Abstract
Lecitase Ultra® solutions are mainly composed of bimolecular aggregates of two open structures of the enzyme. The immobilization and fixation of these bimolecular aggregates onto support surfaces is here proposed as a novel protocol for the immobilization and stabilization of Lecitase. The resulting derivatives of Lecitase aggregates were much more stable than the diluted solutions of the enzyme. The most stable of them was obtained by covalent immobilization of the bimolecular aggregate: 300-fold more stable than the diluted enzyme and 75-fold more stable than open Lecitase adsorbed onto hydrophobic supports. The bimolecular aggregate that adsorbed onto polyethyleneimine-agarose exhibited the best combination of activity and stability for the hydrolysis of krill oil. Omega-3 acids are in the sn-2 position of the krill oil, but they are also released by a phospholipase A1 because of migration issues.
1. Introduction
Enzymes are excellent catalysts throughout the chemical industry, but they have two limitations: they are water-soluble and usually very unstable. Finding a solution to these problems is one of the main objectives of enzyme engineering.
Immobilization converts enzymes into heterogeneous biocatalysts and allows them to be re-used or used continuously. The development of strategies to stabilize enzymes using immobilization techniques enables the preparation of heterogeneous and robust biocatalysts [1].
Lecitase Ultra® (Lecitase) is a phospholipase A1 obtained by the biotechnology company Novozymes by genetic engineering. It is a chimera obtained by the fusion of the genes encoding the Thermomyces lanuginosus lipase (TLL) and the phospholipase A1 [2]. Lecitase is very useful for oil degumming [3,4], racemic mixtures resolution [5], asymmetric hydrolysis [6,7], etc.
There are many papers related to Lecitase immobilization [4,5,8,9,10,11], but relevant stabilizations have not been reported. Additionally, in contrast to most lipases, the open structure of Lecitase that is adsorbed and fixed to hydrophobic supports is hyperactivated, and only four times more stable compared with diluted soluble enzymes [5].
In Section 1.1,Section 1.2, Section 1.3 and Section 1.4, the main aspects of our hypothesis are briefly explained.
1.1. The Structure of Soluble Lecitase
Lipases, and very likely Lecitase, may exhibit a complex behavior in solution (Figure 1). The isolated closed structure of these enzymes is stable in aqueous media and partially in equilibrium with an isolated and highly unstable open structure exhibiting a very large hydrophobic active center. At high concentrations, the isolated open form can be stabilized by forming bimolecular aggregates associated with their active centers that become unexposed to the aqueous medium.
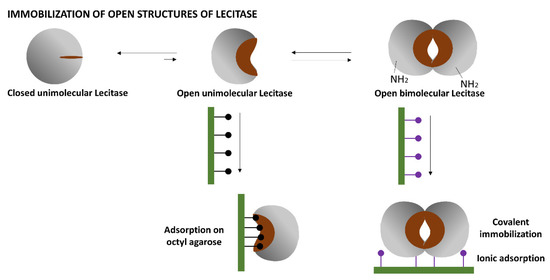
Figure 1.
Possible structures of Lecitase in solution. Mechanisms for immobilization of the open structure of Lecitase.
Using dynamic light scattering, the structure of soluble Lecitase was tested at concentrations higher than 2 mg/mL [12]. If two open structures of soluble Lecitase become associated through their open and hydrophobic active centers, the addition of a surfactant (e.g., cetyltrimethylammonium bromide—CTAB—for Lecitase) promotes the dissociation of the bimolecular aggregate [6,7]. According to results previously reported for several lipases, the complex structure of soluble Lecitase can be represented, as shown in Figure 1.
1.2. Immobilization of the Open Structure of Lecitase
The simplest way to immobilize the open structure of Lecitase, similar to most lipases, is by the interfacial adsorption of the soluble enzyme onto a hydrophobic support (e.g., octyl-agarose). In this way, the open active center becomes fixed and stabilized on the support surface [5].
The other open structures of Lecitase can be immobilized by fixing the bimolecular aggregates of the open structure onto different supports; that is, by simultaneously immobilizing the two enzyme molecules that form the aggregate so that it cannot become dissociated at various pH and temperatures.
Based on the surface of the bimolecular aggregate of TLL (Figure 2), two main immobilization–fixation protocols were chosen to be tested. The first is covalent immobilization of the bimolecular aggregate on cyanogen bromide (CNBr)-activated agarose at pH 8.5 via the two amino termini of the aggregate. At this pH, lysine residues are poorly reactive for the intermolecular reaction with the support. The bimolecular aggregate is fixed when its two amino termini are placed in the same hemisphere and relatively close to each other. This functions for TTL, to which Lecitase should be very similar [2]. The second is ionic adsorption on anionic exchangers through the region of the aggregate having the highest density of carboxyl groups. This region seems to be placed between the two molecules forming the aggregate.
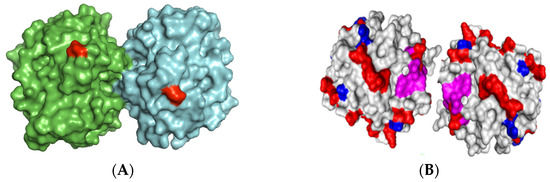
Figure 2.
3D-structure of Thermomyces lanuginosus lipase (TLL) aggregate. Lecitase was designed by genetic modification of TLL [12], so both aggregates should be similar. (A): Amino-terminal residues (red). Both amino termini are placed in the same hemisphere and relatively close to each other. (B). Carboxyl groups (red), Lysine groups (blue), active center (violet). The region involving both enzyme molecules is very rich in carboxyl groups.
The immobilization–fixation of bimolecular aggregates of Lecitase or other lipases has to fulfill three conditions: the immobilization of high concentrations of soluble Lecitase to favor bimolecular aggregation, the absence of surfactants or co-solvents in the immobilization medium to avoid aggregate dissociation, and the correct orientation of the supports to achieve the simultaneous immobilization of the two aggregate molecules that stabilize it under different pH and temperature conditions.
1.3. Immobilization of the Monomolecular Closed Structure of Lecitase
Soluble Lecitase exhibited the monomolecular, hyperactivated open structure in the presence of the surfactant CTAB [13,14]. This monomolecular enzyme can be immobilized through its amino terminus by covalent attachment on an CNBr-activated agarose. After a very mild immobilization process (15 min at 4 °C to avoid multipoint covalent immobilization), the surfactant was removed and the monomolecular closed structure of Lecitase was immobilized. This was a close approximation of the immobilized derivative of the native Lecitase and could be the ideal blank to evaluate the activity and stability of all other Lecitase derivatives in any reaction medium. We checked the thermal stability of this derivative with respect to a very diluted soluble enzyme (in a mainly dissociated state) in a fully aqueous medium (Figure 3).
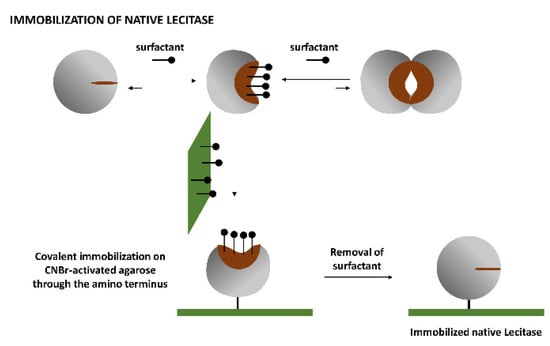
Figure 3.
The immobilization mechanism of the isolated close structure of Lecitase. The hyperactivated, isolated form becomes predominant in the presence of a surfactant (CTAB). This structure was immobilized by one-point covalent immobilization on CNBr-activated agarose at pH 8.5 for 15 min at 4 °C. After immobilization, the surfactant was removed to form the immobilized closed structure of the enzyme.
1.4. Release of All Fatty Acids from Phospholipids by Phospholipase A1
The differently immobilized Lecitase derivatives were tested for the hydrolysis of krill oil. The hydrolysis of phospholipids by phospholipase A1 in an aqueous medium at pH 7.0 and 25 °C firstly releases the fatty acids in the sn-1 position of the phospholipid. However, when the sn-1 hydroxyl is free, the migration of omega 3 fatty acids from sn-2 to sn-1 is very fast under these experimental conditions. In this way, they could now be released by phospholipase A1. Under these conditions (neutral pH and 25 °C), phospholipase A1 apparently behaves like a non-selective phospholipase (Figure 4).
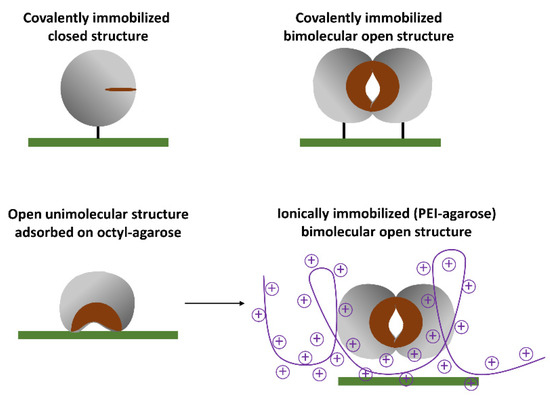
Figure 4.
Different derivatives of the immobilized Lecitase.
In this paper, the search for new open structures of Lecitase entailed studying bimolecular aggregates of soluble Lecitase and their thermal stability. Concentrated soluble Lecitase was studied using dynamic light scattering to detect well-defined aggregates. The aggregates were dissociated by surfactants in order to support the involvement of hydrophobic active centers of open structures of the two enzyme molecules. The thermal stabilities of concentrated solutions of Lecitase were compared with very dilute solutions, where mainly isolated and closed structures occurred.
If the thermal stability of these aggregates proved promising, the immobilization and fixation of aggregates on the surface of solid supports would be intended. Fixation involved the two aggregate molecules in the immobilization process simultaneously; in this way, the bimolecular aggregate was stable at different levels of pH and temperature. To “rationalize” the fixation of the aggregate, the X-ray structure of the TLL aggregate was considered. Except for the active center, the whole structure of Lecitase should be very similar to that of TLL. The open monomolecular structure of the enzyme was immobilized by adsorption onto hydrophobic supports. (Figure 4). Thermal stability of all the derivatives was tested as was their catalytic activity (hydrolysis) on krill oil, which was hydrolyzed with different enzyme derivatives under very mild experimental conditions to maintain stable the labile structure of the omega-3 fatty acids. The ability of phospholipase A1 to release the omega-3 fatty acids at the sn-2 position of the phospholipid was also tested.
2. Results and Discussion
2.1. Study of Soluble Lecitase Using Dynamic Light Scattering (DLS)
Using moderate Lecitase concentrations (2 mg/mL) at pH 7.0 and 25 °C, two well-defined peaks with no intermediate ones were observed using DLS (Figure 5). The peak corresponding to the higher hydrodynamic radius was the maximum (65%). The peak corresponding to the smaller hydrodynamic radius was the minimum (35%). In the presence of 0.1% of the surfactant CTAB, the minimum peak increased to 80% and the maximum peak decreased to 20%.
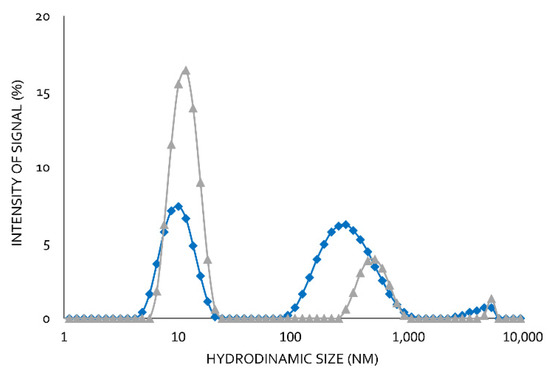
Figure 5.
Dynamic light scattering of soluble Lecitase (2 mg/mL). Blue line: the soluble enzyme in the absence of CTAB. Grey line: the soluble enzyme in the presence of 0.2% CTAB.
In a previous paper, we reported the formation of bimolecular lipase aggregates [15,16]. We therefore assumed that the peak with the higher hydrodynamics corresponded to a bimolecular aggregate associated with hydrophobic interactions, which became dissociated in the presence CTAB. These hydrophobic interactions involved the highly active hydrophobic centers of two open structures of Lecitase. Lipases and Lecitase do not have any additional relevant hydrophobic pockets on their surfaces [17]. Therefore, Lecitase solutions of moderate or high concentrations are very rich in bimolecular enzyme aggregates that can become dissociated into isolated enzyme molecules in the presence of CTAB. DLS is a very effective technique to detect protein aggregations, but the real size of the protein aggregates is difficult to quantify [12].
From these results, the thermal stability of a very diluted Lecitase solution (0.1 mg/mL, mainly composed of dissociated closed structures) was compared with a very concentrated solution of Lecitase (10 mg/mL, mainly composed of bimolecular aggregates) (Table 1). The aggregates were 75-fold more stable than the diluted enzyme. It seems that this open structure of Lecitase was highly stabilized. In addition, bimolecular aggregates of Lecitase were 20-fold more stable than the open structure of Lecitase immobilized on hydrophobic supports [5]. Interestingly, the stability of the Lecitase open structure strongly depended on the surface onto which this open structure was adsorbed. The aggregation of two open structures of Lecitase promoted the highest stabilization. The immobilization and fixation of these new open structures of Lecitase was then intended.

Table 1.
Thermal inactivation of diluted and concentrate Lecitase solutions. Enzyme solutions were incubated at pH 7.0 and 60 °C. At different times, aliquots were withdrawn and assayed according to the standard p-NPB assay. Half-life time is the time necessary to decrease the activity by 50%. Stabilization factor is the ratio of half-life times, taking the half-life of the diluted enzyme as the reference.
2.2. Immobilization of Lecitase Ultra® on Different Supports
Protein concentration was determined with the Pierce BCA assay: 20 mg of Lecitase per mL of commercial preparation was established as the reference concentration. The enzyme load of all the derivatives was 2 mg of Lecitase per gram of support. In Figure 4 the different immobilization approaches are graphically represented.
The immobilization and fixation of bimolecular aggregates was performed as described in the Introduction: (1) covalent immobilization on CNBr-activated agarose through the two amino termini of the aggregate and (2) ionic adsorption on polyethyleneimine-agarose (in the absence of CTAB) through the region with the highest density of carboxy groups belonging to the aggregate. In contrast, covalent immobilization of the completely dissociated enzyme (in the presence of CTAB) on CNBr-activated agarose was also performed. Finally, monomeric Lecitase was immobilized by interfacial adsorption on octyl-agarose, as previously reported [5].
According to the aggregation issue previously explained, we represented the bimolecular aggregate as the maximum conformation of Lecitase immobilized in CNBr–agarose and polyethyleneimine (PEI)-agarose derivatives. It is important to take this feature of lipases into consideration because aggregation may produce changes in activity, stability, or even selectivity [15].
Table 2 shows the yield and recovered activity after the different immobilization methods. The enzymatic load of all the derivatives was 2 mg of enzyme per 1 g of support, which represents less than 5% of the maximum load [15]. This moderate enzyme loading was designed to prevent significant diffusion problems. We obtained a high yield, more than 90%, in all the immobilization protocols. The specific activity of commercial Lecitase, measured with the p-NPB assay, was 3.05 U/mg.

Table 2.
Preparation of different immobilized derivatives of Lecitase.
After immobilization, the CNBr derivative was boiled in the presence of sodium dodecyl sulfate (SDS) and the supernatant was analyzed by SDS-PAGE. No bands of Lecitase were observed (data not shown). These results seemed to indicate that the bimolecular aggregate was effectively fixed on the immobilization support.
Conversely, immobilization on PEI-agarose was much faster for the aggregate (in the absence of CTAB) than the isolated enzyme (in the presence of CTAB) (Figure 6). Again, it seemed that the aggregate was fixed on the immobilization support. If immobilization occurred through only one Lecitase molecule, the immobilization rate should have been similar in the presence or absence of CTAB.
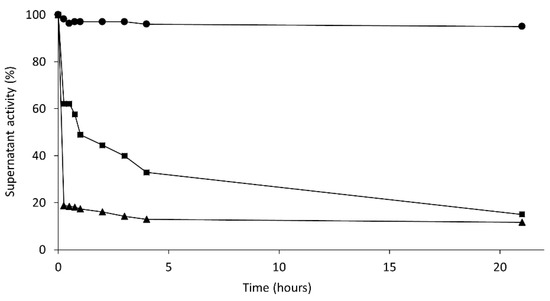
Figure 6.
Time-course of immobilization of Lecitase on PEI-agarose. 100% activity of the soluble enzyme before immobilization (in the presence or absence of CTAB). Circles: activity of enzyme solutions without supports; squares: activity of the supernatant of the immobilization suspension in the presence of CTAB; triangles: activity of the supernatant of the immobilization suspension in the absence of CTAB. Since the activity of the enzyme solution remained unaltered for 20 h, the decay of activity in the immobilization suspension can be exactly associated with the percentage of soluble enzyme that became immobilized.
Recovered activity after immobilization showed interesting results: a drastic reduction in activity in CNBr with only 25% of initial activity, a mild reduction of activity in CNBr–CTAB and PEI, and the hyperactivation of Lecitase immobilized on octyl-agarose (Table 2). This low activity of the covalently immobilized aggregate might be explained by the fact that it was covalently immobilized in CNBr via a certain multipoint covalent attachment. At first glance, immobilization only occurred through the two amino termini of the two molecules of the aggregate. However, the long-term incubation of the derivate immobilized on the activated support could have promoted an additional covalent attachment of lysine (Lys) residues placed in the area of the two amino termini, and this multipoint attachment could cause a significant distortion to the structure of the bimolecular aggregate.
2.3. Thermal Stability of the Different Derivatives
In Table 3, the half-lives obtained for the different derivatives during thermal inactivation at 60 °C and pH 7.0 are shown. A half-life is the time necessary to decrease the initial activity of each derivative (before thermal inactivation) by 50%. Stabilizations were calculated as the ratio between the half-life of each derivative and the covalently immobilized enzyme in the presence of CTAB.

Table 3.
Thermal inactivation of Lecitase derivatives: t1/2: half-life time of the inactivation (in hours); rt1/2: relative half-life, corresponding to the stabilization factor, i.e., t1/2 divided by the one of CNBr–CTAB. Inactivation was carried out at 60 °C and pH 7.
The less stable derivative was the immobilized, isolated, closed structure of Lecitase. This derivative had the same stability as the highly diluted soluble enzyme (Table 1). In addition to this, it exhibited 80% activity (Table 2). This immobilized derivative thus seemed to be a very close approximation to the immobilized native Lecitase.
Conversely, all the derivatives of the open Lecitase structure were more stable than the derivative of the closed structure. Curiously, the open form stabilized by adsorption on octyl-agarose was not as highly stabilized (only 4-fold more stable) as previously reported [5]. Stabilization by interfacial adsorption on hydrophobic supports was much higher with other lipases [14].
However, immobilized and fixed bimolecular aggregates were the most stable derivatives. The PEI-agarose derivative was 75-fold more stable than the enzyme covalently immobilized in the presence of CTAB or the highly diluted soluble enzyme, and they preserved 75% catalytic activity. This stability was very similar to that of the highly concentrated enzyme solutions (also mainly bimolecular aggregates). Conversely, the bimolecular aggregate fixed by multipoint covalent immobilization to the CNBr-activated agarose was the most stable derivative (300-fold stabilization factor), but it preserved only 25% catalytic activity. As mentioned above, in addition to the two amino termini of the aggregate, some Lys residues placed in their proximity could also become attached to the support. This multipoint covalent attachment can promote enzyme distortions, but also highly stabilize the 3D structure. This may be the cause of the highly increased stability as well as the relevant decrease in enzyme activity.
The most representative inactivation progressions are represented in Figure 7. The most stable derivative at first showed rapid inactivation down to 70% of the remaining activity, which could have been due to the percentage of immobilized isolated Lecitase as detected by DLS. CNBr–agarose was able to immobilize both the isolated enzyme and the bimolecular aggregate. However, the second phase of the inactivation was extremely stable, which seemed to be due to the bimolecular aggregate immobilized by multipoint covalent immobilization. Its stabilization could be much higher than the 300-fold factor calculated if the two inactivation phases are taken into account. Similarly, the derivative of the immobilized native enzyme (maximum in isolated closed enzymes) exhibited a second highly stable inactivation phase due to the low percentage of the immobilized bimolecular aggregate, such as what was detected in the soluble Lecitase using DLS. In a forthcoming paper, a novel strategy to selectively immobilize the bimolecular aggregate will be reported.
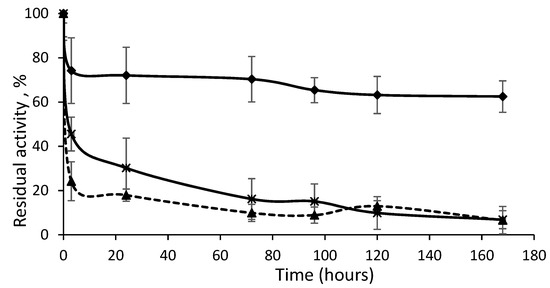
Figure 7.
Time course of thermal inactivation of three Lecitase derivatives. Rhombus: bimolecular aggregate of open enzyme covalently immobilized; triangles: closed enzyme covalently immobilized; crosses: open enzyme adsorbed onto octyl-agarose.
2.4. Release of Omega 3 Fatty Acids by Enzymatic Hydrolysis of Krill Oil
One gram of commercial krill oil capsules contains 240 mg of omega-3 fatty acids that are mainly in the sn-2 position of the phospholipid [18,19,20,21]. The enzymatic hydrolysis of krill oil was performed under very mild experimental conditions (pH 7.0 and 25 °C) to minimize undesirable oxidation of the polyunsaturated fatty acids. The reaction had an initial lag of 5–10 min and then the release of the omega-3 fatty acid was linear for at least 30 min. The activities of the different derivatives are shown in Table 4.

Table 4.
Release of omega-3 fatty acids by the enzymatic hydrolysis of krill oil.
Lecitase adsorbed onto octyl-agarose was the most active derivative, but PEI-Lecitase (ionic adsorption of the bimolecular aggregate) also exhibited relatively high activity (63% with respect to the octyl derivative). The most stable derivative (covalently immobilized derivative of the bimolecular aggregate) was 6-fold less active than the octyl derivative. Under very mild reaction conditions, all derivatives were stable; however, as a compromise solution between activity and stability, PEI-Lecitase was selected as the optimal biocatalyst. Compared with the octyl derivative, it had 63% activity but was 16-fold more stable. With this optimal derivative, consecutive reaction cycles were performed.
In each cycle, 1 g of the biocatalyst was added to 5 mL of the aqueous phase. More than 95% of the omega-3 fatty acids contained in 1 g of krill oil were released in 2 h. Ten reaction cycles were identical in activity (26 UI/g) for the release of omega-3 fatty acids and in the final yield (95%). Omega-3 fatty acids were not released in the absence of biocatalyst.
Under the reaction conditions, it seemed that migration 2 > 1 was very fast. In this way, Lecitase, phospholipase A1 apparently behaved like a non-selective phospholipase. Of course, Lecitase was not able to hydrolyze the ester bonds in position sn2, but after the sn1 position was hydrolyzed, the omega-3 fatty acids were in their new sn1 position from where they were easily released by Lecitase. In this way, phospholipase A1 was able to release all the fatty acids contained in krill oil by hydrolysis in aqueous media, where migration was highly favored.
3. Materials and Methods
3.1. Materials
Lecitase Ultra® was donated by Novozymes (Bagsværd, Denmark). The agarose supports Cyanogen-bromide activated Sepharose 4B (CNBr–agarose) and Octyl-Sepharose 4B (octyl-agarose) were from GE Healthcare (Buckinghamshire, UK). Polyethyleneimine (Mw: ~25 kDa), p-nitrophenyl butyrate (p-NPB), 5 % (w/v) picryl sulfonic acid solution (TNBSA), and hexadecyltrimethylammonium bromide (CTAB) were from Merck (St. Louis, Mo, USA). Pierce™ BCA Protein Assay Kits were from Thermo-Fisher Scientific (Waltham, Ma, USA). Krill oil was obtained from a local market (all other reagents were of analytical grade). One gram (2 capsules) contained 240 mg of omega-3 fatty acids (both EPA and DHA).
3.2. Protein Concentration Determination
A Pierce™ BCA Protein Assay Kit was used following the manufacturer instructions to determine the concentration of the commercial Lecitase Ultra®.
3.3. Characterization of Aggregates with Dynamic Light Scattering
We performed measurements in a ZETASIZER NANO-ZS device (Malvern Instruments Ltd., Malvern, UK) to determine the presence of Lecitase aggregates. Commercial Lecitase samples were diluted in 25 mM phosphate buffer pH 7.0 (2 mg/mL) in the presence or absence of the surfactant CTAB (0.1%). We compared the hydrodynamic radii of both samples to detect possible enzyme aggregates.
3.4. Standard Assay to Determine Lipase Activity (p-NPB Assay)
We prepared a substrate solution composed of 0.5 mM p-nitrophenyl butyrate (p-NPB) and 25 mM phosphate buffer, pH 7.0. The enzyme solution contained 0.1 g of soluble or immobilized biocatalyst per mL in the phosphate buffer. The increased absorbance of released p-nitrophenol at 348 nm (ε = 5.150 mM−1 cm−1) was measured when 0.1 mL of the enzyme solution/suspension was added to 2.5 mL of the reaction mixture (at 25 °C). The amount of enzyme (in µg) catalyzing the release of 1 μmol of p-nitrophenol per minute under these conditions defines one international unit (U) of the lipase activity. The spectrophotometer used was a thermostated JASCO V630 series (Madrid, Spain) with spectrophotometric cells with magnetic stirring.
3.5. Immobilization of Lipases on Different Supports
In all the immobilization processes, initial activity, supernatant, and suspension activity (several times during immobilization), and final activity of the immobilized preparations were measured with the p-NPB assay.
3.5.1. Immobilization on Activated CNBr–Agarose
CNBr–agarose was hydrated by incubation with HCl in H2O solution (pH = 2) for 30 min. Then, 0.1 mL of commercial Lecitase (20 mg/mL) was diluted in 9.9 mL of 25 mM phosphate buffer pH 7. For the immobilization in the presence of CTAB, 0.333 mL of a 3% CTAB solution were added to reach a final concentration of 0.1% CTAB. Finally, 1 g of wet CNBr–agarose was added, and the suspension was gently stirred overnight at 4 °C. After 24 h of immobilization, the enzyme-support solution was filtered, washed and diluted in 10 mL of 25 mM bicarbonate buffer, pH 9. The immobilized enzyme solution was incubated at 4 °C for 3 days to completely deactivate the remaining active groups (cyanates) on the support. The immobilized derivatives were then washed with 25 mM phosphate buffer at pH 7.0 and filtered. Derivatives were stored in a wet state at 4 °C.
After immobilization, a sample of each immobilized derivative was boiled in the presence of sodium dodecyl sulfate (SDS) to dissociate all the aggregates that were not covalently attached to the support. The supernatant of the boiled samples was analyzed by SDS-PAGE. No bands corresponding to soluble Lecitase were observed for all derivatives. This means that the bimolecular aggregate was effectively covalently attached to the support via simultaneous immobilization of the two enzyme molecules.
3.5.2. Immobilization of Lipases on Octyl-Agarose
A volume of 0.1 mL of the commercial preparation of Lecitase-Ultra® was diluted in 9.9 mL of 25 mM phosphate buffer pH = 7. Then, 1 g of octyl-agarose was added to the solution and incubated with gentle stirring for 1 h at 25 °C. After the immobilization process, the suspension with the enzyme immobilized on the octyl-agarose was filtered and washed with the phosphate buffer several times. Derivatives were stored in wet state at 4 °C [22].
3.5.3. Immobilization of Lecitase on PEI-Agarose
One gram of glyoxyl-agarose was functionalized with polyethyleneimine (polymer of 25 kDa). The method was previously described in [23]. Then, 0.1 mL of the commercial preparation of Lecitase-Ultra® (in the absence or presence of 0.1% of CTAB) was diluted in 9.9 mL of 25 mM phosphate buffer at pH 7.0, to which 1 g of the PEI-functionalized support was added. The suspension was incubated for 5 h at 25 °C and gently stirred. During the immobilization, the activities of the suspension and supernatant were measured with the p-NPB assay. The suspension was then filtered, and the biocatalyst washed with the phosphate buffer.
The time-course of immobilization for Lecitase in the absence of CTA (when it was mainly aggregated) was compared with time course of immobilization of Lecitase in the presence of CTAB (when it was mainly dissociated). The activity of both enzyme solutions (absence or presence of CTAB) remained unaltered for 24 h. Therefore, the decrease of activity of the supernatant of both immobilization suspensions could be correctly associated to the increase of soluble enzyme that became immobilized.
3.6. Thermal Inactivation of Soluble Lecitase
Two different solutions of Lecitase were incubated at pH 7.0 and 60 °C. The time-course of aliquot inactivation of a very diluted solution (0.1 mg/mL), where the enzyme was mainly dissociated, was compared with the time-course of inactivation of a highly concentrated solution (10 mg/mL), where the enzyme was mainly aggregated. At a different time, aliquots of both solutions were withdrawn (200 µL for the diluted enzyme and 2 µL for the concentrated one) and analyzed with the standard assay.
3.7. Thermal Inactivation of Different Lecitase Immobilized Preparations
To test the stability of the biocatalysts, 0.5 g of each immobilized preparation was suspended in 5 mL of 25 mM phosphate buffer (pH = 7.0). The samples were incubated at 60 °C. Of each of them, samples (0.1 mL) were periodically withdrawn, and their remaining activity was measured with the p-NPB assay as previously described. The half-life of each derivative was determined as the time necessary to reach a remaining activity of 50% (while 100% was the activity of each derivative before the incubation at a high temperature).
3.8. Hydrolysis of Krill Oil
The activity of Lecitase Ultra® immobilized on different supports was analyzed via hydrolysis of krill oil. It was performed in a two-phase system formed by an aqueous solution (0.1 M Tris-Cl buffer, pH = 7) and an organic phase (cyclohexane) [24]. First, 5 mL of each phase and 0.1 mL of krill oil were placed in a 20 mL reactor and pre-incubated for 30 min with agitation. Afterwards, 1 g of the different derivatives was added to the suspension and located in the aqueous phase. The suspension was stirred during the reaction. Several aliquots of 50 µL of the organic phase were withdrawn at different times to measure the amount of omega-3 fatty acids released in course of the hydrolysis of krill oil.
3.9. Analysis of Free Polyunsaturated Fatty Acids (PUFA) Using HPLC–UV
The aliquots of the hydrolysis reactions were analyzed by HPLC. The PUFA concentration released at different times was analyzed by reverse phase HPLC (RP-HPLC) (Spectra Physic SP4000) coupled with a UV detector (Spectra System P4000) (Waltham, MA, USA) using a C8 Kromasil (0.4 cm × 15 cm) column. The mobile phase was composed of acetonitrile and 10 mM TRIS buffer, pH = 3 (75:25 v/v). The flow rate was 1 mL/min and the UV detection of the PUFAs was performed at 215 nm. The mean retention times for the PUFA were 9.4 min and 10.2 min for the DHA. The pure commercial standards were compared with the PUFAs produced in the reaction to determine their concentration.
4. Conclusions
At concentrations higher than 2 mg/mL, Lecitase mostly formed bimolecular aggregates, where two open structures of two enzyme molecules were associated though their active centers. The highly concentrated soluble enzyme (mostly in the form of bimolecular aggregates of open structures) was 75-fold more stable than the highly diluted soluble enzyme (mostly in the form of monomolecular closed structures). For this reason, the simultaneous immobilization on a solid support of the two molecules of the bimolecular aggregate of the open structure of Lecitase was proposed as a novel strategy to stabilize Lecitase.
This stabilization strategy included the (i) immobilization of a highly concentrated solutions of Lecitase; (ii) absence of surfactants or co-solvents in the immobilization mixture to avoid dissociation of the bimolecular aggregate, and (iii) fixing of the aggregates by simultaneous immobilization on the support surface of both enzyme molecules involved in aggregation to preserve their integrity under a range of experimental conditions.
These bimolecular enzyme aggregates were easily fixed and immobilized by ionic adsorption on anion exchangers (PEI-Lecitase) or by covalent immobilization through the two amino termini of the bimolecular aggregate on CNBr-activated agarose gels (covalent aggregates). Both derivatives exhibited very high stability compared with the diluted soluble enzyme: 75-fold higher for PEI-Lecitase and 300-fold for covalent aggregates. Moreover, these derivatives were much more stable than the open structure of Lecitase adsorbed onto hydrophobic supports.
This monomolecular structure of Lecitase was covalently immobilized (through its amino terminus) in the presence of CTAB. After immobilization, the surfactant was removed and the monomolecular closed structure of Lecitase was obtained. This derivative had almost an identical activity and stability properties as the highly diluted soluble enzyme. This immobilized derivative (immobilized native Lecitase) seemed to be the best reference for the evaluation of activity and stability of all immobilized Lecitase derivatives under non-conventional experimental conditions.
Krill oil has 100% omega-3 fatty acids in the sn-2 position. However, all the immobilized derivatives of Lecitase (phospholipase A1) were able to release more than 95% of them during the hydrolysis reaction at pH 7.0 and 25 °C. Lecitase thus seemingly behaved like a non-selective phospholipase. When the sn1 position was released, the omega-3 fatty acid in the sn-2 position migrated very rapidly to the sn-1 position and was then released. The optimal derivative, as a compromise of activity and stability, was the ionically adsorbed bimolecular enzyme derivative (PEI-Lecitase). Ten consecutive cycles were performed with identical enzyme activity (26 U/mL), and a hydrolytic yield (95%) of omega-3 fatty acids was observed.
Author Contributions
Conceptualization, J.M.G., J.R.-M. and G.F.-L.; methodology, J.M.G. and J.R.-M.; validation, J.M.G. and J.R-M.; formal analysis, J.M.G. and J.R.-M.; investigation, D.A.-S. and C.F.; resources, J.M.G.; data curation, J.M.G. and G.F.-L.; writing—original draft preparation, J.M.G.; writing—review and editing, J.M.G., J.R.-M. and G.F.-L.; supervision, J.M.G. and J.R.-M.; project administration, J.M.G. and G.F.-L.; funding acquisition, J.M.G. and G.F.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Plan Nacional (Spanish Ministerio de Ciencia) Grant No. RTI2018-093583-B-I00.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Ramiro Martinez (Novozymes, Spain) for the generous donation of LECITASE ULTRA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mateo, C.; Jose, M.; Palomo, J.M.; Fernandez-Lorente, G.; Jose, M.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity via Immobilization Techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.; Ortiz, C.; Berenguer-Murcia, A.; Barbosa, O.; Rodrigues, R.; Fernandez-Lafuente, R. Lecitase ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef] [Green Version]
- Manjula, S.; Jose, A.; Divakar, S.; Subramanian, R.N. Degumming rice bran oil using phospholipase-A1. Eur. J. Lipid Sci. Technol. 2011, 113, 658–664. [Google Scholar] [CrossRef]
- Sheelu, G.; Kavitha, G.; Fadnavis, N.W. Efficient Immobilization of Lecitase in Gelatin Hydrogel and Degumming of Rice Bran Oil Using a Spinning Basket Reactor. J. Am. Oil Chem. Soc. 2008, 85, 739–748. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Effect of the immobilization protocol in the activity, stability, and enantioslectivity of Lecitase® Ultra. J. Mol. Catal. B Enzym. 2007, 47, 99–104. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Asymmetric hydrolysis of dimethyl 3-phenylglutarate catalyzed by Lecitase Ultra®: Effect of the immobilization protocol on its catalytic properties. Enzym. Microb. Technol. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Piel, M.S.; Peters, G.H.; Brask, J. Chemoenzymatic synthesis of fluorogenic phospholipids and evaluation in assays of phospholipases A, C and D. Chem. Phys. Lipids 2017, 202, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, J.C.S.; Rueda, N.; Torres, R.; Barbosa, O.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Evaluation of divinyl-sulfone activated agarose to immobilize lipases and to tune their catalytic properties. Process Biochem. 2015, 50, 918–927. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.; Silva, F.F.; Lemos, T.L.; Fernandez-Lafuente, R.; Gonçalves, L.R.; dos Santos, J.C. Modulation of Lecitase properties via immobilization on differently activated Immobead-350: Stabilization and inversion of enantiospecificity. Process. Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Taraban, M.B.; Depaz, R.A.; Lobo, B.; Yu, Y.B. Water Proton NMR: A Tool for Protein Aggregation Characterization. Anal. Chem. 2017, 89, 5494–5502. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Cabrera, Z.; Fernandez-Lafuente, R.; Guisán, J.M. Improved Catalytic Properties of Immobilized Lipases by the Presence of Very Low Concentrations of Detergents in the Reaction Medium. Biotechnol. Bioeng. 2007, 97, 242–250. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fernandez-Lorente, G.; Mateo, C.; Segura, R.L.; Ortiz, C.; Fernandez-Lafuente, R.; Guisán, J.M. Purification, Immobilization, Hyperactivation, and Stabilization of Lipases by Selective Adsorption on Hydrophobic Supports. In Immobilization of Enzymes and Cells. Methods in Biotechnology; Humana Press: Totowa, NJ, USA, 2006; Volume 22, pp. 143–152. [Google Scholar]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General Trend of Lipase to Self-Assemble Giving Bimolecular Aggregates Greatly Modifies the Enzyme Functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Selfassembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Yalagala, P.C.R.; Sugasini, D.; Zaldua, S.B.; Tai, L.M.; Subbaiah, P.V. Lipase Treatment of Dietary Krill Oil, but Not Fish Oil, Enables Enrichment of Brain Eicosapentaenoic Acid and Docosahexaenoic Acid. Mol. Nutr. Food Res. 2020, 64, 2000059. [Google Scholar] [CrossRef]
- Yang, T.; Fruekilde, M.B.; Xu, X. Suppression of acyl migration in enzymatic production of structured lipids through tem-perature programming. Food Chem. 2005, 92, 101–107. [Google Scholar] [CrossRef]
- Pacheco, C.; Crapiste, G.H.; Carrín, M.E. Study of acyl migration during enzymatic interesterification of liquid and fully hydrogenated soybean oil. J. Mol. Catal. B Enzym. 2015, 122, 117–124. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Qu, L.; Wang, X.; Liu, Y. Mechanism and effective factors of acyl migration of lysophospholipid. Chin. J. Org. Chem. 2014, 34, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Mateo, C.; Pessela, B.C.C.; Fuentes, M.; Torres, R.; Ortiz, C.; López-Gallego, F.; Betancor, L.; Alonso-Morales, N.; Guisan, J.M.; Fernandez-Lafuente, R. Very Strong But Reversible Immobilization of Enzymes on Supports Coated With Ionic Polymers. Immobil. Enzym. Cells 2006, 205–216. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Guisán, J.M. Release of omega-3 fatty acids by the hydrolysis of fish oil catalyzed by lipases immobilized on hydrophobic supports. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 1173–1178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).