Synthetic and Post-Synthetic Strategies to Improve Photoluminescence Quantum Yields in Perovskite Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
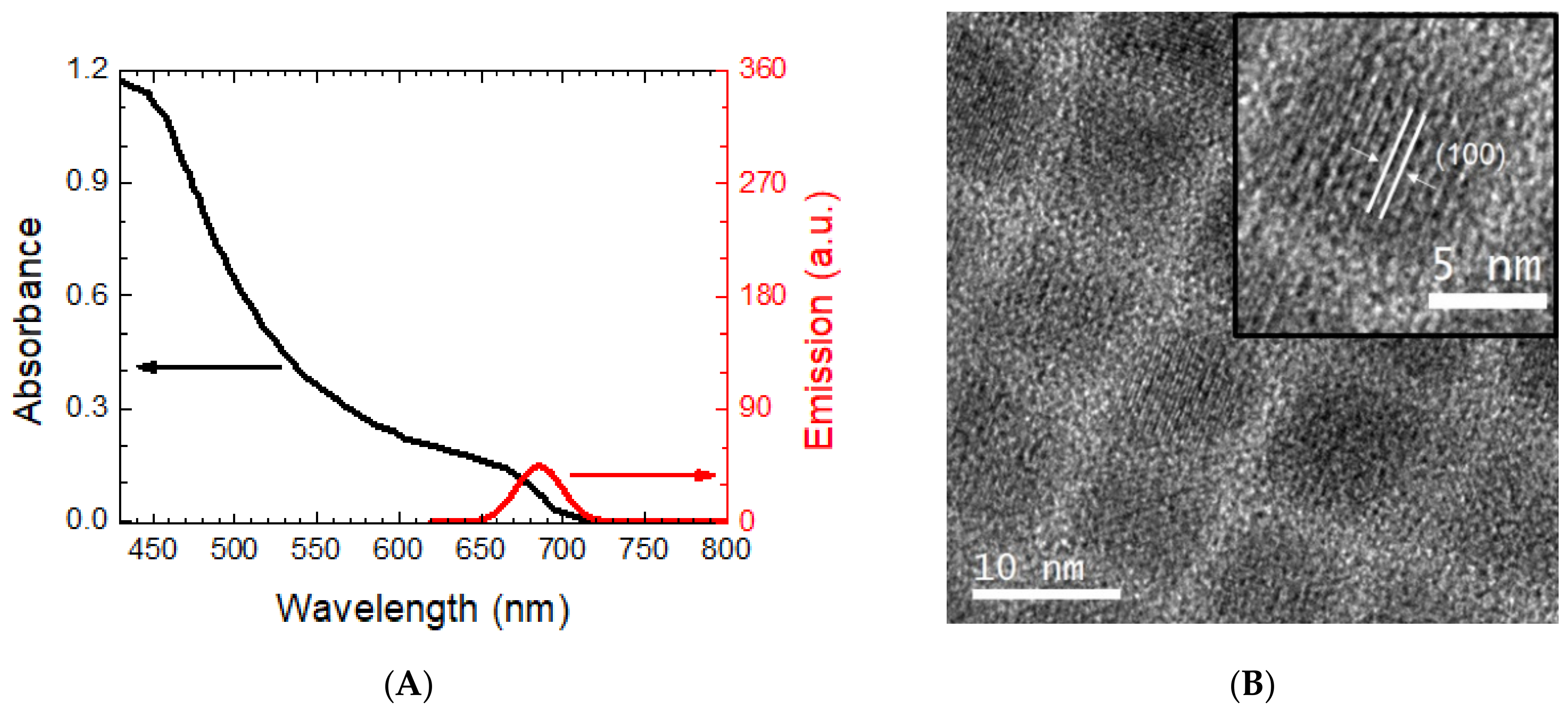
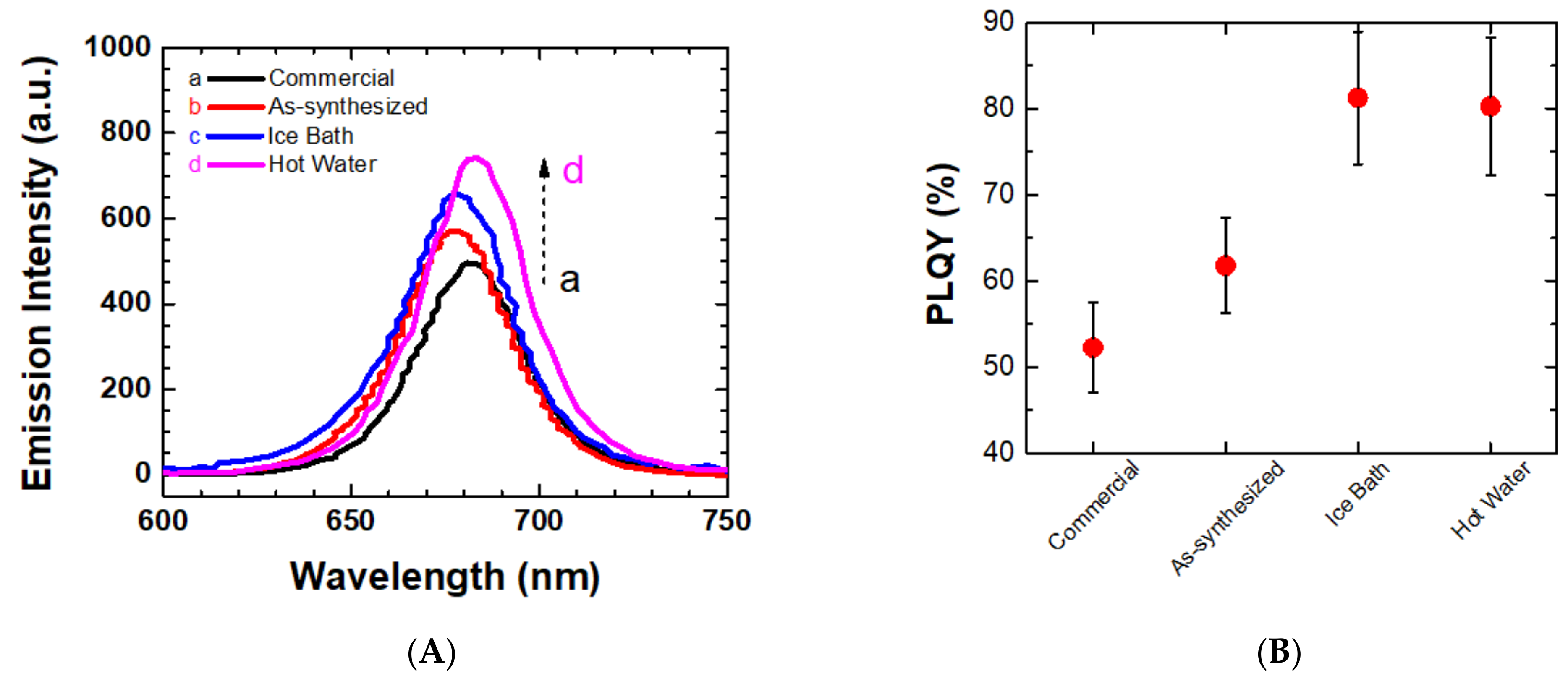
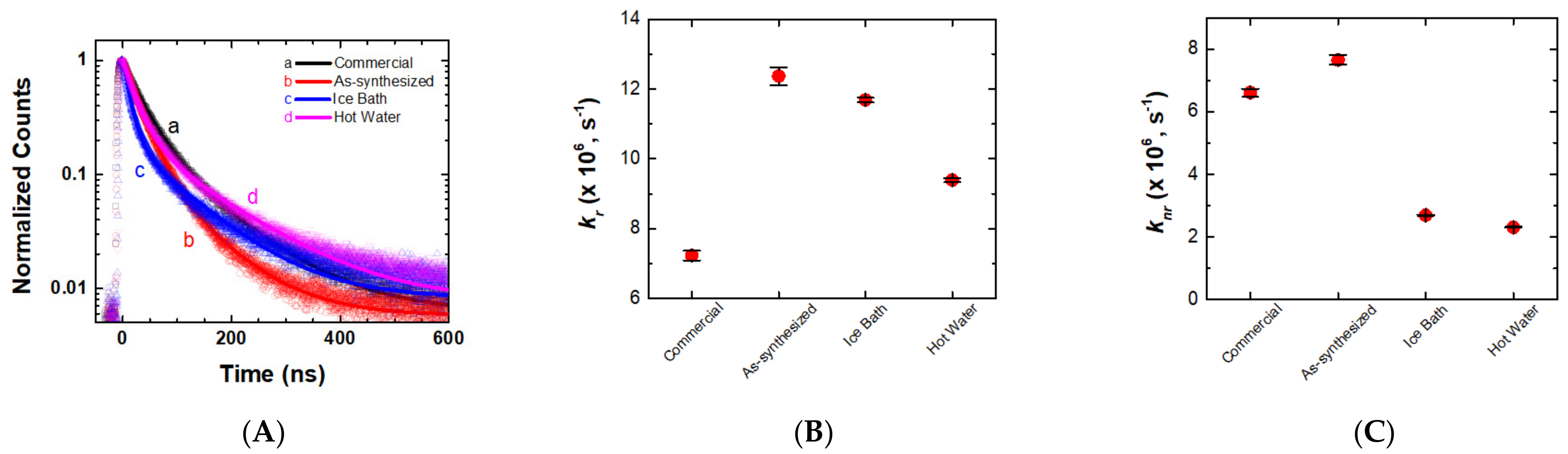
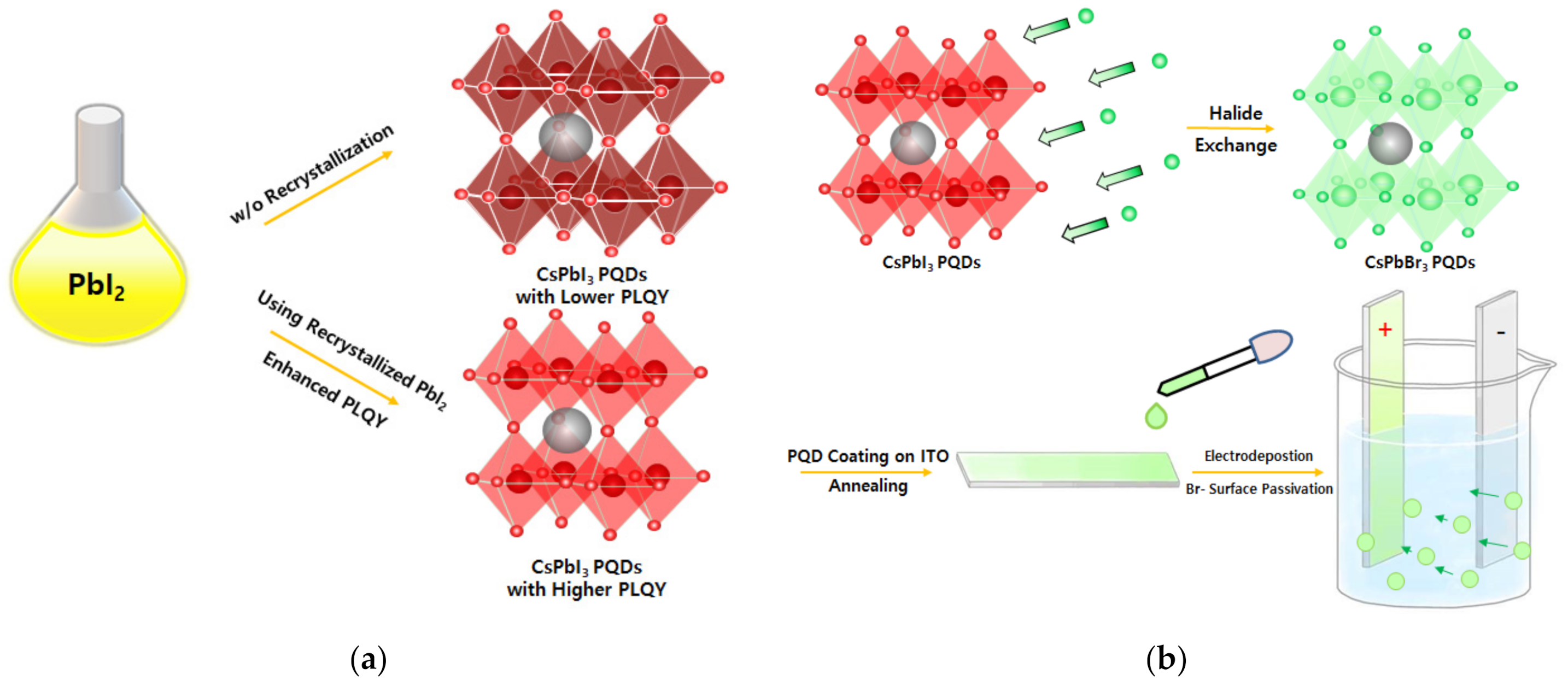
2.1. Impact of Recrystallization of PbI2 Precursor for Photophysical Properties of the PQDs
2.2. Halide Vacancy Mediated Surface Defect Passivation through Electrodeposition
2.3. The Two Different Synthetic and Post-synthetic Strategies to Improve Photoluminescence Quantum Yield of the PQDs
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of the PbI2 Perovskite Precursors and Recrystallization Process
- (1)
- Recrystallization process with slow cooling (called “Hot Water”)
- (2)
- Recrystallization process with fast cooling (called “Ice”)
3.2.2. Synthesis of CsPbI3 Perovskite Quantum Dots
3.2.3. Halide Exchange Process and Deposition of Perovskite Quantum Dots and on ITO to form Stable CsPbBr3 Perovskite/ITO Films in Ambient Condition at Room Temperature
3.2.4. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Min, H.; Lee, K.S.; Lee, D.Y.; Yoon, S.M.; Seok, S.I. Impact of Strain Relaxation on Performance of Alpha-formamidinium Lead Iodide Perovskite Solar Cells. Science 2020, 370, 108–112. [Google Scholar] [CrossRef]
- Gualdron-Reyes, A.F.; Yoon, S.J.; Barea, E.M.; Agouram, S.; Munoz-Sanjose, V.; Melendez, A.M.; Nino-Gomez, M.E.; Mora-Sero, I. Controlling the Phase Segregation in Mixed Halide Perovskites through Nanocrystal Size. ACS Energy Lett. 2019, 4, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, S.G.; Lee, D.K.; Park, N.G. CH3NH3PbI3 and HC(NH2)2PbI3 Powders Synthesized from Low-Grade PbI2: Single Precursor for High-Efficiency Perovskite Solar Cells. Chemsuschem 2018, 11, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, B.W.; Baek, J.; Yun, J.S.; Kwon, H.W.; Seidel, J.; Min, H.; Coelho, S.; Lim, S.; Huang, S.J.; et al. Unveiling the Relationship between the Perovskite Precursor Solution and the Resulting Device Performance. J. Am. Chem. Soc. 2020, 142, 6251–6260. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Shin, Y.; Jeon, G.G.; Kang, D.; Jung, J.; Jeon, B.; Park, J.; Kim, J.; Yoon, S.J. Cost-efficient, Effect of Low-Quality PbI2 Purification to Enhance Performances of Perovskite Quantum Dots and Perovskite Solar Cells. Energies 2021, 14, 201. [Google Scholar]
- Yuan, Y.; Huang, J. Ion Migration in Organometal Trihalide Perovskite and Its Impact on Photovoltaic Efficiency and Stability. Acc. Chem. Res. 2016, 49, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent Engineering for High-performance Inorganic-organic Hybrid Perovskite Solar Cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Tress, W.; Yavari, M.; Domanski, K.; Yadav, P.; Niesen, B.; Correa Baena, J.P.; Hagfeldt, A.; Graetzel, M. Interpretation and Evolution of Open-circuit Voltage, Recombination, Ideality Factor and Subgap Defect States During Reversible Light-soaking and Irreversible Degradation of Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 151–165. [Google Scholar] [CrossRef]
- Zolfaghari, Z.; Hassanabadi, E.; Pitarch-Tena, D.; Yoon, S.J.; Shariatinia, Z.; van de Lagemaat, J.; Luther, J.M.; Mora-Sero, I. Operation Mechanism of Perovskite Quantum Dot Solar Cells Probed by Impedance Spectroscopy. ACS Energy Lett. 2019, 4, 251–258. [Google Scholar] [CrossRef]
- Sum, T.C.; Mathews, N.; Xing, G.; Lim, S.S.; Chong, W.K.; Giovanni, D.; Dewi, H.A. Spectral Features and Charge Dynamics of Lead Halide Perovskites: Origins and Interpretations. Acc. Chem. Res. 2016, 49, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Christians, J.A.; Leighton, D.T.; Kamat, P.V. Rate Limiting Interfacial Hole Transfer in Sb2S3 Solid-state Solar Cells. Energy Environ. Sci. 2014, 7, 1148–1158. [Google Scholar] [CrossRef]
- Konidakis, I.; Maksudov, T.; Serpetzoglou, E.; Kakavelakis, G.; Kymakis, E.; Stratakis, E. Improved Charge Carrier Dynamics of CH3NH3PbI3 Perovskite Films Synthesized by Means of Laser-Assisted Crystallization. ACS Appl. Energy Mater. 2018, 1, 5101–5111. [Google Scholar] [CrossRef]
- Li, F.; Zhu, W.; Bao, C.; Yu, T.; Wang, Y.; Zhou, X.; Zou, Z. Laser-assisted crystallization of CH3NH3PbI3 Films for Efficient Perovskite Solar Cells with a High Open-circuit Voltage. Chem. Commun. 2016, 52, 5394–5397. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Masi, S.; Mora-Seró, I. Progress in Halide-perovskite Nanocrystals with Near-unity Photoluminescence Quantum Yield. Trends Chem. 2021, 3, 499–511. [Google Scholar] [CrossRef]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Golden Rain. Available online: https://edu.rsc.org/exhibition-chemistry/golden-rain/2000048.article (accessed on 20 December 2020).
- Samu, G.F.; Balog, A.; De Angelis, F.; Meggiolaro, D.; Kamat, P.V.; Janaky, C. Electrochemical Hole Injection Selectively Expels Iodide from Mixed Halide Perovskite Films. J. Am. Chem. Soc. 2019, 141, 10812–10820. [Google Scholar] [CrossRef] [Green Version]
- Buin, A.; Pietsch, P.; Xu, J.; Voznyy, O.; Ip, A.H.; Comin, R.; Sargent, E.H. Materials Processing Routes to Trap-free Halide Perovskites. Nano Lett. 2014, 14, 6281–6286. [Google Scholar] [CrossRef]
- Wang, C.; Chesman, A.S.R.; Yin, W.; Frazer, L.; Funston, A.M.; Jasieniak, J.J. Facile Purification of CsPbX3 (X = Cl−, Br−, I−) Perovskite Nanocrystals. J. Chem. Phys. 2019, 151, 121105. [Google Scholar] [CrossRef]
- Draguta, S.; Thakur, S.; Morozov, Y.V.; Wang, Y.; Manser, J.S.; Kamat, P.V.; Kuno, M. Spatially Non-uniform Trap State Densities in Solution-Processed Hybrid Perovskite Thin Films. J. Phys. Chem. Lett. 2016, 7, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.A.; Becker, M.A.; Kamat, P.V. Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 13452–13457. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Hines, D.A.; Kamat, P.V. Quantum Dot Surface Chemistry: Ligand Effects and Electron Transfer Reactions. J. Phys. Chem. C 2013, 117, 14418–14426. [Google Scholar] [CrossRef]
- Bridewell, V.L.; Alam, R.; Karwacki, C.J.; Kamat, P.V. CdSe/CdS Nanorod Photocatalysts: Tuning the Interfacial Charge Transfer Process through Shell Length. Chem. Mater. 2015, 27, 5064–5071. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodríguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattousi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS Core-shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminiscent Nanocrystallines. J. Phys. Chem. B 1997, 101, 9463. [Google Scholar] [CrossRef]
- Kahmann, S.; Sytnyk, M.; Schrenker, N.; Matt, G.J.; Spiecker, E.; Heiss, W.; Brabec, C.J.; Loi, M.A. Revealing Trap States in Lead Sulphide Colloidal Quantum Dots by Photoinduced Absorption Spectroscopy. Adv. Electron. Mater. 2018, 4, 1700348. [Google Scholar] [CrossRef] [Green Version]
- Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A.F.; Masi, S.; Yoon, S.J.; Poyatos, M.; Julián-López, B.; Mora-Seró, I. Ligand & Band Gap Engineering: Tailoring the Protocol Synthesis for Achieving High-quality CsPbI3 Quantum Dots. Nanoscale 2020, 12, 14194–14203. [Google Scholar]
- Yoon, S.J.; Guo, Z.; Claro, P.C.D.; Sheychenko, E.V.; Huang, L.B. Direct Imaging of Long-Range Exciton Transport in Quantum Dot Superlattices by Ultrafast Microscopy. ACS Nano 2016, 10, 7208–7215. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.R.; Eisler, H.-J.; Stott, N.E.; Bawendi, M.G. Emission Intensity Dependence and Single-Exponential Behavior In Single Colloidal Quantum Dot Fluorescence Lifetimes. J. Phys. Chem. B 2004, 108, 143–148. [Google Scholar] [CrossRef]
- Lee, C.; Kim, K.; Shin, Y.; Han, D.; Yoon, S.J. In Situ Spectroelectrochemical Investigation of Perovskite Quantum Dots for Tracking Their Transformation. Front. Energy Res. 2021, 8. [Google Scholar] [CrossRef]
- Hoffman, J.B.; Zaiats, G.; Wappes, I.; Kamat, P.V. CsPbBr3 Solar Cells: Controlled Film Growth through Layer-by-Layer Quantum Dot Deposition. Chem. Mater. 2017, 29, 9767–9774. [Google Scholar] [CrossRef] [Green Version]


| PbI2 | PLQY (%) | kr (s−1) | knr (s−1) | |
|---|---|---|---|---|
| Commercial | 72.3 ± 1.4 | 52.3 ± 5.2 | 7.23 ± 0.14 (×106) | 6.61 ± 0.13 (×106) |
| As-synthesized | 50.0 ± 1.0 | 61.8 ± 5.6 | 1.24 ± 0.03 (×107) | 7.64 ± 0.16 (×106) |
| Ice Bath | 69.5 ± 0.4 | 81.3 ± 7.7 | 1.17 ± 0.07 (×107) | 2.70 ± 0.02 (×106) |
| Hot Water | 85.5 ± 0.6 | 80.3 ± 8.0 | 9.39 ± 0.07 (×106) | 2.31 ± 0.02 (×106) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Lee, S.J.; Shin, Y.; Woo, Y.; Han, S.-H.; Gualdrón-Reyes, A.F.; Mora-Seró, I.; Yoon, S.J. Synthetic and Post-Synthetic Strategies to Improve Photoluminescence Quantum Yields in Perovskite Quantum Dots. Catalysts 2021, 11, 957. https://doi.org/10.3390/catal11080957
Lee C, Lee SJ, Shin Y, Woo Y, Han S-H, Gualdrón-Reyes AF, Mora-Seró I, Yoon SJ. Synthetic and Post-Synthetic Strategies to Improve Photoluminescence Quantum Yields in Perovskite Quantum Dots. Catalysts. 2021; 11(8):957. https://doi.org/10.3390/catal11080957
Chicago/Turabian StyleLee, ChaeHyun, Soo Jeong Lee, YeJi Shin, Yeonsu Woo, Sung-Hwan Han, Andrés Fabián Gualdrón-Reyes, Iván Mora-Seró, and Seog Joon Yoon. 2021. "Synthetic and Post-Synthetic Strategies to Improve Photoluminescence Quantum Yields in Perovskite Quantum Dots" Catalysts 11, no. 8: 957. https://doi.org/10.3390/catal11080957
APA StyleLee, C., Lee, S. J., Shin, Y., Woo, Y., Han, S.-H., Gualdrón-Reyes, A. F., Mora-Seró, I., & Yoon, S. J. (2021). Synthetic and Post-Synthetic Strategies to Improve Photoluminescence Quantum Yields in Perovskite Quantum Dots. Catalysts, 11(8), 957. https://doi.org/10.3390/catal11080957







