Testing PtCu Nanoparticles Supported on Highly Ordered Mesoporous Carbons CMK3 and CMK8 as Catalysts for Low-Temperature Fuel Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization
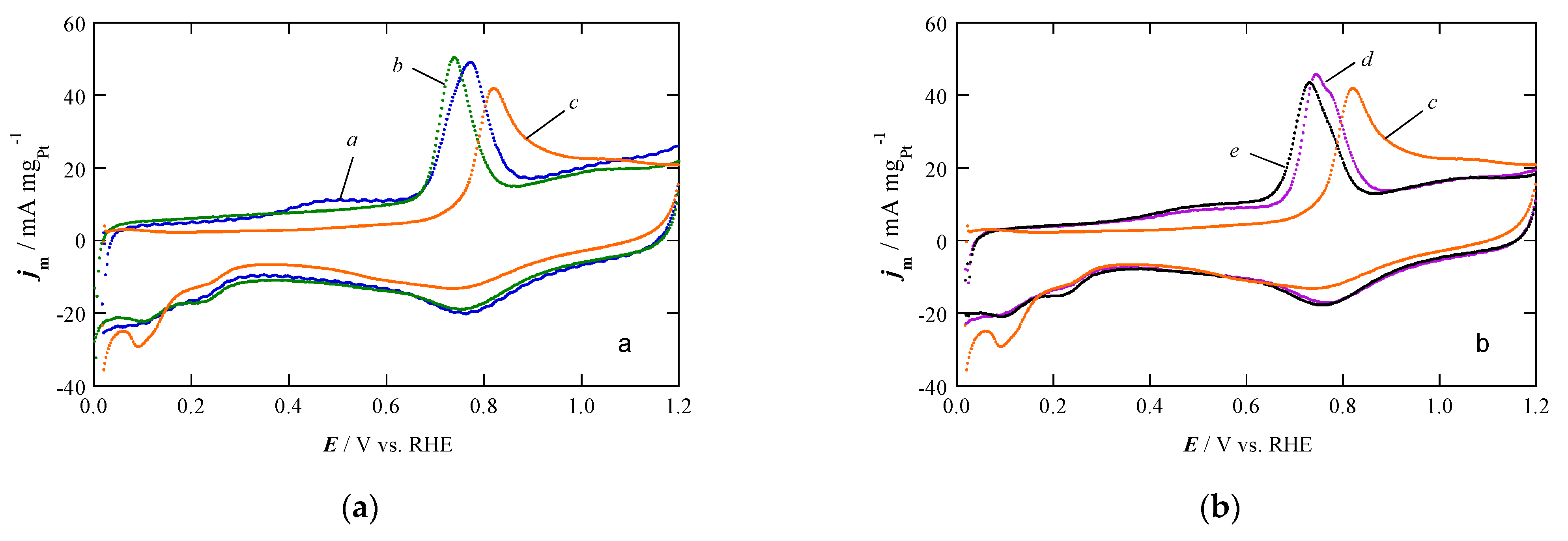
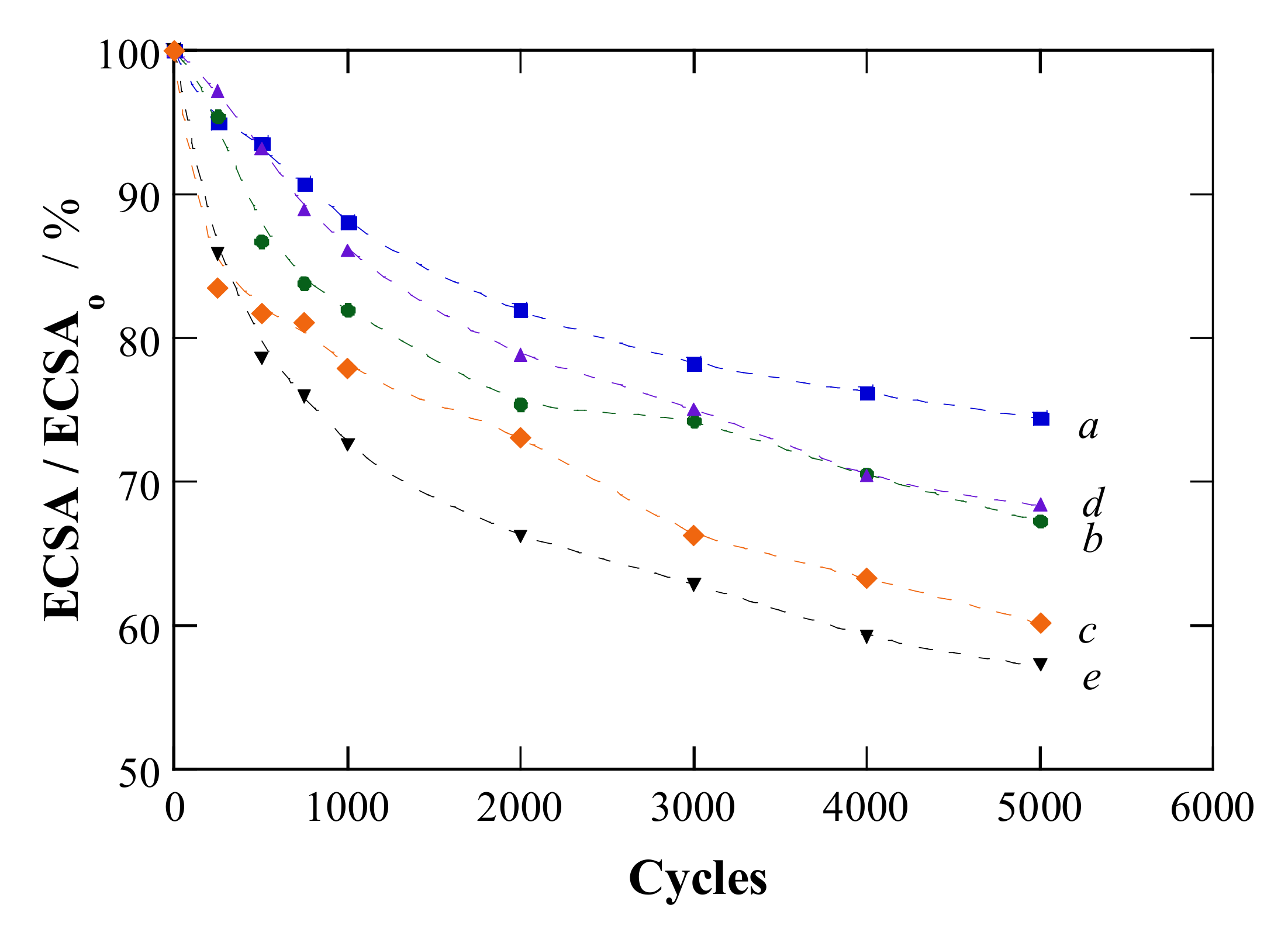
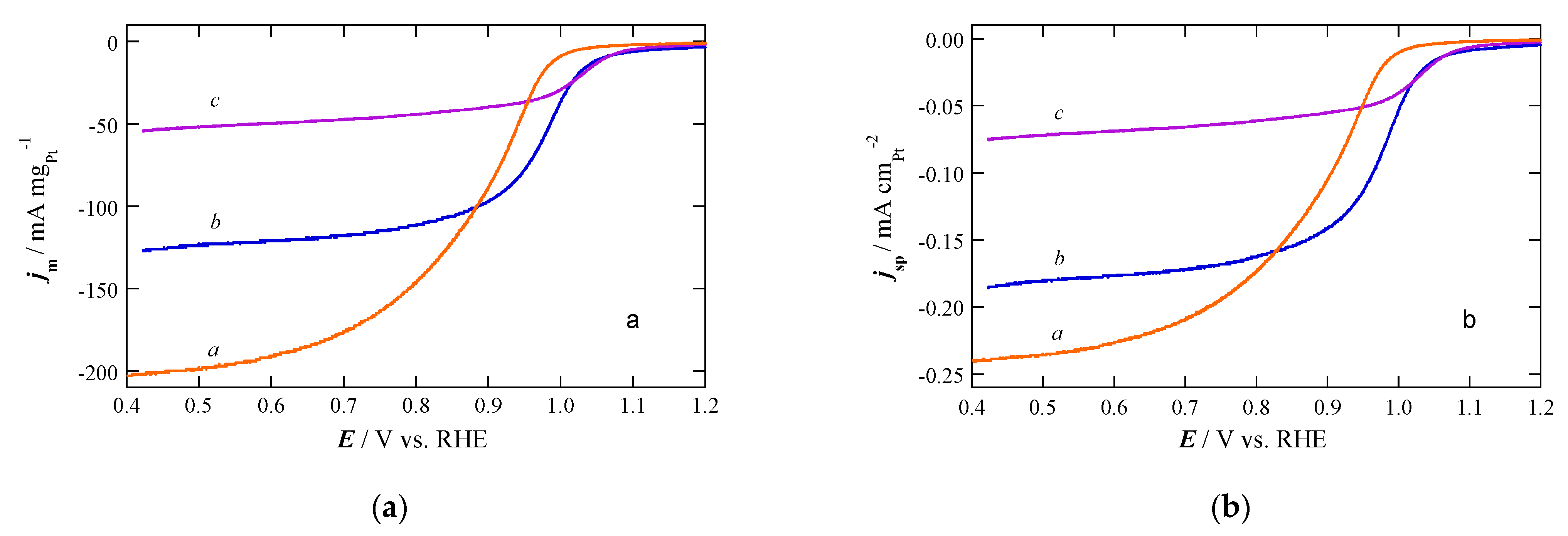
2.2. Electrochemical Characterization
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of the Catalysts
3.3. Structural Characterization
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onat, N.; Bayar, H. The sustainability indicators of power production systems. Renew. Sustain. Energy Rev. 2010, 14, 3108–3115. [Google Scholar] [CrossRef]
- Tie, S.F.; Tan, C.W. A review of energy sources and energy management system in electric vehicles. Renew. Sustain. Energy Rev. 2013, 20, 82–102. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Jouin, M.; Gouriveau, R.; Hissel, D.; Péra, M.C.; Zerhouni, N. Prognostics and health management of PEMFC-State of the art and remaining challenges. Int. J. Hydrog. Energy 2013, 38, 15307–15317. [Google Scholar] [CrossRef]
- Alves, H.J.; Bley, C., Jr.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Sulaiman, N.; Hannan, M.A.; Mohamed, A.; Majlan, E.H.; Wan Daud, W.R. A review on energy management system for fuel cell hybrid electric vehicle: Issues and challenges. Renew. Sustain. Energy Rev. 2015, 52, 802–814. [Google Scholar] [CrossRef]
- Daud, W.R.W.; Rosli, R.E.; Majlan, E.H.; Hamid, S.A.A.; Mohamed, R.; Husaini, T. PEM fuel cell system control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Alcaide, F.; Cabot, P.L.; Brillas, E. Fuel cells for chemicals and energy cogeneration. J. Power Sources 2006, 153, 47–60. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Liu, Z.; Ling, X.Y.; Su, X.; Lee, J.Y. Carbon-supported Pt and PtRu nanoparticles as catalysts for a direct methanol fuel cell. J. Phys. Chem. B 2004, 108, 8234–8240. [Google Scholar] [CrossRef]
- Ruth, K.; Vogt, M.; Zuber, R. Development of CO-tolerant catalysts. In Handbook of Fuel Cells–Fundamentals, Technology and Applications; Vielstich, W., Gasteiger, H.A., Lamm, A., Eds.; John Wiley & Sons: New York, NY, USA, 2010; Volume 3, pp. 489–496. [Google Scholar] [CrossRef]
- Velázquez-Palenzuela, A.; Brillas, E.; Arias, C.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L. Structural analysis of carbon-supported Ru-decorated Pt nanoparticles synthesized using forced deposition and catalytic performance toward CO, methanol, and ethanol electro-oxidation. J. Catal. 2013, 298, 112–121. [Google Scholar] [CrossRef]
- Acres, G.J.K.; Hards, G.A. Electrocatalysts for fuel cells. Catal. Today 1997, 38, 393–400. [Google Scholar] [CrossRef]
- Li, F.; Chan, K.Y.; Yung, H.; Yang, C.; Ting, S.W. Uniform dispersion of 1:1 PtRu nanoparticles in ordered mesoporous carbon for improved methanol oxidation. Phys. Chem. Chem. Phys. 2013, 15, 13570–13577. [Google Scholar] [CrossRef]
- Nilekar, A.U.; Alayoglu, S.; Eichhorn, B.; Mavrikakis, M. Preferential CO oxidation in hydrogen: Reactivity of core-shell nanoparticles. J. Am. Chem. Soc. 2010, 132, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Hwang, J.; Chung, J.S. Characterization and activity correlations of Pt bimetallic catalysts for low temperature fuel cells. Int. J. Hydrog. Energy 2011, 36, 4007–4014. [Google Scholar] [CrossRef]
- Ignaszak, A.; Teo, C.; Ye, S.; Gyenge, E. Pt-SnO2-Pd/C electrocatalyst with enhanced activity and durability for the oxygen reduction reaction at low Pt loading: The effect of carbon support type and activation. J. Phys. Chem. C 2010, 114, 16488–16504. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Norskov, K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, S.; Srinivasan, S.J. Enhanced electrocatalysis of oxygen reduction on platinum alloys in proton exchange membrane fuel cells. J. Electroanal. Chem. 1993, 357, 201–224. [Google Scholar] [CrossRef]
- Srivastava, R.; Mani, P.; Hahn; Strasser, P. Efficient oxygen reduction fuel cell electrocatalysis on voltammetrically dealloyed Pt–Cu–Co nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 8988–8991. [Google Scholar] [CrossRef]
- Caballero-Manrique, G.; Velázquez-Palenzuela, A.; Brillas, E.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L. Electrochemical synthesis and characterization of carbon-supported Pt and Pt-Ru nanoparticles with Cu cores for CO and methanol oxidation in polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2014, 39, 12859–12869. [Google Scholar] [CrossRef]
- Xiong, L.; Kannan, A.M.; Manthiram, A. Pt-M (M = Fe, Co, Ni and Cu) electrocatalysts synthesized by an aqueous route for proton exchange membrane fuel cells. Electrochem. Commun. 2002, 4, 898–903. [Google Scholar] [CrossRef]
- Xiong, L.; Manthiram, A. Effect of atomic ordering on the catalytic activity of carbon supported PtM (M = Fe, Co, Ni, and Cu) alloys for oxygen reduction in PEMFCs. J. Electrochem. Soc. 2005, 152, A697–A703. [Google Scholar] [CrossRef]
- Podlovchenko, B.I.; Krivchenko, V.A.; Maksimov, Y.M.; Gladysheva, T.D.; Yashina, L.V.; Evlashin, S.A.; Pilevsky, A.A. Specific features of the formation of Pt(Cu) catalysts by galvanic displacement with carbon nanowalls used as support. Electrochim. Acta 2012, 76, 137–144. [Google Scholar] [CrossRef]
- Mohl, M.; Dobo, D.; Kukovecz, A.; Konya, Z.; Kordas, K.; Wei, J.; Vajtai, R.; Ajayan, P.M. Formation of CuPd and CuPt bimetallic nanotubes by galvanic replacement reaction. J. Phys. Chem. C 2011, 115, 9403–9409. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Strasser, P. Dealloyed binary PtM3(M = Cu, Co, Ni) and ternary PtNi3M (M = Cu, Co, Fe, Cr) electrocatalysts for the oxygen reduction reaction: Performance in polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 666–673. [Google Scholar] [CrossRef]
- Ding, L.X.; Wang, A.L.; Li, G.R.; Liu, Z.Q.; Zhao, W.X.; Su, C.Y.; Tong, Y.X. Porous Pt-Ni-P composite nanotube arrays: Highly electroactive and durable catalysts for methanol electrooxidation. J. Am. Chem. Soc. 2012, 134, 5730–5733. [Google Scholar] [CrossRef]
- Oezaslan, M.; Strasser, P. Activity of dealloyed PtCo3 and PtCu3 nanoparticle electrocatalyst for oxygen reduction reaction in polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 5240–5249. [Google Scholar] [CrossRef]
- Jayasayee, K.; Van Veen, J.A.R.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.M.; de Bruijn, F.A. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl. Catal. B Environ. 2012, 111–112, 515–526. [Google Scholar] [CrossRef]
- Caballero-Manrique, G.; Nadeem, I.; Brillas, E.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L. Effects of the electrodeposition time in the synthesis of carbon-supported Pt(Cu) and Pt-Ru(Cu) core-shell electrocatalysts for polymer electrolye fuel cells. Catalysts 2016, 6, 125. [Google Scholar] [CrossRef]
- Caballero-Manrique, G.; Brillas, E.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L. Electrochemical oxidation of the carbon support to synthesize Pt(Cu) and Pt-Ru(Cu) core-shell electrocatalysts for low-temperature fuel cells. Catalysts 2015, 5, 815–837. [Google Scholar] [CrossRef]
- Geboes, B.; Mintsouli, I.; Wouters, B.; Georgieva, J.; Kakaroglou, A.; Sotiropoulos, S.; Valova, E.; Armyanov, S.; Hubin, A.; Breugelmans, T. Surface and electrochemical characterisation of a Pt-Cu/C nano-structured electrocatalyst, prepared by galvanic displacement. Appl. Catal. B Environ. 2014, 150–151, 249–256. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Armyanov, S.; Valova, E.; Avdeev, G.; Hubin, A.; Steenhaut, O.; Dille, J.; Tsiplakides, D.; Balomenou, S.; et al. Pt-Cu electrocatalysts for methanol oxidation prepared by partial galvanic replacement of Cu/carbon powder precursors. Appl. Catal. B Environ. 2013, 136–137, 160–167. [Google Scholar] [CrossRef]
- Georgieva, J.; Valova, E.; Mintsouli, I.; Sotiropoulos, S.; Armyanov, S.; Kakaroglou, A.; Hubin, A.; Steenhaut, O.; Dille, J. Carbon-supported Pt(Cu) electrocatalysts for methanol oxidation prepared by Cu electroless deposition and its galvanic replacement by Pt. J. Appl. Electrochem. 2014, 44, 215–224. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Alekseenko, A.A.; Lin, R.; Tabachkova, N.Y.; Safronenko, O.I. Activity and stability of Pt/C and Pt-Cu/C electrocatalysts. Electrocatalysis 2018, 9, 550–562. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Srabionyan, V.V.; Kurzin, A.A.; Bulat, N.V.; Shemet, D.B.; Avakyan, L.A.; Belenov, S.V.; Volochaev, V.A.; Zizak, I.; Guterman, V.E.; et al. Bimetallic PtCu core-shell nanoparticles in PtCu/C electrocatalysts: Structural and electrochemical characterization. Appl. Catal. A Gen. 2016, 525, 226–236. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Belenov, S.V.; Menshikov, V.S.; Guterman, V.E. Pt(Cu)/C electrocatalysts with low platinum content. Russ. J. Electrochem. 2018, 54, 415–425. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Belenov, S.V.; Menshikov, V.S.; Tabachkova, N.Y.; Safronenko, O.I.; Moguchikh, E.A. Pt/C electrocatalysts based on the nanoparticles with the gradient structure. Int. J. Hydrog. Energy 2018, 43, 3676–3687. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Carrera-Cerritos, R.; Sebastián, D.; Ledesma-García, J.; Arriaga, L.G.; Aricò, A.S.; Baglio, V. PtCu catalyst for the electro-oxidation of ethanol in an alkaline direct alcohol fuel cell. Int. J. Hydrog. Energy 2017, 42, 27919–27928. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Bahrami, M.; Fard, Z.S.; Fard, S.F.H.; Roushani, M.; Agahi, B.H.; Fath, R.H.; Sarmoor, S.S. Designing of some platinum or palladium-based nanoalloys as effective electrocatalysts for methanol oxidation reaction. Int. J. Hydrog. Energy 2018, 43, 15095–15111. [Google Scholar] [CrossRef]
- Sarkar, A.; Manthiram, A. Synthesis of Pt@Cu Core-shell nanoparticles by galvanic displacement of Cu by Pt4+ ions and their application as electrocatalysts for oxygen reduction reaction in fuel cells. J. Phys. Chem. C 2010, 114, 4725–4732. [Google Scholar] [CrossRef]
- Coleman, E.J.; Chowdhury, M.H.; Co, A.C. Insights into the oxygen reduction reaction activity of Pt/C and PtCu/C catalysts. ACS Catal. 2015, 5, 1245–1253. [Google Scholar] [CrossRef]
- Garcia-Cardona, J.; Sirés, I.; Alcaide, F.; Brillas, E.; Centellas, F.; Cabot, P.L. Electrochemical performance of carbon-supported Pt(Cu) electrocatalysts for low-temperature fuel cells. Int. J. Hydrog. Energy 2020, 45, 20582–20593. [Google Scholar] [CrossRef]
- Menshchikov, V.; Alekseenko, A.; Guterman, V.E.; Nechitailov, A.; Glebova, N.; Tomasov, A.; Spiridonova, O.; Belenov, S.; Zelenina, N.; Safronenko, O. Effective platinum-copper catalysts for methanol oxidation and oxygen reduction in proton-exchange membrane fuel cell. Nanomaterials 2020, 10, 742. [Google Scholar] [CrossRef]
- Samad, S.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Sunarso, J.; Chong, S.T.; Daud, V.R.W. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrog. Energy 2018, 43, 7823–7854. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts-A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Elangovan, A.; Xu, J.; Brown, E.; Liu, B.; Li, J. Fundamental electrochemical insights of vertically aligned carbon nanofiber architecture as a catalyst support for ORR. J. Electrochem. Soc. 2020, 167, 066523. [Google Scholar] [CrossRef]
- Majlan, E.H.; Rohendi, D.; Daud, W.R.W.; Husaini, T.; Haque, M.A. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 89, 117–134. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, J.; Wang, Y.; Lin, Y. Novel catalyst support materials for PEM fuel cells: Current status and future prospects. J. Mater. Chem. 2009, 19, 46–59. [Google Scholar] [CrossRef]
- Dicks, A.L. The role of carbon in fuel cells. J. Power Sources 2006, 156, 128–141. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC. Part I. Physico-chemical and electronic interaction between Pt and carbon support, and activity enhancement of Pt/C catalyst. J. Power Sources 2007, 172, 133–144. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC. Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Roen, L.M.; Paik, C.H.; Jarvi, T.D. Electrocatalytic corrosion of carbon support in PEMFC cathodes. Electrochem. Solid State Lett. 2004, 7, 8–12. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell. J. Power Sources 2007, 171, 558–566. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.Z.; Hin, J.N.C.; Wang, H.; Friedrich, K.A.; Schulze, M. A review of platinum-based catalyst layer degradation in proton exchange membrane fuel cells. J. Power Sources 2009, 194, 588–600. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Seo, M.H.; Choi, S.M.; Kim, H.J.; Kim, W.B. The graphene-supported Pd and Pt catalysts for highly active oxygen reduction reaction in an alkaline condition. Electrochem. Commun. 2011, 13, 182–185. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, S. Recent progress in graphene-based nanomaterials as advanced electrocatalysts towards oxygen reduction reaction. Nanoscale 2013, 5, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Reddy, A.L.M.; Shaijumon, M.M.; Rajalakshmi, N.; Ramaprabhu, S. Pt-Ru/multi-walled carbon nanotubes as electrocatalysts for direct methanol fuel cell. Int. J. Hydrog. Energy 2008, 33, 427–433. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y.; Shi, P. Durability study of PtC and PtCNTs catalysts under simulated PEM fuel cell conditions. J. Electrochem. Soc. 2006, 153, 1093–1097. [Google Scholar] [CrossRef]
- Devrim, Y.; Arica, E.D. Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell. Int. J. Hydrog. Energy 2019, 44, 18951–18966. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Tammeveski, K.T.; López-Cudero, A.; Solla-Gullón, J.; Feliu, J.M. Electroreduction of oxygen on Pt nanoparticle/carbon nanotube nanocomposites in acid and alkaline solutions. Electrochim. Acta 2010, 55, 794–803. [Google Scholar] [CrossRef]
- Álvarez, G.; Alcaide, F.; Miguel, O.; Cabot, P.L.; Martínez-Huerta, M.V.; Fierro, J.L.G. Electrochemical stability of carbon nanofibers in proton exchange membrane fuel cells. Electrochim. Acta 2011, 56, 9370–9377. [Google Scholar] [CrossRef]
- Sebastián, D.; Ruíz, A.G.; Suelves, I.; Moliner, R.; Lázaro, M.J.; Baglio, V.; Stassi, A.; Aricò, A.S. Enhanced oxygen reduction activity and durability of Pt catalysts supported on carbon nanofibers. Appl. Catal. B Environ. 2012, 115–116, 269–275. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, B.I.; Kim, J. Durability test with fuel starvation using a Pt/CNF catalyst in PEMFC. Nanoscale Res. Lett. 2012, 7, 21–25. [Google Scholar] [CrossRef]
- Zaragoza-Martín, F.; Sopeña-Escario, D.; Morallón, E.; de Lecea, C.S.M. Pt/carbon nanofibers electrocatalysts for fuel cells. Effect of the support oxidizing treatment. J. Power Sources 2007, 171, 302–309. [Google Scholar] [CrossRef]
- Guo, J.; Sun, G.; Wang, Q.; Wang, G.; Zhou, Z.; Tang, S.; Jiang, L.; Zhou, B.; Xin, Q. Carbon nanofibers supported Pt-Ru electrocatalysts for direct methanol fuel cells. Carbon 2006, 44, 152–157. [Google Scholar] [CrossRef]
- El-Deeb, H.; Bron, M. Microwave-assisted polyol synthesis of PtCu/carbon nanotube catalysts for electrocatalytic oxygen reduction. J. Power Sources 2015, 275, 893–900. [Google Scholar] [CrossRef]
- El-Deeb, H.; Bron, M. Electrochemical dealloying of PtCu/CNT electrocatalysts synthesized by NaBH4-assisted polyol-reduction: Influence of preparation parameters on oxygen reduction activity. Electrochim. Acta 2015, 164, 315–322. [Google Scholar] [CrossRef]
- Xia, K.; Gao, Q.; Wu, C.; Song, S.; Ruan, M. Activation, characterization and hydrogen storage properties of the mesoporous carbon CMK-3. Carbon 2007, 45, 1989–1996. [Google Scholar] [CrossRef]
- Ma, T.Y.; Liu, L.; Yuan, Z.Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Ambrosio, E.P.; Dumitrescu, M.A.; Francia, C.; Gerbaldi, C.; Spinelli, P. Ordered mesoporous carbons as catalyst support for PEM fuel cells. Fuel Cells 2009, 9, 197–200. [Google Scholar] [CrossRef]
- Phan, T.N.; Gong, M.K.; Thangavel, R.; Lee, Y.S.; Ko, C.H. Enhanced electrochemical performance for EDLC using ordered mesoporous carbons (CMK-3 and CMK-8): Role of mesopores and mesopore structures. J. Alloy Compd. 2019, 780, 90–97. [Google Scholar] [CrossRef]
- Güneş, S.; Güldür, F.Ç. Synthesis of OMC supported Pt catalysts and the effect of the metal loading technique on their PEM fuel cell performances. Chem. Eng. Commun. 2020, 207, 961–971. [Google Scholar] [CrossRef]
- Álvarez, G.; Alcaide, F.; Miguel, O.; Calvillo, L.; Lázaro, M.J.; Quintana, J.J.; Calderón, J.C.; Pastor, E. Technical electrodes catalyzed with PtRu on mesoporous ordered carbons for liquid direct methanol fuel cells. J. Solid State Electrochem. 2010, 14, 1027–1034. [Google Scholar] [CrossRef]
- Calvillo, L.; Gangeri, M.; Perathoner, S.; Centi, G.; Moliner, R.; Lázaro, M.J. Synthesis and performance of platinum supported on ordered mesoporous carbons as catalyst for PEM fuel cells: Effect of the surface chemistry of the support. Int. J. Hydrog. Energy 2011, 36, 9805–9814. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Alcaide, F.; Álvarez, G.; Calvillo, L.; Lázaro, M.J.; Pastor, E. Pt-Ru electrocatalysts supported on ordered mesoporous carbon for direct methanol fuel cell. J. Power Sources 2010, 195, 4022–4029. [Google Scholar] [CrossRef]
- Gupta, G.; Slanac, D.A.; Kumar, P.; Wiggins-Camacho, J.D.; Wang, X.; Swinnea, S.; More, K.L.; Dai, S.; Stevenson, K.J.; Johnston, K.P. Highly stable and active Pt-Cu oxygen reduction electrocatalysts based on mesoporous graphitic carbon supports. Chem. Mater. 2009, 21, 4515–4526. [Google Scholar] [CrossRef]
- Calvillo, L.; Lázaro, M.J.; García-Bordejé, E.; Moliner, R.; Cabot, P.L.; Esparbé, I.; Pastor, E.; Quintana, J.J. Platinum supported on functionalized ordered mesoporous carbon as electrocatalyst for direct methanol fuel cells. J. Power Sources 2007, 169, 59–64. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Yang, L.; Wang, F.; Yin, J. Unprotected Pt nanoclusters anchored on ordered mesoporous carbon as an efficient and stable catalyst for oxygen reduction reaction. Electrochim. Acta 2019, 297, 539–544. [Google Scholar] [CrossRef]
- Brandiele, R.; Durante, C.; Zerbetto, M.; Vicentini, N.; Kosmala, T.; Badocco, D.; Pastore, P.; Rizzi, G.A.; Isse, A.A.; Gennaro, A. Probing the correlation between Pt-support interaction and oxygen reduction reaction activity in mesoporous carbon materials modified with Pt-N active sites. Electrochim. Acta 2018, 277, 287–300. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Perazzolo, V.; Agnoli, S.; Schneider, O.; Granozzi, G.; Gennaro, A. Metal-support interaction in platinum and palladium nanoparticles loaded on nitrogen-doped mesoporous carbon for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.acsmaterial.com/materials/carbon-series.html (accessed on 22 February 2021).
- Powder Diffraction File, International Centre for Diffraction Data (ICDD), 12 Campus Boulevard Newton Square, Pennsylvania, 19073-3273, USA. 2018. Available online: http://www.icdd.com (accessed on 22 February 2021).
- Shen, Y.; Zhang, Z.; Xiao, K.; Xi, J. Synthesis of Pt, PtRh, and PtRhNi alloys supported by pristine graphene nanosheets for ethanol electrooxidation. ChemCatChem 2014, 6, 3254–3261. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Belenov, S.V.; Shemet, D.B.; Srabionyan, V.V.; Avakyan, L.A.; Volochaev, V.A.; Mikheykin, A.S.; Bdoyan, K.E.; Zizak, I.; Guterman, V.E.; et al. Effect of thermal treatment on the atomic structure and electrochemical characteristics of bimetallic PtCu core-shell nanoparticles in PtCu/C electrocatalysts. J. Phys. Chem. C 2018, 122, 17199–17210. [Google Scholar] [CrossRef]
- Calvillo, L.; Celorrio, V.; Moliner, R.; Cabot, P.L.; Esparbé, I.; Lázaro, M.J. Control of textural properties of ordered mesoporous materials. Micropor. Mesopor. Mater. 2008, 116, 292–298. [Google Scholar] [CrossRef]
- Available online: http://database.iem.ac.ru/mincryst/ (accessed on 22 February 2021).
- Du, X.; Luo, S.; Du, H.; Tang, M.; Huang, X.; Shen, P.K. Monodisperse and self-assembled Pt-Cu nanoparticles as an efficient electrocatalyst for the methanol oxidation reaction. J. Mater. Chem. A 2016, 4, 1579–1586. [Google Scholar] [CrossRef]
- Alcaide, F.; Álvarez, G.; Cabot, P.L.; Genova-Koleva, R.V.; Grande, H.J.; Martínez-Huerta, M.V.; Miguel, O. Supporting PtRh alloy nanoparticle catalysts by electrodeposition on carbon paper for the ethanol electrooxidation in acidic medium. J. Electroanal. Chem. 2020, 861, 113960. [Google Scholar] [CrossRef]
- Long, G.; Li, X.; Wan, K.; Liang, A.; Piao, J.; Tsiakaras, P. Pt/CN-doped electrocatalysts: Superior electrocatalytic activity for methanol oxidation reaction and mechanistic insight into interfacial enhancement. Appl. Catal. B Environ. 2017, 203, 541–548. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hamnett, A.; Kennedy, B.J.; Weeks, S.A. XPS investigation of platinized carbon electrodes for the direct methanol air fuel cell. Electrochim. Acta 1987, 32, 1233–1238. [Google Scholar] [CrossRef]
- Rigsby, M.A.; Zhou, W.P.; Lewera, A.; Duong, H.T.; Bagus, P.S.; Jaegermann, W.; Hunger, R.; Wieckowski, A. Experiment and theory of fuel cell catalysis: Methanol and formic acid decomposition on nanoparticle Pt/Ru. J. Phys. Chem. C 2008, 112, 15595–15601. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; López-Cudero, A.; Solla-Gullón, J.; Sepúlveda-Escribano, A.; Aldaz, A. Hydrogenation of α, β unsaturated aldehydes over polycrystalline, (111) and (100) preferentially oriented Pt nanoparticles supported on carbon. J. Catal. 2008, 253, 159–166. [Google Scholar] [CrossRef]
- Ioroi, T.; Fujiwara, N.; Siroma, Z.; Yasuda, K.; Miyazaki, Y. Platinum and molybdenum oxide deposited carbon electrocatalyst for oxidation of hydrogen containing carbon monoxide. Electrochem. Commun. 2002, 4, 442–446. [Google Scholar] [CrossRef]
- Enterría, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Shanahan, P.V.; Xu, L.; Liang, C.; Waje, M.; Dai, S.; Yan, Y.S. Graphitic mesoporous carbon as a durable fuel cell catalyst support. J. Power Sources 2008, 185, 423–427. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef]
- Corona, B.; Howard, M.; Zhang, L.; Henkelman, G. Computational screening of core@shell nanoparticles for the hydrogen evolution and oxygen reduction reactions. J. Chem. Phys. 2016, 145, 244708. [Google Scholar] [CrossRef] [PubMed]
- Beermann, V.; Gocyla, M.; Willinger, E.; Rudi, S.; Heggen, M.; Dunin-Borkowski, R.E.; Willinger, M.G.; Strasser, P. Rh-doped Pt-Ni octahedral nanoparticles: Understanding the correlation between elemental distribution, oxygen reduction reaction, and shape stability. Nano Lett. 2016, 16, 1719–1725. [Google Scholar] [CrossRef]
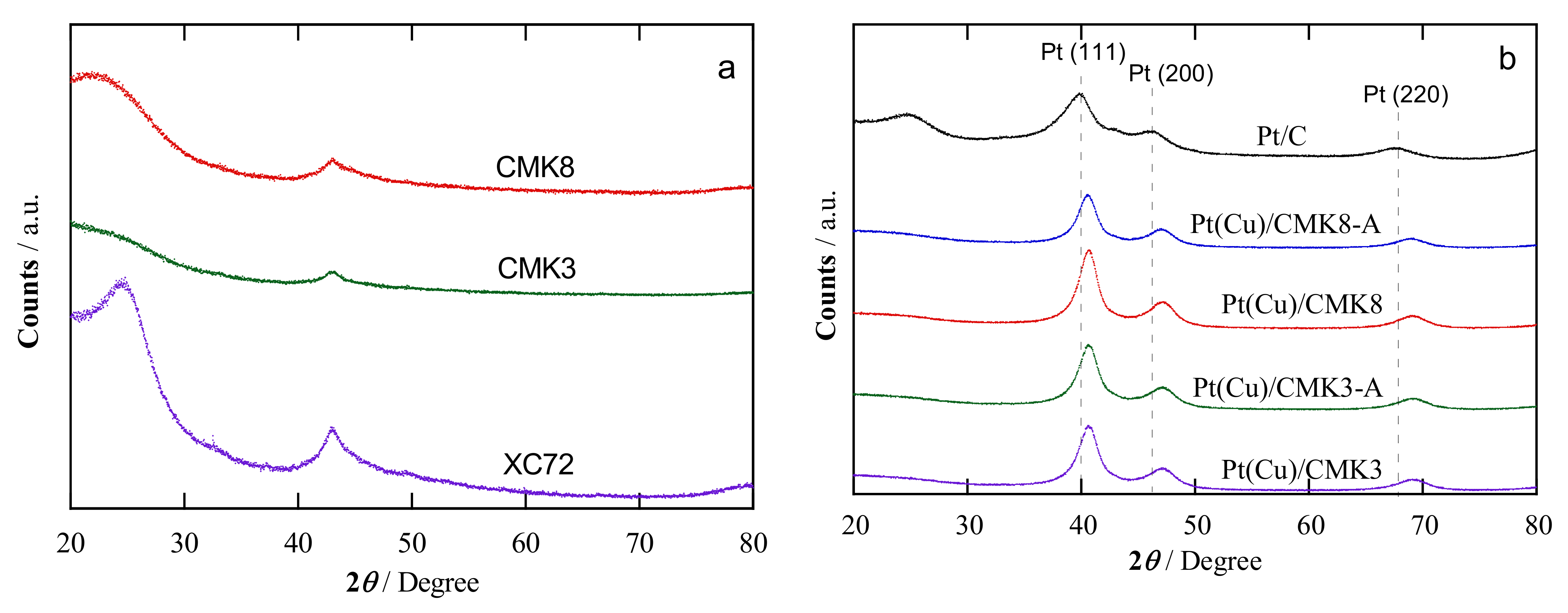
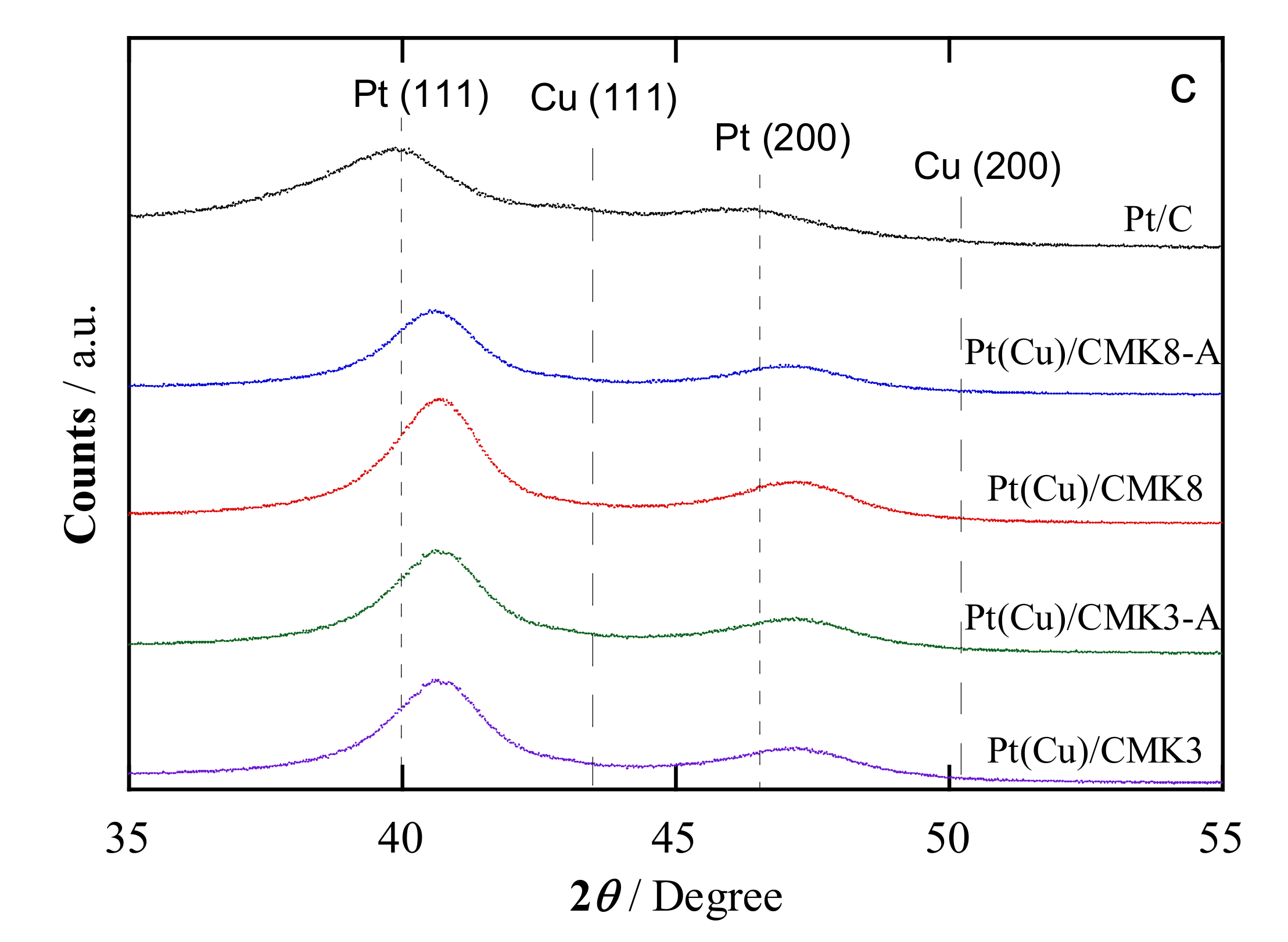
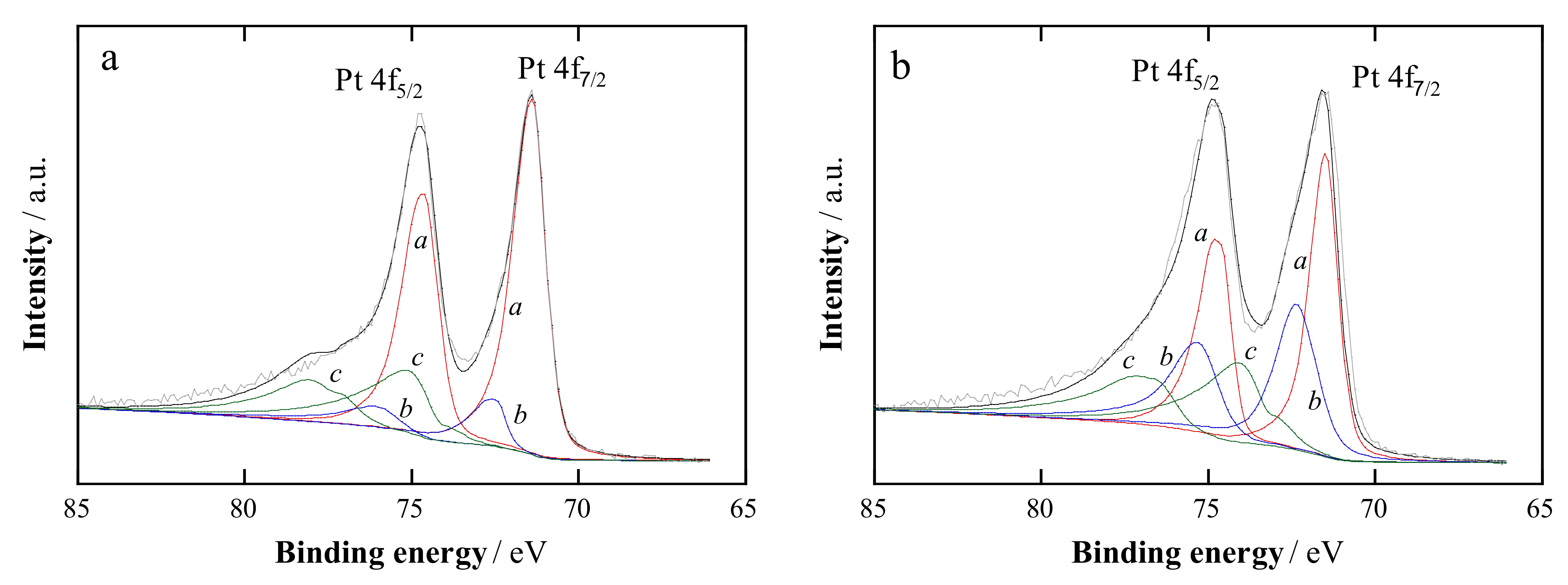
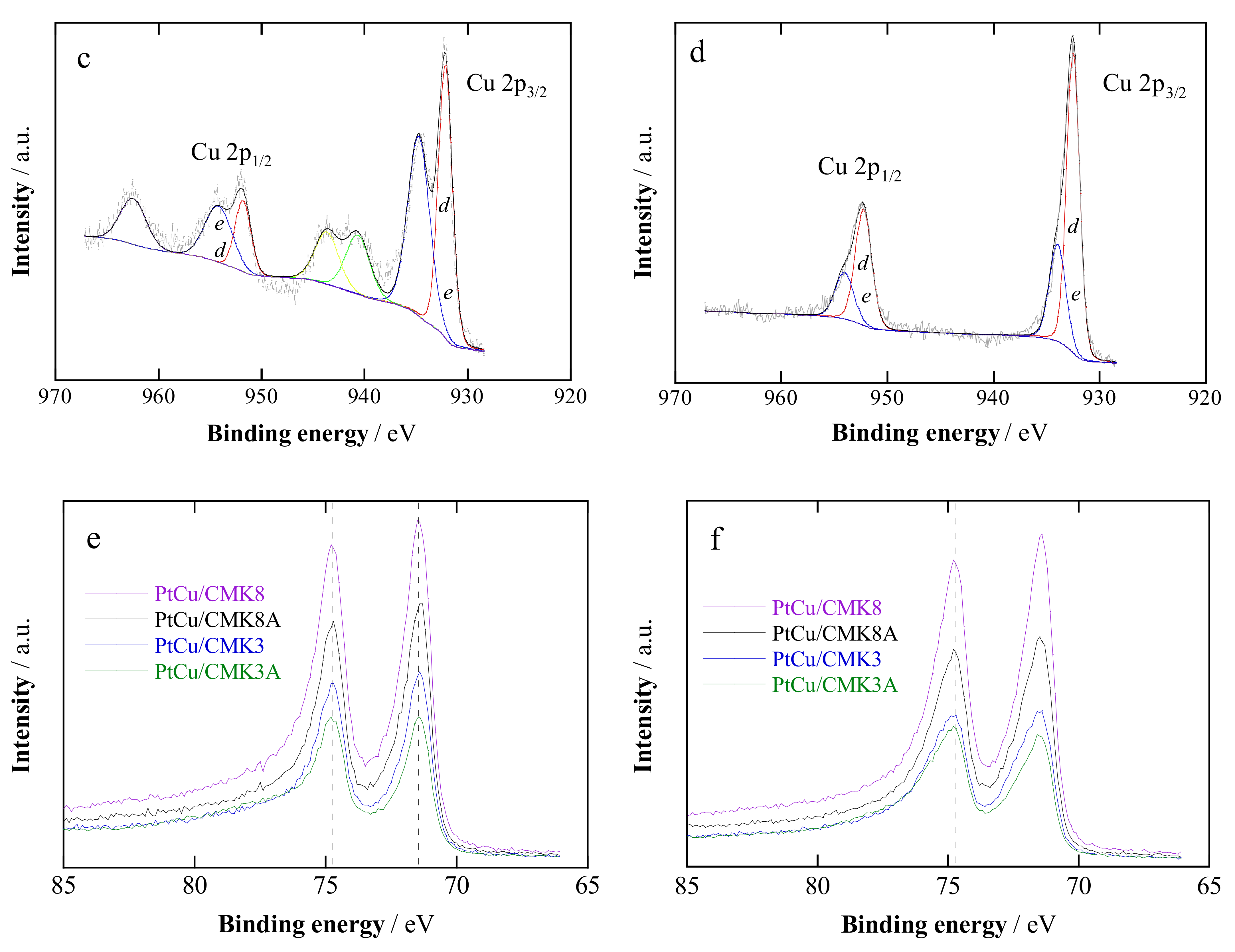

| Catalyst | Crystallite Size a/nm | Pt:Cu Ratio b/at.% | Metal Content c/wt.% | Pt:Cu Ratio d/at.% | Particle Size e/nm | |
|---|---|---|---|---|---|---|
| Pt | Cu | |||||
| Pt(Cu)/CMK3 | 4.4 | 83:17 | 26 | 9 | 48:52 | 4.8 |
| Pt(Cu)/CMK3-A | 4.0 | 72:28 | 19 | 14 | 30:70 | 4.8 |
| Pt(Cu)/CMK8 | 5.1 | 80:20 | 32 | 6 | 64:36 | 5.1 |
| Pt(Cu)/CMK8-A | 4.5 | 83:17 | 38 | 6 | 68:32 | 4.9 |
| Pt/C | 2.6 | 100:0 | 19 | 0 | 100:0 | 2.5 |
| Species | Ar+ | Pt(Cu) /CMK3 | Pt(Cu) /CMK3-A | Pt(Cu) /CMK8 | Pt(Cu) /CMK8-A |
|---|---|---|---|---|---|
| Pt:Cu:C | N | 5:1:94 | 4:3:93 | 14:2:84 | 9:1:90 |
| Pt:Cu:C | Y | 6:3:91 | 6:6:88 | 17:3:80 | 13:2:85 |
| Pt:Cu | N | 77:23 | 58:42 | 86:14 | 90:10 |
| Pt:Cu | Y | 70:30 | 50:50 | 83:17 | 83:17 |
| Pt(0):Pt(II):Pt(IV) a | N | 66:10:24 | 60:3:37 | 43:45:12 | 68:10:22 |
| Pt(0):Pt(II):Pt(IV) a | Y | 43:32:25 | 46:30:24 | 74:10:16 | 63:13:24 |
| Cu(0):Cu(II) b | N | 48:52 | 44:56 | 52:64 | 38:62 |
| Cu(0):Cu(II) b | Y | 71:29 | 73:27 | 78:22 | 70:30 |
| Pt(0):Cu(0) c | N | 82:18 | 65:35 | 84:16 | 94:6 |
| Pt(0):Cu(0) c | Y | 64:36 | 40:60 | 83:17 | 81:19 |
| Catalyst | Pt Loading /µg cm−2 | ECSAH-des /m2 gPt−1 | ECSACO-des /m2 gPt−1 | ECO /V |
|---|---|---|---|---|
| Pt(Cu)/CMK3 | 26.5 | 68.5 | 68.7 | 0.65 |
| Pt(Cu)/CMK3-A | 19.4 | 56.3 | 57.7 | 0.65 |
| Pt(Cu)/CMK8 | 19.6 | 72.8 | 73.2 | 0.66 |
| Pt(Cu)/CMK8-A | 23.3 | 44.6 | 45.2 | 0.64 |
| Pt/C (commercial) | 20.4 | 84.3 | 85.2 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Cardona, J.; Alcaide, F.; Brillas, E.; Sirés, I.; Cabot, P.L. Testing PtCu Nanoparticles Supported on Highly Ordered Mesoporous Carbons CMK3 and CMK8 as Catalysts for Low-Temperature Fuel Cells. Catalysts 2021, 11, 724. https://doi.org/10.3390/catal11060724
Garcia-Cardona J, Alcaide F, Brillas E, Sirés I, Cabot PL. Testing PtCu Nanoparticles Supported on Highly Ordered Mesoporous Carbons CMK3 and CMK8 as Catalysts for Low-Temperature Fuel Cells. Catalysts. 2021; 11(6):724. https://doi.org/10.3390/catal11060724
Chicago/Turabian StyleGarcia-Cardona, Julia, Francisco Alcaide, Enric Brillas, Ignasi Sirés, and Pere L. Cabot. 2021. "Testing PtCu Nanoparticles Supported on Highly Ordered Mesoporous Carbons CMK3 and CMK8 as Catalysts for Low-Temperature Fuel Cells" Catalysts 11, no. 6: 724. https://doi.org/10.3390/catal11060724
APA StyleGarcia-Cardona, J., Alcaide, F., Brillas, E., Sirés, I., & Cabot, P. L. (2021). Testing PtCu Nanoparticles Supported on Highly Ordered Mesoporous Carbons CMK3 and CMK8 as Catalysts for Low-Temperature Fuel Cells. Catalysts, 11(6), 724. https://doi.org/10.3390/catal11060724









