Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization
Abstract
:1. Introduction
2. Novel Techniques for Lipase Immobilization
2.1. Crosslinked Enzyme Aggregates (CLEAs)
2.2. Covalent Organic Frameworks (COFs)
2.3. Metal-Organic Frameworks (MOFs)
2.4. 3D Printing
2.5. Electrospinning
2.6. Electrospraying
2.7. Hybrid Nanoflowers
2.8. Pickering Emulsion Enzyme Encapsulation
2.9. Peptide-Guided Immobilization
3. Novel Carriers for Immobilization
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bilal, M.; Iqbal, H.M.; Hu, H.; Wang, W.; Zhang, X. Metabolic engineering and enzyme-mediated processing: A biotechnological venture towards biofuel production—A review. Renew. Sustain. Energy Rev. 2018, 82, 436–447. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Biotechnological approaches for the production of pharmaceutically important compound: Plumbagin. Curr. Pharm. Biotechnol. 2018, 19, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Silveira, B.M.P.; Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. An overview of biotechnological processes in the food industry. In Bioprocessing for Biomolecules Production; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 1–19. [Google Scholar]
- Ashrafi, A.M.; Sýs, M.; Sedláčková, E.; Farag, A.S.; Adam, V.; Přibyl, J.; Richtera, L.; Sýs, A. Application of the enzymatic electrochemical biosensors for monitoring non-competitive inhibition of enzyme activity by heavy metals. Sensors 2019, 19, 2939. [Google Scholar] [CrossRef] [Green Version]
- Mariz, B.d.P.; Carvalho, S.; Batalha, I.L.; Pina, A.S. Artificial enzymes bringing together computational design and directed evolution. Org. Biomol. Chem. 2021, 19, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Virgen-Ortíz, J.J.; dos Santos, J.C.; Berenguer-Murcia, A.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Dannert, C.; López-Gallego, F. Advances and opportunities for the design of self-sufficient and spatially organized cell-free biocatalytic systems. Curr. Opin. Chem. Biol. 2018, 49, 97–104. [Google Scholar] [CrossRef]
- Schmieg, B.; Döbber, J.; Kirschhöfer, F.; Pohl, M.; Franzreb, M. Advantages of hydrogel-based 3D-printed enzyme reactors and their limitations for biocatalysis. Front. Bioeng. Biotechnol. 2019, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Bolina, I.C.A.; Gomes, R.A.B.; Mendes, A.A. Biolubricant production from several oleaginous feedstocks using lipases as catalysts: Current scenario and future perspectives. BioEnergy Res. 2021, 1–19. [Google Scholar] [CrossRef]
- Pohanka, M. Biosensors and bioassays based on lipases, principles and applications: A review. Molecules 2019, 24, 616. [Google Scholar] [CrossRef] [Green Version]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Melani, N.; Tambourgi, E.B.; Silveira, E. Lipases: From production to applications. Sep. Purif. Rev. 2019, 49, 143–158. [Google Scholar] [CrossRef]
- Valério, R.B.R.; Cavalcante, A.L.G.; Mota, G.F.; de Sousa, I.G.; da Silva Souza, J.E.; Cavalcante, F.T.T.; de Aguiar Falcão, I.R.; da Silva Moreira, K. Understanding the biocatalytic potential of lipase from rhizopus chinensis. Biointerface Res. Appl. Chem. 2021, 12, 4230–4260. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial activation’ of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst–many applications: The use of candida antarctica b-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Reetz, M.T. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Kublicki, M.; Koszelewski, D.; Brodzka, A.; Ostaszewski, R. Wheat germ lipase: Isolation, purification and applications. Crit. Rev. Biotechnol. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Rani, K.Y.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2017, 34, 5–28. [Google Scholar] [CrossRef]
- Miguez, J.P.; Gama, R.S.; Bolina, I.C.; de Melo, C.C.; Cordeiro, M.R.; Hirata, D.B.; Mendes, A.A. Enzymatic synthesis optimization of a cosmetic ester catalyzed by a homemade biocatalyst prepared via physical adsorption of lipase on amino-functionalized rice husk silica. Chem. Eng. Res. Des. 2018, 139, 296–308. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2020, 164, 1566–1587. [Google Scholar] [CrossRef]
- Moreira, K.S.; Júnior, L.S.M.; Monteiro, R.R.C.; De Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; De Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the production of enzymatic biodiesel from residual babassu oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, F.T.T.; Neto, F.S.; Falcão, I.R.D.A.; Souza, J.E.D.S.; Junior, L.S.D.M.; Sousa, P.D.S.; Rocha, T.G.; de Sousa, I.G.; Gomes, P.H.D.L.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2020, 288, 119577. [Google Scholar] [CrossRef]
- Lima, G.V.; da Silva, M.R.; Fonseca, T.D.S.; de Lima, L.B.; Oliveira, M.D.C.F.D.; de Lemos, T.L.G.; Zampieri, D.; dos Santos, J.C.S.; Rios, N.S.; Gonçalves, L.R.B.; et al. Chemoenzymatic synthesis of (S)-Pindolol using lipases. Appl. Catal. A Gen. 2017, 546, 7–14. [Google Scholar] [CrossRef]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Quayson, E.; Amoah, J.; Hama, S.; Kondo, A.; Ogino, C. Immobilized lipases for biodiesel production: Current and future greening opportunities. Renew. Sustain. Energy Rev. 2020, 134, 110355. [Google Scholar] [CrossRef]
- Contesini, F.J.; Davanço, M.G.; Borin, G.P.; Vanegas, K.G.; Cirino, J.P.G.; De Melo, R.R.; Mortensen, U.H.; Hildén, K.; Campos, D.R.; Carvalho, P.D.O. Advances in recombinant lipases: Production, engineering, immobilization and application in the pharmaceutical industry. Catalysts 2020, 10, 1032. [Google Scholar] [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; e Silva, N.C.G.; Souza, J.E.D.S.; Nunes, Y.L.; dos Santos, J.C.S. An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef]
- Rocha, T.G.; Gomes, P.H.D.L.; de Souza, M.C.M.; Monteiro, R.R.C.; dos Santos, J.C.S. Lipase cocktail for optimized biodiesel production of free fatty acids from residual chicken oil. Catal. Lett. 2020, 151, 1155–1166. [Google Scholar] [CrossRef]
- Souza, J.E.S.; Monteiro, R.R.C.; Rocha, T.G.; Moreira, K.S.; Cavalcante, F.T.T.; Braz, A.K.D.S.; de Souza, M.C.M.; dos Santos, J.C.S. Sonohydrolysis using an enzymatic cocktail in the preparation of free fatty acid. 3 Biotech 2020, 10, 1–10. [Google Scholar] [CrossRef]
- De Souza, T.C.; Fonseca, T.D.S.; Silva, J.D.S.; Lima, P.J.M.; Neto, C.A.C.G.; Monteiro, R.R.C.; Rocha, M.V.P.; De Mattos, M.C.; Dos Santos, J.C.S.; Gonçalves, L.R.B. Modulation of lipase B from Candida antarctica properties via covalent immobilization on eco-friendly support for enzymatic kinetic resolution of rac-indanyl acetate. Bioprocess Biosyst. Eng. 2020, 43, 2253–2268. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.; Silva, F.F.; Lemos, T.L.; Fernandez-Lafuente, R.; Gonçalves, L.R.; dos Santos, J.C. Modulation of Lecitase properties via immobilization on differently activated Immobead-350: Stabilization and inversion of enantiospecificity. Process. Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Paulino, B.N.; Pessôa, M.G.; Molina, G.; Neto, A.A.K.; Oliveira, J.V.C.; Mano, M.C.R.; Pastore, G.M. Biotechnological production of value-added compounds by ustilaginomycetous yeasts. Appl. Microbiol. Biotechnol. 2017, 101, 7789–7809. [Google Scholar] [CrossRef]
- Maldonado, R.R. A review on geotrichum lipases: Production, purification, immobilization and applications. Chem. Biochem. Eng. Q. 2017, 30, 439–454. [Google Scholar] [CrossRef]
- Riegler-Berket, L.; Leitmeier, A.; Aschauer, P.; Dreveny, I.; Oberer, M. Identification of lipases with activity towards monoacylglycerol by criterion of conserved cap architectures. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 679–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, T.L.; Buchholz, P.C.F.; Pleiss, J. The modular structure of α/β-hydrolases. FEBS J. 2019, 287, 1035–1053. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.; Pinheiro, B.; Pinheiro, M.P.; Bezerra, R.M.; dos Santos, J.C.S.; Gonçalves, L.R.B. Biotechnological potential of lipases from pseudomonas: Sources, properties and applications. Process. Biochem. 2018, 75, 99–120. [Google Scholar] [CrossRef]
- Cheng, C.; Jiang, T.; Wu, Y.; Cui, L.; Qin, S.; He, B. Elucidation of lid open and orientation of lipase activated in interfacial activation by amphiphilic environment. Int. J. Biol. Macromol. 2018, 119, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Noda, H.; Kondo, A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr. Opin. Biotechnol. 2018, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zheng, X.; Zhang, X.; Zhang, K.; Lin, Y.; Liang, S. Combined strategies for engineering a novel whole-cell biocatalyst of Candida rugosa lipase with improved characteristics. Biochem. Eng. J. 2019, 151. [Google Scholar] [CrossRef]
- Bresolin, D.; Estrella, A.S.; Da Silva, J.R.P.; Valerio, A.; Sayer, C.; de Araujo, P.H.H.; De Oliveira, D. Synthesis of a green polyurethane foam from a biopolyol obtained by enzymatic glycerolysis and its use for immobilization of lipase NS. Bioprocess. Biosyst. Eng. 2018, 42, 213–222. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in enzymatic biodiesel production and commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Monteiro, R.R.C.; Neto, D.M.A.; da Silva, F.F.; de Paula, R.; de Lemos, T.L.; Fechine, P.B.; Correa, M.; Bohn, F.; Gonçalves, L.R.; et al. A new heterofunctional support for enzyme immobilization: PEI functionalized Fe3O4 MNPs activated with divinyl sulfone. Application in the immobilization of lipase from thermomyces lanuginosus. Enzym. Microb. Technol. 2020, 138, 109560. [Google Scholar] [CrossRef]
- Melo, A.D.Q.; Silva, F.F.M.; Dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Filho, F.A.D. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef] [Green Version]
- Bonazza, H.L.; Manzo, R.M.; Mammarella, E.J.; Dos Santos, J.C.S. Operational and thermal stability analysis of thermomyces lanuginosus lipase covalently immobilized onto modified chitosan supports. Appl. Biochem. Biotechnol. 2017, 184, s12010–s12017. [Google Scholar] [CrossRef]
- Villalba, M.; Verdasco-Martín, C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Otero, C. Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzym. Microb. Technol. 2016, 90, 35–44. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; dos Santos, J.C.; Barbosa, O.; Torres, R.; Pereira, E.B.; Corberan, V.C.; Gonçalves, L.R.; Fernandez-Lafuente, R. Tuning of Lecitase features via solid-phase chemical modification: Effect of the immobilization protocol. Process. Biochem. 2014, 49, 604–616. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.; Ana, H.B.D.S.; Goncalves, L.R.B.; Fernandez-Lafuente, R. Improving the catalytic properties of immobilized Lecitase via physical coating with ionic polymers. Enzym. Microb. Technol. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Da Fonseca, A.M.; Colares, R.P.; De Oliveira, M.M.; De Souza, M.C.M.; Monteiro, R.R.C.; Araújo, R.D.S.; Amorim, A.V.; Dos Santos, J.C.S.; Alcócer, J.C.A.; Pinto, O.R.D.O. Enzymatic biocatalyst using enzymes from pineapple (Ananas comosus) peel immobilized in hydrogel beads. Rev. Eletrônica Gestão Educ. Tecnol. Ambient. 2019, 23, 32. [Google Scholar] [CrossRef]
- Monteiro, R.; Dos Santos, J.; Alcántara, A.; Fernandez-Lafuente, R. Enzyme-coated micro-crystals: An almost forgotten but very simple and elegant immobilization strategy. Catalysts 2020, 10, 891. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of lipases on heterofunctional octyl–glyoxyl agarose supports. Meth. Enzymol. 2016, 571, 73–85. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; Dos Santos, J.C.S.; De Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl butyrate synthesis catalyzed by lipases A and B from candida antarctica immobilized onto magnetic nanoparticles. improvement of biocatalysts’ performance under ultrasonic irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, N.S.; Morais, E.G.; Galvão, W.D.S.; Neto, D.M.A.; dos Santos, J.C.S.; Bohn, F.; Correa, M.A.; Fechine, P.B.A.; Fernandez-Lafuente, R.; Gonçalves, L.R.B. Further stabilization of lipase from Pseudomonas fluorescens immobilized on octyl coated nanoparticles via chemical modification with bifunctional agents. Int. J. Biol. Macromol. 2019, 141, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; dos Santos, J.C.; Ortiz, C.; Berenguer-Murcia, A.; Barbosa, O.; Rodrigues, R.; Fernandez-Lafuente, R. Lecitase ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef] [Green Version]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of lipases by the unfolding and refolding of covalently immobilized biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef] [Green Version]
- Tom, R.J.; Sankaranarayanan, S.; Rodrigues, J.J.P.C. Smart energy management and demand reduction by consumers and utilities in an IoT-fog-based power distribution system. IEEE Internet Things J. 2019, 6, 7386–7394. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Rosset, D.V.; Tres, M.V.; Oliveira, J.V.; Mazutti, M.A.; Jahn, S.L. Production of biodiesel catalyzed by lipase from Thermomyces lanuginosus in its soluble form. Can. J. Chem. Eng. 2018, 96, 2361–2368. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.T.R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Rueda, N.; Albuquerque, T.L.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; Dos Santos, J.C.S.; Barbosa, O.; Fernandez-Lafuente, R. Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads prevents enzyme desorption. Molecules 2016, 21, 646. [Google Scholar] [CrossRef] [Green Version]
- Rios, N.S.; Neto, D.M.A.; dos Santos, J.C.S.; Fechine, P.B.A.; Fernández-Lafuente, R.; Gonçalves, L.R.B. Comparison of the immobilization of lipase from Pseudomonas fluorescens on divinylsulfone or p-benzoquinone activated support. Int. J. Biol. Macromol. 2019, 134, 936–945. [Google Scholar] [CrossRef]
- De Souza, T.C.; Fonseca, T.D.S.; da Costa, J.A.; Rocha, M.V.P.; de Mattos, M.C.; Fernandez-Lafuente, R.; Gonçalves, L.R.; dos Santos, J.C.S. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: Application to the chemoenzymatic production of (R)-Indanol. J. Mol. Catal. B Enzym. 2016, 130, 58–69. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Rueda, N.; Bartolome-Cabrero, R.; Rodriguez, M.D.; Albuquerque, T.L.; dos Santos, J.C.; Barbosa, O.; Fernandez-Lafuente, R. Improved immobilization and stabilization of lipase from Rhizomucor miehei on octyl-glyoxyl agarose beads by using CaCl. Process. Biochem. 2016, 51, 48–52. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Goncalves, L.R.B.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzym. Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R.T.R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2016, 259, 107–118. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of lipases—A Review. Part I: Enzyme immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Moreira, K.D.S.; De Oliveira, A.L.B.; Júnior, L.S.D.M.; Monteiro, R.R.C.; Da Rocha, T.N.; de Menezes, F.L.; Fechine, L.; DeNardin, J.C.; Michea, S.; Freire, R.M.; et al. Lipase from rhizomucor miehei immobilized on magnetic nanoparticles: Performance in Fatty Acid Ethyl Ester (FAEE) optimized production by the taguchi method. Front. Bioeng. Biotechnol. 2020, 8, 693. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.; Freire, T.M.; Dutra, L.M.U.; Fechine, L.; Gonçalves, L.R.B.; De Souza, M.C.M.; Dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of lipase A from candida antarctica onto chitosan-coated magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Lopez, L.; Bartolome-Cabrero, R.; Rodriguez, M.D.; Dos Santos, C.S.; Rueda, N.; Fernandez-Lafuente, R. Stabilizing effects of cations on lipases depend on the immobilization protocol. RSC Adv. 2015, 5, 83868–83875. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Lorente, F.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2017, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, A.; Sindhu, R.; Binod, P.; Sukumaran, R.K.; Pandey, A. Strategies for design of improved biocatalysts for industrial applications. Bioresour. Technol. 2017, 245, 1304–1313. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Parra, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants—A review. Sci. Total Environ. 2016, 576, 646–659. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 2018, 381, 151–160. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Cohen, J.L.; Barile, D.; Bell, J.M.L.N. Recent advances in immobilization strategies for glycosidases. Biotechnol. Prog. 2016, 33, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Kim, A.M. An overview of techniques in enzyme immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- De Oliveira, U.M.F.; de Matos, L.J.B.L.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Efficient biotechnological synthesis of flavor esters using a low-cost biocatalyst with immobilized Rhizomucor miehei lipase. Mol. Biol. Rep. 2018, 46, 597–608. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.; de Sant’Ana, H.B.; Goncalves, L.R.B.; Fernandez-Lafuente, R. Stabilizing hyperactivated lecitase structures through physical treatment with ionic polymers. Process. Biochem. 2014, 49, 1511–1515. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Villalba, M.; dos Santos, J.C.; Tobajas, M.; Fernandez-Lafuente, R.; Otero, C. Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil. Biochem. Eng. J. 2016, 111, 75–86. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Dos Santos, J.C.S.; Rodrigues, R.C.; Fernandez-Lafuente, R. Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef] [Green Version]
- Chi, M.-C.; Huang, Y.-F.; Lu, B.-Y.; Lin, M.-G.; Wang, T.-F.; Lin, L.-L. Magnetic cross-linked enzyme aggregates of a transpeptidase-specialized variant (N450D) of Bacillus licheniformis γ-Glutamyl transpeptidase: An efficient and stable biocatalyst for l-theanine synthesis. Catalysts 2021, 11, 243. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process. Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Liu, W.; Wang, J.; Chen, H. A novel cross-linked enzyme aggregates (CLEAs) of papain and neutrase-production, partial characterization and application. Int. J. Biol. Macromol. 2017, 95, 650–657. [Google Scholar] [CrossRef]
- Jin, W.; Xu, Y.; Yu, X.-W. Formation lipase cross-linked enzyme aggregates on octyl-modified mesocellular foams with oxidized sodium alginate. Colloids Surf. B Biointerfaces 2019, 184, 110501. [Google Scholar] [CrossRef]
- Ramos, M.D.; Miranda, L.P.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. Improving the yields and reaction rate in the ethanolysis of soybean oil by using mixtures of lipase CLEAs. Molecules 2019, 24, 4392. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Ma, P.; Liu, Y.; Xiaoyan, M.; Chen, F.; Li, M. 3D coral-like gold/carbon paper electrode modified with covalent and cross-linked enzyme aggregates for electrochemical sensing of glucose. Microchem. J. 2020, 159, 105347. [Google Scholar] [CrossRef]
- De Sousa, M.; Gurgel, B.S.; Pessela, B.C.; Gonçalves, L.R. Preparation of CLEAs and magnetic CLEAs of a recombinant l-arabinose isomerase for d-tagatose synthesis. Enzym. Microb. Technol. 2020, 138, 109566. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Su, Y.; Liu, Y.; Sun, L.; Yu, M.; Wu, Y. Preparation and characterization of cross-linked enzyme aggregates (CLEAs) of recombinant thermostable alkylsulfatase (SdsAP) from Pseudomonas sp. Process. Biochem. 2016, 51, 2084–2089. [Google Scholar] [CrossRef]
- Hong, J.; Jung, D.; Park, S.; Oh, Y.; Oh, K.K.; Lee, S.H. Immobilization of laccase via cross-linked enzyme aggregates prepared using genipin as a natural cross-linker. Int. J. Biol. Macromol. 2021, 169, 541–550. [Google Scholar] [CrossRef]
- Mehde, A.A.; Mehdi, W.A.; Özacar, M.; Özacar, Z.Z. Evaluation of different saccharides and chitin as eco-friendly additive to improve the magnetic cross-linked enzyme aggregates (CLEAs) activities. Int. J. Biol. Macromol. 2018, 118, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.K.H.A.; El-Enshasy, H.A.; Abu Bakar, F.D.; Murad, A.M.A.; Jahim, J.M.; Illias, R.M. Improvement of cross-linking and stability on cross-linked enzyme aggregate (CLEA)-xylanase by protein surface engineering. Process. Biochem. 2019, 86, 40–49. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.; Golubovic, M.; Ottens, M.; Kieboom, A.; van Rantwijk, F.; van der Wielen, L.; Sheldon, R. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, N.; Sarathi, M.; Pennathur, G. Cross-linked esterase aggregates (CLEAs) using nanoparticles as immobilization matrix. Prep. Biochem. Biotechnol. 2019, 49, 270–278. [Google Scholar] [CrossRef]
- Matijošytė, I.; Arends, I.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; de María, P.D. Immobilization of pseudomonas stutzeri lipase through cross-linking aggregates (CLEA) for reactions in deep eutectic solvents. J. Biotechnol. 2021, 337, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Rathod, V.K. Magnetic macromolecular cross linked enzyme aggregates (CLEAs) of glucoamylase. Enzym. Microb. Technol. 2016, 83, 78–87. [Google Scholar] [CrossRef]
- Carneiro, E.; Bastos, A.; De Oliveira, U.; De Matos, L.; Adriano, W.; Monteiro, R.; Dos Santos, J.; Gonçalves, L. improving the catalytic features of the lipase from rhizomucor miehei immobilized on chitosan-based hybrid matrices by altering the chemical activation conditions. Química Nova 2020, 43, 1234–1239. [Google Scholar] [CrossRef]
- Reis, C.; Sousa, E.; Serpa, J.; Oliveira, R.; Santos, J. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Química Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2013, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Betancor, L.; López-Gallego, F.; Alonso-Morales, N.; Dellamora, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisán, J.M. Glutaraldehyde in protein immobilization. Phytoremediation 2006, 22, 57–64. [Google Scholar] [CrossRef]
- De Oliveira, A.L.B.; Cavalcante, F.T.T.; Moreira, K.S.; Monteiro, R.R.C.; Rocha, T.G.; Souza, J.E.S.; da Fonseca, A.M.; Lopes, A.A.S.; Guimarães, A.P.; de Lima, R.K.C.; et al. Lipases immobilized onto nanomaterials as biocatalysts in biodiesel production: Scientific context, challenges, and opportunities. Rev. Virtual Quim. 2021, 13, 875–891. [Google Scholar] [CrossRef]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; Moreira, K.d.S.; de Oliveira, A.L.B.; Mota, G.F.; Souza, J.E.D.S.; Falcão, I.R.D.A.; et al. Chemical and physical chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Neto, D.M.A.; Galvão, W.; Rios, N.; Carvalho, A.C.L.D.M.; Correa, M.; Bohn, F.; Fernandez-Lafuente, R.; Fechine, P.; de Mattos, M.C.; et al. Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem. Eng. J. 2017, 125, 104–115. [Google Scholar] [CrossRef]
- Betancor, L.; Gallego, F.L.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.-O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzym. Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Liao, Q.; Du, X.; Jiang, W.; Tong, Y.; Zhao, Z.; Fang, R.; Feng, J.; Tang, L. Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1: Preparation, characterization and application as a biocatalyst. J. Biotechnol. 2018, 272-273, 48–55. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, D.; Yin, L.; Wang, F. Preparation, activity and structure of cross-linked enzyme aggregates (CLEAs) with nanoparticle. Enzym. Microb. Technol. 2017, 107, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Grajales-Hernández, D.; Armendáriz-Ruiz, M.; Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C. Chitosan-based CLEAs from Aspergillus niger type A feruloyl esterase: High-productivity biocatalyst for alkyl ferulate synthesis. Appl. Microbiol. Biotechnol. 2020, 104, 10033–10045. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, B.B.; Sulman, E.M.; Stadol’Nikova, P.Y.; Golikova, E.P.; Sidorov, A.I.; Matveeva, V.G. Immobilized enzymes from the class of oxidoreductases in technological processes: A review. Catal. Ind. 2019, 11, 251–263. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, Y.; Tan, Z.; Zhong, C.; Han, P.; Jia, S. Mesoporous phenylalanine ammonia lyase microspheres with improved stability through calcium carbonate templating. Int. J. Biol. Macromol. 2017, 98, 887–896. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, G.; Liu, Z. Synthesis and characterization of cross linked enzyme aggregates of serine hydroxyl methyltransferase from Idiomerina leihiensis. Int. J. Biol. Macromol. 2018, 117, 683–690. [Google Scholar] [CrossRef]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, Combi-CLEAs and ‘Smart’ magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Da Fonseca, A.M.; Dos Santos, J.C.S.; De Souza, M.C.M.; de Oliveira, M.M.; Colares, R.P.; De Lemos, T.L.G.; Filho, R.B. The use of new hydrogel microcapsules in coconut juice as biocatalyst system for the reaction of quinine. Ind. Crop. Prod. 2019, 145, 111890. [Google Scholar] [CrossRef]
- Muley, A.B.; Awasthi, S.; Bhalerao, P.P.; Jadhav, N.L.; Singhal, R.S. Preparation of cross-linked enzyme aggregates of lipase from Aspergillus niger: Process optimization, characterization, stability, and application for epoxidation of lemongrass oil. Bioprocess Biosyst. Eng. 2021, 44, 1383–1404. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Covalent organic framework-based materials: Synthesis, modification, and application in environmental remediation. Coord. Chem. Rev. 2021, 441, 213989. [Google Scholar] [CrossRef]
- Ning, J.; Gao, Y.; Cao, X.; Wei, H.; Wang, B.; Hao, L. Substituent engineering of covalent organic frameworks modulates the crystallinity and electrochemical reactivity. J. Energy Chem. 2021, 65, 490–496. [Google Scholar] [CrossRef]
- Xin, J.; Wang, X.; Li, N.; Liu, L.; Lian, Y.; Wang, M.; Zhao, R.-S. Recent applications of covalent organic frameworks and their multifunctional composites for food contaminant analysis. Food Chem. 2020, 330, 127255. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.S.B.; de Oliveira, A.B.V.; Sindra, H.C.; Archanjo, B.S.; Mendoza, M.E.; Carneiro, L.S.A.; Buarque, C.D.; Esteves, P.M. Heterogeneous catalysis by covalent organic frameworks (COF): Pd(OAc)2@COF-300 in cross-coupling reactions. ChemCatChem 2016, 8, 743–750. [Google Scholar] [CrossRef]
- Abuzeid, H.R.; El-Mahdy, A.F.; Kuo, S.-W. Covalent organic frameworks: Design principles, synthetic strategies, and diverse applications. Giant 2021, 6, 100054. [Google Scholar] [CrossRef]
- Gendy, E.A.; Ifthikar, J.; Ali, J.; Oyekunle, D.T.; Elkhlifia, Z.; Shahib, I.I.; Khodair, A.I.; Chen, Z. Removal of heavy metals by covalent organic frameworks (COFs): A review on its mechanism and adsorption properties. J. Environ. Chem. Eng. 2021, 9, 105687. [Google Scholar] [CrossRef]
- Wang, H.; Wanga, T.; Maa, R.; Wua, K.; Lia, H.; Fengb, B.; Lib, C.; Shenb, Y. Facile synthesis of sulfonated covalent organic framework for the adsorption of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2020, 112, 122–129. [Google Scholar] [CrossRef]
- Bao, T.; Wang, S.; Zhang, N.; Zhang, J. Facile synthesis and immobilization of functionalized covalent organic framework-1 for electrochromatographic separation. J. Chromatogr. A 2021, 1645, 462130. [Google Scholar] [CrossRef]
- Gong, K.; Zhang, D.; Wang, Y.; Li, C.; Zhang, H.; Li, H.; Feng, H. Biguanide-functionalized hierarchical porous covalent organic frameworks for efficient catalysis of condensation reactions. Mol. Catal. 2021, 509, 111663. [Google Scholar] [CrossRef]
- Khan, N.A.; Wu, H.; Jinqiu, Y.; Mengyuan, W.; Yang, P.; Long, M.; Rahman, A.U.; Ahmad, N.M.; Zhang, R.; Jiang, Z. Incorporating covalent organic framework nanosheets into polyamide membranes for efficient desalination. Sep. Purif. Technol. 2021, 274, 119046. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Aramesh, N.; Sher, F.; Bilal, M. Covalent organic frameworks as robust materials for mitigation of environmental pollutants. Chemosphere 2021, 270, 129523. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Luo, B.; Zhang, H.; Li, Z.; Zhu, N.; Lan, F.; Wu, Y. Surfactant-free synthesis of covalent organic framework nanospheres in water at room temperature. J. Colloid Interface Sci. 2021, 606, 1333–1339. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent organic frameworks for photocatalytic applications. Appl. Catal. B Environ. 2020, 276, 119174. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Tong, C.; Wang, Z.; Xu, F.; Wang, X.; Weng, B.; Pan, D.; Zhu, R. Novel sulfhydryl functionalized covalent organic frameworks for ultra-trace Hg2+ removal from aqueous solution. J. Mater. Sci. Technol. 2021, 93, 89–95. [Google Scholar] [CrossRef]
- Huang, M.; Chong, J.; Hu, C.; Yang, Y. Ratiometric fluorescent detection of temperature and MnO4—Using a modified covalent organic framework. Inorg. Chem. Commun. 2020, 119, 108094. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Q. Recent progress in covalent organic frameworks as light-emitting materials. Mater. Today Energy 2021, 20, 100635. [Google Scholar] [CrossRef]
- Gan, J.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review. Int. J. Biol. Macromol. 2020, 167, 502–515. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, C.-W.; Aguila, B.; Perman, J.A.; Wang, S.; Huang, H.-Y.; Xiao, F.-S.; Ma, S. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 984–992. [Google Scholar] [CrossRef]
- Oliveira, F.L.; França, A.D.S.; De Castro, A.M.; Souza, R.O.M.A.; Esteves, P.M.; Goncalves, R.S.B. Enzyme immobilization in covalent organic frameworks: Strategies and applications in biocatalysis. Chem. Plus Chem. 2020, 85. [Google Scholar] [CrossRef] [PubMed]
- Yusran, Y.; Li, H.; Guan, X.; Fang, Q.; Qiu, S. Covalent Organic Frameworks for Catalysis. Energy Chem. 2020, 2, 100035. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Lan, P.C.; Ma, S. Tuning pore heterogeneity in covalent organic frameworks for enhanced enzyme accessibility and resistance against denaturants. Adv. Mater. 2019, 31, e1900008. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.L.; De Souza, S.P.; Bassut, J.; Álvarez, H.M.; Garcia-Basabe, Y.; De Souza, R.O.M.A.; Esteves, P.M.; Goncalves, R.S.B. Enzyme-decorated covalent organic frameworks as nanoporous platforms for heterogeneous biocatalysis. Chem. A Eur. J. 2019, 25, 15863–15870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-W.; Cai, C.-X.; Xing, X.; Li, J.; Hu, Z.-E.; Xie, Z.-B.; Wang, N.; Yu, X.-Q. Magnetic COFs as satisfied support for lipase immobilization and recovery to effectively achieve the production of biodiesel by great maintenance of enzyme activity. Biotechnol. Biofuels 2021, 14, 1–12. [Google Scholar] [CrossRef]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zbořil, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent development of covalent organic frameworks (COFs): Synthesis and catalytic (organic-electro-photo) applications. Mater. Horizons 2019, 7, 411–454. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Gropp, C.; Yaghi, O.M. Reticulating 1D ribbons into 2D covalent organic frameworks by imine and imide linkages. J. Am. Chem. Soc. 2020, 142, 2771–2776. [Google Scholar] [CrossRef]
- Wen, A.; Li, G.; Wu, D.; Yu, Y.; Yang, Y.; Hu, N.; Wang, H.; Chen, J.; Wu, Y. Sulphonate functionalized covalent organic framework-based magnetic sorbent for effective solid phase extraction and determination of fluoroquinolones. J. Chromatogr. A 2019, 1612, 460651. [Google Scholar] [CrossRef]
- Su, D.; Feng, B.; Xu, P.; Zeng, Q.; Shan, B.; Song, Y. Covalent organic frameworks and electron mediator-based open circuit potential biosensor for in vivo electrochemical measurements. Anal. Methods 2018, 10, 4320–4328. [Google Scholar] [CrossRef]
- Samui, A.; Happy; Sahu, S.K. Integration of α-amylase into covalent organic framework for highly efficient biocatalyst. Microporous Mesoporous Mater. 2020, 291. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Deng, C. Rational synthesis of novel recyclable Fe3O4@MOF nanocomposites for enzymatic digestion. Chem. Commun. 2015, 51, 8116–8119. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Mann, M. Consecutive proteolytic digestion in an enzyme reactor increases depth of proteomic and phosphoproteomic analysis. Anal. Chem. 2012, 84, 2631–2637. [Google Scholar] [CrossRef]
- Wang, L.; Liang, H.; Xu, M.; Wang, L.; Xie, Y.; Song, Y. Ratiometric electrochemical biosensing based on double-enzymes loaded on two-dimensional dual-pore COFETTA-TPAL. Sens. Actuators B Chem. 2019, 298. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, L.; Zhang, R.; Bai, Y.; Zhu, R.; Pang, H. Recent advances in the development of electronically and ionically conductive metal-organic frameworks. Coord. Chem. Rev. 2021, 439, 213915. [Google Scholar] [CrossRef]
- Whelan, É.; Steuber, F.W.; Gunnlaugsson, T.; Schmitt, W. Tuning photoactive metal–organic frameworks for luminescence and photocatalytic applications. Coord. Chem. Rev. 2021, 437, 213757. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; D’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic framework nanoparticles. Adv. Mater. 2018, 30, e1800202. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Chuhadiya, S.; Himanshu; Suthar, D.; Patel, S.; Dhaka, M. Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review. Coord. Chem. Rev. 2021, 446, 214115. [Google Scholar] [CrossRef]
- Li, R.; Zhang, W.; Zhou, K. Metal–Organic-framework-based catalysts for photoreduction of CO. Adv. Mater. 2018, 30, e1705512. [Google Scholar] [CrossRef]
- Li, D.-Z.; Chen, L.; Liu, G.; Yuan, Z.-Y.; Li, B.-F.; Zhang, X.; Wei, J.-Q. Porous metal–organic frameworks for methane storage and capture: Status and challenges. New Carbon Mater. 2021, 36, 468–496. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, R.; Jiao, L.; Jiang, H.-L. Metal–organic framework-derived porous materials for catalysis. Coord. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.-Q.; Kumar, A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Bryliakov, K.P. Asymmetric catalysis using metal-organic frameworks. Coord. Chem. Rev. 2021, 437, 213845. [Google Scholar] [CrossRef]
- Guo, W.; Cheng, J.; Song, Y.; Liu, S.; Ali, K.A.; Kumar, S. Three-dimensional numerical simulation of light penetration in an optimized flow field composed of microalgae cells, carbon dioxide bubbles and culture medium. Bioresour. Technol. 2019, 292, 121979. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-J.; Li, J.-R. An Overview of Metal–Organic frameworks for green chemical engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Biomimetic mineralization inducing lipase–metal–organic framework nanocomposite for pickering interfacial biocatalytic system. ACS Sustain. Chem. Eng. 2019, 7, 7127–7139. [Google Scholar] [CrossRef]
- Qin, Y.; Wan, Y.; Guo, J.; Zhao, M. Two-dimensional metal-organic framework nanosheet composites: Preparations and applications. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Nemiwal, M.; Gosu, V.; Zhang, T.C.; Kumar, D. Metal organic frameworks as electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 10216–10238. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.; Shieh, F.-K.; Wu, K.C.-W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Lin, C.; Xu, K.; Zheng, R.; Zheng, Y. Immobilization of amidase into a magnetic hierarchically porous metal–organic framework for efficient biocatalysis. Chem. Commun. 2019, 55, 5697–5700. [Google Scholar] [CrossRef]
- Marsh, C.; Shearer, G.C.; Knight, B.T.; Paul-Taylor, J.; Burrows, A.D. Supramolecular aspects of biomolecule interactions in metal–organic frameworks. Coord. Chem. Rev. 2021, 439, 213928. [Google Scholar] [CrossRef]
- Vahabi, A.H.; Norouzi, F.; Sheibani, E.; Rahimi-Nasrabadi, M. Functionalized Zr-UiO-67 metal-organic frameworks: Structural landscape and application. Coord. Chem. Rev. 2021, 445, 214050. [Google Scholar] [CrossRef]
- He, J.; Sun, S.; Zhou, Z.; Yuan, Q.; Liu, Y.; Liang, H. Thermostable enzyme-immobilized magnetic responsive Ni-based metal–organic framework nanorods as recyclable biocatalysts for efficient biosynthesis of S-adenosylmethionine. Dalton Trans. 2019, 48, 2077–2085. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.-L. Metal-organic-framework-based single-atom catalysts for energy applications. Chem 2019, 5, 786–804. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Hu, H.; Wang, Z.; Du, Y.; Zhong, L.; Zhang, C.; Jiang, Y.; Jia, S.; Cui, J. Three-dimensional ordered magnetic macroporous metal-organic frameworks for enzyme immobilization. J. Colloid Interface Sci. 2021, 590, 436–445. [Google Scholar] [CrossRef]
- Farmakes, J.; Schuster, I.; Overby, A.; Alhalhooly, L.; Lenertz, M.; Li, Q.; Ugrinov, A.; Choi, Y.; Pan, Y.; Yang, Z. Enzyme immobilization on graphite oxide (GO) surface via one-pot synthesis of GO/metal–organic framework composites for large-substrate biocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 23119–23126. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wu, Y.; Wang, J.; Chen, Z.; Liu, W.; Su, W.; Liu, F. Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal–organic framework for efficient biocatalysis. Nanoscale 2019, 12, 967–972. [Google Scholar] [CrossRef]
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Construction of multiple enzyme metal–organic frameworks biocatalyst via DNA scaffold: A promising strategy for enzyme encapsulation. Chem. Eng. J. 2019, 363, 174–182. [Google Scholar] [CrossRef]
- Cui, J.D.; Feng, Y.; Jia, S. Silica encapsulated catalase@metal-organic framework composite: A highly stable and recyclable biocatalyst. Chem. Eng. J. 2018, 351, 506–514. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Bai, S.; Shao, X.; Jiang, L.; Li, Q. Immobilized lipase in bio-based metal-organic frameworks constructed by biomimetic mineralization: A sustainable biocatalyst for biodiesel synthesis. Colloids Surfaces B Biointerfaces 2020, 188, 110812. [Google Scholar] [CrossRef]
- Li, P.; Modica, J.A.; Howarth, A.J.; Vargas L., E.; Moghadam, P.Z.; Snurr, R.Q.; Mrksich, M.; Hupp, J.T.; Farha, O.K. Toward Design Rules for Enzyme Immobilization in Hierarchical Mesoporous Metal-Organic Frameworks. Chem 2016, 1, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Benahmed, L.; Bourdreux, F.; Zhang, Q.; Serre, C.; Mahy, J.; Steunou, N. Enzyme encapsulation in mesoporous metal—Organic frameworks for selective biodegradation of harmful dye molecules. Angew. Chem. Int. Ed. 2018, 57, 16141–16146. [Google Scholar] [CrossRef]
- Li, P.; Chen, Q.; Wang, T.C.; Vermeulen, N.A.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; Shen, D.; Anderson, R.; Gómez-Gualdrón, D.A.; et al. Hierarchically engineered mesoporous metal-organic frameworks toward cell-free immobilized enzyme systems. Chem 2018, 4, 1022–1034. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Liu, X.; Qi, W.; Wang, Y.; Su, R.; He, Z. A facile strategy for enzyme immobilization with highly stable hierarchically porous metal–organic frameworks. Nanoscale 2017, 9, 17561–17570. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortes, J.; O’Keeffe, M.; Yaghi, O. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Wu, C.-D. Designed fabrication of biomimetic metal–organic frameworks for catalytic applications. Coord. Chem. Rev. 2019, 378, 445–465. [Google Scholar] [CrossRef]
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D printing to innovate biopolymer materials for demanding applications: A review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D printing: Approaches and bioapplications. Biosens. Bioelectron. 2020, 175, 112849. [Google Scholar] [CrossRef] [PubMed]
- Jayapiriya, U.S.; Goel, S. Surface modified 3D printed carbon bioelectrodes for glucose/O2 enzymatic biofuel cell: Comparison and optimization. Sustain. Energy Technol. Assessments 2020, 42, 100811. [Google Scholar] [CrossRef]
- Jiang, P.; Ji, Z.; Wang, X.; Zhou, F. Surface functionalization—A new functional dimension added to 3D printing. J. Mater. Chem. C 2020, 8, 12380–12411. [Google Scholar] [CrossRef]
- Sans, V. Emerging trends in flow chemistry enabled by 3D printing: Robust reactors, biocatalysis and electrochemistry. Curr. Opin. Green Sustain. Chem. 2020, 25, 100367. [Google Scholar] [CrossRef]
- Belgrano, F.D.S.; Diegel, O.; Pereira, N.; Hatti-Kaul, R. Cell immobilization on 3D-printed matrices: A model study on propionic acid fermentation. Bioresour. Technol. 2018, 249, 777–782. [Google Scholar] [CrossRef]
- Yoon, H.S.; Yang, K.; Kim, Y.M.; Nam, K.; Roh, Y.H. Cellulose nanocrystals as support nanomaterials for dual droplet-based freeform 3D printing. Carbohydr. Polym. 2021, 272, 118469. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Iii, R.G.; Panhuis, M.I.H. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Peng, M.; Mittmann, E.; Wenger, L.; Hubbuch, J.; Engqvist, M.K.M.; Niemeyer, C.M.; Rabe, K.S. 3D-Printed phenacrylate decarboxylase flow reactors for the chemoenzymatic synthesis of 4-hydroxystilbene. Chem. A Eur. J. 2019, 25, 15998–16001. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Yang, M.; Cui, C.; Cao, H. In situ immobilization of glucose oxidase and catalase in a hybrid interpenetrating polymer network by 3D bioprinting and its application. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 411–418. [Google Scholar] [CrossRef]
- Ye, J.; Chu, T.; Chu, J.; Gao, B.; He, B. A Versatile Approach for Enzyme Immobilization Using Chemically Modified 3D-Printed Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 18048–18054. [Google Scholar] [CrossRef]
- De Santis, P.; Meyer, L.-E.; Kara, S. The rise of continuous flow biocatalysis – fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Valotta, A.; Maier, M.C.; Soritz, S.; Pauritsch, M.; Koenig, M.; Brouczek, D.; Schwentenwein, M.; Gruber-Woelfler, H. 3D printed ceramics as solid supports for enzyme immobilization: An automated DoE approach for applications in continuous flow. J. Flow Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Lva, K.; Kumissaya, L.; Wub, B.; Chua, J.; Heab, B. Specific immobilization of lipase on functionalized 3D printing scaffolds via enhanced hydrophobic interaction for efficient resolution of racemic 1-indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.K.; Botti, R.F.; Innocentini, M.D.D.M.; Marques, R.F.C.; Colombo, P.; de Paula, A.V.; Flumignan, D.L. 3D printed geopolymer: An efficient support for immobilization of Candida rugosa lipase. Chem. Eng. J. 2021, 414, 128843. [Google Scholar] [CrossRef]
- Molinero-Fernández, A.; López, M.; Escarpa, A. Electrochemical microfluidic micromotors-based immunoassay for C-Reactive protein determination in preterm neonatal samples with sepsis suspicion. Anal. Chem. 2020, 92, 5048–5054. [Google Scholar] [CrossRef]
- Lin, F.; Zhao, X.; Wang, J.; Yu, S.; Deng, Y.; Geng, L.; Li, H. A novel microfluidic chip electrophoresis strategy for simultaneous, label-free, multi-protein detection based on a graphene energy transfer biosensor. Analyst 2014, 139, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Herr, A. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7, 041501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, E.J.; Mazzeo, A.D.; Whitesides, G.M. Paper-based electroanalytical devices for accessible diagnostic testing. MRS Bull. 2013, 38, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Pereiro, I.; Tabnaoui, S.; Fermigier, M.; du Roure, O.; Descroix, S.; Viovy, J.-L.; Malaquin, L. Magnetic fluidized bed for solid phase extraction in microfluidic systems. Lab a Chip 2017, 17, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, I.; Bendali, A.; Tabnaoui, S.; Alexandre, L.; Srbova, J.; Bilkova, Z.; Deegan, S.; Joshi, L.; Viovy, J.-L.; Malaquin, L.; et al. A new microfluidic approach for the one-step capture, amplification and label-free quantification of bacteria from raw samples. Chem. Sci. 2016, 8, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Sasso, L.A.; Johnston, I.H.; Zheng, M.; Gupte, R.K.; Undar, A.; Zahn, J.D. Automated microfluidic processing platform for multiplexed magnetic bead immunoassays. Microfluid. Nanofluidics 2012, 13, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcante, F.; Falcão, I.d.A.; Souza, J.D.S.; Rocha, T.; de Sousa, I.; Cavalcante, A.; de Oliveira, A.; de Sousa, M.; dos Santos, J. Designing of nanomaterials-based enzymatic biosensors: Synthesis, properties, and applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Aldhahri, M.M.; Almulaiky, Y.Q.; El-Shishtawy, R.M.; Al-Shawafi, W.; Alngadh, A.; Maghrabi, R. Facile immobilization of enzyme via co-electrospinning: A simple method for enhancing enzyme reusability and monitoring an activity-based organic semiconductor. ACS Omega 2018, 3, 6346–6350. [Google Scholar] [CrossRef]
- Brites, M.D.M.; Cerón, A.A.; Costa, S.M.; Oliveira, R.C.; Ferraz, H.G.; Catalani, L.H.; Costa, S.A. Bromelain immobilization in cellulose triacetate nanofiber membranes from sugarcane bagasse by electrospinning technique. Enzym. Microb. Technol. 2019, 132, 109384. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Huang, F.; Wei, Q. Electrospun nanofibers for enzyme immobilization. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 765–781. ISBN 9780323512701. [Google Scholar]
- Alonso-González, M.; Corral-González, A.; Felix, M.; Romero, A.; Martin-Alfonso, J. Developing active poly(vinyl alcohol)-based membranes with encapsulated antimicrobial enzymes via electrospinning for food packaging. Int. J. Biol. Macromol. 2020, 162, 913–921. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Fan, Y.; Tian, X.; Zheng, L.; Jin, X.; Zhang, Q.; Xu, S.; Liu, P.; Yang, N.; Bai, H.; Wang, H. Yeast encapsulation in nanofiber via electrospinning: Shape transformation, cell activity and immobilized efficiency. Mater. Sci. Eng. C 2020, 120, 111747. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Aldhahri, M.; Almulaiky, Y.Q. Dual immobilization of α-amylase and horseradish peroxidase via electrospinning: A proof of concept study. Int. J. Biol. Macromol. 2020, 163, 1353–1360. [Google Scholar] [CrossRef]
- Jun, S.-H.; Yang, J.; Jeon, H.; Kim, H.S.; Pack, S.P.; Jin, E.; Kim, J. Stabilized and immobilized carbonic anhydrase on electrospun nanofibers for enzymatic CO2 conversion and utilization in expedited microalgal growth. Environ. Sci. Technol. 2020, 54, 1223–1231. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Peksel, A. enhanced catalytic activity of immobilized phytase into polyvinyl alcohol-sodium alginate based electrospun nanofibers. Catal. Lett. 2020, 151, 821–831. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, K.; Hsu, R.; Hsieh, C.; Wang, H.; Ting, Y. Enzymatic degradation of ginkgolic acid by laccase immobilized on novel electrospun nanofiber mat. J. Sci. Food Agric. 2020, 100, 2705–2712. [Google Scholar] [CrossRef]
- Jhuang, J.-R.; Lin, S.-B.; Chen, L.-C.; Lou, S.-N.; Chen, S.-H.; Chen, H.-H. Development of immobilized laccase-based time temperature indicator by electrospinning zein fiber. Food Packag. Shelf Life 2019, 23, 100436. [Google Scholar] [CrossRef]
- Syukri, M.S.M.; Rahman, R.A.; Mohamad, Z.; Illias, R.M.; Mahmood, N.A.N.; Jaafar, N.R. Optimization strategy for laccase immobilization on polyethylene terephthalate grafted with maleic anhydride electrospun nanofiber mat. Int. J. Biol. Macromol. 2020, 166, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Staszak, M.; Jankowska, K.; Kaźmierczak, K.; Degórska, O.; Nguyen, L.N.; Kijeńska-Gawrońska, E.; Pinelo, M.; Jesionowski, T. The response surface methodology for optimization of tyrosinase immobilization onto electrospun polycaprolactone–chitosan fibers for use in bisphenol A removal. Int. J. Biol. Macromol. 2020, 165, 2049–2059. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. Exploring current tendencies in techniques and materials for immobilization of laccases—A review. Int. J. Biol. Macromol. 2021, 181, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Zhang, Q.; Li, J.; Tian, H. Preparation of lipase–electrospun SiO2 nanofiber membrane bioreactors and their targeted catalytic ability at the macroscopic oil–water interface. J. Agric. Food Chem. 2020, 68, 8362–8369. [Google Scholar] [CrossRef]
- Işik, C.; Arabaci, G.; Doğaç, Y.I.; Deveci, I.; Teke, M. Synthesis and characterization of electrospun PVA/Zn2+ metal composite nanofibers for lipase immobilization with effective thermal, pH stabilities and reusability. Mater. Sci. Eng. C 2019, 99, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Anandharamakrishnan, C.; Parthasarathi, S. (Eds.) Food Nanotechnology; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781315153872. [Google Scholar]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2020, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Morato, N.M.; Holden, D.T.; Cooks, R.G. High-throughput label-free enzymatic assays using desorption electrospray-ionization mass spectrometry. Angew. Chem. Int. Ed. 2020, 59, 20459–20464. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.M.; Siqueira, N.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Ting, Y.; Kuo, H.-C.; Hsieh, C.-W.; Hsu, H.-Y.; Wu, C.-N.; Cheng, K.-C. Enzymatic degradation of ginkgolic acids by laccase immobilized on core/shell Fe3O4/nylon composite nanoparticles using novel coaxial electrospraying process. Int. J. Biol. Macromol. 2021, 172, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Wang, J.; Ji, X.; Su, Z.; Wang, S.; Zhang, S. Positional assembly of multi-enzyme cascade reaction in polyelectrolyte doped microcapsule through electrospray and layer-by-layer assembly. Synth. Syst. Biotechnol. 2020, 5, 206–213. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.D.L.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Valdespino-León, M.; Calderón-Domínguez, G. Glucose oxidase release of stressed chia mucilage-sodium alginate capsules prepared by electrospraying. J. Food Process. Preserv. 2021, 45, e15484. [Google Scholar] [CrossRef]
- Ibili, H.; Dasdemir, M.; Çankaya, T.; Orhan, M.; Güneşoğlu, C.; Anul, S.A. Investigation of poly(lactic acid) nanocapsules containing the plant extract via coaxial electrospraying method for functional nonwoven applications. J. Ind. Text. 2021. [Google Scholar] [CrossRef]
- Xing, X.; Han, Y.; Jiang, Q.; Sun, Y.; Wang, X.; Qu, G.; Sun, G.; Li, Y. Immobilization of laccases onto cellulose nanocrystals derived from waste newspaper: Relationship between immobilized laccase activity and dialdehyde content. Cellulose 2021, 1–13. [Google Scholar] [CrossRef]
- Lim, L.-T. Electrospinning and electrospraying technologies for food and packaging applications. In Electrospun Polymers and Composites; Woodhead Publishing: Cambridge, UK, 2020; pp. 217–259. [Google Scholar] [CrossRef]
- Liu, N.; Li, D.; Wang, W.; Hollmann, F.; Xu, L.; Ma, Y.; Yang, B.; Bai, W.; Sun, X.; Wang, Y. Production and immobilization of lipase PCL and its application in synthesis of α-linolenic acid-rich diacylglycerol. J. Food Biochem. 2018, 42, e12574. [Google Scholar] [CrossRef] [Green Version]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Su, Z. Enzyme-based hybrid nanoflowers with high performances for biocatalytic, biomedical, and environmental applications. Coord. Chem. Rev. 2020, 416, 213342. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, L.; Tan, X.; Jiao, L.; Wei, Q.; Li, H. Bioinspired synthesis of organic–inorganic hybrid nanoflowers for robust enzyme-free electrochemical immunoassay. Biosens. Bioelectron. 2019, 133, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; Adhikari, M.D.; Chung, M.; Tran, T.D.; Kim, J.; Kim, M.I. Magnetic nanoparticles-embedded enzyme-inorganic hybrid nanoflowers with enhanced peroxidase-like activity and substrate channeling for glucose biosensing. Adv. Heal. Mater. 2019, 8, e1801507. [Google Scholar] [CrossRef]
- Chui, C.-Y.; Ye, H. Fundamental concepts and insights into electrospraying for biomedical applications. In Biomedical Applications of Electrospinning and Electrospraying; Woodhead Publishing: Cambridge, UK, 2021; pp. 185–206. [Google Scholar] [CrossRef]
- Li, H.; Hou, J.; Duan, L.; Ji, C.; Zhang, Y.; Chen, V. Graphene oxide-enzyme hybrid nanoflowers for efficient water soluble dye removal. J. Hazard. Mater. 2017, 338, 93–101. [Google Scholar] [CrossRef]
- Lee, S.W.; Cheon, S.A.; Kim, M.I.; Park, T.J. Organic–inorganic hybrid nanoflowers: Types, characteristics, and future prospects. J. Nanobiotechnology 2015, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- An, S.S.; Park, H.G.; Kim, M.I.; Batule, B.; Park, K.S. Ultrafast sonochemical synthesis of protein-inorganic nanoflowers. Int. J. Nanomed. 2015, ume 10, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Batule, B.S.; Park, K.S.; Gautam, S.; Cheon, H.J.; Kim, M.I.; Park, H.G. Intrinsic peroxidase-like activity of sonochemically synthesized protein copper nanoflowers and its application for the sensitive detection of glucose. Sens. Actuators B Chem. 2018, 283, 749–754. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. Enzyme-inorganic hybrid nanoflowers: Classification, synthesis, functionalization and potential applications. Chem. Eng. J. 2021, 415, 129075. [Google Scholar] [CrossRef]
- Soni, S.; Dwivedee, B.P.; Banerjee, U.C. An ultrafast sonochemical strategy to synthesize lipase-manganese phosphate hybrid nanoflowers with promoted biocatalytic performance in the kinetic resolution of β-Aryloxyalcohols. Chem. Nano Mat. 2018, 4, 1007–1020. [Google Scholar] [CrossRef]
- Chung, M.; Nguyen, T.L.; Tran, T.Q.N.; Yoon, H.H.; Kim, I.T.; Kim, M.I. Ultrarapid sonochemical synthesis of enzyme-incorporated copper nanoflowers and their application to mediatorless glucose biofuel cell. Appl. Surf. Sci. 2018, 429, 203–209. [Google Scholar] [CrossRef]
- Park, K.S.; Batule, B.S.; Chung, M.; Kang, K.S.; Park, T.J.; Kim, M.I.; Park, H.G. A simple and eco-friendly one-pot synthesis of nuclease-resistant DNA–inorganic hybrid nanoflowers. J. Mater. Chem. B 2017, 5, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, J.; Zhang, Z.; Jiang, Y.; Bilal, M.; Jiang, Y.; Jia, S.; Cui, J. Self-assembly of activated lipase hybrid nanoflowers with superior activity and enhanced stability. Biochem. Eng. J. 2020, 158, 107582. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, X.; Kong, D.; Li, G.; Li, Q. Immobilization of thermophilic lipase in inorganic hybrid nanoflower through biomimetic mineralization. Colloids Surfaces B Biointerfaces 2020, 197, 111450. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Qi, L.; Luo, Z. Pickering emulsion-based microreactors for size-selective interfacial enzymatic catalysis. Front. Bioeng. Biotechnol. 2020, 8, 950. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yin, W.; Chen, J.; Wang, W.; Guo, T.; Meng, T. Hollow colloidosomes with an enzyme confined in a porous shell as Pickering interfacial biocatalysts for efficient bioconversions. Green Chem. 2020, 23, 740–744. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Li, Y.; Hong, L.; Ngai, T. Submicron inverse pickering emulsions for highly efficient and recyclable enzymatic Catalysis. Chem. Asian J. 2018, 13, 3533–3539. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Shi, J.; Tang, H.; Xiang, X.; Huang, F.; Zheng, M.-M. Carbon nanoparticle-stabilized pickering emulsion as a sustainable and high-performance interfacial catalysis platform for enzymatic esterification/transesterification. ACS Sustain. Chem. Eng. 2019, 7, 7619–7629. [Google Scholar] [CrossRef]
- Sun, T.; Dong, Z.; Wang, J.; Huang, F.-H.; Zheng, M.-M. Ultrasound-assisted interfacial immobilization of lipase on hollow mesoporous silica spheres in a pickering emulsion system: A hyperactive and sustainable biocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 17280–17290. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Oriented enzyme immobilization at the oil/water interface enhances catalytic activity and recyclability in a pickering emulsion. Langmuir 2017, 33, 12317–12325. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, M.; Zhang, X.; Xin, H.; Yang, H. Pickering emulsion as an efficient platform for enzymatic reactions without stirring. ACS Sustain. Chem. Eng. 2016, 4, 6838–6843. [Google Scholar] [CrossRef]
- Lou, W.-Y.; Fernández-Lucas, J.; Ge, J.; Wu, C. Editorial: Enzyme or whole cell immobilization for efficient biocatalysis: Focusing on novel supporting platforms and immobilization techniques. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.; Holmes, M.; Ettelaie, R. Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics. Adv. Colloid Interface Sci. 2018, 263, 195–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, T.; Liu, B.; Wang, R.; Huang, Y.; Luo, J.; Li, Y. The enhanced fatty acids flavor release for low-fat cheeses by carrier immobilized lipases on O/W Pickering emulsions. Food Hydrocoll. 2021, 116, 106651. [Google Scholar] [CrossRef]
- Wang, T.; Chen, K.; Li, Z.; Jiang, L.; Yu, D.; Cheng, J.; Wang, L. Construction of an enzyme—Pickering emulsion catalytic system and its application in the interfacial catalytic reaction of rice bran oil deacidification. LWT 2021, 150, 111921. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Q.; Zhu, L.; Ning, J.; Yang, H.; Ning, K.; He, Y. Emulsion hydrogel microbeads encapsulating extractants prepared by O/W/O double pickering emulsions for the recovery of Cu(II) from water. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126932. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, L.; Li, Y.; Yin, S.-W.; Ngai, T. Inverse pickering emulsion stabilized by binary particles with contrasting characteristics and functionality for interfacial biocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 4989–4997. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zhou, S.; Huang, J.; Wang, P. Engineering bioscaffolds for enzyme assembly. Biotechnol. Adv. 2021, 107721. [Google Scholar] [CrossRef]
- Zhang, Q.; Bolisetty, S.; Cao, Y.; Handschin, S.; Adamcik, J.; Peng, Q.; Mezzenga, R. Selective and efficient removal of fluoride from water: In situ engineered amyloid fibril/ZrO 2 hybrid membranes. Angew. Chem. Int. Ed. 2019, 58, 6012–6016. [Google Scholar] [CrossRef]
- Torres, M.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhu, M. Reconstitution of cellulosome: Research progress and its application in biorefinery. Biotechnol. Appl. Biochem. 2019, 66, 720–730. [Google Scholar] [CrossRef]
- Smith, S.P.; A Bayer, E. Insights into cellulosome assembly and dynamics: From dissection to reconstruction of the supramolecular enzyme complex. Curr. Opin. Struct. Biol. 2013, 23, 686–694. [Google Scholar] [CrossRef]
- Kumada, Y.; Kuroki, D.; Yasui, H.; Ohse, T.; Kishimoto, M. Characterization of polystyrene-binding peptides (PS-tags) for site-specific immobilization of proteins. J. Biosci. Bioeng. 2010, 109, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, W.; Wang, Y.; Liu, D.; Wang, P. Biofilm polysaccharide display platform: A natural, renewable, and biocompatible material for improved lipase performance. J. Agric. Food Chem. 2020, 68, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, W.; Xuan, Q.; Zhou, Y.; Zhou, S.; Huang, J.; Wang, P. Binding peptide-guided immobilization of lipases with significantly improved catalytic performance using escherichia coli BL21(DE3) biofilms as a platform. ACS Appl. Mater. Interfaces 2021, 13, 6168–6179. [Google Scholar] [CrossRef]
- Mulinari, J.; Oliveira, J.V.; Hotza, D. Lipase immobilization on ceramic supports: An overview on techniques and materials. Biotechnol. Adv. 2020, 42, 107581. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef]
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H.M. Graphene and graphene oxide: Functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int. J. Biol. Macromol. 2018, 120, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 2018, 276, 153–162. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Mokhtar, N.F.; Rahman, R.N.Z.R.A.; Noor, N.D.M.; Shariff, F.M.; Ali, M.S.M. The immobilization of lipases on porous support by adsorption and hydrophobic interaction method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Cui, J.D.; Ren, S.; Sun, B.; Jia, S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, challenges and opportunities of enzyme immobilization on porous silicon for biosensing applications. J. Environ. Chem. Eng. 2020, 8, 104266. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Dos Santos, L.A.; De Almeida, J.M.; Krieger, N.; Mateo, C. Recent trends in biomaterials for immobilization of lipases for application in non-conventional media. Catalysts 2020, 10, 697. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.-T.; Shi, Y.-P. “Recent advances on support materials for lipase immobilization and applicability as biocatalysts in inhibitors screening methods”-A review. Anal. Chim. Acta 2019, 1101, 9–22. [Google Scholar] [CrossRef]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, X.; Wang, L.; Zhou, L.; Huang, Z.; Ma, L.; He, Y.; Shi, L.; Gao, J. Virus-like organosilica nanoparticles for lipase immobilization: Characterization and biocatalytic applications. Biochem. Eng. J. 2019, 144, 125–134. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Zare, A.; Bordbar, A.-K.; Jafarian, F.; Tangestaninejad, S. Candida rugosa lipase immobilization on various chemically modified Chromium terephthalate MIL-101. J. Mol. Liq. 2018, 254, 137–144. [Google Scholar] [CrossRef]
- Xiang, X.; Suo, H.; Xu, C.; Hu, Y. Covalent immobilization of lipase onto chitosan-mesoporous silica hybrid nanomaterials by carboxyl functionalized ionic liquids as the coupling agent. Colloids Surfaces B Biointerfaces 2018, 165, 262–269. [Google Scholar] [CrossRef]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef]
- Wan, D.; Tian, L.; Li, X.; Li, B.; Zhang, Q. A versatile strategy for enzyme immobilization: Fabricating lipase/inorganic hybrid nanostructures on macroporous resins with enhanced catalytic properties. Biochem. Eng. J. 2018, 139, 101–108. [Google Scholar] [CrossRef]
- Asmat, S.; Husain, Q. Exquisite stability and catalytic performance of immobilized lipase on novel fabricated nanocellulose fused polypyrrole/graphene oxide nanocomposite: Characterization and application. Int. J. Biol. Macromol. 2018, 117, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Y. Poly(carboxybetaine methacrylate)-grafted silica nanoparticle: A novel carrier for enzyme immobilization. Biochem. Eng. J. 2018, 132, 122–129. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, C.; Yang, N.; Wang, Q.; Wang, J.; Pan, H.; Hu, Y.; Ruan, C. Enhanced activity and stability of industrial lipases immobilized onto spherelike bacterial cellulose. Int. J. Biol. Macromol. 2017, 109, 1174–1181. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Y.; Yang, X.; Li, Y.; Wang, C. Electrospun nanofibrous membranes containing epoxy groups and hydrophilic polyethylene oxide chain for highly active and stable covalent immobilization of lipase. Chem. Eng. J. 2018, 336, 456–464. [Google Scholar] [CrossRef]
- Gricajeva, A.; Kazlauskas, S.; Kalėdienė, L.; Bendikienė, V. Analysis of Aspergillus sp. lipase immobilization for the application in organic synthesis. Int. J. Biol. Macromol. 2018, 108, 1165–1175. [Google Scholar] [CrossRef]
- Orrego, A.H.; Ghobadi, R.; Moreno-Perez, S.; Mendoza, A.J.; Fernández-Lorente, G.; Guisan, J.M.; Rocha-Martin, J. Stabilization of immobilized lipases by intense intramolecular cross-linking of their surfaces by using aldehyde-dextran polymers. Int. J. Mol. Sci. 2018, 19, 553. [Google Scholar] [CrossRef] [Green Version]
- Sipponen, M.H.; Farooq, M.; Koivisto, J.; Pellis, A.; Seitsonen, J.; Österberg, M. Spatially confined lignin nanospheres for biocatalytic ester synthesis in aqueous media. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Razak, F.I.A.; Jamalis, J.; Widodo, N.; Wahab, R.A. Characterization, optimization and stability studies on Candida rugosa lipase supported on nanocellulose reinforced chitosan prepared from oil palm biomass. Int. J. Biol. Macromol. 2018, 114, 306–316. [Google Scholar] [CrossRef]
- Otari, S.; Patel, S.K.; Kalia, V.C.; Lee, J.-K. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour. Technol. 2020, 302, 122887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Zhu, Y.; Hong, L.; Li, T.; Wang, X.; Fu, Y. Lipases immobilized on the modified polyporous magnetic cellulose support as an efficient and recyclable catalyst for biodiesel production from Yellow horn seed oil. Renew. Energy 2019, 145, 1246–1254. [Google Scholar] [CrossRef]
- Manan, F.M.A.; Attan, N.; Widodo, N.; Aboul-Enein, H.Y.; Wahab, R.A. Rhizomucor miehei lipase immobilized on reinforced chitosan–chitin nanowhiskers support for synthesis of eugenyl benzoate. Prep. Biochem. Biotechnol. 2018, 48, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Kim, D.; Nah, J.-W.; Jeon, Y.-J. Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chem. Eng. J. 2018, 355, 33–48. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Khademian, E.; Salehi, E.; Sanaeepur, H.; Galiano, F.; Figoli, A. A systematic review on carbohydrate biopolymers for adsorptive remediation of copper ions from aqueous environments-part A: Classification and modification strategies. Sci. Total. Environ. 2020, 738, 139829. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Qi, H.; Du, Y.; Hu, G.; Zhang, L. Poly(carboxybetaine methacrylate)-functionalized magnetic composite particles: A biofriendly support for lipase immobilization. Int. J. Biol. Macromol. 2017, 107, 2660–2666. [Google Scholar] [CrossRef]
- Khan, N.; Maseet, M.; Basir, S.F. Synthesis and characterization of biodiesel from waste cooking oil by lipase immobilized on genipin cross-linked chitosan beads: A green approach. Int. J. Green Energy 2019, 17, 84–93. [Google Scholar] [CrossRef]
- Motaung, T.E.; Linganiso, L.Z. Critical review on agrowaste cellulose applications for biopolymers. Int. J. Plast. Technol. 2018, 22, 185–216. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Attan, N.; Mahat, N.A.; Razak, F.I.A.; Jamalis, J.; Wahab, R.A. Structure and properties of oil palm-based nanocellulose reinforced chitosan nanocomposite for efficient synthesis of butyl butyrate. Carbohydr. Polym. 2017, 176, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, J.; Senthil, T.; Wu, L. Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
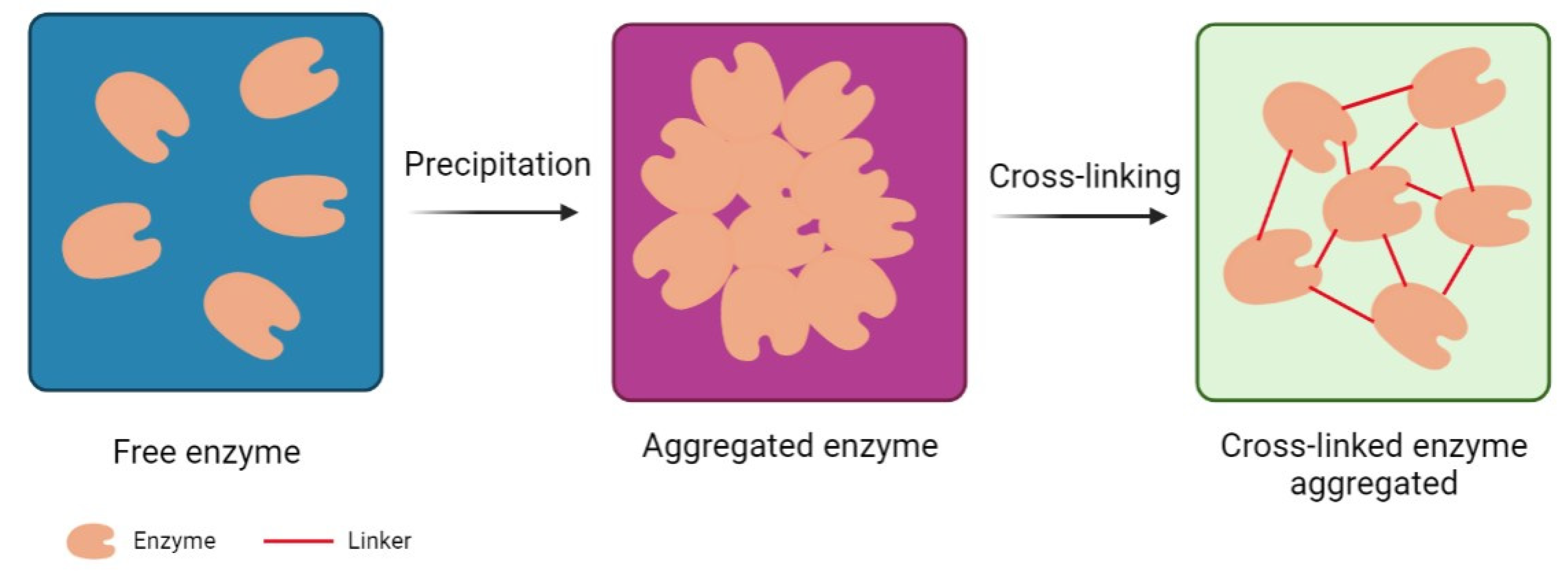
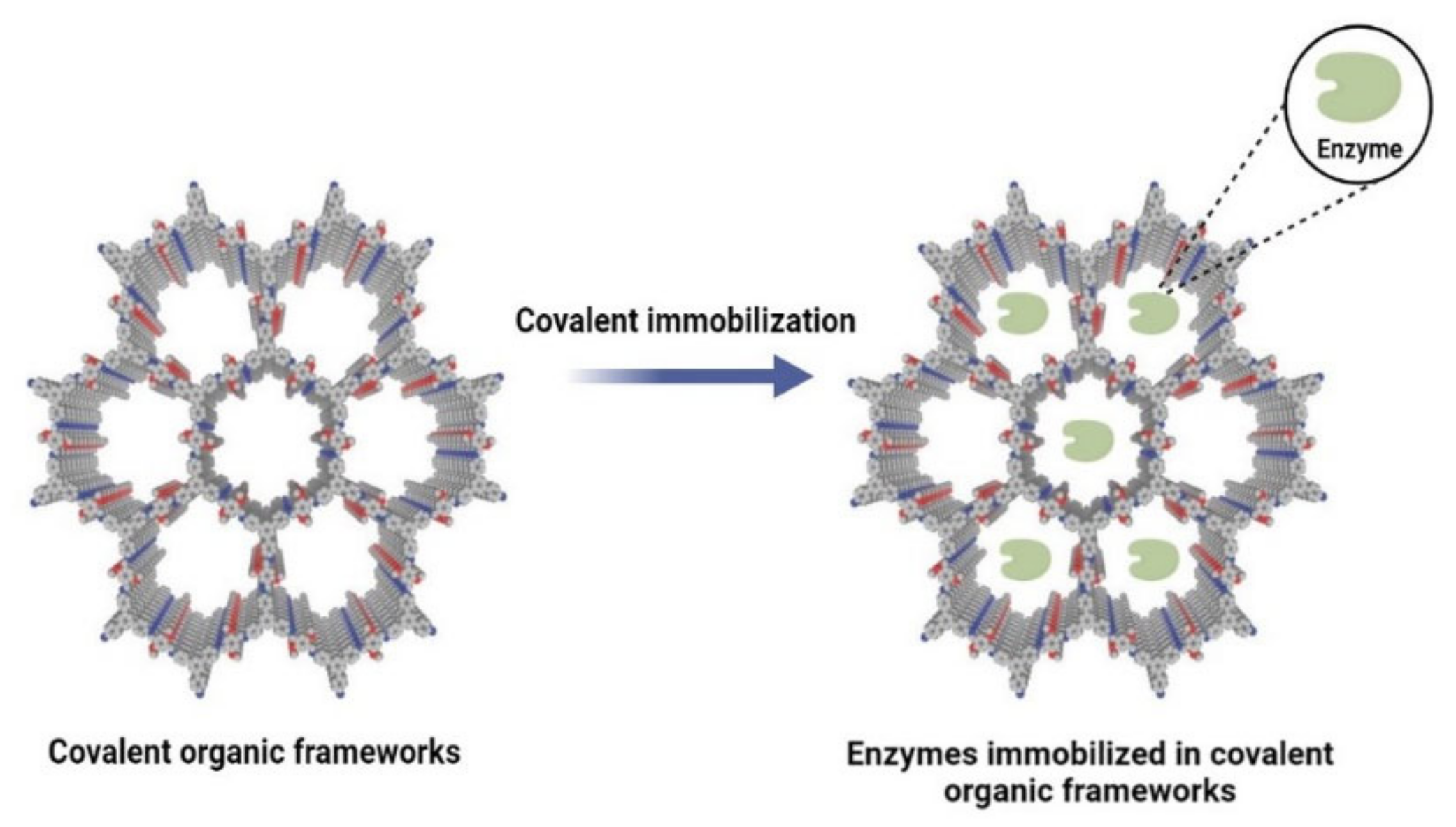
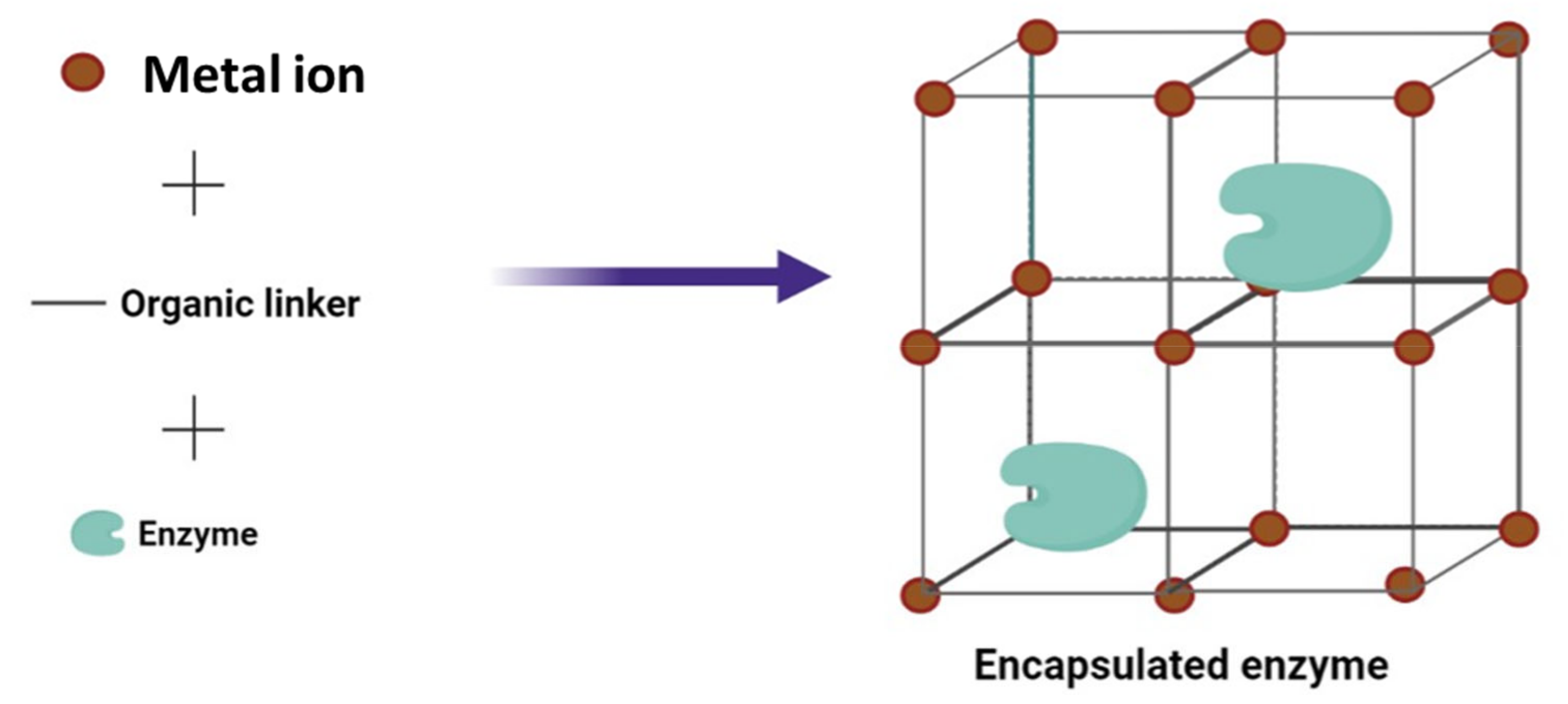
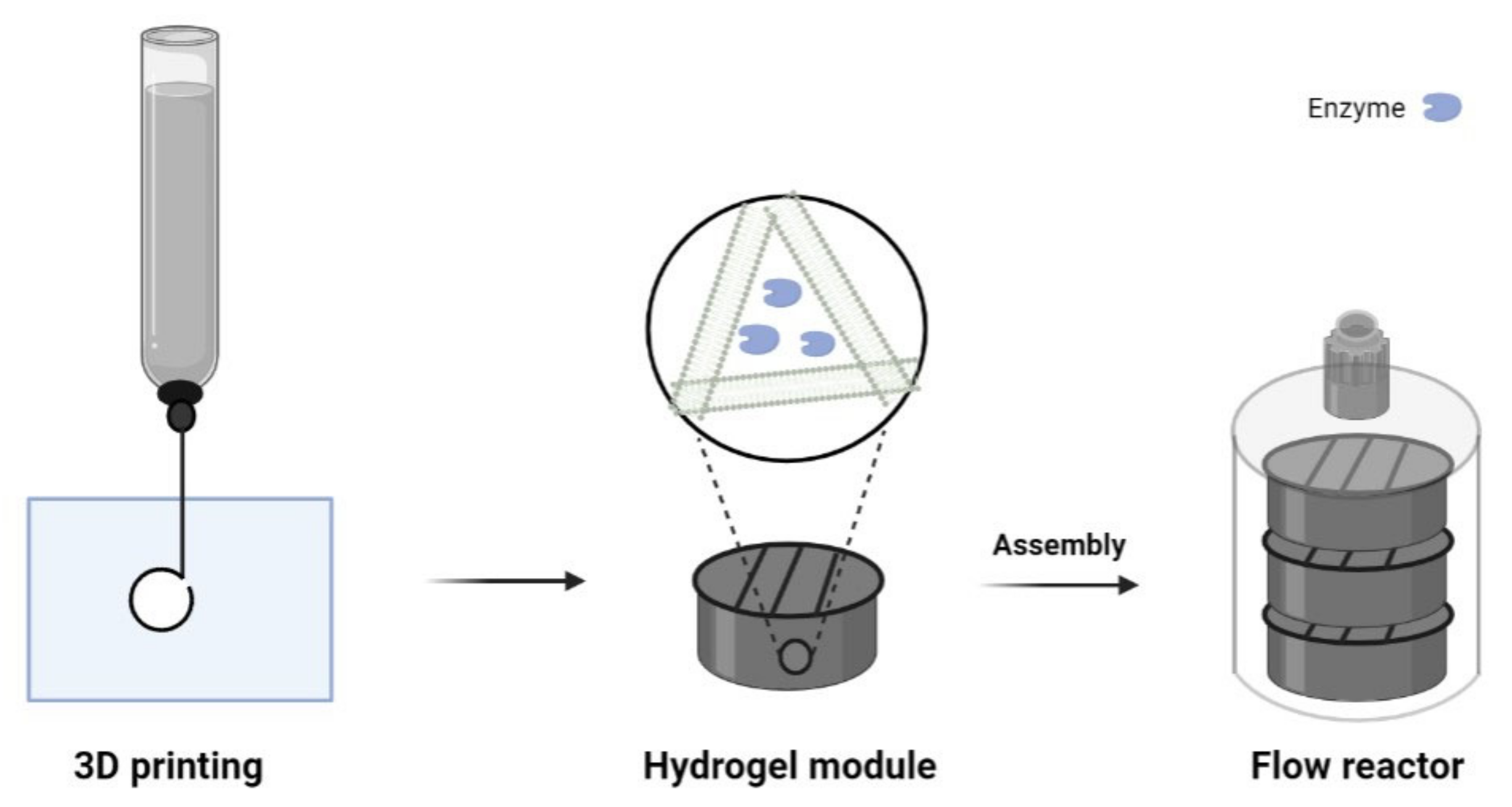
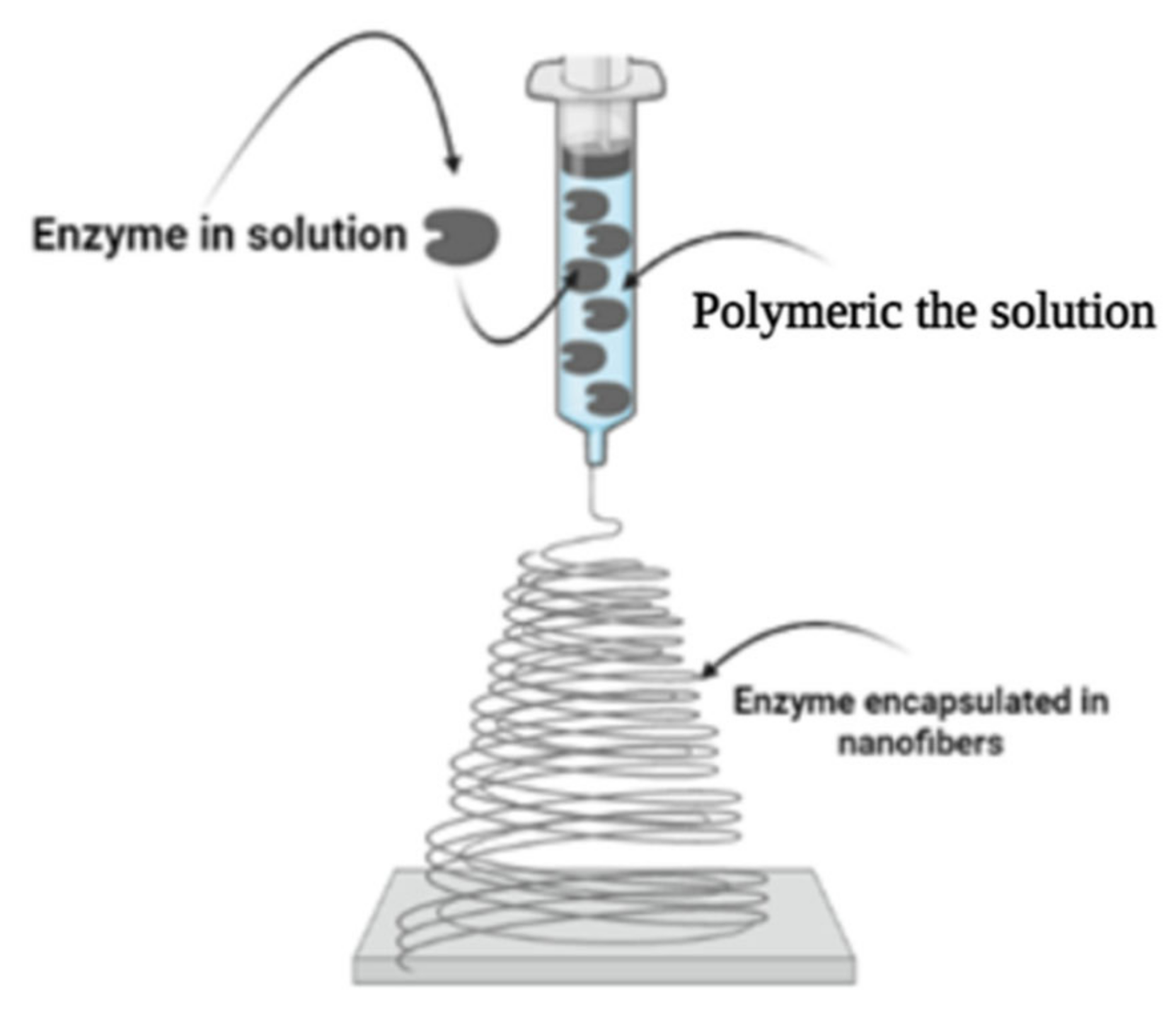
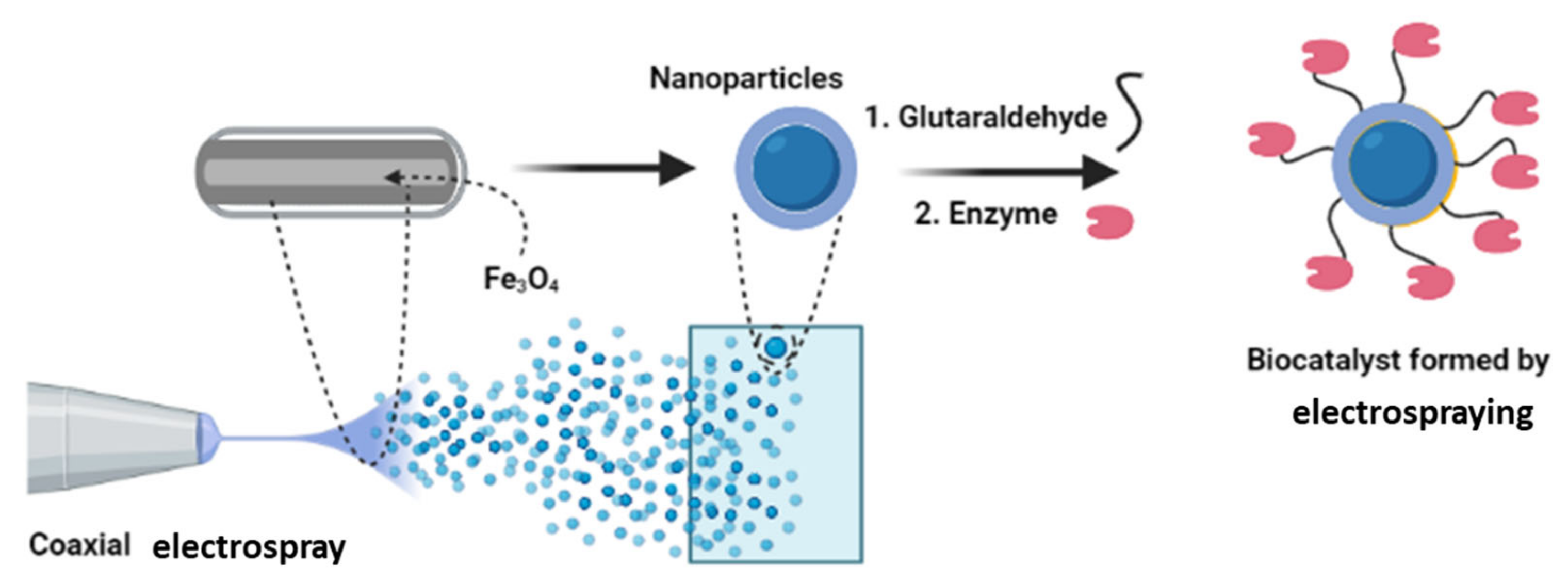
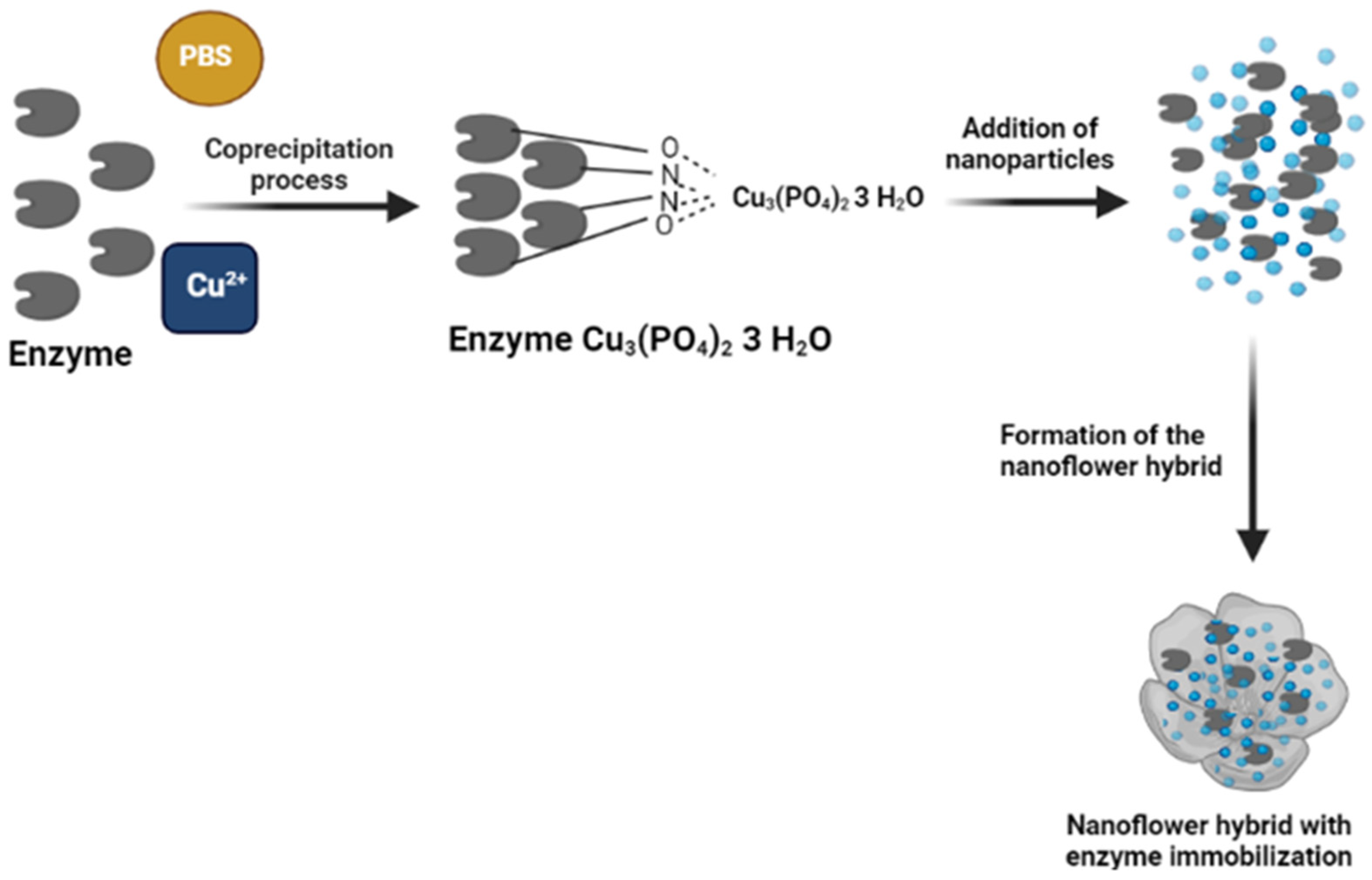
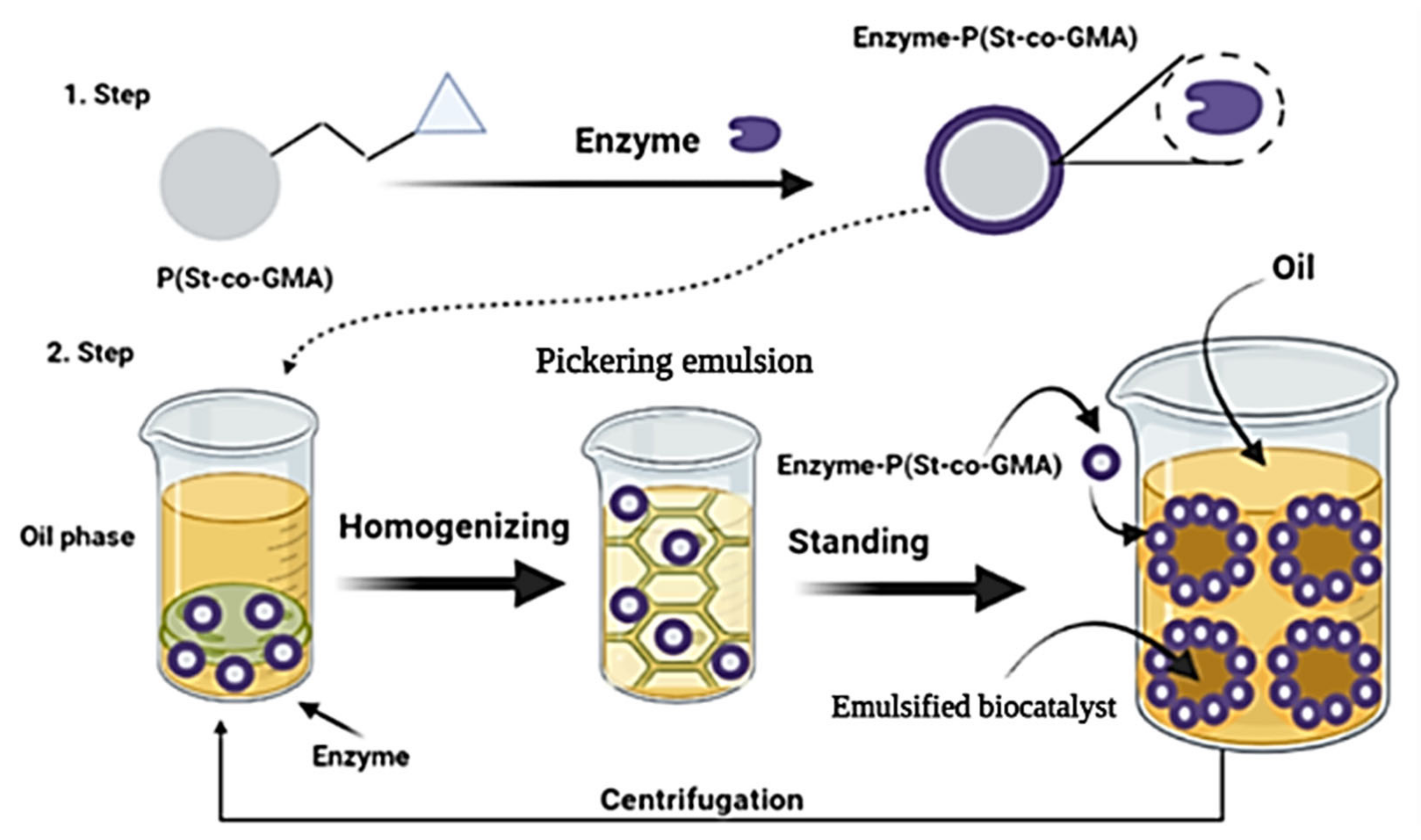
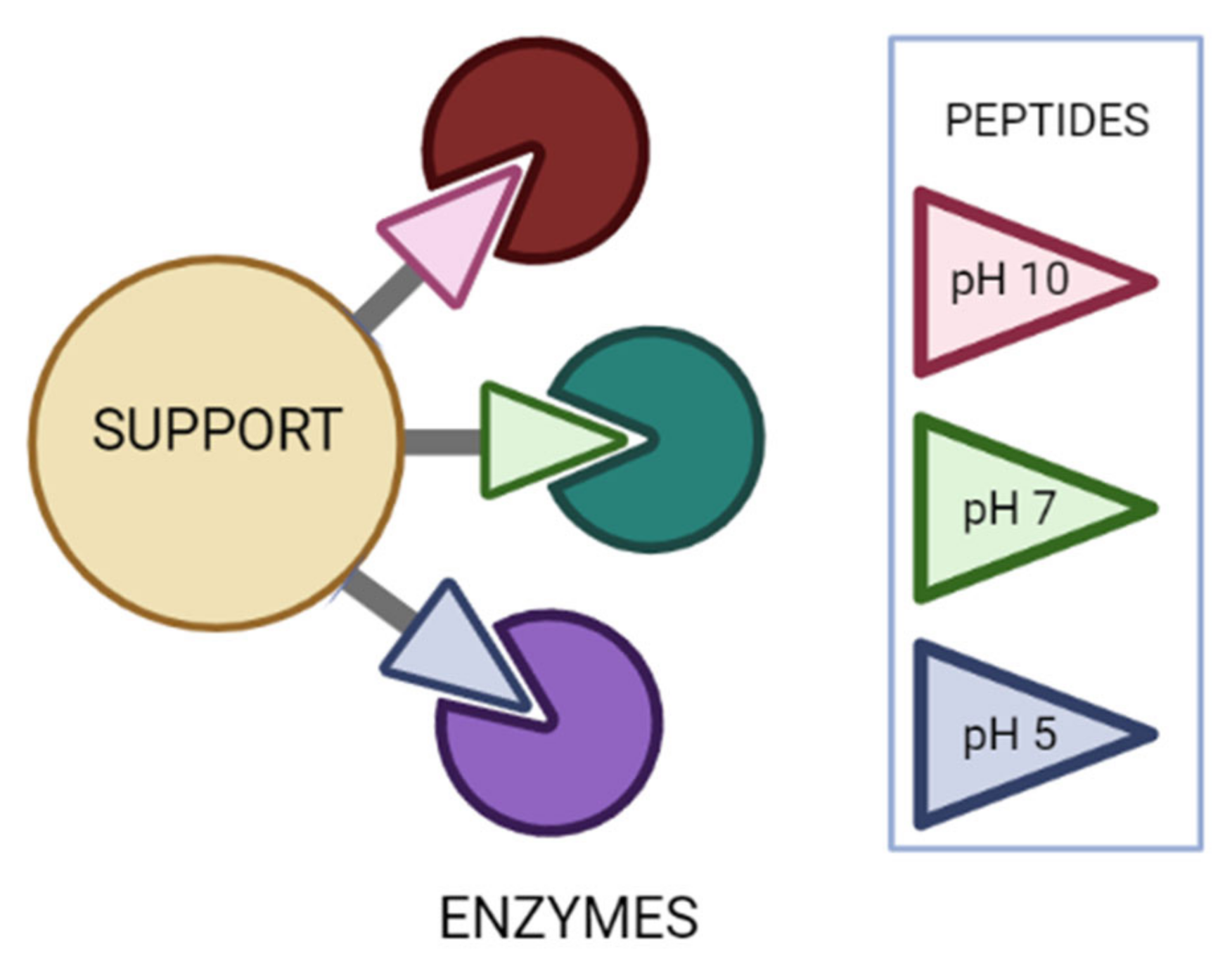
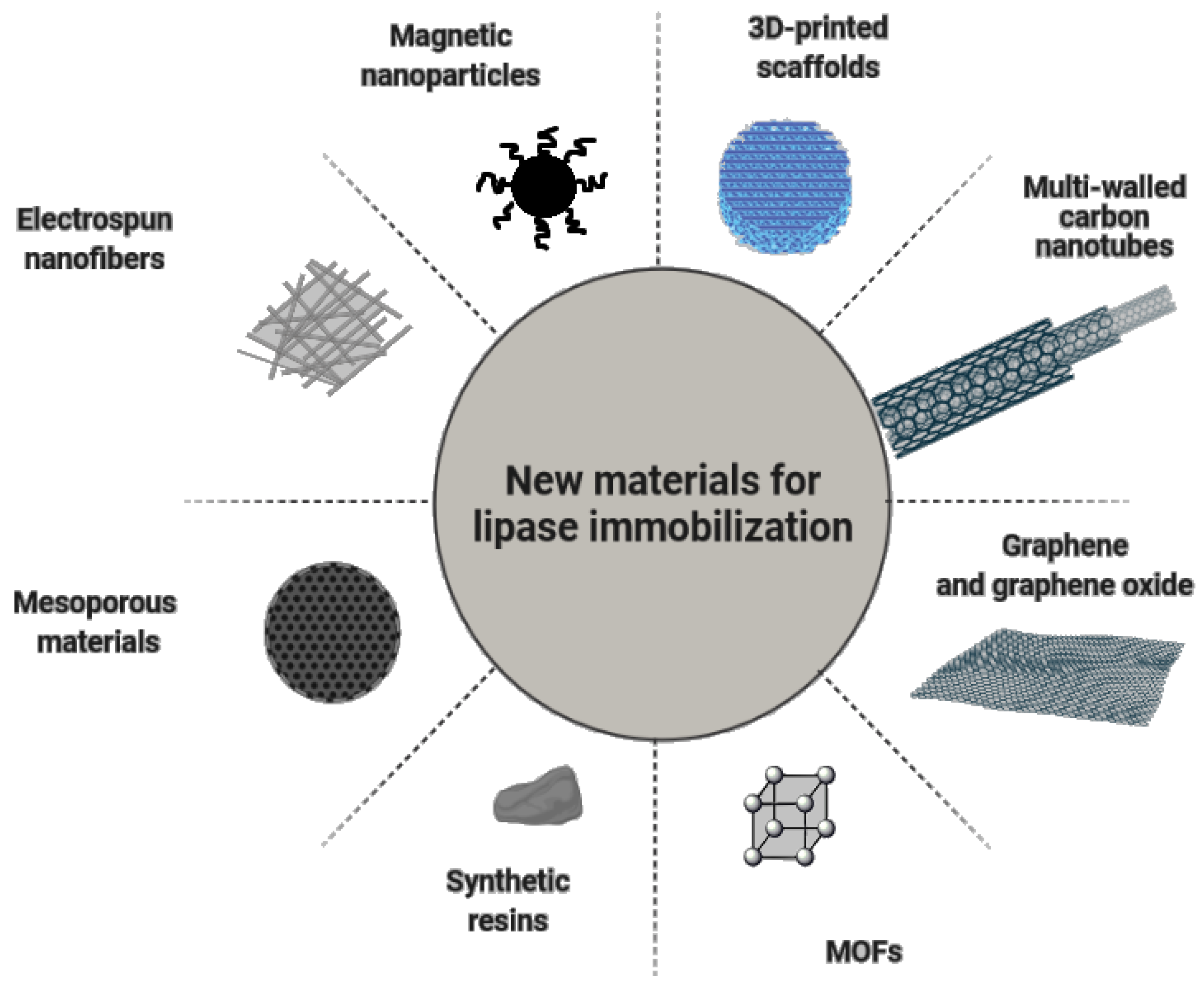
| Support | Lipase Source | Application | Immobilization Technique | Ref. |
|---|---|---|---|---|
| Virus-like mesoporous organosilica nanoparticles | Lipase B from Candida antarctica | Synthesis of levulinate esters | Covalent bonding | [297] |
| LifetechTM methacrylic synthetic resins | Thermomyces lanuginosus | Biodiesel synthesis | Physical adsorption | [298] |
| MIL-101(Cr) MOFs | Candida rugosa | Hydrolysis of p-nitrophenyl palmitate | Covalent bonding | [299] |
| Chitosan–mesoporous silica hybrid nanomaterials | Porcine pancreatic PPL | Triacetin hydrolysis | Covalent bonding | [300] |
| Magnetic multiwalled carbon nanotubes | Candida rugosa | Synthesis of fruit flavors | Covalent bonding | [301] |
| 3D-printed carbon fiber-reinforced polylactic acid scaffolds | Burkholderia ambifaria | Hydrolysis of p-nitrophenyl palmitate | Adsorption | [203] |
| Inorganic hybrid nanosheets on sulfonated macroporous resins | Candida rugosa | Hydrolysis of the olive oil emulsion | Encapsulation | [302] |
| Nanocellulose-fused polypyrrole/graphene oxide nanocomposites | Candida rugosa | Synthesis of fruit flavors | Adsorption | [303] |
| Poly(carboxybetaine methacrylate)-grafted silica nanoparticles | Candida rugosa | Hydrolysis of p-nitrophenyl acetate | Covalent bonding | [304] |
| Spherelike bacterial cellulose | Rhizopus chinensis | Hydrolysis of the olive oil emulsion | Covalent bonding and physical adsorption | [305] |
| Electrospun nanofibrous membranes containing epoxy groups and a hydrophilic polyethylene oxide chain | Lipase B from Candida antarctica | Hydrolysis of olive oil | Covalent bonding | [244] |
| Pyrolyzed sugar industry waste | Aspergillus sp. lipase | Synthesis of 2-phenylethyl butanoate | Adsorption | [306] |
| Octyl Sepharose crosslinked with dextran aldehyde polymers | Thermomyces lanuginosus, Rhizomucor miehiei, and lipase B from Candida antarctica | Hydrolysis of p-nitrophenyl butyrate | Covalent bonding | [307] |
| Colloidal lignin particles | Lipase M from Mucor javanicus | Synthesis of butyl butyrate | Entrapment | [308] |
| Chitosan/nanocellulose biocomposites | Candida rugosa | Synthesis of butyl butyrate | Covalent bonding | [309] |
| Magnetic rice straws | Thermomyces lanuginosus | Synthesis of biodiesel | Covalent bonding | [310] |
| Diethylenetriamine-modified magnetic cellulose beads | Lipase B from Candida antarctica | Synthesis of biodiesel | Covalent bonding | [311] |
| Chitosan–chitin nanowhiskers | Rhizomucor miehei | Synthesis of eugenyl benzoate | Covalent bonding | [312] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. https://doi.org/10.3390/catal11101222
Cavalcante FTT, Cavalcante ALG, de Sousa IG, Neto FS, dos Santos JCS. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts. 2021; 11(10):1222. https://doi.org/10.3390/catal11101222
Chicago/Turabian StyleCavalcante, Francisco T. T., Antônio L. G. Cavalcante, Isamayra G. de Sousa, Francisco S. Neto, and José C. S. dos Santos. 2021. "Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization" Catalysts 11, no. 10: 1222. https://doi.org/10.3390/catal11101222
APA StyleCavalcante, F. T. T., Cavalcante, A. L. G., de Sousa, I. G., Neto, F. S., & dos Santos, J. C. S. (2021). Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts, 11(10), 1222. https://doi.org/10.3390/catal11101222








