Abstract
This paper describes the investigation of electrocatalytic activity of the AuCeO2/C catalyst, prepared using the microwave irradiation method, towards the oxidation of sodium borohydride and oxygen reduction reactions in an alkaline medium. It was found that the obtained AuCeO2/C catalyst with Au loading and electrochemically active surface area of Au nanoparticles (AuNPs) equal to 71 µg cm−2 and 0.05 cm2, respectively, showed an enhanced electrocatalytic activity towards investigated reactions, compared with the Au/C catalyst with an Au loading and electrochemically active surface area of AuNPs equal to 78 µg cm−2 and 0.19 cm2, respectively. The AuCeO2/C catalyst demonstrated ca. 4.5 times higher current density values for the oxidation of sodium borohydride compared with those of the bare Au/C catalyst. Moreover, the onset potential of the oxygen reduction reaction (0.96 V) on the AuCeO2/C catalyst was similar to the commercial Pt/C (0.98 V).
1. Introduction
Conventional combustion-based technologies with high emission rates pose a significant threat regarding air pollution, health, and the climate. Their operation requires enormous natural resources that are not eternal. Therefore, the demand for alternative energy sources has mobilized scientists around the world to search for and research such energy sources. One of them is fuel cells, which can operate at higher efficiencies than combustion engines and can convert the chemical energy in the fuel to electrical energy with efficiencies of up to 60%; it is notable that fuel cells have lower emissions than combustion engines [1]. Different kinds of fuel cells such as direct alcohol fuel cells (DAFC), alkali fuel cells (AFC), direct borohydride fuel cells (DBFC), Proton Exchange Membrane fuel cells (PEMFC), Solid Oxide fuel cells (SOFC), and others have been investigated intensely within the past few decades [2]. All of them have their advantages and disadvantages, however the principle of their operation is similar—fuel oxidation on the anode side and oxygen reduction on the cathode side [3]. Lately, the most attention has attracted DAFC, DBFC, PEMFC because the materials used in these fuel cells can be more easily produced, stored, and transported compared to the other fuel cells, making them more advantageous. DBFC has attracted the attention of researchers because of sodium borohydride (sodium borohydride anion BH4−) potential to generate extremely pure hydrogen on demand or just be directly oxidized in a DBFC. Moreover, the BH4− has higher volumetric (7314 Whdm−3) and gravimetric (7100 Whkg−1) energy density than methanol (4800 Whdm−3) and (6000 Whkg−1), respectively [4,5,6,7]. BH4− is also more stable in an alkaline medium (pH > 14) [8,9]. Additionally, an alkaline BH4− solution is easy and safe to transport and the final product BO2−anion (boric acid) of BH4− oxidation (BOR) is environmentally safe, relatively inert, and non-toxic [9]. The oxygen reduction reaction (ORR) kinetics are facile under alkaline conditions [10,11,12]. In the light of these advantages, DBFC technology is still attractive for investigation regarding its use as a potential power generator technology in energy systems [13,14,15,16].
One more reason for the efficient operation of fuel cells is the selection of a functional, efficient, and relatively inexpensive catalytic material that initiates the onset of a particular electrochemical reaction. It is known that, regarding reducing the amount of the pure noble metal in the catalysts used for fuel oxidation and oxygen reduction, the studies of bimetals (PtCo, PdRh, AuCu, etc.) [17,18,19,20,21] or metal oxides-based (Co3O4, SnOx, MnO2, RuO2, etc.) catalysts were initiated [22,23,24,25,26,27,28]. In general, the composite catalysts have better catalytic properties compared to the pure catalysts from the same metal. Lately, we have been interested in gold interaction with metal oxides such as CeO2, Co3O4 or Nb2O5 because electrocatalysts of such composition are only sparsely described. It has been found that AuCo3O4/C and AuCeO2/C catalysts prepared by the adsorption method showed an enhanced activity toward ORR [29,30]. Moreover, AuCeO2/C prepared by this method exhibited an enhanced activity toward the oxidation of ethylene glycol [30]. It was found that the AuCeO2/C catalyst, which was prepared by the microwave irradiation method, has also a great electrocatalytic activity toward the oxidation of alcohols [31] compared to the Au/C catalyst. Therefore, continuing the research of this catalyst, it was decided to investigate its electrocatalytic properties for direct BOR and ORR.
This paper describes the electrocatalytic properties of the AuCeO2/C catalyst prepared by the microwave irradiation method for the oxidation of borohydride and reduction of oxygen. The composition, morphology, and structure of the prepared AuCeO2/C and Au/C catalysts were characterized using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES), Transmission Electron Microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Field Emission Scanning Electron Microscopy (FESEM), and X-ray diffraction (XRD). The electrochemical measurements were carried out in an alkaline medium using a rotating disk electrode (RDE), linear sweep voltammetry (LSV), and cyclic voltammetry (CV).
2. Results and Discussion
In our previous study, AuCeO2/C prepared by microwave irradiation method has been found as an efficient catalyst toward ethanol, methanol, ethylene glycol, and glycerol oxidation reaction [31]. Therefore, we decided to investigate this catalyst more widely. This paper presents an investigation of the electrocatalytic activity of as-prepared AuCeO2/C and Au/C catalysts toward sodium borohydride oxidation and oxygen reduction reactions. For comparison, CeO2/C substrate (called as CeO2/C catalyst) was also investigated for BOR and ORR. The AuCeO2/C, Au/C, and CeO2/C catalysts were characterized using various methods of materials characterization. The data obtained were described in detail in Ref. [31]. The XRD data confirmed the crystallographic planes of cubic Au and CeO2 phases for the investigated CeO2/C, AuCeO2/C, and Au/C catalysts. The diffraction peaks at the 2θ = 38.18, 44.39, and 64.58° were assigned to the 111, 200, and 220 crystallographic planes of the cubic Au phase for the AuCeO2/C and Au/C catalysts. The diffraction peaks at the 2θ = 28.58, 33.12, 47.54, and 56.41° corresponding to the (111), (200) (220), and (311) crystallographic planes of the cubic CeO2 phase were established for the AuCeO2/C and CeO2/C catalysts [31]. The changes in the Au and CeO2 lattice parameters were not detected, indicating that no metal alloy or metal solid solution was formed in the AuCeO2/C catalyst. The estimated size of Au crystallites was equal to 9.3 ± 0.6 nm and 3.6 ± 0.4 for the Au/C and AuCeO2/C catalysts, respectively.
The TEM examination showed spherical AuNPs with a diameter less than 20 nm for the Au/C (Figure 1a) and AuCeO2/C (Figure 1b) catalysts. The high-resolution TEM (HRTEM) images [31] revealed the differences between the size of Au crystallites and AuNPs. It has been found that AuNP was composed of several Au crystallites of different orientations. Additionally, the AuNPs presented a polycrystalline structure with the characteristic lattice spacing of ~0.23 nm, corresponding to the (111) plane of Au [31,32].
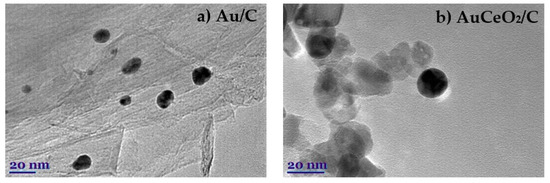
Figure 1.
TEM images of the Au/C (a) and AuCeO2/C (b) catalysts prepared by the microwave irradiation method.
It has been determined that Au in a metallic phase for both Au/C and AuCeO2/C catalysts exists [31,33,34]. The investigation of the O 1s XPS spectra elucidated the lattice oxygen species for the CeO2/C and AuCeO2/C catalysts [31,34]. A Ce was found to correspond to Ce4+for both CeO2/C and AuCeO2/C catalysts [31,35,36]. These XPS results show that CeO2 in CeO2/C and AuCeO2/C exists in oxide form. Obviously, C 1s XPS spectra confirmed the presence of carbon for all catalysts studied [31,37,38].
To provide comprehensive information about the structure and dispersion of active components on the surface of the prepared catalysts, the Au/C and AuCeO2/C catalysts were additionally investigated by SEM. Figure 2 shows surface topography images of the synthesized AuCeO2/C (Figure 2a) and Au/C (Figure 2b) catalysts and total spectra of Au, Ce, O, and C elements (Figure 2(a2,b2)). From the enlarged pictures of the EDS area it can be seen that Au is evenly distributed on the surface of the substrate (Figure 2(a1,b1)). Mass spectra reveal the elemental composition of investigated catalysts (Figure 2(a2,b2)).
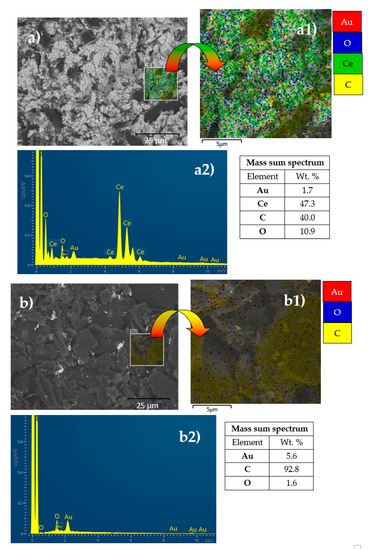
Figure 2.
SEM images of the AuCeO2/C (a) and Au/C (b) catalysts, enlarged images of the EDS area (a1,b1), and total spectra of elements (a2,b2).
The electrochemically active surface areas (ESA) of AuNPs were estimated from the integrated charge of the Au oxide reduction region (QAuO) in the cyclic voltammograms [31,39] and calculated according to Equation (1):
where 400 µC cm−2 is the charge required to reduce a monolayer of Au oxide.
ESA (cm2) = QAuO (µC)/400 (µC cm−2),
The obtained ESA values were 0.05 and 0.19 cm2 for AuCeO2/C and Au/C, respectively [31]. The Au loading in the prepared catalysts determined using ICP-OES was equal to 71 and 78 µg cm−2 for AuCeO2/C and Au/C, respectively [31].
Furthermore, Figure 3 presents the cyclic voltammograms of the investigated catalysts recorded in 1 M NaOH and 0.05 M BH4− + 1 M NaOH solutions at a sweep rate of 50 mV s−1. The obtained data correspond to the theoretical ones. Firstly, it can be clearly seen that there were no evident peaks in a 1 M NaOH solution for all investigated catalysts. Meanwhile, the CVs of Au/C and AuCeO2/C recorded in a 0.05 M BH4− + 1 M NaOH solution showed three typical peaks of the BH4− oxidation. As is evident, on the forward scan the obtained peak A1 at approximately −0.26 V was attributed to the direct potentially eight-electron oxidation of BH4− [9,13]. However, there was no response to the direct oxidation of BH4− associated with the peak A1 in the CV of CeO2/C. The obtained peak A2 at approximately 0.26 V was related to the oxidized surface of the catalysts followed by an immediate decrease in the activity of the electrode, which reveals that the oxidized surface is relatively inactive towards the BH4− oxidation reaction [9]. In addition, the decrease in current density at 0.3–0.4 V may be attributed to the passivation of the electrode surface because of the adsorption of intermediates of BH4− oxidation. On the reverse scan, a sharp peak B that appeared at approximately 0.2 V was attributed to the reactivation of the electrode surface [9,13]. As seen in Figure 3, the AuCeO2/C catalyst outperformed Au/C for the oxidation of BH4−.
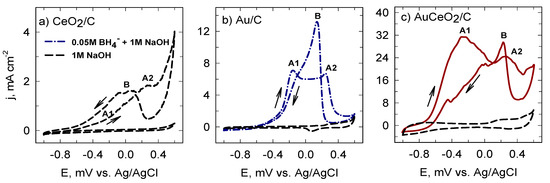
Figure 3.
Comparison of cyclic voltammetry’s (CVs) of the investigated CeO2/C (a), Au/C (b) and AuCeO2/C (c) catalysts recorded in a 1 M NaOH and 0.05 M BH4− + 1 M NaOH solutions at 50 mV s−1; 25 °C.
Comparison of current density, mass, and specific activities for the Au/C and AuCeO2/C catalysts is illustrated in Figure 4. It is clear that the AuCeO2/C catalyst exhibited more negative onset potential. Current density values that correspond to the direct BH4− oxidation were approximately 4.5 times higher at the AuCeO2/C compared to the Au/C (cf. Figure 4b,c). The BH4− oxidation current densities normalized by the Au loading (mA mgAu−1) and electrochemically active surface area of AuNPs (mA cm−2) represent the mass and specific activity of the AuCeO2/C and Au/C catalysts, respectively (Figure 4b,c). As could be expected due to similar Au loading in the catalysts, the mass activity for BH4− oxidation was approximately five times higher at the AuCeO2/C compared with that for the Au/C catalyst (Figure 4b). The values of specific activities for BH4− oxidation were approximately 17 times higher at the AuCeO2/C catalyst than those at the Au/C catalyst (Figure 4c). The electrocatalytic enhancement of the CeO2 supported catalyst was attributed to the CeO2 synergistic electronic effect with Au.
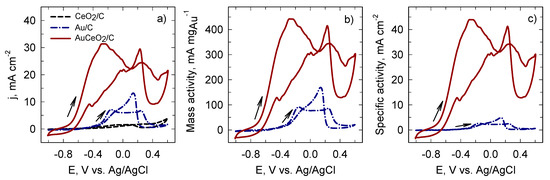
Figure 4.
Comparison of current density (a), mass (b), and specific (c) activities of the investigated catalysts recorded in a 0.05 M BH4− + 1 M NaOH solution at 50 mV s−1; 25 °C.
Figure 5 presents the electrochemical durability of the AuCeO2/C, Au/C, and CeO2/C catalysts investigated using chronoamperometry. The chronoamperometric measurement (CA) curves were recorded at constant voltage equal to −0.26 V. As seen in Figure 5a, current density slightly decreases over time (t < 200 s) and gradually reaches the steady-state of the oxidation of BH4− reaction on the investigated catalysts. The current density remains constant throughout the experiment. Moreover, at the end of the experimental period, the BH4− oxidation current density is ca. 5.5 times larger on the AuCeO2/C catalyst than that on the Au/C catalyst. The higher CA current density indicates better electrocatalytic activity. Additionally, the current density values at the end of the experimental period were normalized by the Au loading and ESA of AuNPs for AuCeO2/C and Au/C catalysts. The results obtained showed the same tendency the AuCeO2/C catalyst exhibited: approximately 6- and 21-times higher mass and specific activity values compared with those of the Au/C catalyst.
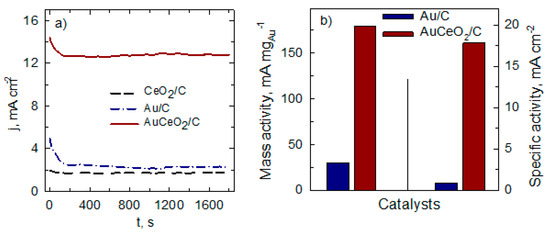
Figure 5.
Chronoamperometric data of the investigated catalysts studied in a 0.05 M BH4− + 1 M NaOH solution at the potential value of −0.26 V (a), mass, and specific activity of the AuCeO2/C and Au/C catalysts calculated from the current density values at the end of the experimental period (b).
Hereinafter, the electrocatalytic activity of the prepared AuCeO2/C, Au/C, and CeO2/C catalysts toward ORR are presented. Firstly, the cyclic voltammograms were recorded in an Ar or O2 saturated 0.1 M NaOH solution by comparing the ORR performance of the catalysts (Figure 6). As seen in Figure 6a–c, all investigated catalysts showed an evident reduction peak in the O2 saturated solution, however, different oxygen reduction potential and current density values. The most positive oxygen reduction potential together with a higher peak current density was observed for the AuCeO2/C catalyst if compared with that for Au/C and CeO2/C (Figure 6d).
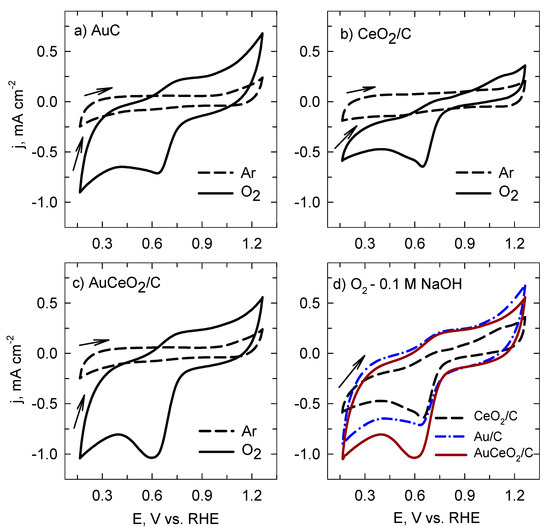
Figure 6.
CVs of the investigated catalysts were recorded in an Ar or O2-saturated 0.1 M NaOH solution (a–c) with a scan rate of 5 mV s−1 at 0 rpm. Comparison of the CVs of the investigated catalysts recorded in an O2-saturated 0.1 M NaOH solution (d).
Then, liner sweep voltammograms were scanned in the O2− saturated 0.1 M NaOH solution from 1.0 V in the cathodic direction to 0.2 V vs. the reversible hydrogen electrode (RHE) at a scan rate of 5 mV s−1. The rotation speed was varied from 400 to 2000 rpm (Figure 7). The obtained ORR response coincides with that reported in the literature of 0.7 and 0.9 V, indicating that the ORR response was dominated by the kinetics of electrocatalysts (Figure 7a–c) [29,30,40]. Furthermore, the ORR polarization curves were used to obtain the Koutecky–Levich (K−L) plots for each catalyst (Figure 7d–f). In general, a good linear fitting was seen between j−1 and w−1/2, indicating the first-order dependence of the ORR kinetics at different potentials (0.4–0.7 V). The calculated electron transfer number (n) for the CeO2/C and Au/C catalysts was approximately two, indicating that these catalysts favor a two-electron transfer reaction path and O2 is reduced to HO2− and OH− [41,42]. Meanwhile, the calculated n for the AuCeO2/C catalysts was approximately 3.8, indicating the 4 e− transfer reaction and direct reduction of O2 to H2O on the surface of this catalyst [43,44].
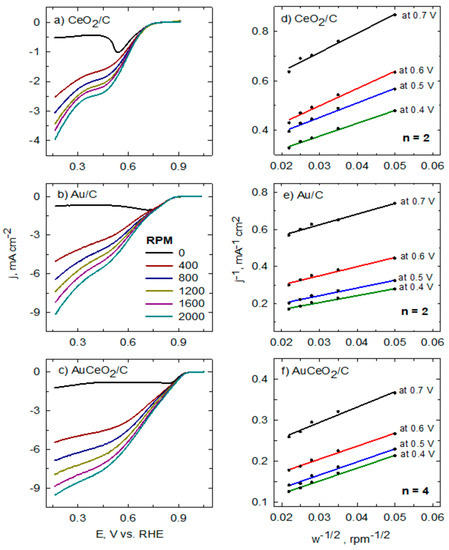
Figure 7.
Linear sweep voltammetry scans (a–c) at the CeO2/C, Au/C, and AuCeO2/C catalysts investigated catalysts recorded in O2-saturated 0.1 M NaOH solution at 5 mV s−1. Koutecky–Levich plots (d–f) were obtained for the investigated catalysts on different potentials in the same solution.
Figure 8 presents a comparison of LSV scans, recorded in an O2-saturated 0.1 M NaOH solution at a rotation speed of 1600 rpm, alongside the mass and specific activities (Figure 8a,b), and chronoamperometric data of ORR (Figure 8c,d). Additionally, the LSV scan of the commercial Pt/C catalyst that has the Pt loading of 0.071 mg Pt cm−2 was recorded in the same manner. From the scans in Figure 8a, it can be clearly seen that the ORR onset potential at 0.96 V for AuCeO2/C was similar to the ORR onset potential of 0.98 V for the commercial Pt/C. Meanwhile, the Au/C and CeO2/C catalysts demonstrated the ORR onset potential at 0.88 and 0.75 V, respectively. Noteworthily, AuCeO2/C and Au/C prepared by the microwave irradiation method exhibited more positive potential compared with our earlier reported AuCeO2/C (0.80 V) [30] and AuCo3O4/C (0.88 V) [29] catalysts. Figure 8b reveals the comparison of mass and specific activities for AuCeO2/C and Au/C calculated from LSV at a potential value of 0.8 V at a rotation speed of 1600 rpm. As seen from the data obtained, the mass and specific activity are approximately 3.5 and 12 times higher, respectively, at the AuCeO2/C than that obtained at Au/C. From the data obtained can be assumed that when Au and CeO2 are together in the composition the electrocatalytic activity of the AuCeO2/C catalyst towards the oxygen reduction reaction could be significantly enhanced. This enhancement could be attributed to the synergistic effect of present metal and metal oxide in the catalyst composition. The durability of the prepared CeO2/C, Au/C, and AuCeO2/C catalysts was evaluated by CA measurements at a potential value of 0.55 V for 1900 s. (Figure 8c). Initially, in the first 100–200 s, a decrease in current density in the ORR was observed for all investigated catalysts. However, after approximately 200 s, the current density settled down and remained constant throughout the experiment (Figure 8c). Figure 8d presents the normalized ORR current densities (percentage of current density retained vs. operation time) for each catalyst after 200 s. It was calculated that investigated catalysts can maintain 94.0, 92.0, 87.0, and 80% in the order Pt/C, AuCeO2/C, Au/C, and CeO2/C, respectively, of the initial current density under continuous operation for 1900 s under a constant voltage of 0.55 V. This indicates that the prepared AuCeO2/C catalyst demonstrated significantly high stability during the ORR process in the alkaline medium compared to commercial Pt/C as well as to Au/C and CeO2/C.
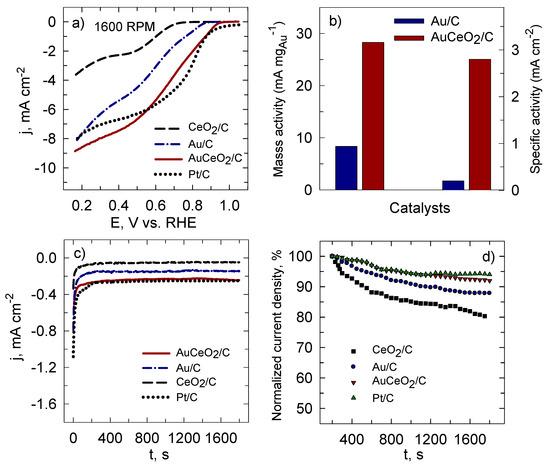
Figure 8.
Comparison of linear sweep voltammetry (LSV) curves recorded on the CeO2/C, Au/C, AuCeO2/C, and Pt/C catalysts in an O2-saturated 0.1 M NaOH solution at 1600 rpm (a); LSV oxygen reduction current densities at 0.8 V normalized by Au loadings and electrochemically active surface areas (ESAs) of AuNPs for the Au/C and AuCeO2/C catalysts (b); chronoamperometric curves recorded on the investigated catalysts at 0.55 V in an O2-saturated 0.1 M NaOH solution (c); chronoamperometric responses (percentage of current density retained vs. operation time) of all the catalysts (d).
Finally, the performance of the NaBH4-H2O2 fuel cell was evaluated using the AuCeO2/C and Au/C catalysts as the anode material. The fuel cell polarization curves and the corresponding power densities against the current density at temperatures in a range of 25–55 °C are given in Figure 9. The fuel cell maintained an open circuit voltage approximately at 1.55 V for the Au/C catalyst (Figure 9a) and 1.7 V for the AuCeO2/C catalyst (Figure 9b). It can be seen that NaBH4-H2O2 fuel cell power density values increased exponentially with increasing temperature. The achieved peak power density of 69.8 mW cm−2 at 25 °C for AuCeO2/C was two times greater as compared with that (31.6 mW cm−2) obtained for Au/C. Increasing the temperature from 25 to 55 °C, the power density increases approximately 1.5 and 2 times for the AuCeO2/C and Au/C catalysts, respectively. More detailed electrochemical parameters of the NaBH4-H2O2 fuel cell using Au/C and AuCeO2/C catalysts as an anode are listed in Table 1. In general, power density peak values obtained in this study were higher compared with those for fuel cells described in the literature [45,46,47].
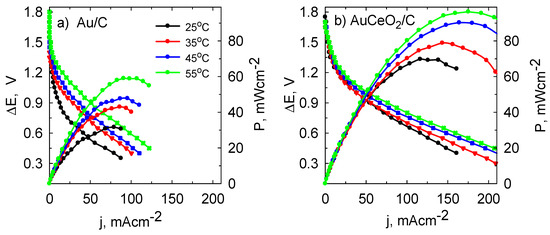
Figure 9.
Cell polarization and power density curves were obtained with the employed Au/C (a) and AuCeO2/C (b) catalysts as the anode at different temperatures for the NaBH4-H2O2 using the anolyte of 0.05 M BH4− in 4 M NaOH and catholyte of 5 M H2O2 in 1.5 M HCl.

Table 1.
Electrochemical parameters of the NaBH4-H2O2 fuel cell at different temperatures employing the Au/C and AuCeO2/C catalysts as the anode.
3. Materials and Methods
3.1. Chemicals
HAuCl4 (99.995%), NaBH4 (>96%), CeO2 powder (99.9%), and graphite powder (99.999%) were purchased from Sigma-Aldrich and Alfa-Aesar Supplies. Pt/C wt. 46.4% Pt (TEC10E50E) was purchased from Tanaka Kikinzoku Kogyo K.K. Supplier. Polyvinylidenefluoride, N-methyl-2-pyrrolydone, ethanol (96%), H2SO4 (96%), and NaOH (98.8%) were purchased from Chempur Company. Oxygen gas (99.999%) was used for the saturation of the NaOH solution. All chemicals were of analytical grade. Ultra-pure water with a resistivity of 18.2 MΩ cm−1 was used for preparing the solutions.
3.2. Preparation of Catalysts
The AuCeO2/C catalyst was prepared using the microwave irradiation method as described in [31]. Briefly, the CeO2/C substrate was obtained by mixing dry powder of CeO2 with carbon powder (the mass ratio being 1:1) in a 2-propanol solution by ultrasonication for 30 min with further desiccation of the mixture. Then, 100 mg of CeO2/C substrate was used for the deposition of AuNPs. The CeO2/C was added to the 6 mL solution containing 1.9 mM of HAuCl4, 1 M of glycerol, and 14 mM NaOH and sonicated for 1 h. The prepared mixture was heated at a temperature of 170 °C for 30 s in the microwave reactor Monowave 300 (Anton Paar). The synthesized AuCeO2/C catalyst powder was filtered and dried in a vacuum oven at 80 °C for 2 h. The Au/C catalyst was prepared in the same way.
3.3. Characterization of Catalysts
The crystal structure and lattice parameters of the synthesized catalysts were characterized using powder X-ray diffraction. XRD patterns of the prepared catalysts were recorded using a D8 diffractometer (Bruker AXS) equipped with a Cu Kα radiation using a Ni/graphite monochromator. A step-scan mode was used in the 2-theta range from 20° to 90° with a step length of 0.02° and a counting time of 5 s per step.
The shape, size, and dispersion of Au nanoparticles on the substrate were examined by TEM analysis using a transmission electron microscope Tecnai G2 F20 X-TWIN equipped with an EDAX spectrometer with an r-TEM detector. For microscopic examinations, 10 mg of sample was first sonicated in 1 mL of ethanol for 1 h, and then the obtained mixture was deposited on the Cu grid covered with a continuous carbon film.
The elemental composition and state of the prepared catalysts were explored by X-ray photoelectron spectroscopy. The XPS analysis was carried out using an “ESCALABMKII” spectrometer (VG Scientific, UK) equipped with an Al Kα X-ray radiation source (1486.6 eV) operated at a fixed pass energy of 20 eV.
The surface morphology of the synthesized catalysts and the distribution of elements in the catalyst was analyzed by SEM using a scanning electron microscope (TM4000Plus, Hitachi). The measurements were made using double-sided adhesive tape (12 mm OD, Pelco Tabs, Ted Pella), which was glued to a pallet for measurements. The surface of the adhesive tape was coated with the catalyst powder.
The Au loading in the catalysts was estimated employing Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). The ICP optical emission spectra were recorded using an ICP optical emission spectrometer Optima 7000 DV (Perkin Elmer).
3.4. Electrochemical Measurements
Electrochemical measurements were undertaken on the electrochemical workstation AUTOLAB and rotating disc electrode (RDE) in a conventional three-electrode cell. A Pt sheet was used as a counter electrode and an Ag/AgCl/KCl electrode was used as a reference. The glassy carbon RDE with a geometric area of 0.07 cm2 coated by catalyst ink was used as the working electrode. The catalyst ink was obtained according to the following steps: at first, 10 mg of the investigated catalysts were dispersed ultrasonically for 1 h in a 100 µL 2% polyvinylidenefluoride in N-methyl-2-pyrrolydone solution. With the aim to keep a similar amount of Au during the measurement, 71 and 78 µg cm−2 for AuCeO2/C and Au/C, respectively, the required amount of the prepared suspension mixture was pipetted onto the polished surface of a glassy carbon electrode and dried in an oven at a temperature of 80 °C for 4 h.
The cyclic voltammetry (CV) measurements were performed in a 0.5 M H2SO4 solution for the determination of electrochemically active surface areas of the prepared catalysts. The electrode potential was cycled in a range of 0–1.5 V with a scan rate of 50 mV s−1. The electro-oxidation of sodium borohydride (BH4−) in an alkaline medium was investigated by CV in a 0.05 M BH4− + 1 M NaOH solution in a range of −0.9–0.6 V at a sweep rate of 50 mV s−1. Chronoamperometric measurements (CA) were carried out in the same solution at a constant potential value of −0.2 V for 1900 s. The electrode potential values are quoted vs. the silver/silver chloride electrode (Ag/AgCl/KClsat).
The CV measurements of ORR were recorded in an Ar or O2 saturated 0.1 M NaOH solutions in the electrode potential range from 0.1 to 1.2 V at a scan rate of 5 mV s−1. A 0.1 M NaOH solution was saturated with Ar or O2 for 10 min before each CV test of ORR. An RDE linear sweep voltammetry curves were recorded in an O2-saturated 0.1 M NaOH solution (saturated with O2 for 10 min before each ORR test) in the electrode potential range from 1 to 0.2 V in the cathodic direction at a scan rate of 5 mV s−1, varying rotation speed from 0 to 2000 rpm. CA for ORR was recorded at a potential value of 0.55 V in an O2-saturated 0.1 M NaOH solution for 1900 s. The electrode potential values for all ORR measurements are quoted vs. the reversible hydrogen electrode (RHE).
Koutecky–Levich (K–L) Equations (2)–(4) were used for calculation of the electron transfer number per oxygen molecule (n) in ORR:
where, j, jk, and jd are the measured current density, kinetic current density, and the diffusion limiting current density (mA cm−2), respectively; n is the number of electrons transferred in the reaction; F is the Faraday constant (F = 96,485 C mol−1); D is the diffusion coefficient of the reactant (1.9 × 10−5 cm2s−1); Co2 is the concentration of the reactant in the bulk electrolyte (1.2 × 10−3 mol L−1); V is the kinetic viscosity of the electrolyte (1.13 × 10−2 cm2s−1); w is the rotation rate; A is the slope of the linear plot of j−1 vs. w−1/2 (K-L plot) [48].
j−1 = jk−1 + jd−1
jd = 0.62nFD2/3 Co2V−1/6ω1/2
j−1 = jk−1 + w−1/2 × A
All measurements were carried out at a temperature of 25 °C. All solutions were deaerated with Ar before each measurement except after O2 saturation for ORR measurements. To facilitate comparison of the obtained data with those described in the literature, the electrode potential for the BH4− oxidation measurements is quoted vs. Ag/AgCl. Electrode potential for ORR measurements is quoted vs. RHE. The presented current densities are normalized concerning the geometric area (0.07 cm2) of the working electrode.
3.5. Fuel Cell Test Measurements
The performance of direct borohydride–hydrogen peroxide fuel cell (NaBH4/H2O2) was evaluated using an electrochemical fuel cell testing system at a temperature in a range of 25–55 °C. A 0.7 cm2 geometric area glassy carbon electrode coated with the AuCeO2/C catalyst was employed as the anode. Catalyst loading was calculated to be the same as for CV measurements. A Pt sheet was used as the cathode. The anode and cathode chambers were separated using a Nafion N117 membrane and filled with 100 mL of the corresponding aqueous electrolyte. The anolyte was composed of an alkaline mixture of 0.05 M BH4− in 4 M NaOH. The catholyte was composed of 5 M H2O2 in 1.5 M HCl. The anolyte and catholyte solutions were prepared immediately before the measurements. The presented current densities are normalized to the geometric area of the anode electrode. The cell testing was performed using a Zennium electrochemical workstation (ZAHNER-Elektrik GmbH & Co. KG).
4. Conclusions
In summary, we deposited AuNPs on the CeO2/C and C substrates preparing the AuCeO2/C and Au/C catalysts. Because of the synergistic effect between CeO2 and Au in the AuCeO2/C catalyst, this catalyst exhibited an enhanced electrocatalytic activity toward the BOR and ORR compared with that of the bare Au/C and Pt/C catalysts. It has been found that the AuCeO2/C catalyst showed approximately 4.5 times higher electrocatalytic activity toward the oxidation of BH4−. Ca. 1.5 times greater power density values in the direct NaBH4-H2O2 fuel cell were obtained by employing AuCeO2/C as the anode compared with those for Au/C. Moreover, the synthesized AuCeO2/C catalyst demonstrated similar onset potential (0.96 V vs. 0.98 V) of ORR and maintenance of initial ORR current density (92% vs. 94%) compared to commercial Pt/C.
Author Contributions
Conceptualization, V.K.; writing—review and editing, V.K.; data curation, V.K.; electrochemical measurements, R.S.; electrochemical measurements, A.B.; materials characterization (TEM) A.D.; materials characterization (XRD) V.P.; materials characterization (XPS) V.J.; supervision, L.T.-T.; supervision, E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
There is no conflict of interest exist.
References
- Fuel Cells. Available online: https://www.energy.gov/eere/fuelcells/fuel-cells (accessed on 13 December 2020).
- Akay, R.G.; Yurtcan, A.B. Direct Liquid Fuel Cells, 1st ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Fuel Cell Basics. Available online: https://americanhistory.si.edu/fuelcells/basics.htm (accessed on 13 December 2020).
- Liu, B.H.; Li, Z.P.; Arai, K.; Suda, S. Performance improvement of a micro borohydride fuel cell operating at ambient conditions. Electrochim. Acta 2005, 50, 3719–3725. [Google Scholar] [CrossRef]
- Li, Z.P.; Liu, B.H.; Arai, K.; Suda, S. Development of the direct borohydride fuel cell. J. Alloys Compd. 2005, 404–406, 648–652. [Google Scholar] [CrossRef]
- Jamard, R.; Latour, A.; Salomon, J.; Capron, P.; Martinent-Beaumont, A. Study of fuel efficiency in a direct borohydride fuel cell. J. Power Sour. 2008, 176, 287–292. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Development of high-performance planar borohydride fuel cell modules for portable applications. J. Power Sour. 2008, 175, 226–231. [Google Scholar] [CrossRef]
- Mirkin, M.V.; Yang, H.; Bard, A.J. Borohydride oxidation at a gold electrode. J. Electrochem. Soc. 1992, 139, 2212–2217. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Cyclic voltammetry investigation of borohydride oxidation at a gold electrode. Electrochim. Acta 2010, 55, 6775–6781. [Google Scholar] [CrossRef]
- Pinto, A.M.R.R.; Oliveira, V.B.; Falcao, D.S. Direct Alcohol Fuel Cells for Portable Applications: Fundamentals, Engineering and Advances, 1st ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 209–244. [Google Scholar]
- Yu, E.H.; Wang, X.; Krewer, U.; Li, L.; Scott, K. Direct oxidation alkaline fuel cells: From materials to systems. Energy Environ. Sci. 2011, 5, 5668–5680. [Google Scholar] [CrossRef]
- Vielstich, A.W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells: Fundamentals, Technology, Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Milikić, J.; Martins, M.; Dobrota, A.S.; Bozkurt, G.; Soylu, G.S.P.; Yurtcane, A.B.; Skorodumova, N.V.; Paštia, I.A.; Šljukić, B.; Santos, D.M.F. A Pt/MnV2O6 nanocomposite for the borohydride oxidation reaction. J. Energy Chem. 2021, 55, 428–436. [Google Scholar] [CrossRef]
- Braesch, G.; Bonnefont, A.; Martin, V.; Savinova, E.R.; Chatenet, M. Borohydride oxidation reaction mechanisms and poisoning effects onAu, Pt and Pd bulk electrodes: From model (low) to direct borohydridefuel cell operating (high) concentrations. Electrochim. Acta 2018, 273, 483–494. [Google Scholar] [CrossRef]
- Backović, G.; Sljukić, B.; Kanberoglu, G.S.; Yurderi, M.; Bulut, A.; Zahmakiran, M.; Santos, D.M.F. Ruthenium(0) nanoparticles stabilized by metal-organic framework as an efficient electrocatalyst for borohydride oxidation reaction. Int. J. Hydrogen Energy 2020, 45, 27056–27066. [Google Scholar] [CrossRef]
- Bortoloti, F.; Angelo, A.C.D. Ordered PtSn/C electrocatalyst: A high performance material for the borohydride electrooxidation reaction. Catalysts 2017, 7, 198. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Kintzel, B.; Bär, R.; Cremers, C.; Tübke, J. Methanol oxidation on PdRh/C electrocatalyst in alkaline media: Temperature and methanol concentration dependencies. J. Electroanal. Chem. 2016, 766, 49–52. [Google Scholar] [CrossRef]
- Li, X.; Qin, X.; Yan, B.; Huang, H.; Zhang, W.; Piao, Y. Pt Nanoclusters anchored on hollow Ag-Au nanostructures for electrochemical oxidation of methanol. Catalysts 2020, 10, 1440. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, D.; Du, C.; Zou, Z.; Zhang, X.; Xia, B.; Yang, H.; Akins, D.L. Carbon-supported Pd-Co bimetallic nanoparticles as electrocatalysts for the oxygen reduction reaction. J. Power Sour. 2007, 167, 243–249. [Google Scholar] [CrossRef]
- Tamašauskaitė-Tamašiūnaitė, L.; Balčiūnaitė, A.; Zabielaitė, A.; Stankevičienė, I.; Kepenienė, V.; Selskis, A.; Juškėnas, R.; Norkus, E. Investigation of electrocatalytic activity of the nanostructured Au-Cu catalyst deposited on the titanium surface towards borohydride oxidation. J. Electroanal. Chem. 2013, 700, 1–7. [Google Scholar] [CrossRef]
- Kepenienė, V.; Tamašauskaitė-Tamašiūnaitė, L.; Jablonskienė, J.; Vaičiūnienė, J.; Kondrotas, R.; Juškėnas, R.; Norkus, E. Investigation of graphene supported platinum-cobalt nanocomposites as electrocatalysts for ethanol oxidation. J. Electrochem. Soc. 2014, 161, F1354–F1359. [Google Scholar] [CrossRef]
- Yongprapat, S.; Therdthianwong, A.; Therdthianwong, S. Au/C catalysts promoted with metal oxides for ethylene glycol electro-oxidation in alkaline solution. J. Electroanal. Chem. 2013, 697, 46–52. [Google Scholar] [CrossRef]
- Van Dao, D.; Adilbish, G.; Le Duc, T.; Nguyen, T.T.D.; Lee, I.H.; Yu, Y.T. Au@CeO2 nanoparticles supported Pt/C electrocatalyst to improve the removal of CO in methanol oxidation reaction. J. Catal. 2019, 377, 589–599. [Google Scholar] [CrossRef]
- Gao, H.; Cao, Y.; Chen, Y.; Lai, X.; Ding, S.; Tu, J.; Qi, J. Au nanoparticle-decorated NiCo2O4 nanoflower with enhanced electrocatalytic activity toward methanol oxidation. J. Alloy Compd. 2018, 732, 460–469. [Google Scholar] [CrossRef]
- Karuppasamy, L.; Chen, C.Y.; Anandan, S.; Wu, J.J. High index surfaces of Au-nanocrystals supported on one-dimensional MoO3-nanorod as a bifunctional electrocatalyst for ethanol oxidation and oxygen reduction. Electrochim. Acta 2017, 246, 75–88. [Google Scholar] [CrossRef]
- Kaskow, I.; Decyk, P.; Sobczak, I. The effect of copper and silver on the properties of Au-ZnO catalyst and its activity in glycerol oxidation. Appl. Surf. Sci. 2018, 444, 197–207. [Google Scholar] [CrossRef]
- Ostojic, N.; Duan, Z.; Galyamova, A.; Henkelman, G.; Crooks, R.M. Electrocatlytic study of the oxygen reduction reaction at gold nanoparticles in the absence and presence of interactions with SnOx supports. J. Am. Chem. Soc. 2018, 140, 13775–13785. [Google Scholar] [CrossRef]
- Kepenienė, V.; Tamašauskaitė-Tamašiūnaitė, L.; Vaičiūnienė, J.; Kondrotas, R.; Pakštas, V.; Norkus, E. Platinum-niobium(V) oxide/carbon nanocomposites prepared by microwave synthesis for ethanol oxidation. Mater. Sci. 2016, 22, 243–248. [Google Scholar] [CrossRef]
- Kepenienė, V.; Stagniūnaitė, R.; Tamašauskaitė-Tamašiūnaitė, L.; Pakštas, V.; Jasulaitienė, V.; Léger, B.; Rousseau, J.; Ponchel, A.; Monflier, E.; Norkus, E. Co3O4/C and Au supported Co3O4/C nanocomposites—Peculiarities of fabrication and application towards oxygen reduction reaction. Mat. Chem. Phys. 2020, 241, 122332–122340. [Google Scholar] [CrossRef]
- Kepenienė, V.; Stagniūnaitė, R.; Upskuvienė, D.; Tamašauskaitė-Tamašiūnaitė, L.; Pakštas, V.; Drabavičius, A.; Andrulevičius, M.; Norkus, E. Electrocatalytic activity of AuCeO2/C towards ethylene glycol oxidation and oxygen reduction reactions. Chemija 2020, 31, 57–68. [Google Scholar] [CrossRef][Green Version]
- Kepenienė, V.; Stagniūnaitė, R.; Balčiūnaitė, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Microwave-assisted synthesis of AuCeO2/C catalyst and its application for oxidation of alcohols in an alkaline medium. New J. Chem. 2020, 44, 18308–18318. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, J.; Shen, P.K.; Li, C.M.; Jiang, S.P. Self-assembled CeO2 on carbon nanotubes supported Au nanoclusters as superior electrocatalysts for glycerol oxidation reaction of fuel cells. Electrochim. Acta 2016, 190, 817–828. [Google Scholar] [CrossRef]
- Fuggle, J.C.; Kallne, E.; Watson, L.M.; Fabian, D.J. Electronic structure of aluminum and aluminum-noble-metal alloys studied by soft-x-ray and x-ray photoelectron spectroscopies. Phys. Rev. B 1977, 16, 750–761. [Google Scholar] [CrossRef]
- Battistoni, C.; Mattogno, G.; Zanoni, R.; Naldini, L.J. Characterisation of some gold clusters by X-ray photoelectron spectroscopy. Electron. Spectrosc. Relat. Phenom. 1982, 28, 23–31. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Wu, J.; Xu, A.; Zhang, Z.; Liao, J.; Lin, S.; Ren, X.; Zhang, P. Synthesis and characterization of Cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrates. Mat. Chem. Phys. 2016, 182, 365–371. [Google Scholar] [CrossRef]
- Beche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Jeong, H.K.; Echeverria, E.; Chakraborti, P.; Le, H.T.; Dowben, P.A. Electronic structure of cyclodextrin-carbon nanotube composite films. RSC Adv. 2017, 7, 10968–10972. [Google Scholar] [CrossRef]
- Baker, M.A.; Hammer, P. A study of the chemical bonding and microstructure if ion beam-deposited cnxfilms including an XPS C 1s peak simulation. Surf. Interface Anal. 1998, 25, 629–642. [Google Scholar] [CrossRef]
- Angerstein-Kozlowska, H.; Conway, B.E.; Hamelin, A.; Stoicoviciu, L. Elementary steps of electrochemical oxidation of single-crystal planesof Au-I. Chemical basis of processes involving geometry of anions and the electrode surfaces. Electrochim. Acta 1986, 31, 1051–1061. [Google Scholar] [CrossRef]
- Paulus, U.; Schmidt, T.; Gasteiger, H.; Behm, R. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disc electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers; Springer: London, UK, 2008. [Google Scholar]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Thomas Goh, F.W.; Andy Hor, T.S.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- He, Q.; Li, Q.; Khene, S.; Ren, X.; López-Suárez, F.E.; Lozano-Castelló, D.; Bueno-López, A.; Wu, G. High-loading cobalt oxide coupled with nitrogen-doped graphene for oxygen reduction in anion-exchange-membrane alkaline fuel cells. J. Phys. Chem. C 2013, 117, 8697–8707. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, S.; Zheng, M.; Tang, J.; Liu, L.; Chen, J.; Wang, X. Graphitic-N-rich N-doped graphene as a high performance catalyst for oxygen reduction reaction in alkaline solution. Int. J. Hydrogen Energy 2020, 45, 32402–32412. [Google Scholar] [CrossRef]
- Yi, L.; Song, Y.; Liu, X.; Wang, X.; Zou, G.; He, P.; Yi, W. High activity of Au-Cu/C electrocatalyst as anodic catalyst for direct borohydride-hydrogen peroxide fuel cell. Int. J. Hydrogen Energy 2011, 36, 15775–15782. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Yang, S.; Lu, H.; Liu, L.; Wang, R. Ultrathin veil-like SnO2 supported Co3O4 nanoparticles for direct borohydride fuel cell anode. J. Power Sour. 2020, 453, 227866. [Google Scholar] [CrossRef]
- Yi, L.; Wei, W.; Zhao, C.; Tian, L.; Liu, J.; Wang, X. Enhanced activity of Au-Fe/C anodic electrocatalyst for direct borohydride-hydrogen peroxide fuel cell. J. Power Sour. 2015, 285, 325–333. [Google Scholar] [CrossRef]
- Shi, K.M.; Cheng, X.; Jia, Z.Y.; Guo, J.W.; Wang, C.; Wang, J. Oxygen reduction reaction of Fe-polyaniline/carbon nanotube and Pt/C catalysts in alkali media. Int. J. Hydrogen Energy 2016, 41, 16903–16912. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).