Synthesis, Characterization and Photoelectric Properties of Fe2O3 Incorporated TiO2 Photocatalyst Nanocomposites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis
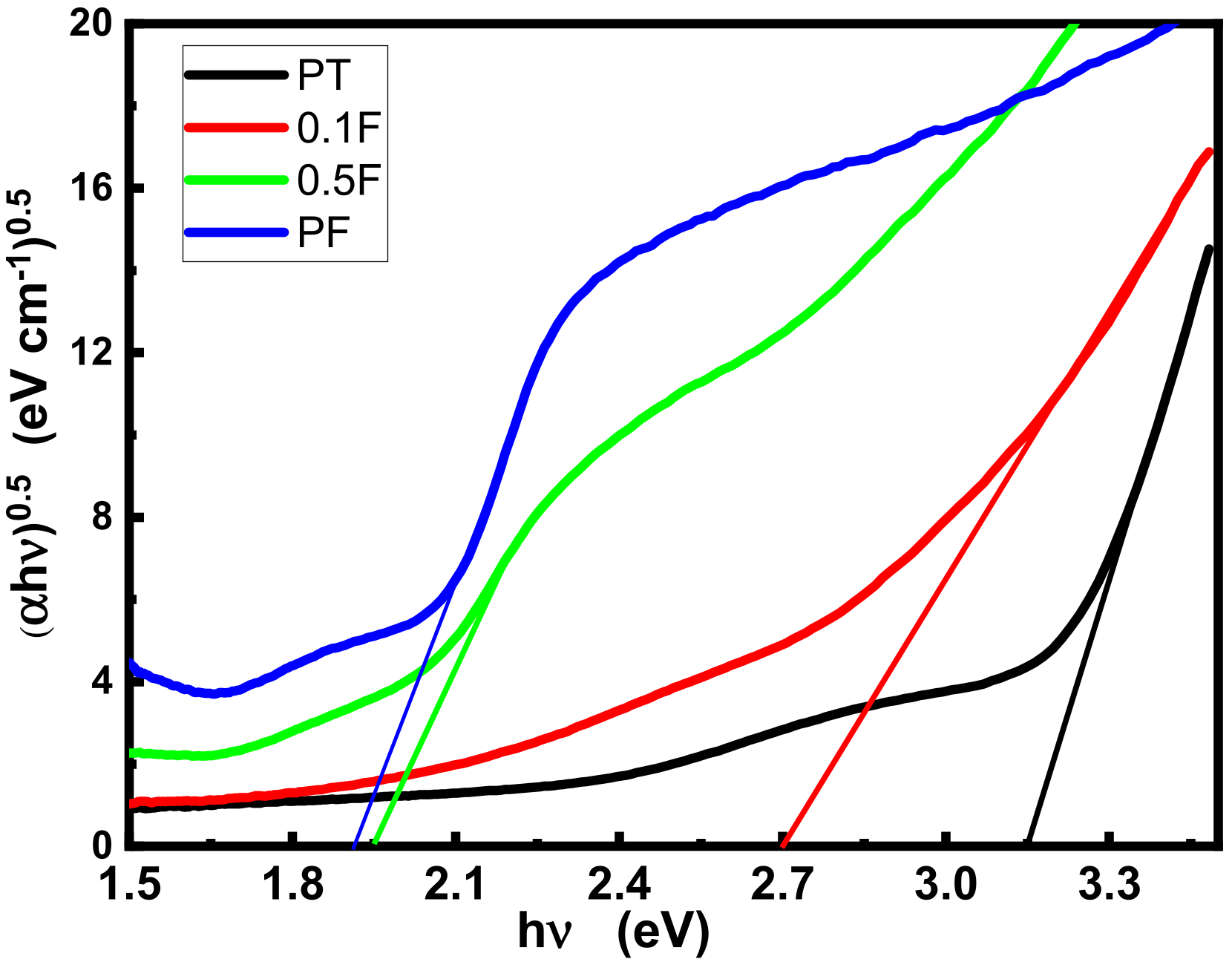
2.2. Optical Properties
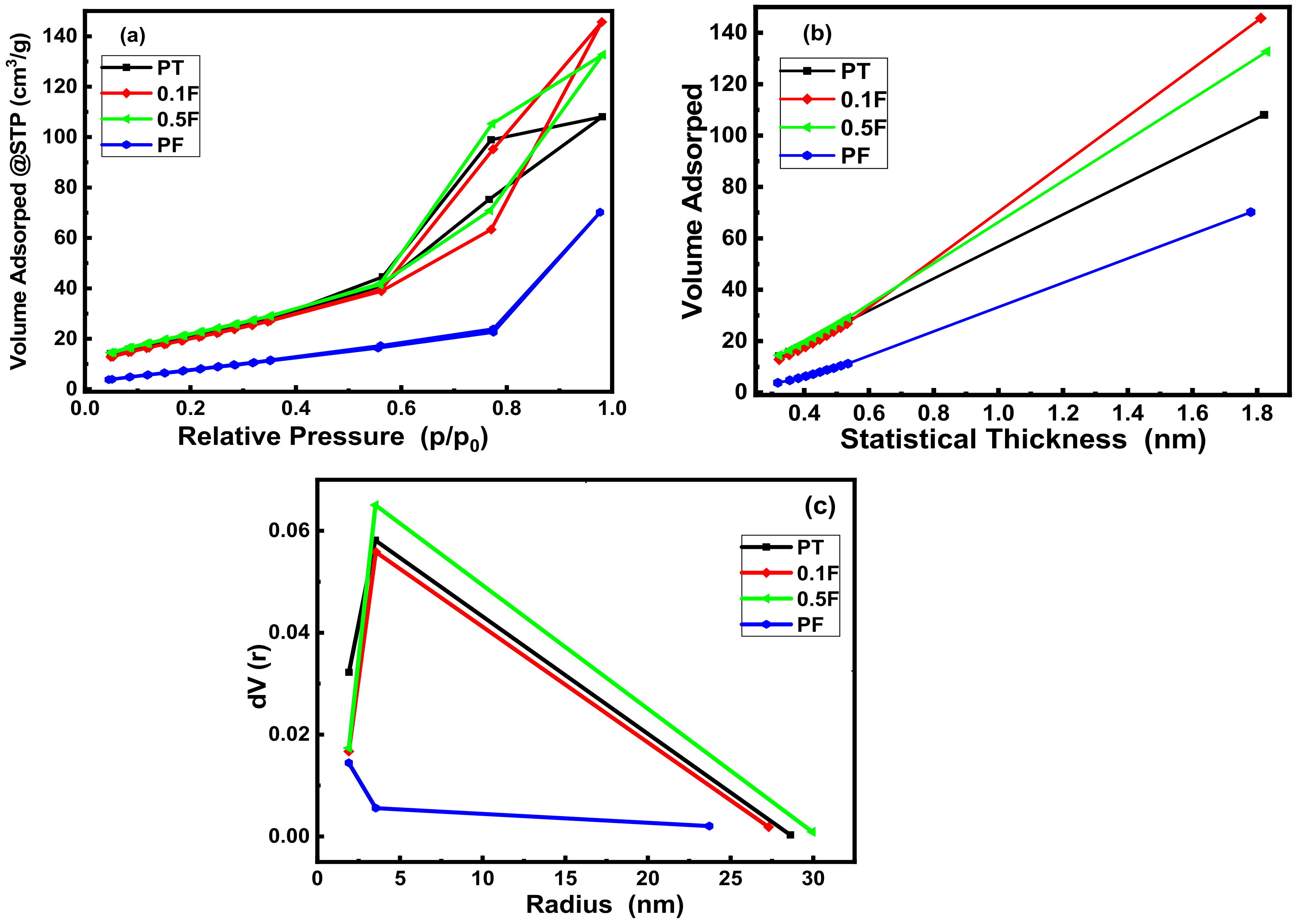
2.3. N2-Physorption Analysis
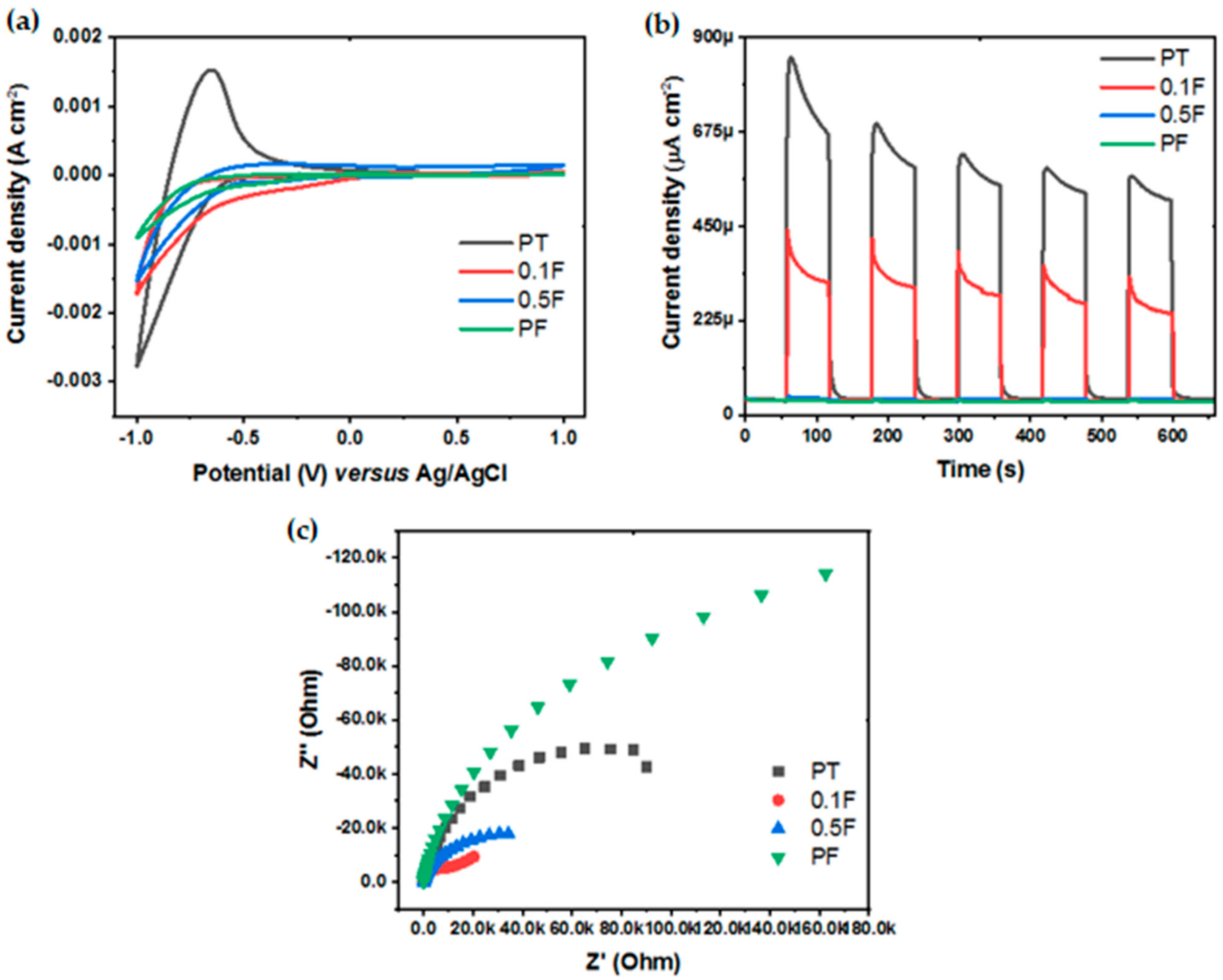
2.4. Photoelectrochemical Properties
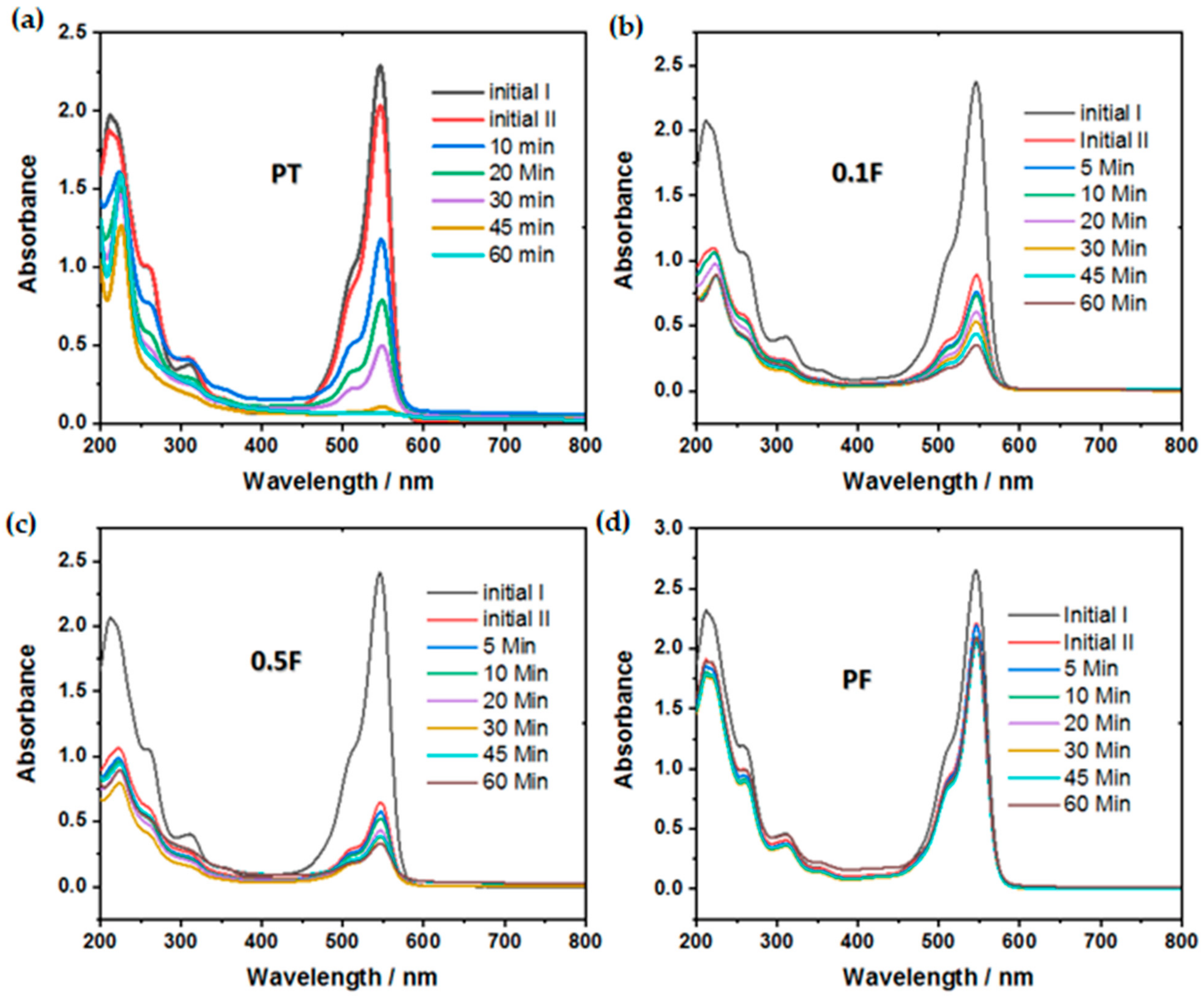
2.5. Photocatalytic Degradation of Rose Bengal Dye
3. Experimental Details
3.1. Synthesis of (TiO2)1−x (Fe2O3)x Nanoparticles (NPs)
3.2. Characterization of (TiO2)1−x(Fe2O3)x Nanocomposites
3.3. Photoelectrochemical Measurements
3.4. Photocatalytic Dye Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, B.M.; Ganesh, I.; Khan, A. Stabilization of nanosized titania-anatase for high temperature catalytic applications. J. Mol. Catal. A Chem. 2004, 223, 295–304. [Google Scholar] [CrossRef]
- Heringa, M.B.; Geraets, L.; van Eijkeren, J.C.; Vandebriel, R.J.; de Jong, W.H.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Zhang, Y.; Tang, M.; Wang, B. Health effects of exposure to nano-TiO2: A meta-analysis of experimental studies. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.A.; Ibrahim, I.A. Impact of supercritical drying and heat treatment on physical properties of titania/silica aerogel monolithic and its applications. Appl. Catal. A Gen. 2008, 346, 200–205. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Nasralla, N.; Yeganeh, M.; Astuti, Y.; Piticharoenphun, S.; Shahtahmasebi, N.; Kompany, A.; Karimipour, M.; Mendis, B.G.; Poolton, N.R.; Šiller, L. Structural and spectroscopic study of Fe-doped TiO2 nanoparticles prepared by sol-gel method. Sci. Iran. 2013, 20, 1018–1022. [Google Scholar]
- Assadi, M.H.N.; Hanaor, D.A.H. The effects of copper doping on the photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zheng, W.; He, B.; Zhang, J.; Anpo, M. Characterization of Fe-TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J. Mol. Catal. A Chem. 2004, 216, 35–43. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J.; Cheng, B. Effects of Fe-doping on the photocatalytic activity of mesoporous TiO2 powders prepared by an ultrasonic method. J. Hazard. Mater. 2006, 137, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.M.; Sanchez, L.C.; Arboleda, J.D.; Beltran, J.J.; Barrero, C.A.; Osorio, J.; Nomura, K. Mixtures of iron and anatase TiO2 by mechanical alloying. Microelectron. J. 2008, 39, 1322–1323. [Google Scholar] [CrossRef]
- Eadi, S.B.; Kim, S.; Jeong, S.W.; Jeon, H.W. Novel preparation of Fe doped TiO2 nanoparticles and their application for gas sensor and photocatalytic degradation. Adv. Mater. Sci. Eng. 2017, 2017, 2191659. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.T.; Siddiqa, A.; Siddiq, M.; Ali, S. Iron-doped titanium dioxide nanotubes: A study of electrical, optical, and magnetic properties. J. Nanopart. Res. 2011, 13, 6517–6525. [Google Scholar] [CrossRef]
- Hirano, M.; Joji, T.; Inagaki, M.; Iwata, H. Direct Formation of Iron (III)-Doped Titanium Oxide (Anatase) by Thermal Hydrolysis and Its Structural Property. J. Am. Ceram. Soc. 2004, 87, 35–41. [Google Scholar] [CrossRef]
- Wang, C.Y.; Böttcher, C.; Bahnemann, D.W.; Dohrmann, J.K. A comparative study of nanometer sized Fe (III)-doped TiO2 photocatalysts: Synthesis, characterization and activity. J. Mater. Chem. 2003, 13, 2322–2329. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Kadi, M.W.; Ismail, A.A. A Facile synthesis of mesoporous α-Fe2O3/TiO2 nanocomposites for hydrogen evolution under visible light. Ceram. Int. 2020, 46, 15604–15612. [Google Scholar] [CrossRef]
- Gareso, P.L.; Sampe, N.; Palentek, V.; Taba, P. Influence of annealing on Fe-doped TiO2 powders using co-precipitation technique. AIP Conf. Proc. 2017, 1801, 020002. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, P.A.; Pecchi, G.A.; Casuscelli, S.G.; Elías, V.R.; Eimer, G.A. A simple synthesis way to obtain iron-doped TiO2 nanoparticles as photocatalytic surfaces. Chem. Phys. Lett. 2019, 732, 136643. [Google Scholar] [CrossRef]
- Umar, A.; Harraz, F.A.; Ibrahim, A.A.; Almas, T.; Kumar, R.; Al-Assiri, M.S.; Baskoutas, S. Iron-doped titanium dioxide nanoparticles as potential scaffold for hydrazine chemical sensor applications. Coatings 2020, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Lourduraj, S.; Williams, R.V. Effect of iron doping on structural and optical properties of TiO2 thin film by sol-gel routed spin coating technique. J. Adv. Dielectr. 2017, 7, 1750024. [Google Scholar] [CrossRef] [Green Version]
- El-Bery, H.M.; Salah, M.R.; Ahmed, S.M.; Soliman, S.A. Efficient non-metal based conducting polymers for photocatalytic hydrogen production: Comparative study between polyaniline, polypyrrole and PEDOT. RSC Adv. 2021, 11, 13229–13244. [Google Scholar] [CrossRef]
- Nabil, S.; Hammad, A.S.; El-Bery, H.M.; Shalaby, E.A.; El-Shazly, A.H. The CO2 photoconversion over reduced graphene oxide based on Ag/TiO2 photocatalyst in an advanced meso-scale continuous-flow photochemical reactor. Environ. Sci. Pollut. Res. 2021, 28, 36157–36173. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Abdelhamid, H.N. Photocatalytic hydrogen generation via water splitting using ZIF-67 derived Co3O4@C/TiO2. J. Environ. Chem. Eng. 2021, 9, 105702. [Google Scholar] [CrossRef]
- Singh, A.P.; Wang, R.B.; Tossi, C.; Tittonen, I.; Wickman, B.; Hellman, A. Hydrogen induced interface engineering in Fe2O3–TiO2 heterostructures for efficient charge separation for solar-driven water oxidation in photoelectrochemical cells. RSC Adv. 2021, 11, 4297–4307. [Google Scholar] [CrossRef]
- Mei, Q.; Zhang, F.; Wang, N.; Yang, Y.; Wu, R.; Wang, W. TiO2/Fe2O3 heterostructures with enhanced photocatalytic reduction of Cr(VI) under visible light irradiation. RSC Adv. 2019, 9, 22764–22771. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.Q.; Zi, T.Q.; Zhao, X.R.; Liu, C.; Ren, Q.; Fang, J.P.; Li, W.M.; Li, A.D. Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition. Sci. Rep. 2020, 10, 13437. [Google Scholar] [CrossRef]
- Hanaor, D.A.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.L.; Hotza, D.; Castro, R.H. Surface energy effects on the stability of anatase and rutile nanocrystals: A predictive diagram for Nb2O5-doped-TiO2. Appl. Surf. Sci. 2017, 393, 103–109. [Google Scholar] [CrossRef]
- Aslam, M.M.; Ali, S.M.; Fatehmulla, A.; Farooq, W.A.; Atif, M.; Al-Dhafiri, A.M.; Shar, M.A. Growth and characterization of layer by layer CdS–ZnS QDs on dandelion like TiO2 microspheres for QDSSC application. Mater. Sci. Semicond. Process. 2015, 36, 57–64. [Google Scholar] [CrossRef]
- Mahadik, M.A.; Shinde, S.S.; Mohite, V.S.; Kumbhar, S.S.; Moholkar, A.V.; Rajpure, K.Y.; Ganesan, V.; Nayak, J.; Barman, S.R.; Bhosale, S.H. Visible light catalysis of rhodamine B using nanostructured Fe2O3, TiO2 and TiO2/Fe2O3 thin films. J. Photochem. Photobiol. B Biol. 2014, 133, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, Y.; Chen, X.; Zeng, X. Fe2O3/TiO2 film electrodes prepared by the forced hydrolysis method and their photoelectrocatalytic performance. Mater. Lett. 2018, 217, 109–112. [Google Scholar] [CrossRef]
- Barkhade, T.; Mishra, S.; Chander, H.; Mahapatra, S.K.; Banerjee, I. Effect of TiO2 and Fe doped TiO2 nanoparticles on mitochondrial membrane potential in HBL-100 cells. Biointerphases 2019, 14, 041003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kuang, M.; Wang, J.; Liu, R.; Xie, S.; Ji, Z. Fabrication of carbon quantum dots/TiO2/Fe2O3 composites and enhancement of photocatalytic activity under visible light. Chem. Phys. Lett. 2019, 730, 391–398. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Xiaoc, J.; Li, X.; Wang, X.; Zhao, T. Characterization and photocatalytic properties of nano-Fe2O3–TiO2 composites prepared through the gaseous detonation method. Ceram. Int. 2017, 43, 14334–14339. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol-gel synthesis of TiO2-Fe2O3 systems: Effects of Fe2O3 content and their photocatalytic properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fís. 2007, 53, 18–22. [Google Scholar]
- Barton, D.G.; Shtein, M.; Wilson, R.D.; Soled, S.L.; Iglesia, E. Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J. Phys. Chem. B 1999, 103, 630–640. [Google Scholar] [CrossRef]
- Shkir, M.; AlFaify, S.; Yahia, I.S.; Ganesh, V.; Shoukry, H. Microwave-assisted synthesis of Gd3+ doped PbI2 hierarchical nanostructures for optoelectronic and radiation detection applications. Phys. B Condens. Matter 2017, 508, 41–46. [Google Scholar] [CrossRef]
- Shkir, M.; Yahia, I.S.; Ganesh, V.; Algarni, H.; AlFaify, S. Facile hydrothermal-assisted synthesis of Gd3+ doped PbI2 nanostructures and their characterization. Mater. Lett. 2016, 176, 135–138. [Google Scholar] [CrossRef]
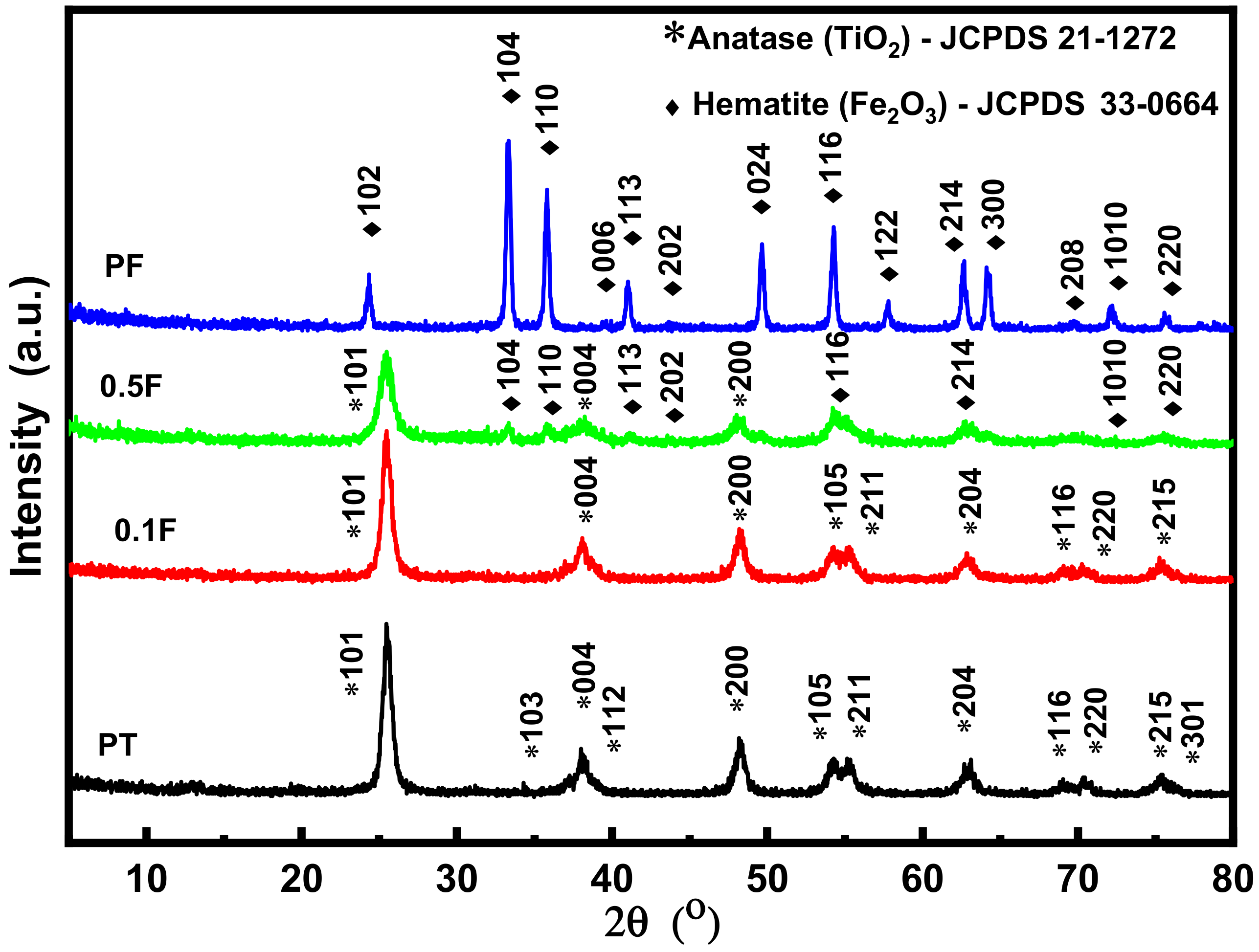
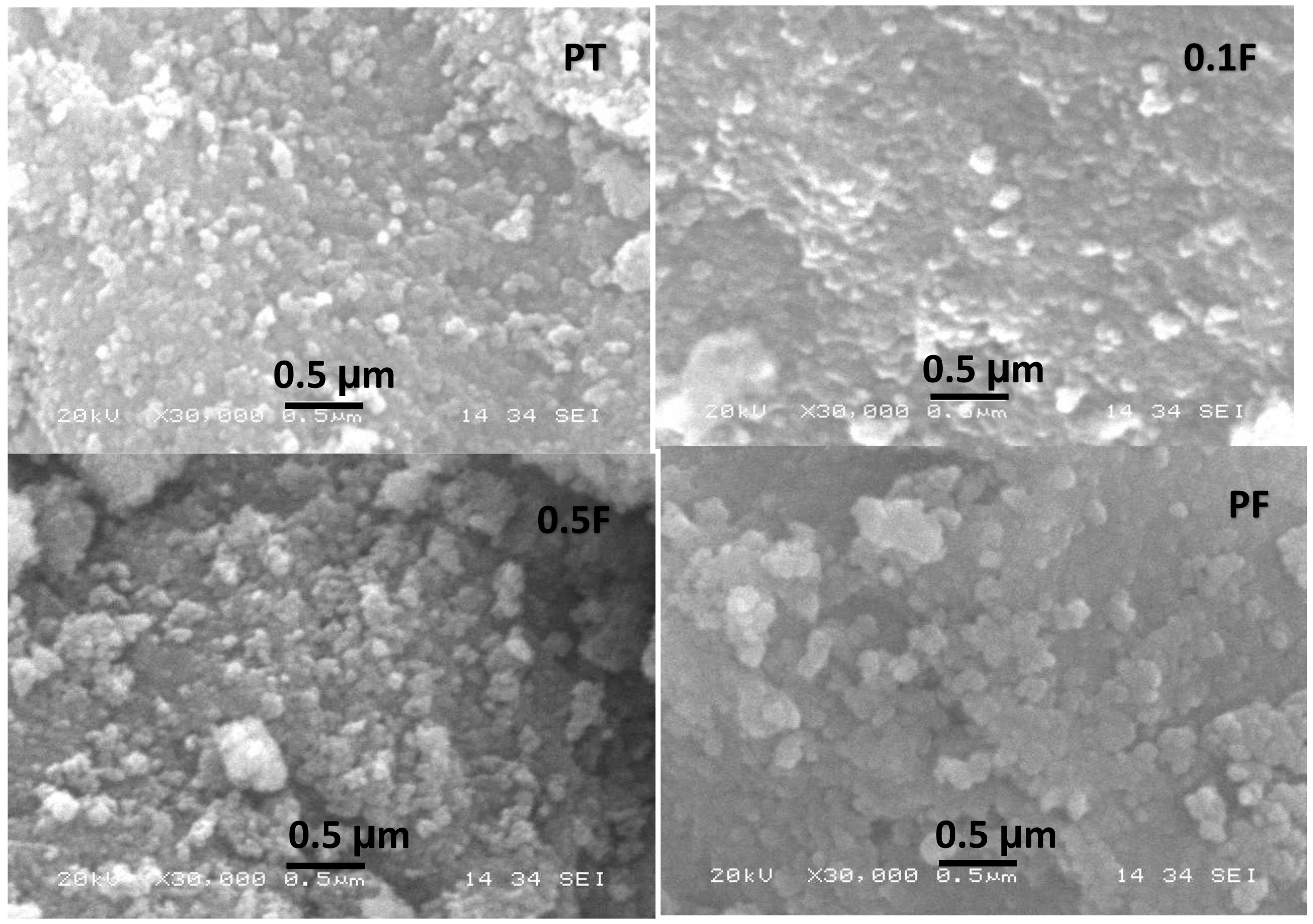

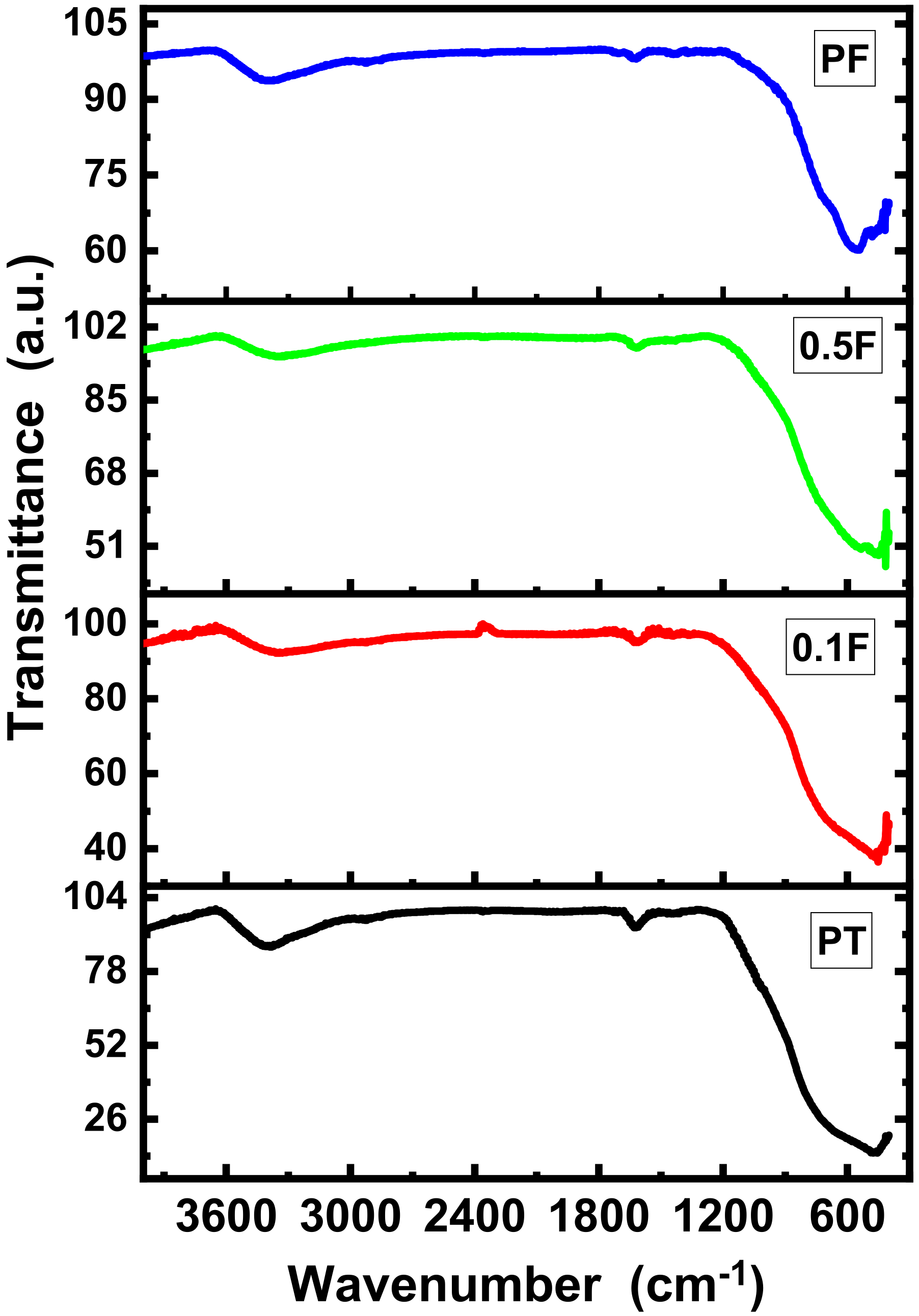

| Sample Name | Crystallite Size (D) (nm) | Dislocation Density (δ) | Microstrain (ε) |
|---|---|---|---|
| PT | 15.6 | 0.006 | 0.002 |
| 0.1F | 21.6 | 0.004 | 0.002 |
| 0.5F | 34.4 | 0.006 | 0.002 |
| PF | 33.7 | 0.001 | 0.001 |
| Sample Name | The Bandgap (eV) |
|---|---|
| PT | 3.15 |
| 0.1F | 2.71 |
| 0.5F | 1.95 |
| PF | 1.91 |
| Sample/Parameter | SBET m2/g | Total Pore Volume cm3/g | Average Pore Radius nm |
|---|---|---|---|
| PT | 82 | 0.1675 | 4.1 |
| 0.1 F | 80.3 | 0.2258 | 5.6 |
| 0.5 F | 86 | 0.2058 | 4.8 |
| PF | 35.6 | 0.1088 | 6.1 |
| Parameter/Sample | * Removal Efficiency % (Dark Adsorption) | * Removal Efficiency % (Photocatalysis) | Rate Constant K /min−1 | ** R2 |
|---|---|---|---|---|
| PT | 11.2 | 97 | 0.058 | 0.97 |
| 0.1F | 62.5 | 61 | 0.016 | 0.98 |
| 0.5F | 79 | 53 | 0.014 | 0.94 |
| PF | 16.3 | 7.3 | 0.0014 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.M.; Sayed, M.A.; Algarni, H.; Ganesh, V.; Aslam, M.; Ismail, A.A.; El-Bery, H.M. Synthesis, Characterization and Photoelectric Properties of Fe2O3 Incorporated TiO2 Photocatalyst Nanocomposites. Catalysts 2021, 11, 1062. https://doi.org/10.3390/catal11091062
Ali AM, Sayed MA, Algarni H, Ganesh V, Aslam M, Ismail AA, El-Bery HM. Synthesis, Characterization and Photoelectric Properties of Fe2O3 Incorporated TiO2 Photocatalyst Nanocomposites. Catalysts. 2021; 11(9):1062. https://doi.org/10.3390/catal11091062
Chicago/Turabian StyleAli, Atif Mossad, Mahmoud Ahmed Sayed, Hamed Algarni, Vanga Ganesh, Muhd Aslam, Adel Ali Ismail, and Haitham Mohamed El-Bery. 2021. "Synthesis, Characterization and Photoelectric Properties of Fe2O3 Incorporated TiO2 Photocatalyst Nanocomposites" Catalysts 11, no. 9: 1062. https://doi.org/10.3390/catal11091062
APA StyleAli, A. M., Sayed, M. A., Algarni, H., Ganesh, V., Aslam, M., Ismail, A. A., & El-Bery, H. M. (2021). Synthesis, Characterization and Photoelectric Properties of Fe2O3 Incorporated TiO2 Photocatalyst Nanocomposites. Catalysts, 11(9), 1062. https://doi.org/10.3390/catal11091062







