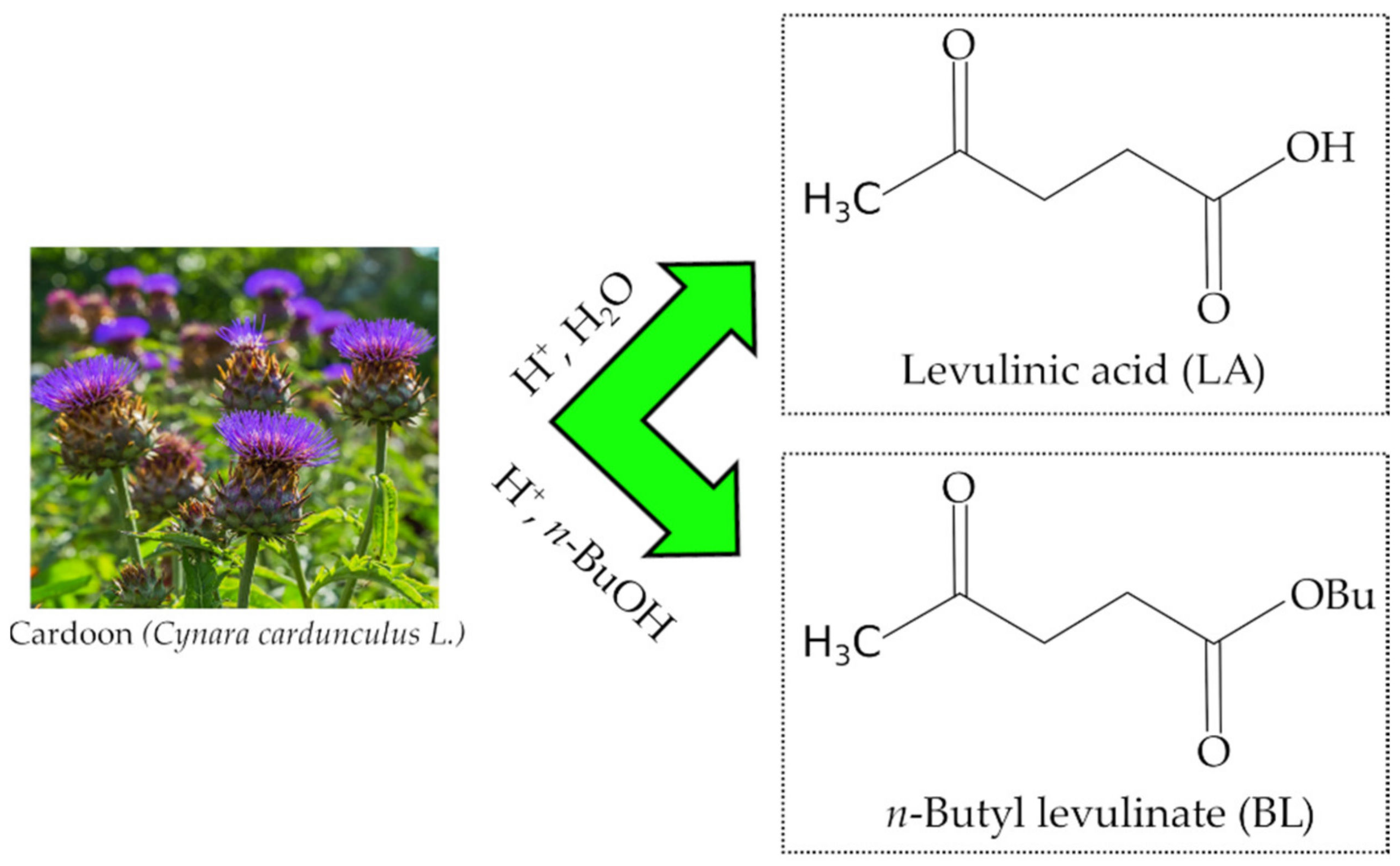
Sustainable Exploitation of Residual Cynara cardunculus L. to Levulinic Acid and n-Butyl Levulinate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Compositional Analysis of Crude Cardoon Samples
2.2. MW-Assisted Hydrolysis of C and E Cardoon Samples
2.3. Hydrolysis of E Cardoon under Conventional Heating
2.4. MW-Assisted Alcoholysis of E Cardoon Sample
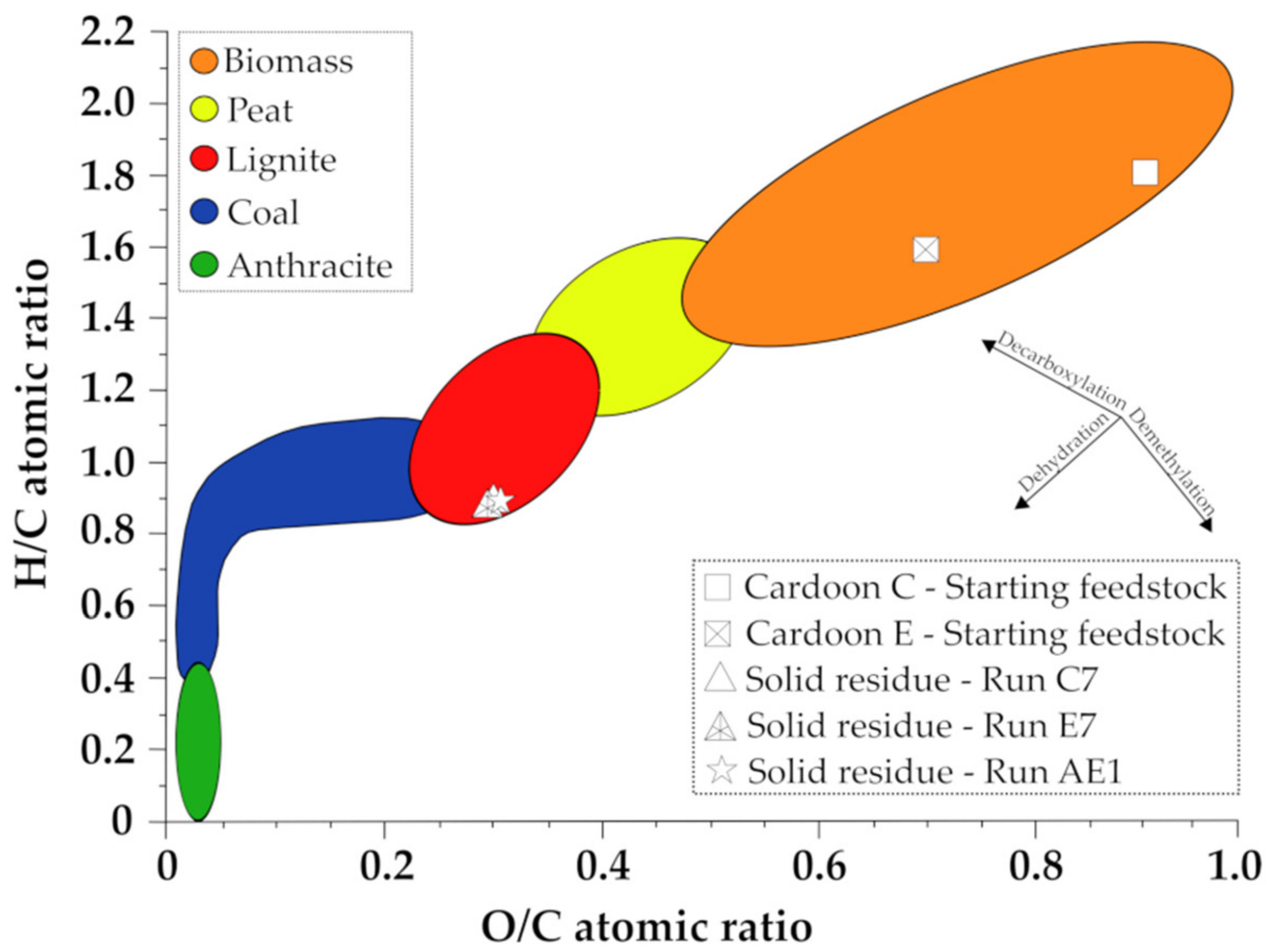
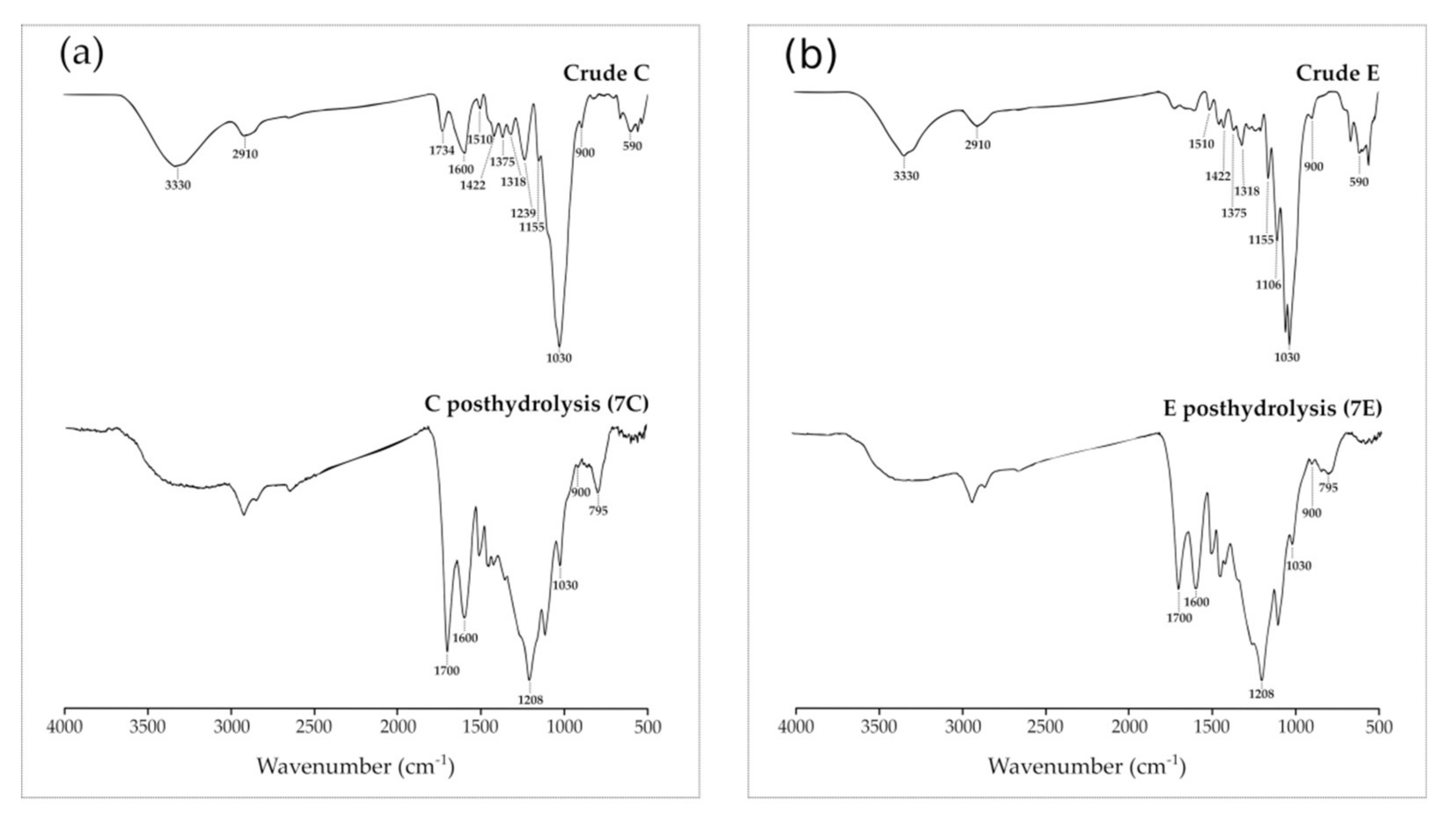
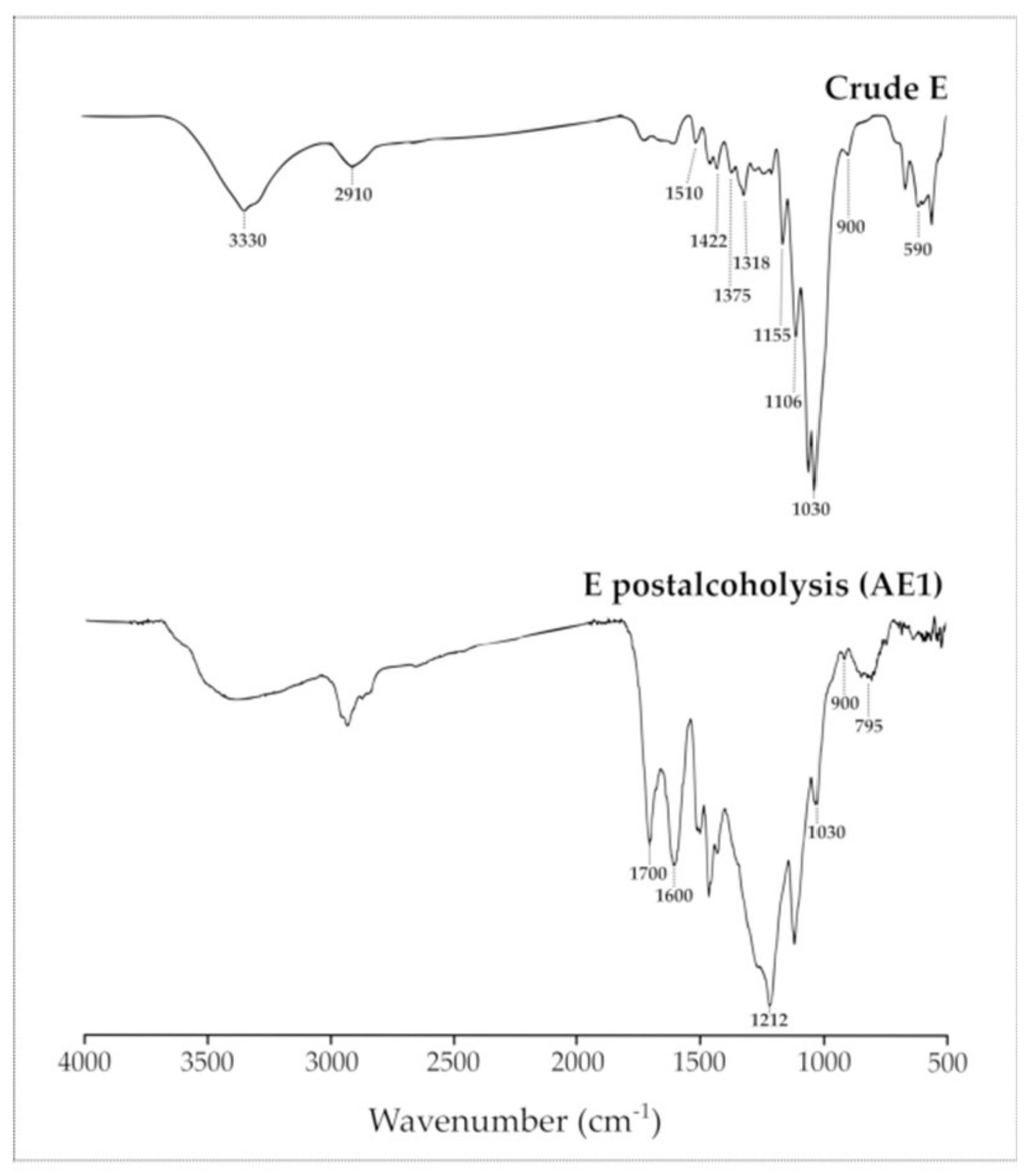
2.5. Characterization of Postreaction Solid Residues
3. Materials and Methods
3.1. Materials
3.2. Steam-Explosion Process Conditions
3.3. Compositional Analysis of Raw Biomass
3.4. Acid-Catalyzed Hydrolysis of Cardoon
3.5. Acid-Catalyzed Alcoholysis of Cardoon
3.6. Analytical Techniques
3.7. Purification of the Crude Mother Liquor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puricelli, S.; Cardellini, G.; Casadei, S.; Faedo, D.; Van den Oever, A.E.M.; Grosso, M. A review on biofuels for light-duty vehicles in Europe. Renew. Sustain. Energy Rev. 2021, 137, 110398. [Google Scholar] [CrossRef]
- Falcone, P.M.; Lopolito, A.; Sica, E. The networking dynamics of the Italian biofuel industry in time of crisis: Finding an effective instrument mix for fostering a sustainable energy transition. Energy Policy 2018, 112, 334–348. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 24 June 2021).
- Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R.K., Meyer, L.A., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Coccia, V.; Cotana, F.; Cavalaglio, G.; Gelosia, M.; Petrozzi, A. Cellulose Nanocrystals Obtained from Cynara Cardunculus and Their Application in the Paper Industry. Sustainability 2014, 6, 5252. [Google Scholar] [CrossRef] [Green Version]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a Sustainable Crop for Biomass and Bioactive Compounds Production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- Grammelis, P.; Malliopoulou, A.; Basinas, P.; Danalatos, N.G. Cultivation and Characterization of Cynara Cardunculus for Solid Biofuels Production in the Mediterranean Region. Int. J. Mol. Sci. 2008, 9, 1241. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crop. Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Espada, J.J.; Villalobos, H.; Rodríguez, R. Environmental assessment of different technologies for bioethanol production from Cynara cardunculus: A Life Cycle Assessment study. Biomass Bioenergy 2021, 144, 105910. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 526–533. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Raspolli Galletti, A.M. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Leal Silva, J.F.; Grekin, R.; Mariano, A.P.; Maciel Filho, R. Making Levulinic Acid and Ethyl Levulinate Economically Viable: A Worldwide Technoeconomic and Environmental Assessment of Possible Routes. Energy Technol. 2018, 6, 613–639. [Google Scholar] [CrossRef]
- Christensen, E.; Williams, A.; Paul, S.; Burton, S.; McCormick, R. Properties and Performance of Levulinate Esters as Diesel Blend Components. Energy Fuels 2011, 25, 5422–5428. [Google Scholar] [CrossRef]
- Howard, M.S.; Issayev, G.; Naser, N.; Sarathy, S.M.; Farooq, A.; Dooley, S. Ethanolic gasoline, a lignocellulosic advanced biofuel. Sustain. Energy Fuels 2019, 3, 409–421. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Giannoni, M.; Raspolli Galletti, A.M. Cascade Strategy for the Tunable Catalytic Valorization of Levulinic Acid and γ-Valerolactone to 2-Methyltetrahydrofuran and Alcohols. Catalysts 2018, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Rivas, S.; Raspolli Galletti, A.M.; Antonetti, C.; Licursi, D.; Santos, V.; Parajó, J.C. A Biorefinery Cascade Conversion of Hemicellulose-Free Eucalyptus Globulus Wood: Production of Concentrated Levulinic Acid Solutions for γ-Valerolactone Sustainable Preparation. Catal. 2018, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Rackemann, D.W.; Doherty, W. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Biorefining 2011, 5, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Timokhin, B.V.; A Baransky, V.; Eliseeva, G.D. Levulinic acid in organic synthesis. Russ. Chem. Rev. 1999, 68, 73–84. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J.R.H. The Biofine Process–Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks. In Biorefineries–Industrial Processes and Product: Status Quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; Volume 1, pp. 139–164. [Google Scholar]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Yamaguchi, M.; Kubo, S.; Yamada, T. Direct preparation of butyl levulinate by a single solvolysis process of cellulose. J. Wood Sci. 2013, 59, 179–182. [Google Scholar] [CrossRef]
- Démolis, A.; Eternot, M.; Essayem, N.; Rataboul, F. Influence of butanol isomers on the reactivity of cellulose towards the synthesis of butyl levulinates catalyzed by liquid and solid acid catalysts. New J. Chem. 2016, 40, 3747–3754. [Google Scholar] [CrossRef]
- Antonetti, C.; Gori, S.; Licursi, D.; Pasini, G.; Frigo, S.; López, M.; Parajó, J.C.; Raspolli Galletti, A.M. One-Pot Alcoholysis of the Lignocellulosic Eucalyptus nitens Biomass to n-Butyl Levulinate, a Valuable Additive for Diesel Motor Fuel. Catalysis 2020, 10, 509. [Google Scholar] [CrossRef]
- Matrìca. Green Chemicals. Available online: https://www.matrica.it/Default.asp?ver=en (accessed on 29 June 2021).
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Coccia, V.; Petrozzi, A.; Nicolini, A. Effect of Double-Step Steam Explosion Pretreatment in Bioethanol Production from Softwood. Appl. Biochem. Biotechnol. 2014, 174, 156–167. [Google Scholar] [CrossRef]
- Novamont. (BIT3G) Project—Third Generation Biorefinery Spread on the Territory, Supported by the Italian Ministry of Education (MIUR) and Coordinated by Novamont; Novamont: Novara, Italy, 2014. [Google Scholar]
- Asghari, F.S.; Yoshida, H. Acid-Catalyzed Production of 5-Hydroxymethyl Furfural from d-Fructose in Subcritical Water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Flannelly, T.; Lopes, M.; Kupiainen, L.; Dooley, S.; Leahy, J.J. Non-stoichiometric formation of formic and levulinic acids from the hydrolysis of biomass derived hexose carbohydrates. RSC Adv. 2015, 6, 5797–5804. [Google Scholar] [CrossRef] [Green Version]
- Shi, N.; Liu, Q.; Ju, R.; He, X.; Zhang, Y.; Tang, S.; Ma, L. Condensation of α-Carbonyl Aldehydes Leads to the Formation of Solid Humins during the Hydrothermal Degradation of Carbohydrates. ACS Omega 2019, 4, 7330–7343. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Rodriguez, M.E.; York, S.W.; Preston, J.F.; Ingram, L.O. Use of UV Absorbance To Monitor Furans in Dilute Acid Hydrolysates of Biomass. Biotechnol. Prog. 2000, 16, 637–641. [Google Scholar] [CrossRef]
- Glińska, K.; Lerigoleur, C.; Giralt, J.; Torrens, E.; Bengoa, C. Valorization of Cellulose Recovered from WWTP Sludge to Added Value Levulinic Acid with a Brønsted Acidic Ionic Liquid. Catalysts 2020, 10, 1004. [Google Scholar] [CrossRef]
- Ibáñez, A.B.; Bauer, S. Analytical method for the determination of organic acids in dilute acid pretreated biomass hydrolysate by liquid chromatography-time-of-flight mass spectrometry. Biotechnol. Biofuels 2014, 7, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Iroba, K.L.; Tabil, L.G.; Sokhansanj, S.; Dumonceaux, T. Pretreatment and fractionation of barley straw using steam explosion at low severity factor. Biomass Bioenergy 2014, 66, 286–300. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Licursi, D.; Antonetti, C.; Bernardini, J.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A.; Martinelli, M.; Raspolli Galletti, A.M. Characterization of the Arundo Donax L. solid residue from hydrothermal conversion: Comparison with technical lignins and application perspectives. Ind. Crop. Prod. 2015, 76, 1008–1024. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Vitolo, S.; Puccini, M.; Ribechini, E.; Bernazzani, L.; Raspolli Galletti, A.M. In-depth characterization of valuable char obtained from hydrothermal conversion of hazelnut shells to levulinic acid. Bioresour. Technol. 2017, 244, 880–888. [Google Scholar] [CrossRef]
- Düdder, H.; Wütscher, A.; Stoll, R.; Muhler, M. Synthesis and characterization of lignite-like fuels obtained by hydrothermal carbonization of cellulose. Fuel 2016, 171, 54–58. [Google Scholar] [CrossRef]
- Di Fidio, N.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D.; Liuzzi, F.; De Bari, I.; Antonetti, C. Multi-Step Exploitation of Raw Arundo donax L. for the Selective Synthesis of Second-Generation Sugars by Chemical and Biological Route. Catalysts 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Li, H.-Y.; Li, M.-F. Cornstalk liquefaction in sub- and super-critical ethanol: Characterization of solid residue and the liquefaction mechanism. J. Energy Inst. 2017, 90, 734–742. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Orella, M.J.; Lin, Z.; Cheng, Z.; Zheng, W.; Nikolakis, V.; Vlachos, D.G. Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins. Green Chem. 2016, 18, 1983–1993. [Google Scholar] [CrossRef] [Green Version]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Dee, S.J.; Bell, A.T. A Study of the Acid-Catalyzed Hydrolysis of Cellulose Dissolved in Ionic Liquids and the Factors Influencing the Dehydration of Glucose and the Formation of Humins. ChemSusChem 2011, 4, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Raspolli Galletti, A.M. Turning Point toward the Sustainable Production of 5-Hydroxymethyl-2-furaldehyde in Water: Metal Salts for Its Synthesis from Fructose and Inulin. ACS Sustain. Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Nasir, N.; Davies, G.; McGregor, J. Tailoring product characteristics in the carbonisation of brewers’ spent grain through solvent selection. Food Bioprod. Process. 2019, 120, 41–47. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Mattonai, M.; Pérez-Armada, L.; Rivas, S.; Ribechini, E.; Raspolli Galletti, A.M. Multi-valorisation of giant reed (Arundo Donax L.) to give levulinic acid and valuable phenolic antioxidants. Ind. Crop. Prod. 2018, 112, 6–17. [Google Scholar] [CrossRef]
- Yu, L.; Falco, C.; Weber, J.; White, R.J.; Howe, J.Y.; Titirici, M.-M. Carbohydrate-Derived Hydrothermal Carbons: A Thorough Characterization Study. Langmuir 2012, 28, 12373–12383. [Google Scholar] [CrossRef] [PubMed]
- Rasrendra, C.; Windt, M.; Wang, Y.; Adisasmito, S.; Makertihartha, I.G.B.N.; van Eck, E.R.H.; Meier, D.; Heeres, H.J. Experimental studies on the pyrolysis of humins from the acid-catalysed dehydration of C6-sugars. J. Anal. Appl. Pyrolysis 2013, 104, 299–307. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Raspolli Galletti, A.M. New Intensification Strategies for the Direct Conversion of Real Biomass into Platform and Fine Chemicals: What Are the Main Improvable Key Aspects? Catalysts 2020, 10, 961. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Mastrolonardo, G.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 2: A review of the biochar roles in growing media, composting and as soil amendment. Chem. Biol. Technol. Agric. 2020, 7, 16–25. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. NREL/TP-510-42618; Scarlata, C., Sluiter, J., Templeton, D., Eds.; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. NREL/TP-510-42619; Sluiter, J., Ed.; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass., NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Woestenborghs, P.L.; Altink, R.L. Process for the Isolation of Levulinic Acid. Patent No WO 2015/007602 A1, 22 January 2015. [Google Scholar]

| Run | Catalyst (wt %) | Sub/Cat (mol/mol) a | Products (g/L) b | LA Ponderal Yield (wt %) | LA Molar Yield (mol %) | |||
|---|---|---|---|---|---|---|---|---|
| Glu | AA | FA | LA | |||||
| C1 (dry) c | HCl (1.6 wt %) | 0.9 | 0.2 | 5.6 | 8.2 | 15.5 | 13.3 | 49.3 |
| C2 (dry) c | H2SO4 (2.1 wt %) | 1.8 | 0.1 | 6.0 | 7.1 | 13.0 | 11.2 | 41.5 |
| E1 (dry) c | HCl (1.6 wt %) | 0.9 | 0.1 | 7.9 | 26.9 | 23.0 | 48.4 | |
| E2 (dry) c | H2SO4 (2.1 wt %) | 1.8 | 0.5 | 7.1 | 22.5 | 19.3 | 40.7 | |
| C3 (wet) d | HCl (1.6 wt %) | 0.9 | 0.2 | 5.6 | 8.5 | 15.9 | 13.6 | 50.7 |
| E3 (wet) d | HCl (1.6 wt %) | 0.9 | 0.1 | 9.4 | 25.5 | 23.6 | 49.7 | |
| Run | Biomass Loading (wt %) a | Sub/Cat (mol/mol) b (HCl wt %) | Products (g/L)c | LA Ponderal Yield (wt %) | LA Molar Yield (mol %) | |||
|---|---|---|---|---|---|---|---|---|
| Glu | AA | FA | LA | |||||
| C3 | 10 | 0.9 (1.6 wt %) | 0.2 | 5.6 | 8.5 | 15.9 | 13.6 | 50.7 |
| C4 | 15 | 0.9 (2.4 wt %) | 0.5 | 8.6 | 12.7 | 23.9 | 13.0 | 48.1 |
| C5 | 20 | 0.9 (3.2 wt %) | 9.3 | 12.7 | 34.6 | 13.0 | 48.5 | |
| C6 | 20 | 1.5 (1.9 wt %) | 9.7 | 13.4 | 27.8 | 10.7 | 39.8 | |
| C7 | 20 | 2.0 (1.4 wt %) | 7.5 | 12.7 | 34.3 | 13.3 | 49.6 | |
| E3 | 10 | 0.9 (1.6 wt %) | 0.1 | 9.4 | 25.5 | 23.6 | 49.7 | |
| E4 | 15 | 0.9 (2.4 wt %) | 11.7 | 47.0 | 24.6 | 51.8 | ||
| E5 | 20 | 0.9 (3.2 wt %) | 12.5 | 58.9 | 21.1 | 44.5 | ||
| E6 | 20 | 1.5 (1.9 wt %) | 15.9 | 55.4 | 20.7 | 43.6 | ||
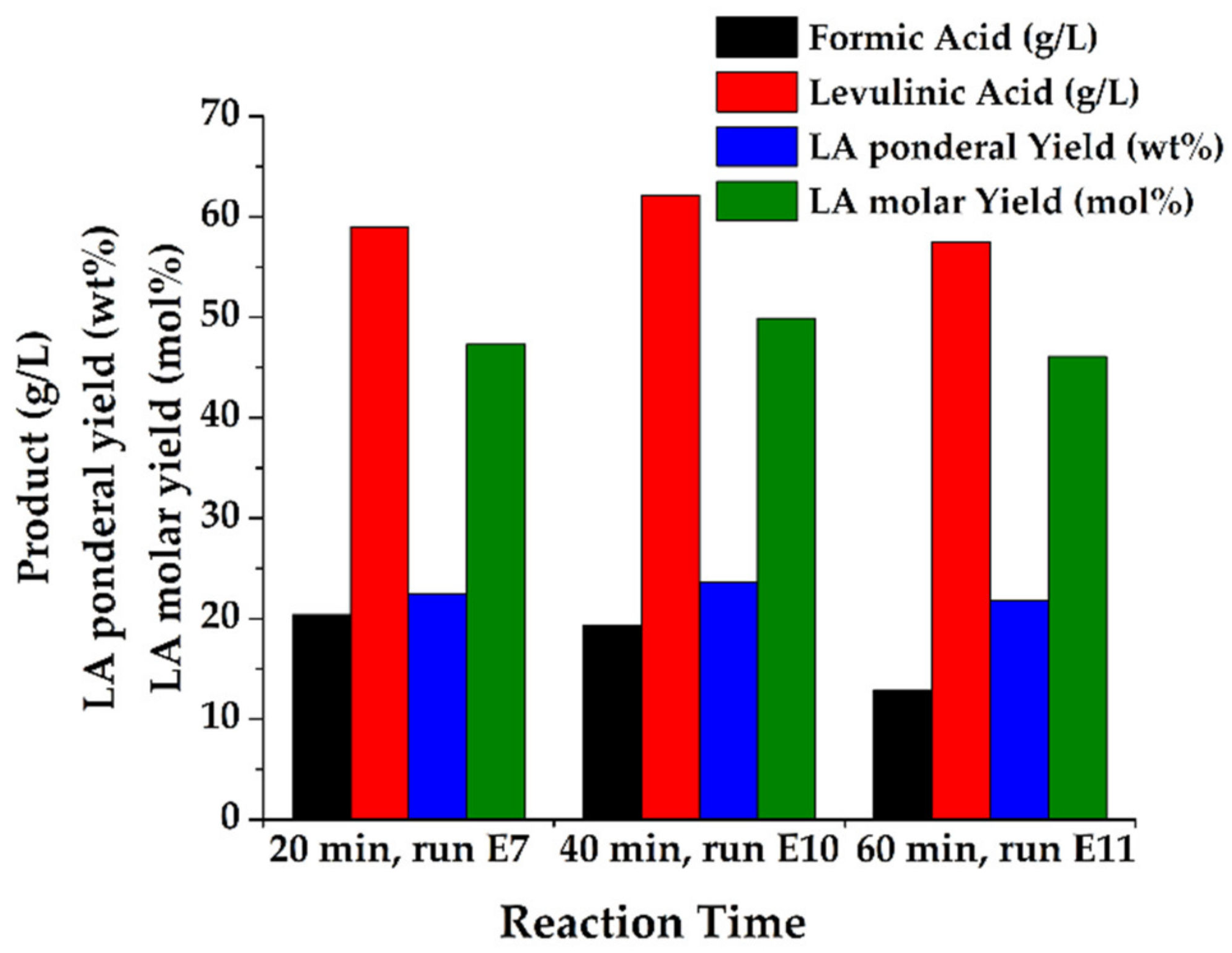
| E7 | 20 | 2.0 (1.4 wt %) | 20.4 | 59.0 | 22.4 | 47.3 | ||
| Run | Temperature (°C) | Products (g/L) a | LA Ponderal Yield (wt %) | LA Molar Yield (mol %) | |||
|---|---|---|---|---|---|---|---|
| Glu | AA | FA | LA | ||||
| E8 | 180 | 19.4 | 46.4 | 18.6 | 39.8 | ||
| E7 | 190 | 20.4 | 59.0 | 22.4 | 47.3 | ||
| E9 | 200 | 13.8 | 48.0 | 19.2 | 41.2 | ||
| Run | Biomass Loading (wt %) a | Sub/Cat (mol/mol) b (HCl wt %) | Heating System | Time (min) | LA Ponderal Yield (wt %) | LA Molar Yield (mol %) |
|---|---|---|---|---|---|---|
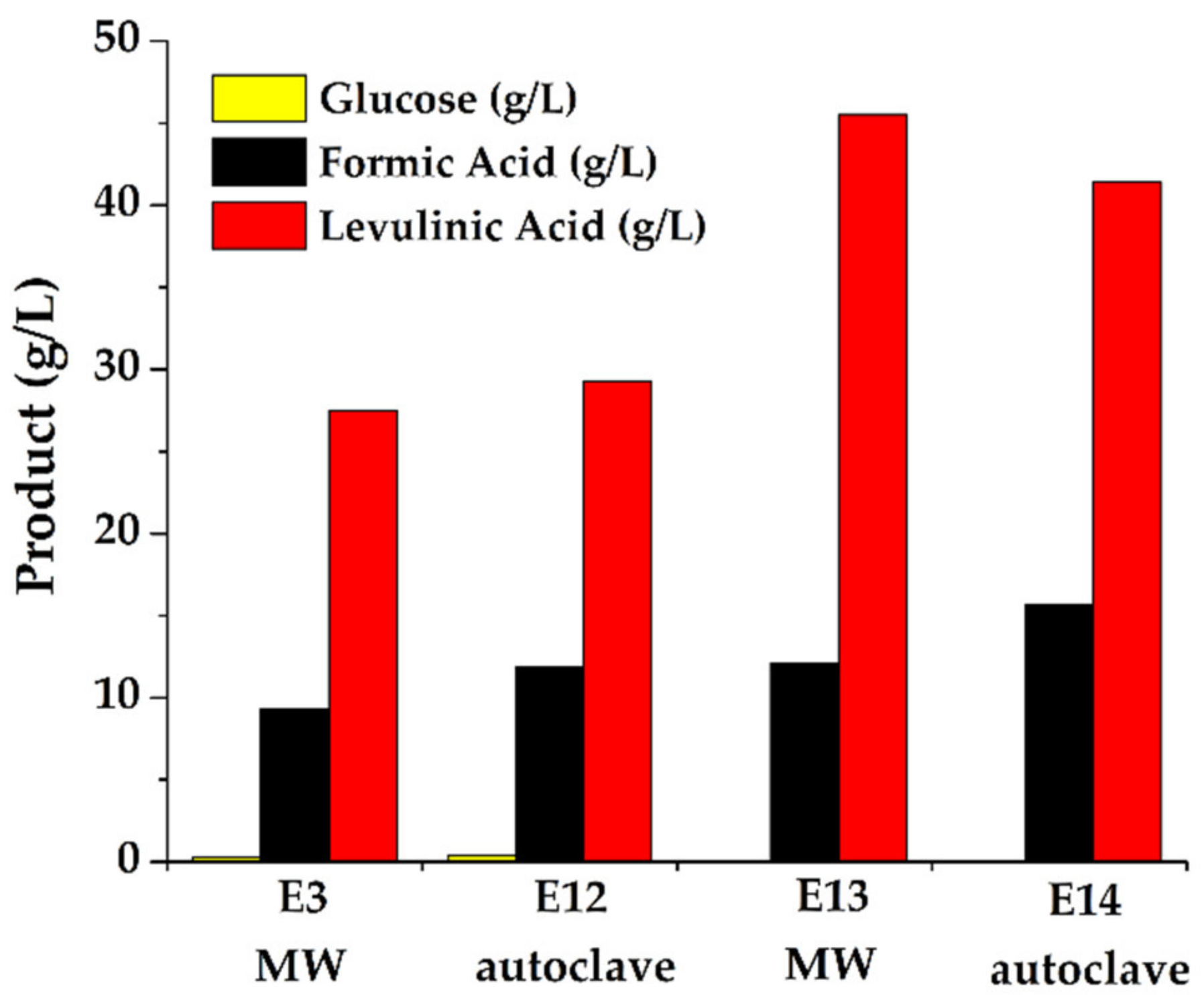
| E3 | 10 | 0.9 (1.6 wt %) | MW | 20 | 23.6 | 49.7 |
| E12 | 10 | 0.9 (1.6 wt %) | autoclave | 120 | 25.2 | 53.1 |
| E13 | 15 | 1.5 (1.4 wt %) | MW | 40 | 24.6 | 51.8 |
| E14 | 15 | 1.5 (1.4 wt %) | autoclave | 120 | 22.3 | 47.1 |
| Run | Biomass Loading (wt %) a | Sub/Cat (mol/mol) b (H2SO4 wt %) | Time (min) | BL (g/L) | BL Ponderal Yield (wt %) | BL Molar Yield (mol %) |
|---|---|---|---|---|---|---|
| AE1 | 8 | 2.4 (1.3 wt %) | 15 | 27.5 | 26.4 | 42.5 |
| AE2 | 15 | 2.4 (2.4 wt %) | 15 | 44.2 | 15.3 | 22.1 |
| AE3 | 15 | 4.7 (1.3 wt %) | 15 | 37.9 | 12.8 | 19.8 |
| AE4 | 8 | 2.4 (1.3 wt %) | 30 | 22.0 | 25.3 | 36.6 |
| AE5 | 8 | 2.4 (1.3 wt %) | 45 | 20.9 | 24.2 | 35.1 |
| Sample | C (%) | H (%) | N (%) | S (%) | O (%) a | Ash (%) | H/C | O/C | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Cardoon C—starting feedstock | 43.5 | 6.4 | 0.2 | 0.3 | 49.6 | 7.2 | 1.8 | 0.9 | 17.47 |
| Cardoon E—starting feedstock | 47.8 | 6.4 | 0.2 | 0.2 | 45.4 | 0.0 | 1.6 | 0.7 | 19.57 |
| Solid residue, run C7 | 66.9 | 5.1 | 0.1 | 0.1 | 27.8 | 0.2 | 0.9 | 0.3 | 26.49 |
| Solid residue, run E7 | 66.4 | 5.2 | 0.1 | 0.1 | 28.2 | 0.3 | 0.9 | 0.3 | 26.39 |
| Solid residue, run AE1 | 65.3 | 5.2 | 0.2 | 0.1 | 29.2 | 0.3 | 0.9 | 0.3 | 25.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raspolli Galletti, A.M.; Licursi, D.; Ciorba, S.; Di Fidio, N.; Coccia, V.; Cotana, F.; Antonetti, C. Sustainable Exploitation of Residual Cynara cardunculus L. to Levulinic Acid and n-Butyl Levulinate. Catalysts 2021, 11, 1082. https://doi.org/10.3390/catal11091082
Raspolli Galletti AM, Licursi D, Ciorba S, Di Fidio N, Coccia V, Cotana F, Antonetti C. Sustainable Exploitation of Residual Cynara cardunculus L. to Levulinic Acid and n-Butyl Levulinate. Catalysts. 2021; 11(9):1082. https://doi.org/10.3390/catal11091082
Chicago/Turabian StyleRaspolli Galletti, Anna Maria, Domenico Licursi, Serena Ciorba, Nicola Di Fidio, Valentina Coccia, Franco Cotana, and Claudia Antonetti. 2021. "Sustainable Exploitation of Residual Cynara cardunculus L. to Levulinic Acid and n-Butyl Levulinate" Catalysts 11, no. 9: 1082. https://doi.org/10.3390/catal11091082
APA StyleRaspolli Galletti, A. M., Licursi, D., Ciorba, S., Di Fidio, N., Coccia, V., Cotana, F., & Antonetti, C. (2021). Sustainable Exploitation of Residual Cynara cardunculus L. to Levulinic Acid and n-Butyl Levulinate. Catalysts, 11(9), 1082. https://doi.org/10.3390/catal11091082












