Influence of PtCu/C Catalysts Composition on Electrochemical Characteristics of Polymer Electrolyte Fuel Cell and Properties of Proton Exchange Membrane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Bimetallic Catalysts
2.2. Stress-Testing of MEA
2.3. Membrane Properties in Mixed H+/Cu2+ Ion Form
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Membrane Transport Properties
3.4. MEA Formation and Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CV | Cyclic voltammetry |
| CVC | Current-voltage curve |
| EMF | Electromotive force |
| ESA | Electrometrically active surface area |
| GDL | Gas diffusion layer |
| LSV | Linear sweep voltammetry |
| MEA | Membrane electrode assembly |
| ORR | Oxygen reduction reaction |
| PEMFC | Proton exchange membrane fuel cell |
| RDE | Rotating disk electrode |
| RHE | Reversible hydrogen electrode |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence analysis |
References
- Daud, W.R.W.; Rosli, R.E.; Majlan, E.H.; Hamid, S.A.A.; Mohamed, R.; Husaini, T. PEM fuel cell system control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Gröger, O.; Gasteiger, H.A.; Suchsland, J.P. Review—Electromobility: Batteries or Fuel Cells? J. Electrochem. Soc. 2015, 162, A2605–A2622. [Google Scholar] [CrossRef]
- Banham, D.W.H.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-performance Platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 2018, 8, 3591–3606. [Google Scholar] [CrossRef]
- Prokop, M.; Drakselova, M.; Bouzek, K. A Review of the experimental study and prediction of Pt-based catalyst degradation during PEM fuel cell operation. Curr. Opin. Electrochem. 2020, 20, 204–207. [Google Scholar] [CrossRef]
- Auer, A.A.; Cap, S.; Antonietti, M.; Cherevko, S.; Deng, X.; Papakonstantinou, G.; Sundmacher, K.; Brüller, S.; Antonyshyn, I.; Dimitratos, N.; et al. MAXNET energy—Focusing research in chemical energy conversion on the electrocatlytic oxygen evolution. Green 2015, 5, 7–21. [Google Scholar] [CrossRef]
- Mahata, A.; Nair, A.S.; Pathak, B. Recent advancements in Pt-nanostructure-based electrocatalysts for the oxygen reduction reaction. Catal. Sci. Technol. 2019, 9, 4835–4863. [Google Scholar] [CrossRef]
- Jung, N.; Chung, D.Y.; Ryu, J.; Yoo, S.J.; Sung, Y.E. Pt-based nanoarchitecture and catalyst design for fuel cell applications. Nano Today 2014, 9, 433–442. [Google Scholar] [CrossRef]
- Chen, S.; Ferreira, P.J.; Sheng, W.; Yabuuchi, N.; Allard, L.F.; Shao-Horn, Y. Enhanced activity for oxygen reduction reaction on “Pt3Co” nanoparticles: Direct evidence of percolated and sandwich-segregation structures. J. Am. Chem. Soc. 2008, 130, 13818–13819. [Google Scholar] [CrossRef] [PubMed]
- Bele, M.; Gatalo, M.; Jovanovič, P.; Ruiz-Zepeda, F.; Šala, M.; Šest, E.; Hodnik, N.; Hočevar, S.; Gatto, I.; Saccà, A.; et al. Insight on Single Cell Proton Exchange Membrane Fuel Cell Performance of Pt-Cu/C Cathode. Catalysts 2019, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.; Hwang, J.; Chung, J.S. Characterization and activity correlations of Pt bimetallic catalysts for low temperature fuel cells. Int. J. Hydrogen Energy 2011, 36, 4007–4014. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, Y.; Zhang, W.; Tang, Y.; Wang, P. Reviews on the effects of contaminations and research methodologies for PEMFC. Int. J. Hydrogen Energy 2020, 45, 23174–23200. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Gallardo-López, B.; Chaparro, A.M.; Daza, L. Physico-chemical study of the degradation of membrane-electrode assemblies in a proton exchange membrane fuel cell stack. J. Power Sources 2011, 196, 4242–4250. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, D.C.; Park, S.H.; Kim, W.J. Preparation of Nafion/various Pt-containing SiO2 composite membranes sulfonated via different sources of sulfonic group and their application in self-humidifying PEMFC. J. Membr. Sci. 2013, 443, 210–218. [Google Scholar] [CrossRef]
- Filippov, A.; Petrova, D.; Falina, I.; Kononenko, N.; Ivanov, E.; Lvov, Y.; Vinokurov, V. Transport asymmetry of novel bi-layer hybrid perfluorinated membranes on the base of MF-4SC modified by halloysite nanotubes with platinum. Polymers 2018, 10, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.A.; Wang, X.; Ozdemir, M.O.; Qi, J.; Bonville, L.J.; Pasaogullari, U.; Molter, T. Distributed PEFC performance during cationic contamination. ECS Trans. 2014, 61, 49–55. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 5258. [Google Scholar] [CrossRef] [Green Version]
- Pryadchenko, V.V.; Srabionyan, V.V.; Kurzin, A.A.; Bulat, N.V.; Shemet, D.D.; Avakyan, L.A.; Belenov, S.V.; Volochaev, V.A.; Zizak, I.; Guterman, V.F.; et al. Bimetallic PtCu nanoparticles in PtCu/C electrocatalysts: Structural and electrochemical characterization. Appl. Catal. A 2016, 525, 226–236. [Google Scholar] [CrossRef]
- Pavlets, A.; Alekseenko, A.; Menshchikov, V.; Belenov, S.; Volochaev, V.; Pankov, I.; Safronenko, O.; Guterman, V. Influence of Electrochemical Pretreatment Conditions of PtCu/C Alloy Electrocatalyst on Its Activity. Nanomaterials 2021, 11, 1499. [Google Scholar] [CrossRef]
- Pavlets, A.S.; Alekseenko, A.A.; Tabachkova, N.Y.; Safronenko, O.I.; Nikulin, A.Y.; Alekseenko, D.V.; Guterman, V.E. A novel strategy for the synthesis of Pt–Cu uneven nanoparticles as an efficient electrocatalyst toward oxygen reduction. Int. J. Hydrogen Energy 2021, 46, 5355–5368. [Google Scholar] [CrossRef]
- Rudi, S.; Cui, C.; Gan, L.; Strasser, P. Comparative Study of the Electrocatalytically Active Surface Areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and CO-stripping voltammetry. Electrocatalysis 2014, 5, 408–418. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, H.J.; Lee, H.J.; Kim, J.; Choi, S.-I. Ni(OH)2 Decorated Pt-Cu octahedra for ethanol electrooxidation reaction. Front. Chem. 2019, 7, 608. [Google Scholar] [CrossRef] [PubMed]
- Ciapina, E.G.; Santos, S.F.; Gonzalez, E.R. Electrochemical CO stripping on nanosized Pt surfaces in acid media: A review on the issue of peak multiplicity. J. Electroanal. Chem. 2018, 815, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Manrique, G.; Garcia-Cardona, J.; Brillas, E.; Jaén, J.A.; Sánchez, J.M.; Cabot, P.L. Synthesis and Evaluation of PtNi Electrocatalysts for CO and Methanol Oxidation in Low Temperature Fuel Cells. Catalysts 2020, 10, 563. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovič, P.; Petek, U.; Šala, M.; Šelih, V.S.; Ruiz-Zepeda, F.; Gaberscek, M. Comparison of Pt-Cu/C with Benchmark Pt-Co/C: Metal Dissolution and Their Surface Interactions. ACS Appl. Energy Mater. 2019, 2, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Durst, J.; Chatenet, M.; Maillard, F. Impact of metal cations on the electrocatalytic properties of Pt/C nanoparticles at multiple phase interfaces. Phys. Chem. Chem. Phys. 2012, 14, 13000–13009. [Google Scholar] [CrossRef]
- Garsany, Y.; Ge, J.; St-Pierre, J.; Rocheleau, R.; Swider-Lyons, K.E. Analytical procedure for accurate comparison of rotating disk electrode results for the oxygen reduction activity of Pt/C. J. Electrochem. Soc. 2014, 161, F628–F640. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef] [PubMed]
- Sulek, M.; Adams, J.; Kaberline, S.; Ricketts, M.; Waldecker, J.R. In situ metal ion contamination and the effects on proton exchange membrane fuel cell performance. J. Power Sources 2011, 196, 8967–8972. [Google Scholar] [CrossRef]
- Aliev, A.D.; Beketaeva, L.A.; Bogdanovskaya, V.A.; Burkovskii, E.V.; Kazanskii, L.P.; Korchagin, O.V.; Ivanitskii, A.A.; Kazanskii, L.P.; Kapustin, A.V.; Korchagin, O.V.; et al. Study of degradation of membrane-electrode assemblies of hydrogen-oxygen (air) fuel cell under the conditions of life tests and voltage cycling. Russ. J. Electrochem. 2014, 50, 773–788. [Google Scholar] [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of cation exchange membranes modified by polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Cai, Y.; Kongkanand, A.; Gu, W.; Moylan, T.M. Effects of Cobalt Cation on Low Pt-loaded PEM Fuel Cell Performance. ECS Trans. 2015, 69, 1047–1061. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs. structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef]
- Stariha, S.; Macauley, N.; Brian, T.S.; Lang, D. Recent Advances in Catalyst Accelerated Stress Tests for Polymer Electrolyte Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, F492–F501. [Google Scholar] [CrossRef]

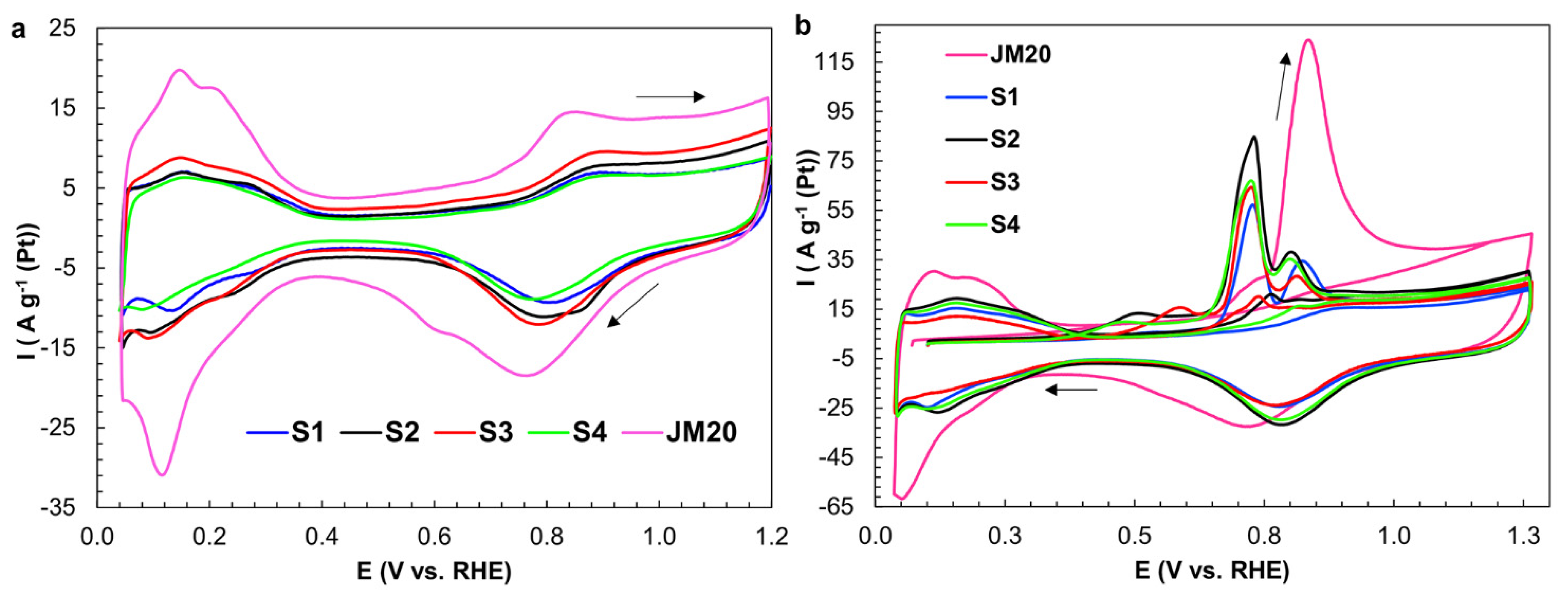
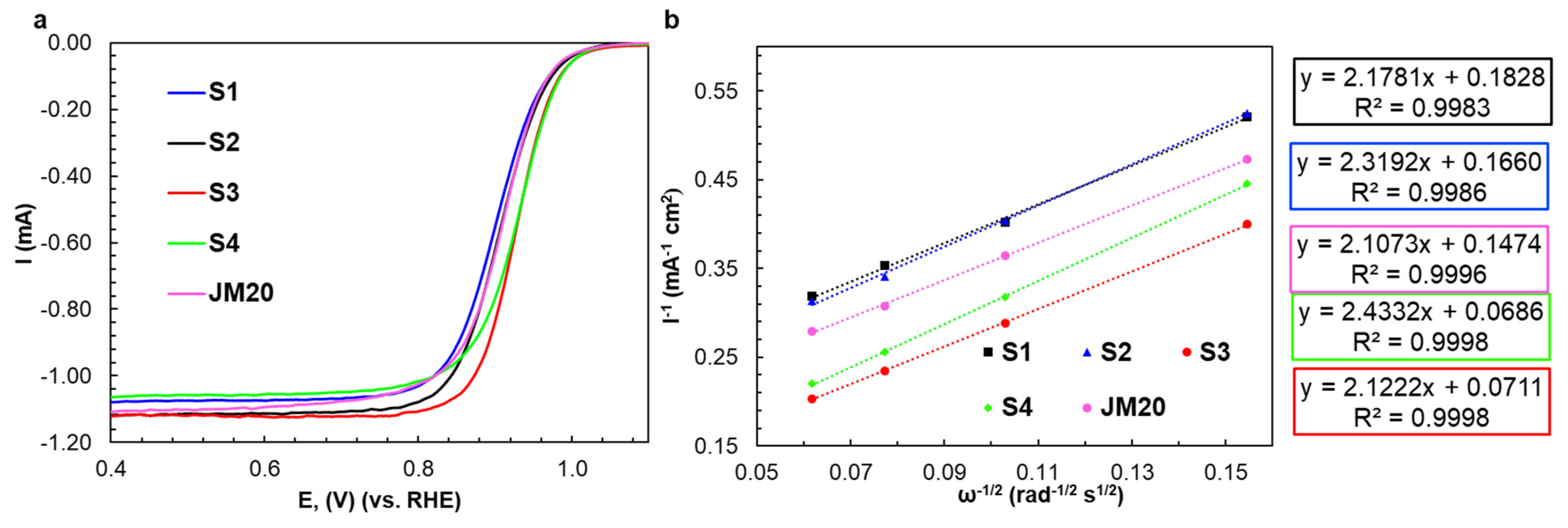
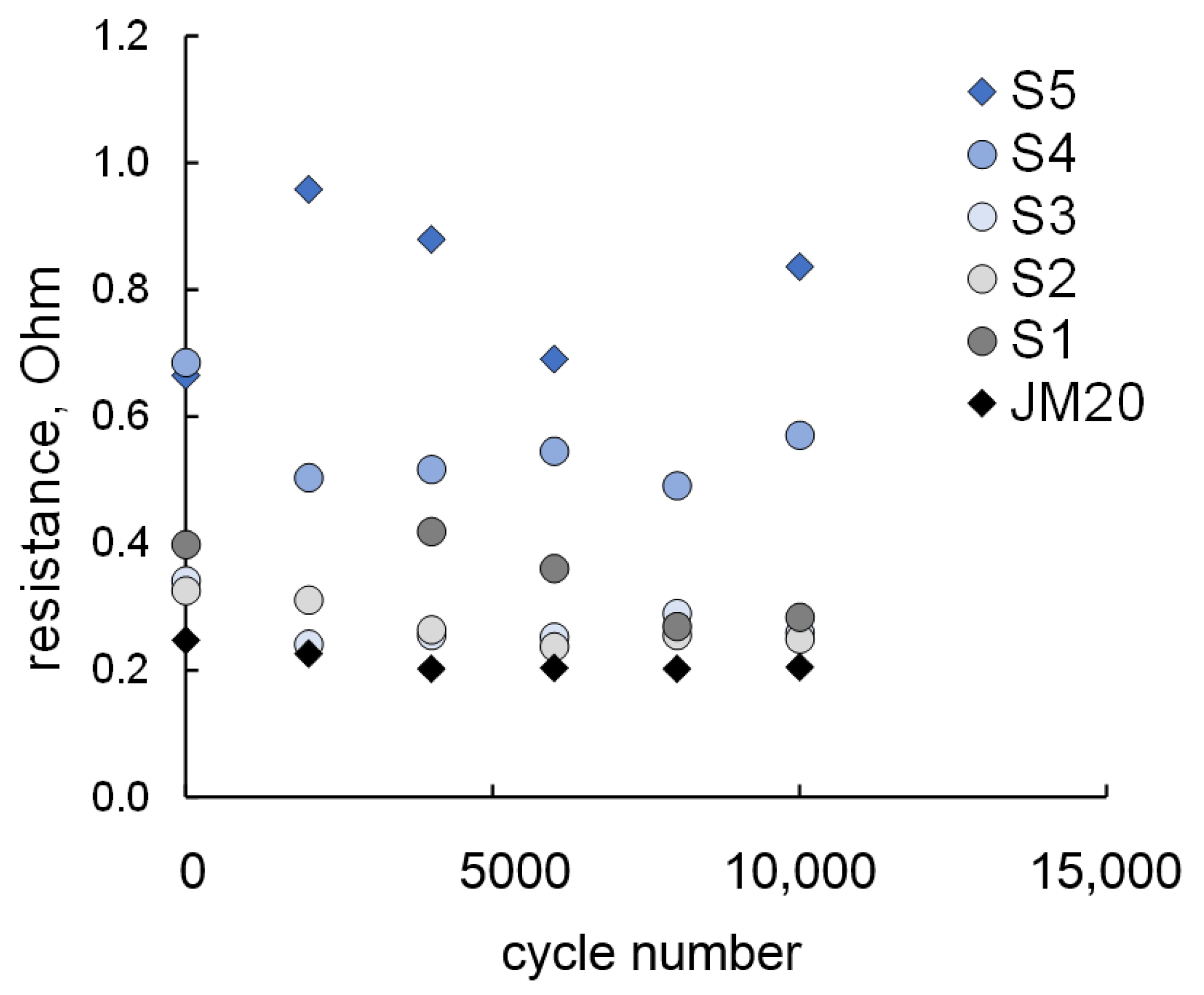
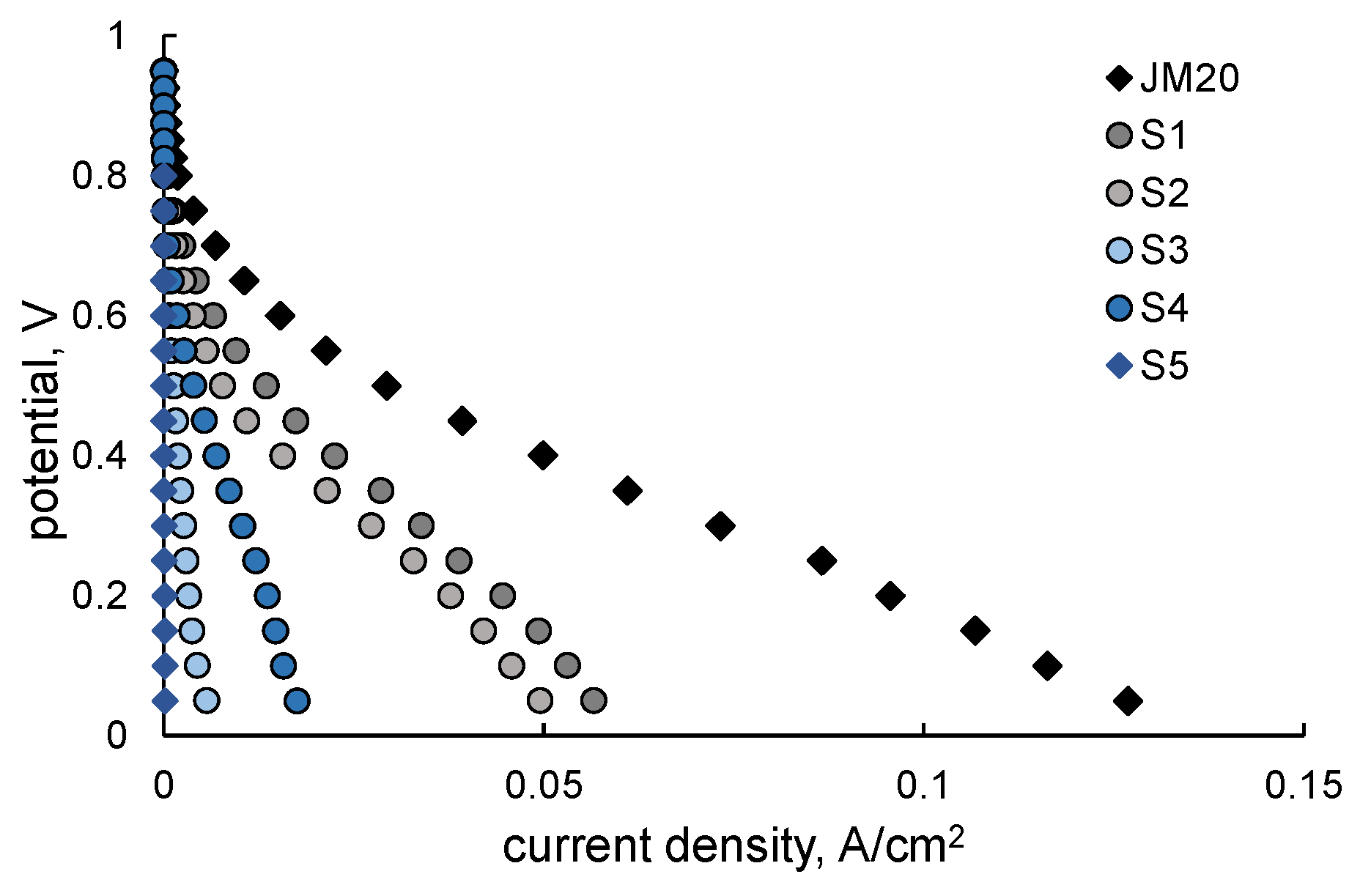
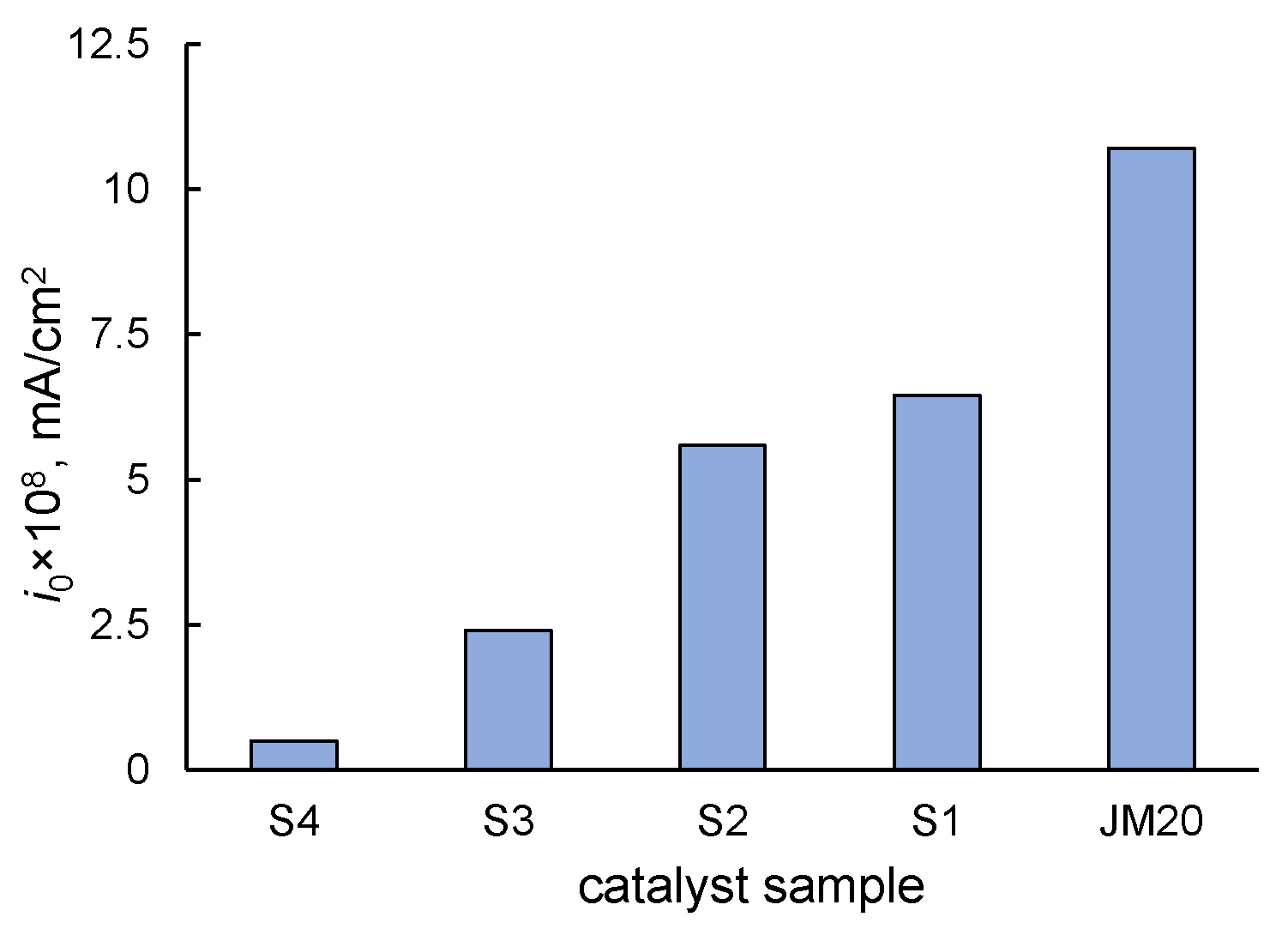

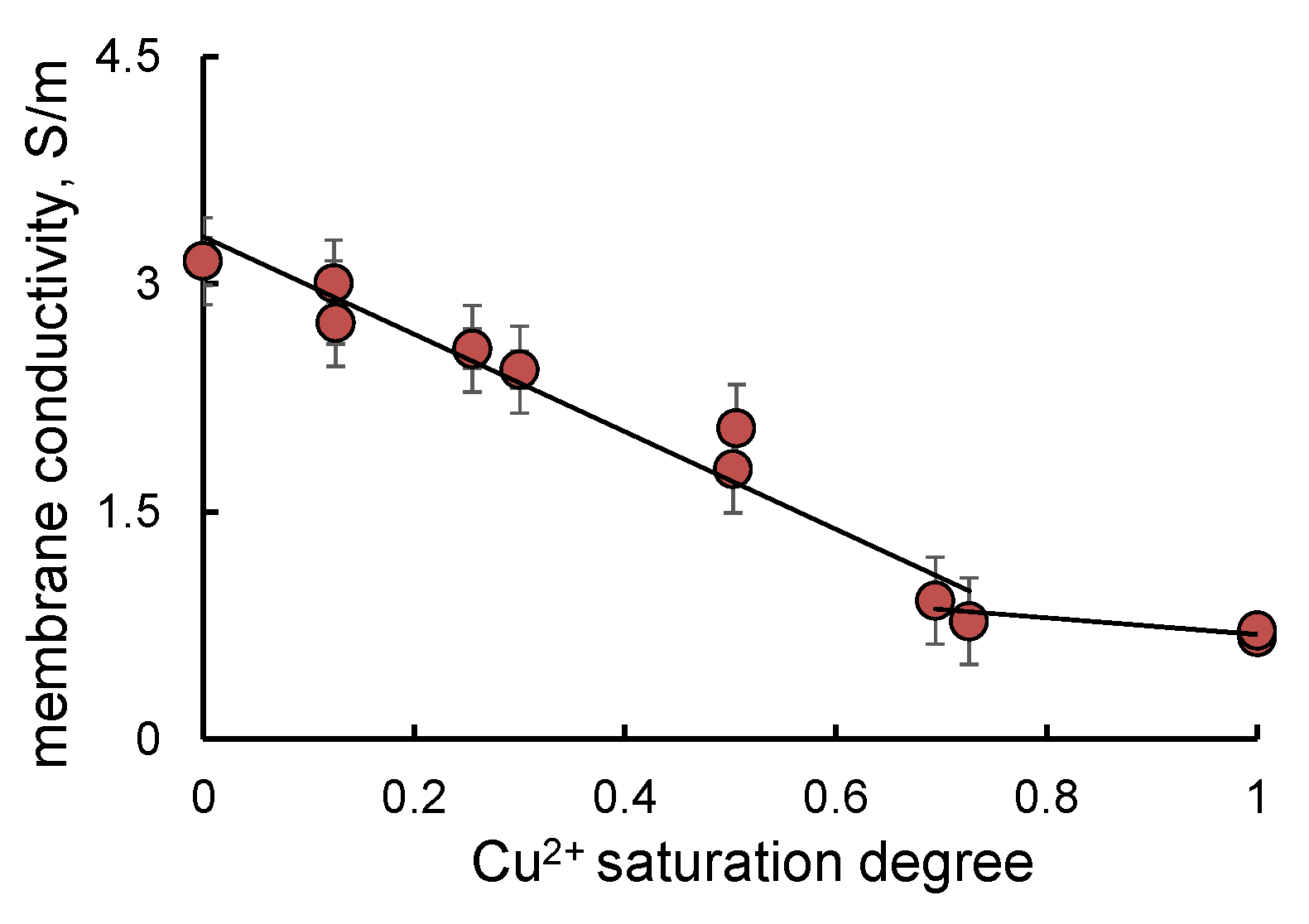
| Sample | ω(M), % | ω(Pt), % | Dav, nm (XRD) | Position of 111 Maximum, 2θ, Degrees | Atomic Ratio Pt:Cu (XRF) |
|---|---|---|---|---|---|
| S1 | 23.6 | 21.6 | 3.1 | 40.7 | PtCu0.3 |
| S2 | 21.4 | 18.8 | 2.6 | 41.0 | PtCu0.4 |
| S3 | 25.7 | 17.9 | 2.6 | 41.1 | PtCu1.3 |
| S4 | 41.0 | 25.0 | 2.3 | 41.1 | PtCu2.0 |
| S5 | 30 | - | - | - | Cu |
| Sample | Pt:Cu Atomic Ratio after Activation (XRF) | ESA, m2/g (Pt) on Hads/Hdes | ESA, m2/g (Pt) on CO | Imass, A·g−1 (Pt) | Isp, A·m−2 (Pt) | Number of ē | E1/2 |
|---|---|---|---|---|---|---|---|
| S1 | PtCu0.22 | 32 | 28 | 140 | 4.4 | 4.1 | 0.89 |
| S2 | PtCu0.32 | 33 | 33 | 169 | 5.1 | 3.9 | 0.91 |
| S3 | PtCu0.25 | 41 | 45 | 417 | 7.2 | 4.3 | 0.93 |
| S4 | PtCu0.24 | 33 | 32 | 329 | 10.0 | 3.7 | 0.94 |
| JM20 | Pt | 80 | 181 | 2.2 | 4.2 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falina, I.; Pavlets, A.; Alekseenko, A.; Titskaya, E.; Kononenko, N. Influence of PtCu/C Catalysts Composition on Electrochemical Characteristics of Polymer Electrolyte Fuel Cell and Properties of Proton Exchange Membrane. Catalysts 2021, 11, 1063. https://doi.org/10.3390/catal11091063
Falina I, Pavlets A, Alekseenko A, Titskaya E, Kononenko N. Influence of PtCu/C Catalysts Composition on Electrochemical Characteristics of Polymer Electrolyte Fuel Cell and Properties of Proton Exchange Membrane. Catalysts. 2021; 11(9):1063. https://doi.org/10.3390/catal11091063
Chicago/Turabian StyleFalina, Irina, Angelina Pavlets, Anastasia Alekseenko, Ekaterina Titskaya, and Natalia Kononenko. 2021. "Influence of PtCu/C Catalysts Composition on Electrochemical Characteristics of Polymer Electrolyte Fuel Cell and Properties of Proton Exchange Membrane" Catalysts 11, no. 9: 1063. https://doi.org/10.3390/catal11091063
APA StyleFalina, I., Pavlets, A., Alekseenko, A., Titskaya, E., & Kononenko, N. (2021). Influence of PtCu/C Catalysts Composition on Electrochemical Characteristics of Polymer Electrolyte Fuel Cell and Properties of Proton Exchange Membrane. Catalysts, 11(9), 1063. https://doi.org/10.3390/catal11091063







