1. Introduction
Every modern vehicle equipped with an internal combustion engine possesses a catalytic system (Three-Way Catalytic converters (TWCs) for petrol engines or Diesel Oxidation Catalysts (DOCs) for diesel engines) to reduce efficiently the emissions of harmful compounds such as carbon monoxide (CO), unburned hydrocarbons (HC), and nitrogen oxides (NOx). Car manufacturers mainly use Platinum Group Metals (PGMs) such as platinum, palladium, and rhodium to perform these catalytic functions.
In general, Platinum Group Metals comprise six similar elements: iridium, osmium, palladium, platinum, rhodium, and ruthenium. These elements are included by the European Commission in the list of critical raw materials, based on their economic importance and supply risk [
1]. The long-term demand for PGMs is strong, with their consumption closely related to the global green energy transition and the imposition of stricter emissions standards—particularly in the automotive sector. Recycling could contribute to the reduction of supply risk and increasingly cover the future PGM demands in the EU and globally [
2]. Moreover, the disadvantages of mining over recovering precious metals include their limited resources, scarcity, expensive and energy intensive mining processes, and the significant amount of waste generated during this process. Platinum Group Metal ores contain very small amounts of these metals. For example, in South Africa (the largest producer of platinum), PGM bearing ores have a low content of between 2 and 6 g/t [
3]. At the same time, it should be noted that automotive catalytic converters typically contain up to 2000 g/t PGM in the ceramic catalyst brick, the active part of the converter [
4]. Therefore, due to the high value of PGMs and the fact that autocatalysts comprise a rich source of PGMs, the attractiveness to recover those metals from end-of-life products, such as spent autocatalysts, is extremely high. In this respect, the very restrictive legal regulations regarding the obligation to obtain specific recovery rates for end-of-life (ELV) vehicles and the mandatory removal of catalysts have improved the situation of the European Union in the area of PGM recovery [
5].
Currently, there are several PGM refining facilities across Europe. However, the recovery of automotive catalysts is conventionally carried out with the use of pyrometallurgical processes, including smelting, as the precursor to hydrometallurgical chemical separation and purification processes. These processes may be effective at upgrading the PGM content; however, they are highly energy intensive methods due to their operation at high temperatures [
6,
7].
In this respect, over the last few decades, various technologies have been investigated for autocatalysts recycling in order to reduce the environmental impact of PGM recycling, compared to the pyrometallurgical processes [
6,
8,
9,
10]. Platinum Group Metals Recovery Using Secondary raw materials (PLATIRUS), is a European Union (EU), Horizon 2020 project whose aim is to bring a complete feed-to-product flowsheet for the separation and purification of PGMs without the use of smelting, while using novel as well as modified traditional processes, thus addressing the Platinum Group Metal supply security within Europe. A relevant paper that can be regarded as a PLATIRUS project overview has been published recently [
11], summarizing the most promising technologies explored during the project. The authors concluded that the PLATIRUS project has successfully achieved its objective to research, evaluate, and upscale novel and greener PGM recycling technologies.
Moreover, new automotive catalysts consist of several components: a monolith substrate, a high surface area washcoat with oxygen storage promoter materials (usually CeO
2-ZrO
2 mixed oxides), the precious metals (Platinum Group Metals, PGMs) that are the active catalyst, and the canning [
12,
13]. Monolith substrates have a honeycomb structure and are usually made from ceramic cordierite, while metallic monoliths are also used. Al
2O
3 is a material used in the washcoat due to its thermal stability, high surface area, and resistance to sintering. These characteristics are needed in order to obtain a higher dispersion of the metal particles of precious metals, thus increasing their exposed surface area. CeO
2 or ceria-based materials (Ce, Zr) are used as promoters due to ceria’s Oxygen Storage Capacity (OSC) [
12]. Cerium ions can easily switch between Ce
3+ and Ce
4+ oxidation states, allowing the catalyst to either release oxygen to the exhaust stream when it is deficient, promoting oxidation reactions, or promote the reduction of NOx to N
2 by storing oxygen when O
2 in the stream is excessive [
12,
14,
15]. Platinum, palladium, and rhodium are the PGMs most commonly used in TWCs. Pt and Pd are mainly used for their ability to promote the oxidation of carbon monoxide and hydrocarbons to CO
2 and water, while Rh is needed for the simultaneous reduction of nitrogen oxides to N
2 gas. Besides, DOCs are used mainly to oxidize hydrocarbon and CO emissions, while another chemical reaction that occurs in a DOC is the oxidation of NO to NO
2, as the concentration of NO
x is important for the function of the diesel particulate filter (DPF) and the selective catalytic reduction (SCR) [
16]. The most widely used DOC catalysts contain Pt and Pd in loadings that usually vary between 50 and 90 g/ft
3 [
13,
16].
Considering the aforementioned, it can be easily concluded that the potential of using recycled PGMs (recovered from end-of-life spent catalytic converters), without further refinement steps, for the production of new autocatalysts can be considered as a very attractive alternative for autocatalyst manufacturers, as it could greatly reduce the production costs.
Moreover, the nature of automotive applications demands the reliable performance of catalytic converters over an extended period with transient operating conditions [
17,
18,
19]. To achieve this, one of the key challenges is dealing with the chemical and thermal ageing effects that reduce the catalytic activity during the operating life of the catalyst. Catalyst deactivation, the loss of catalytic activity and/or selectivity over time, is a problem of great concern in the practice of industrial catalytic processes [
19,
20,
21]. Catalyst deactivation may occur by several mechanisms; nevertheless, the most relevant mechanisms are sintering, fouling/coking, and poisoning. These mechanisms can be classified according to their nature as thermal, mechanical, or chemical. Thermal ageing is the major cause of catalyst deactivation. The high temperature induces a series of physical processes that lead to sintering of washcoat components and PGMs particles, reducing in this way the access of reactants to the active catalytic sites [
17,
21]. The presence of water vapor accelerates the sintering process.
The present paper deals with the validation of the performances of new automotive catalysts prepared using recycled PGMs obtained through the PLATIRUS approach. In this respect, different types of small-scale autocatalysts (monolithic carrots) were prepared with different PGM loadings as well as types of cordierite supports (900 cells per square inch (cpsi) hexagonal and 400 cpsi). The performance of the produced catalysts was evaluated, both as fresh and aged catalysts, for the steady-state as well as transient operation. To the best of our knowledge, this is the first study performed on the evaluation of the performance of autocatalysts produced from recycled PGMs solutions containing impurities (without further refinement steps), thus closing the PGMs recycling loop. Several characterization techniques have been conducted (XRF, Optical Microscopy, and N2 physisorption) for the determination of the physicochemical characteristics of the synthesized catalysts, and their catalytic performance has been evaluated by measuring their conversion efficiency for the main pollutant gases as a function of the temperature on a synthetic gas bench: CO, unburned hydrocarbons, and NOx.
3. Discussion
The imposition of continuously stricter EURO protocols regarding the acceptable emission levels of vehicles has led the automotive industry towards the use of higher quantities of PGMs for the development of catalysts that can comply with these regulations. This paper reports, for the first time, the successful preparation of new TWC and DOC catalysts using PGM containing solutions coming from the recovery of precious metals from spent catalytic converters as metal precursors. These new catalysts were found to exhibit similar catalytic performances to commercial catalysts, both as fresh and aged samples.
Concerning the recycled PGM solutions (metal nitrates) that were used, they were found to contain identified impurities that are commonly found in end-of-life autocatalysts such as Zn, Ca, Pb, P etc. [
13,
21,
22,
23]. Such elements are known to be deposited on catalysts during their use in cars, as they are present in the engine out gases due to the combustion of oil and fuel additives [
21,
22]. Thus, the detection of those impurities in the metal solutions coming from the recycling of spent catalytic converters can be justified. It should be noted that the investigation of the effect of the impurities present in the PGMs solutions on the structural configuration and the chemical interactions between the impurities and the active metals and washcoat materials is beyond the scope of this paper. The present study focuses on the ability to use recovered PGMs without further refinement steps for the synthesis of automotive catalysts that perform adequately well concerning toxic gases abatement; the detailed physicochemical characterization of the materials and the possible role of the impurities on fresh and aged catalysts will be studied in subsequent publications.
The successful synthesis of new autocatalysts (TWCs and DOCs) was confirmed in terms of desired metal loading by measuring the weight increase of the monoliths before and after their impregnation with the washcoat, as well as with the use of XRF analysis of the catalyst, with the slightly higher PGM loading of the catalysts synthesized using recycled PGMs solutions being due to the presence of PGM impurities that were found in the solutions (e.g., Pd was found to be present in the recycled Pt nitrate solution used for the preparation of the catalysts). Moreover, optical microscopy confirmed the uniform deposition of the washcoat on the cordierite cells, while no blocked cells were observed.
Catalyst ageing is one of the most important aspects taken into consideration in autocatalyst development because there is legislation that defines the required ability of the catalytic systems to meet the emission standards up to a specific mileage on vehicles [
17]. Ageing is related to catalyst deactivation over time. This phenomenon can be due to the development of high temperatures in the catalyst (thermal ageing), or poisoning and fouling due to phosphorus, sulfur, and other impurities that can be found in the gas stream (chemical ageing) [
17,
21].
Thermal ageing is accompanied by a series of physical processes that lead to the sintering of the washcoat, with a decrease in the surface area of the carrier [
21,
24,
25]. Alumina, the most common washcoat material for catalytic devices, has many different polymorphs. High-temperature exposures cause undesired phase transformations for alumina that change the washcoat properties; 03B3-Alumina, having a high surface area (a strong desired property for the catalyst support), is gradually transformed into other forms of alumina with lower surface areas (δ, θ, α), increasing the rate by increasing the temperature [
20,
26]. Ceria-based composite oxides (mainly CeO
2-ZrO
2 mixed oxides) are used as supports to provide oxygen storage ability. The role of the Zr promoter is to increase the ceria’s thermal stability and favor metal dispersion [
27]. However, CeO
2-ZrO
2 has also been found to inactivate in increased temperatures under ageing conditions [
28]. Noble metal particles are also subjected to similar processes. Upon temperature increase, large metal agglomerates are formed, leading to a decrease of the active surface that can be accessed by the reactants [
17,
29]. Platinum Group Metal (PGM) particles can be affected by the sintering process of the support, suffering encapsulation due to the collapse of the porous structure [
30]. It should be noted that the presence of water can accelerate these reactions (hydrothermal ageing).
As a result, automotive companies need to test the performance of after-treatment systems under such conditions to ensure that the vehicles perform well according to legal requirements. Various ageing techniques have been applied by automotive companies and in the literature as well, such as vehicle or bench ageing of the catalysts, in an attempt to simulate the real conditions a catalyst is subjected to during its lifetime [
17,
21,
22]. Such methods are characterized by disadvantages related to difficulties and increased costs and time consumption. Alternatively, static ageing is often used. In the latter case, the catalyst is placed in an oven (under air conditions or in controlled gas mixtures) and is heated at a specified temperature (often >1000 °C), where they are left for a predetermined time. Even though this method is not representative of the dynamic conditions or the chemical alterations that can be caused by the function of a vehicle engine, it is advantageous due to its low operating cost and the ability to control the gas composition seen by the catalyst [
17].
3.1. Three-Way Catalysts (TWCs)
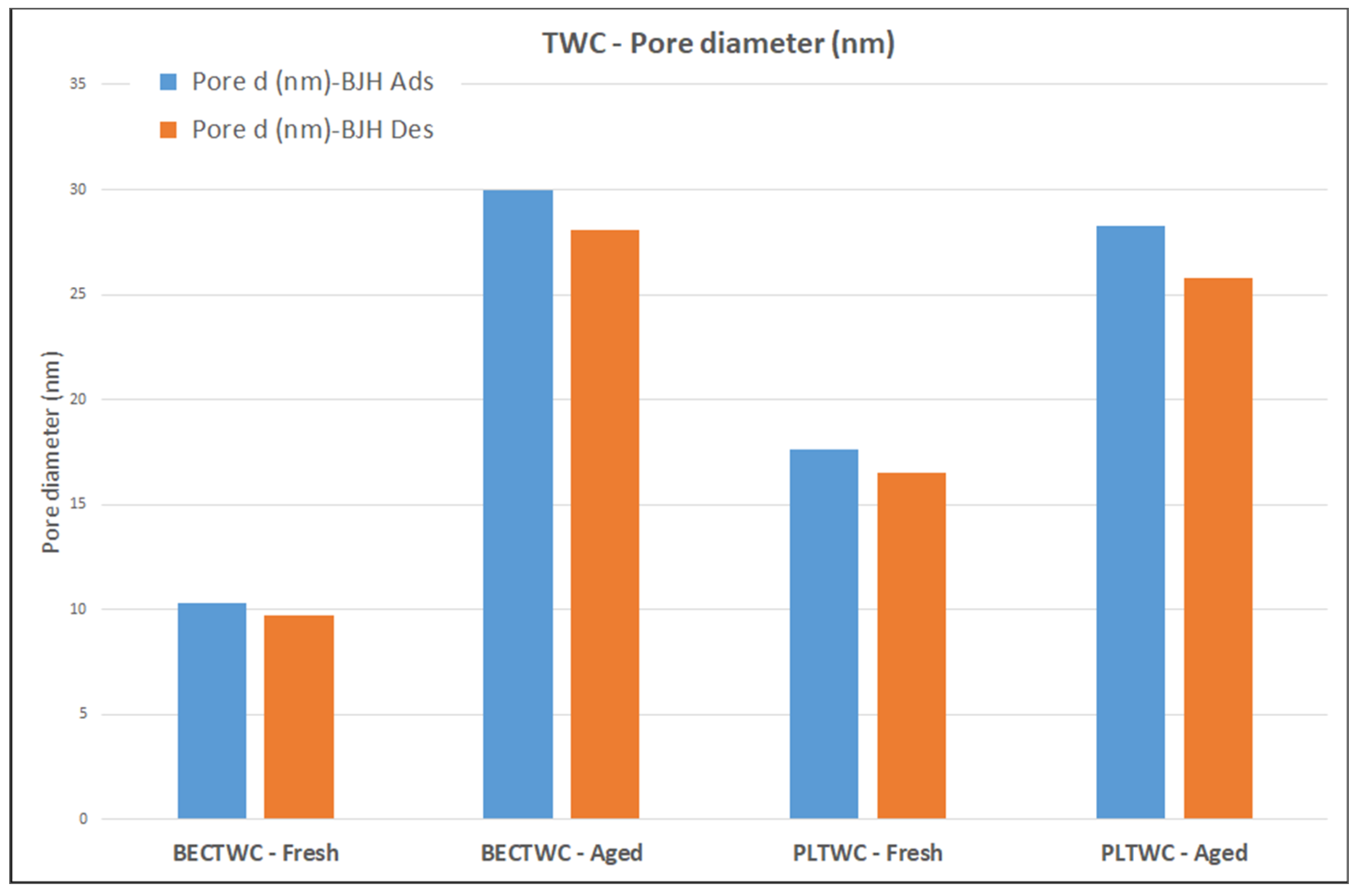
The results concerning the specific surface areas of the TWCs and the pore diameters showed that fresh catalysts presented relatively low BET values. Thermal ageing was found to have a detrimental effect on these values as they were decreased by 92% and 85% upon ageing in the case of BECTWC and PLTWC, respectively. A possible effect of the impurities present in the recycled PGM solutions used may be implied by these results. These impurities could lead to lower SSAs for the fresh PLTWC catalyst compared to the benchmark reference catalyst (BECTWC), but at the same time they may have a positive effect during the thermal treatment of the catalysts, leading to lower losses of surface area. In general, the results confirm that structural changes took place following the exposure of the samples at high temperatures, causing a decrease of the surface area and a loss of porosity due to sintering of both the support material and metal particles.
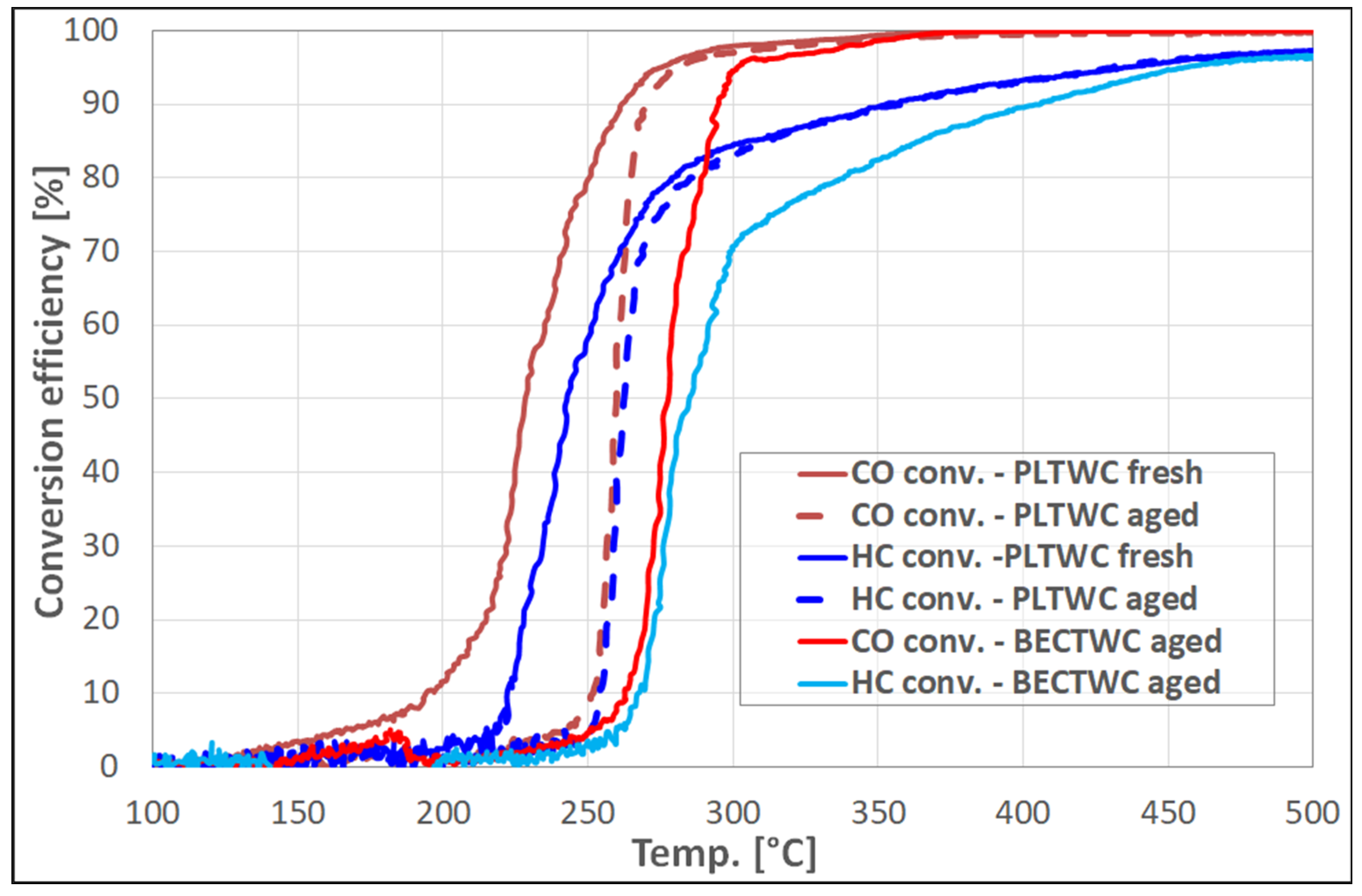
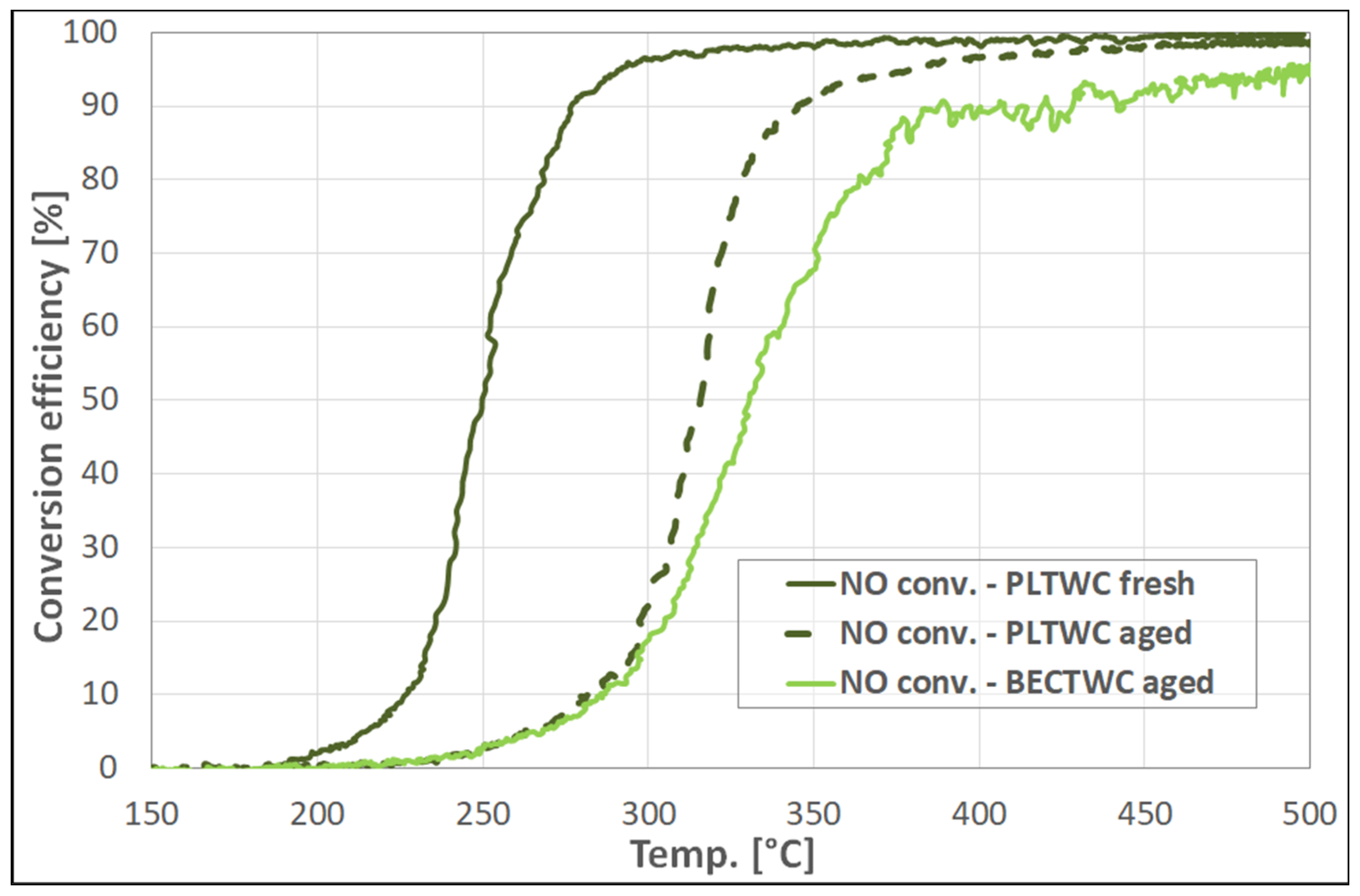
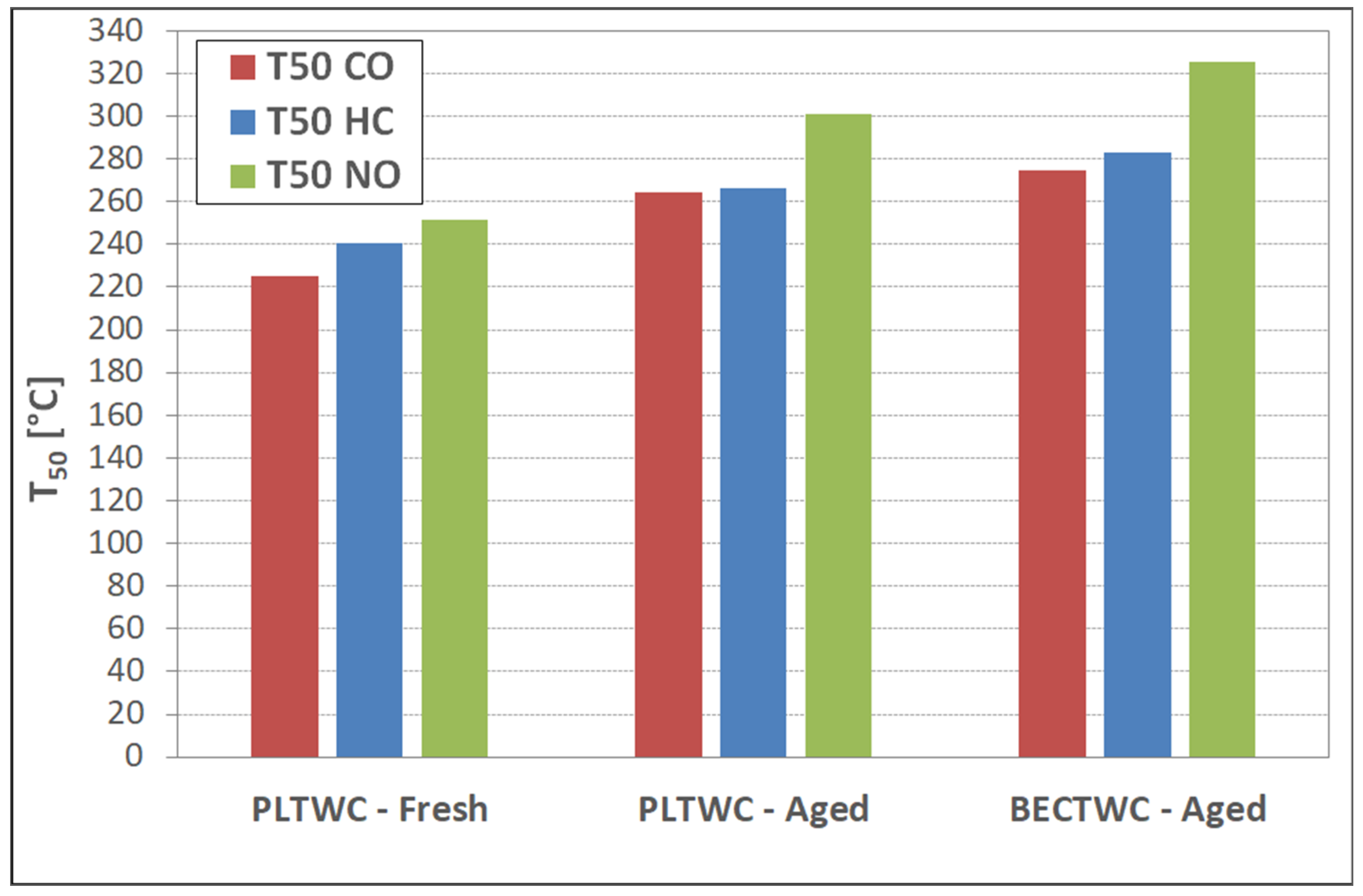
Regarding the light-off temperatures of the PLTWC catalyst, concerning the oxidation of CO and HC, as well as the reduction of NO, the T
50 values of the samples obtained from PGMs recycled are in line with commercial catalysts compliant with the EURO 6b emission standards, with similar metal loadings [
21,
22,
31]. TWC benchmark (BECTWC) performances are slightly worse (not presented). One possible reason for this discrepancy could be the imperfect control of the washcoat loading because benchmark catalysts were manufactured using small monolith carrots characterized by a non-flat external surface that can support washcoat material, as described in
Section 4.3.
Concerning the performance of the aged catalysts, the structural effects observed upon ageing were found to also influence the catalytic efficiency of the samples, reducing their ability for the abatement of pollutant emissions emitted from the vehicle engines. Again, it should be highlighted that the results concerning the performance of the aged catalysts (BECTWC and PLTWC) were found to be similar or even better than those of the commercial samples reported in the literature, for catalysts that have been subjected to similar thermal treatment [
21,
22,
31].
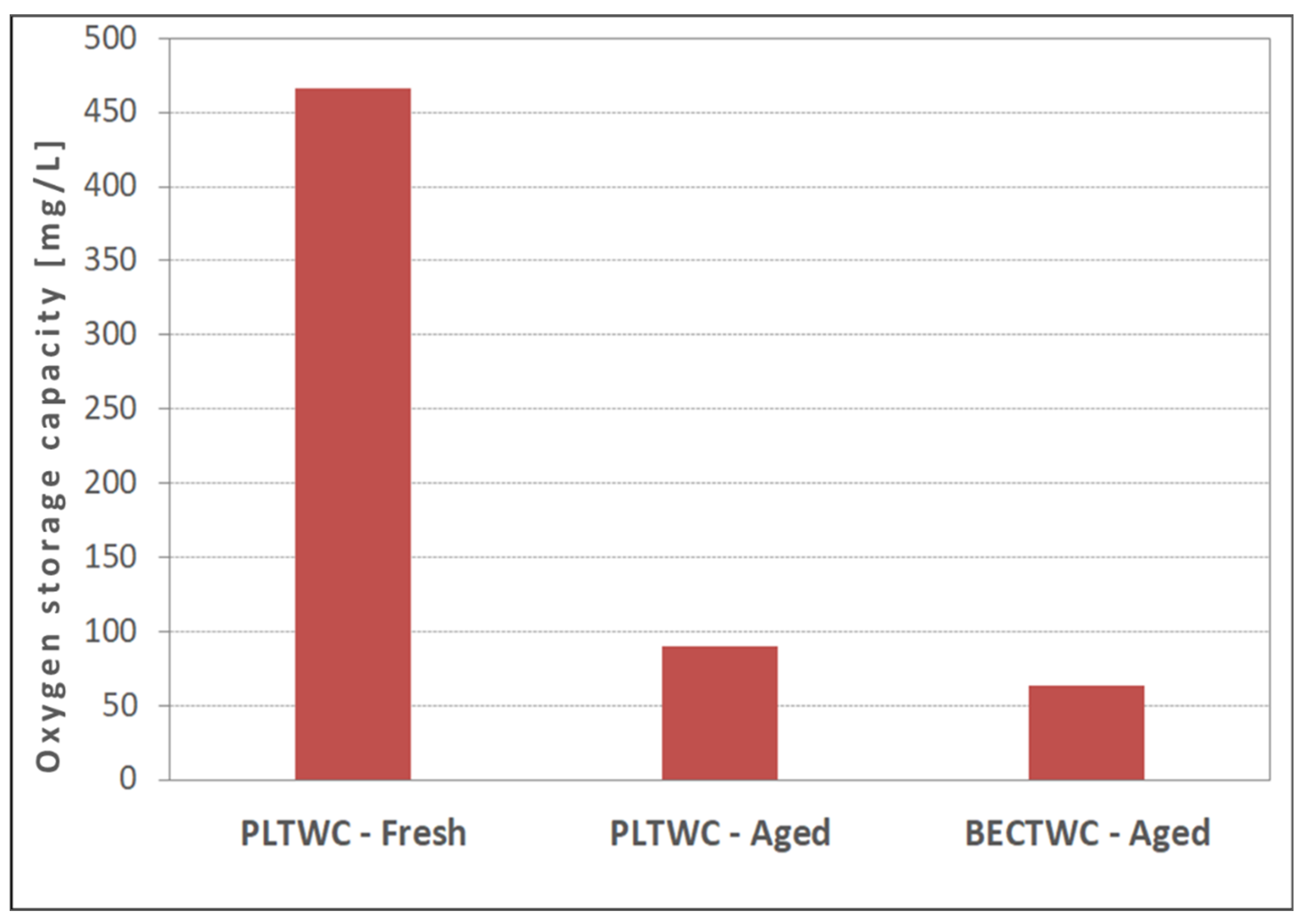
It has been reported that the conversion efficiency of the TWCs is dependent on the Oxygen Storage Capacity (OSC), the storage and release kinetics, and the current oxidation state of the material. Regarding this parameter, all samples showed OSC values lower than the typical values reported for commercial catalysts, especially those in fresh condition [
21,
22]. The latter may be an implication that sample preparation needs further optimization. Regarding the effects of ageing on catalysts OSC, this parameter decreased significantly upon thermal treatment. This finding is related to the redox properties of the oxygen storage components, which were found to change significantly with ageing. The sintering of active components can hinder the Ce
4+ to Ce
3+ reaction, decreasing the contact between the material and the engine out gases. Similar results have been reported in the literature [
22,
28]. The interaction of the materials with Ce sharing O atom can be altered due to the sintering of metal particles, leading to the decrease of OSC and catalytic activity of TWC as the catalyst deactivation proceeds [
32,
33]. Moreover, the disappearance of the active metal components on the catalyst surface by encapsulation with alumina and ceria further reduces the OSC of TWCs [
28,
30,
34].
In addition, in the case of PLTWC, the impurities present in the recycled PGMs solutions used may contribute to the low values of OSC due to intervention of the chemical ageing. For example, it has been reported that the phosphate compounds present in the engine out gas (P is an identified impurity of the Pt nitrate solution) can react with Ce, forming a stable CePO
4, unable to participate in the redox reaction [
23]. However, it should be emphasized that, even though the OSC of the aged PLTWC catalyst decreased significantly compared to value obtained for the fresh sample, it was still higher than the corresponding value of the aged BECTWC catalyst. Thus, results of the present study indicate that the better efficiency of the PLTWC aged catalyst can be related to the higher OSC value of this catalyst compared to the BECTWC aged catalyst.
3.2. Diesel Oxidation Catalysts (DOCs)
The calculated specific surface areas of the DOC catalysts (both fresh and aged) showed, similarly to the TWC case, that the catalysts produced using solutions containing recycled PGMs were found to have slightly lower BET values compared to the ones prepared with the use of commercial metal precursors, probably due to the presence of impurities in the recycled PGMs solutions. Concerning the structural changes that take place upon thermal ageing, the results of the SSA measurements revealed, in accordance with the characterization results of TWCs, a decrease of the porous structure of DOCs due to sintering of the support material exposed at high temperatures. In the case of DOCs, lower differences were observed between fresh and aged samples (47% and 19% for BECDOC and PLDOC, respectively), indicating a good stability of the washcoat when exposed to high temperatures. The results of BJH adsorption and desorption measurements suggested a loss of small pores over the catalysts when the samples were exposed to high temperatures, due to support sintering. It should be noted that the results showed that TWC catalysts were more sensitive to the ageing conditions compared to the DOCs.
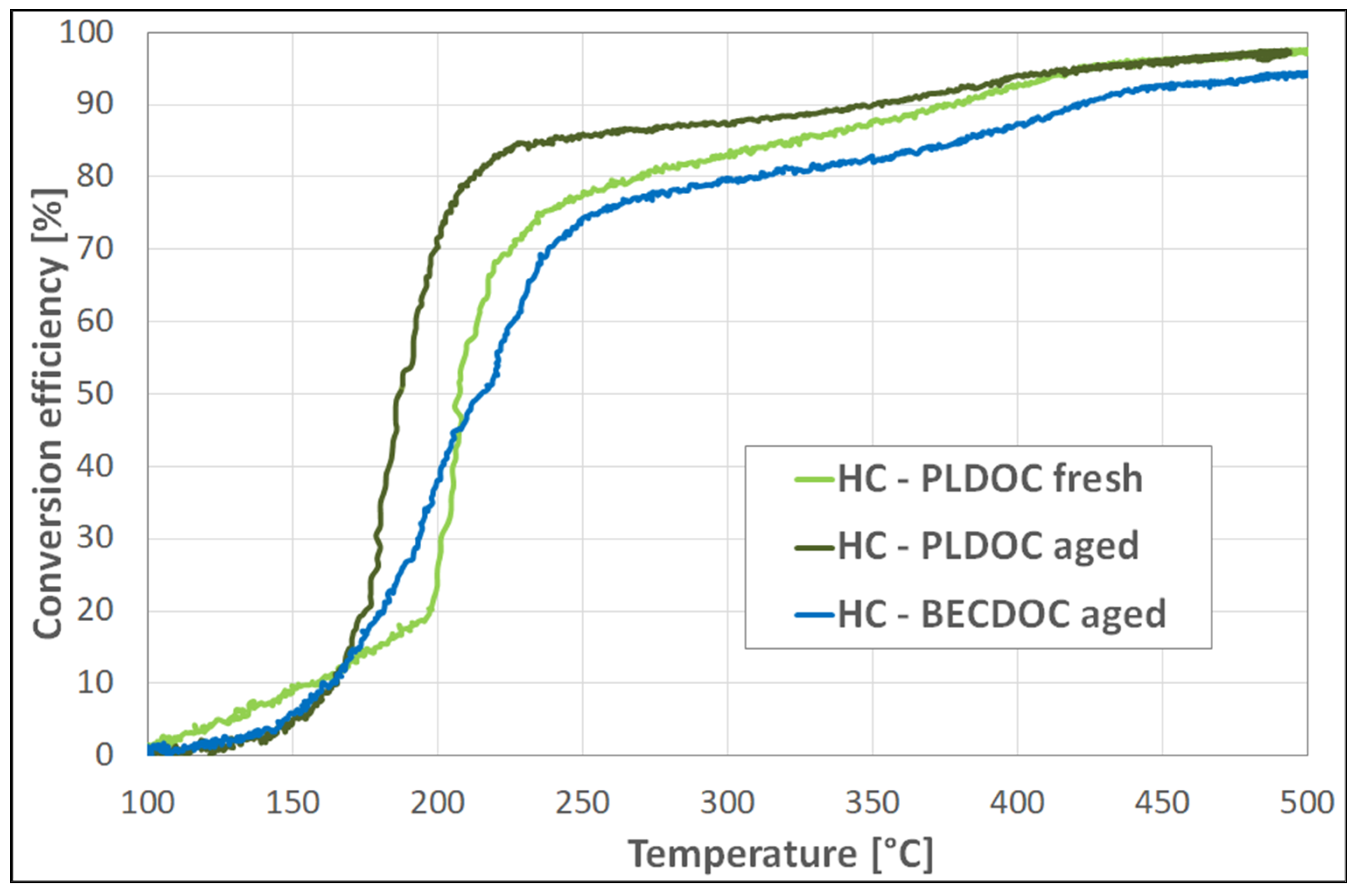
Even in this case, DOC obtained from recycled PGMs showed better performances than the reference samples prepared by commercial metal nitrates. Fresh PLDOC showed worst performances, particularly for HC conversion and NO oxidation; this behavior is probably related to the inhomogeneity of the samples. A possible reason for the discrepancy between PLDOC fresh and aged is related to the manufacturing process; due to the low amount of recycled PGMs available, the PLDOC samples manufactured in a very small scale (length 2.0″), incorporating recycled PGMs, may be affected by the high external surface of the carrot, resulting in an uneven washcoat distribution. Benchmark catalyst (BECDOC) results are similar to expected commercial performance (although slightly worse).
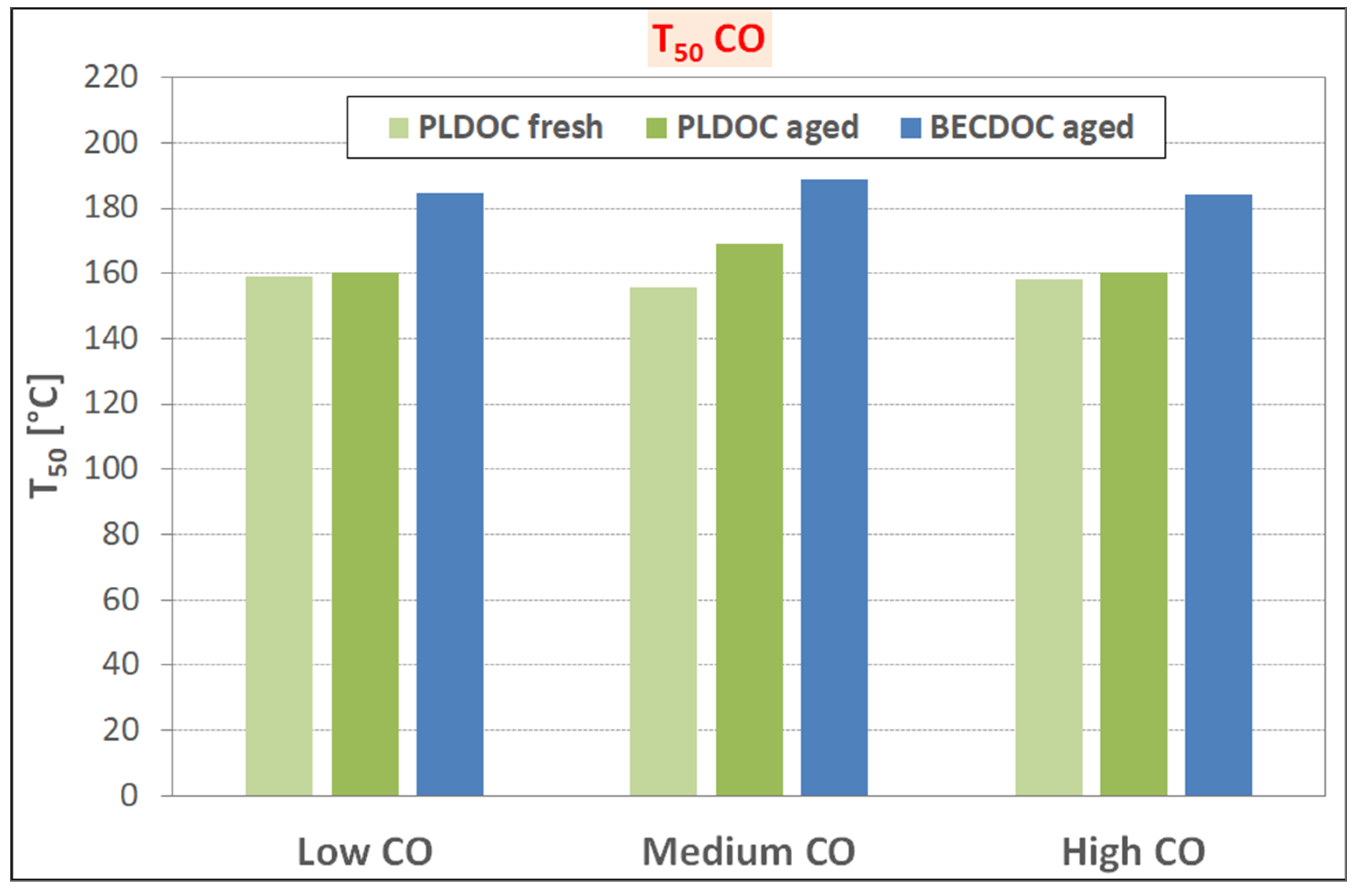
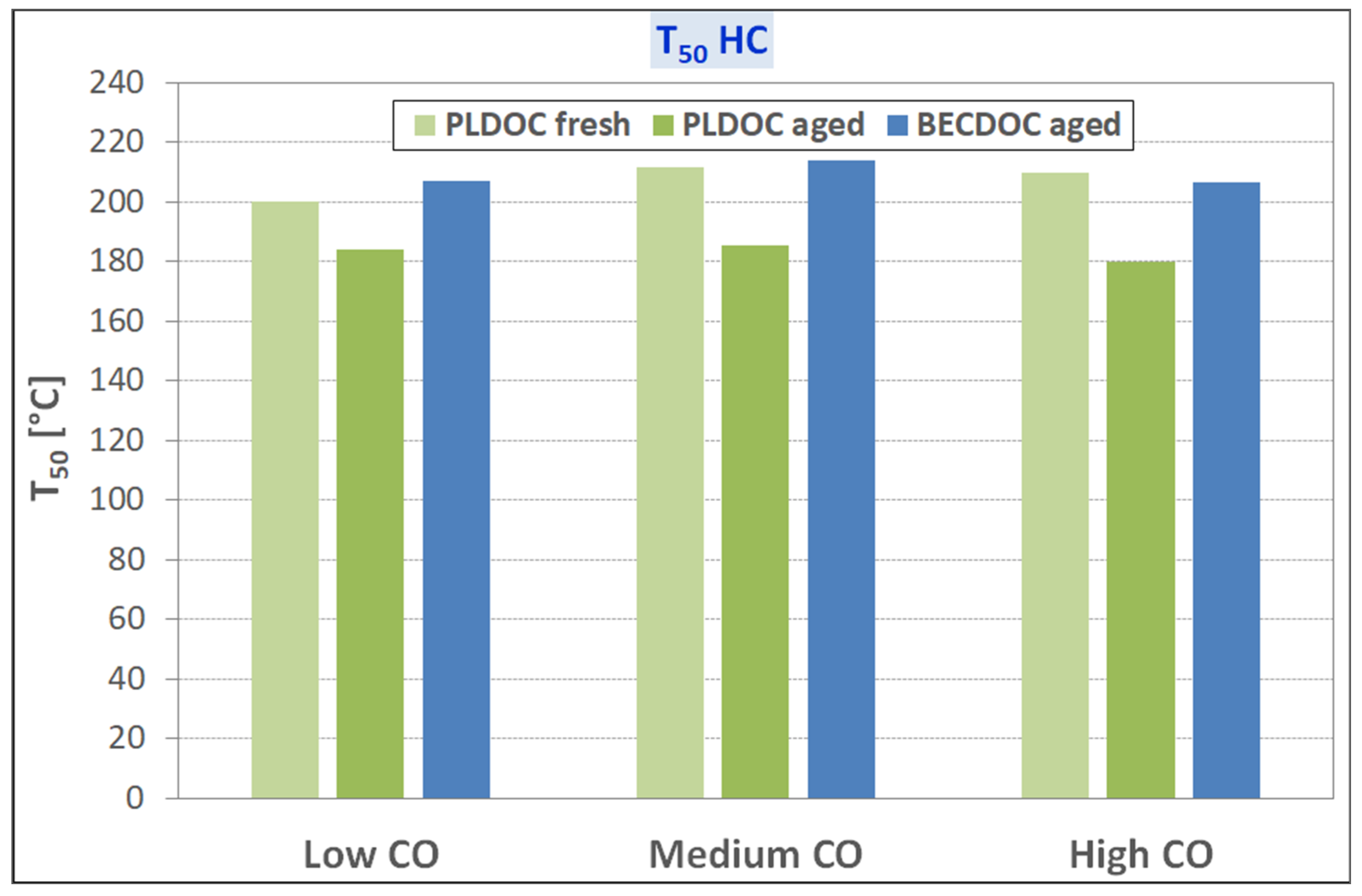
Considering the light-off temperatures for CO and unburned HC oxidation, the recycled catalysts have unusual behavior in terms of T50; the expected would be that T50 increases with temperature whereas the recycled catalysts show stable behavior. Further investigation is required in identifying some of the impurities that work as a stabilizer for the washcoat of the catalyst, preventing aggregation and or oxidation of the active catalytic sides and the needs of some refining of process parameters. Finally, PLDOC shows good overall performances that are comparable to commercial ones with similar loading.
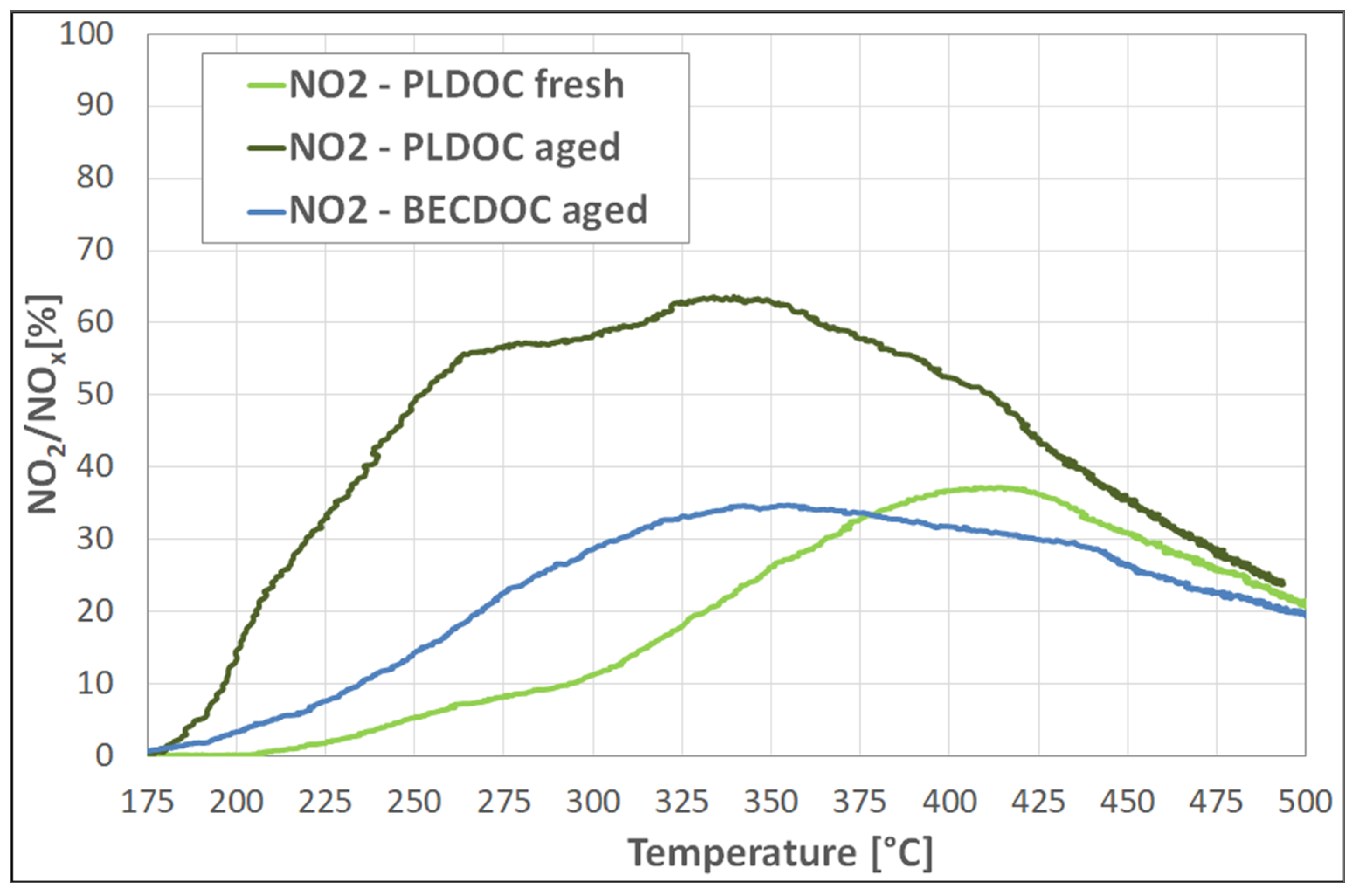
Another reaction taking place in the DOC catalysts is the NO oxidation towards NO
2. The concentration of NO
x is of major importance for the function of downstream components such as DPF and SCR. With the use of DOCs, the concentration of NO from the engine exhaust gas (which is about 90% in the untreated stream) can be reduced, leading to an increase of the NO
2/NO ratio by inducing thermodynamic equilibrium [
13,
16,
35]. Similarly to the catalytic performance results, aged PLDOC catalyst exhibited the best performance concerning the NO
2/NO
x ratio, amongst the catalyst investigated.
4. Materials and Methods
A series of small scale (monolithic carrot) autocatalysts was prepared starting from fresh PGMs (benchmark catalysts for performance comparison) and PGMs recycled through the PLATIRUS hydrometallurgical recycling route. The types of catalysts are Three-Way Catalytic Converters (for Petrol Engine Applications) and Oxidation Catalysts (for Diesel Engine applications), and the methods followed for their production are described below.
4.1. PGM Precursors
In the case of the benchmark catalysts, commercially available aqueous solutions of palladium (II) nitrate (Heraeus, solution assay 17.94 wt.%) and rhodium (III) nitrate (Hereaus, solution assay 9.27 wt.%) were used, while platinum (II) nitrate was used in powder form (58 wt.% provided by Yurui Chemical Co. Ltd., Shanghai, China). On the other hand, for the production of catalysts with recycled metals, the nitrate metal solutions came from the recovery of PGMs from secondary source materials.
The nitrate metal precursors that were used for the preparation of the catalysts from recycled PGMs were produced through the PLATIRUS project process and came from the recovery of PGMs from secondary source materials. The processes used for the recovery of PGMs are described in detail in a previous publication [
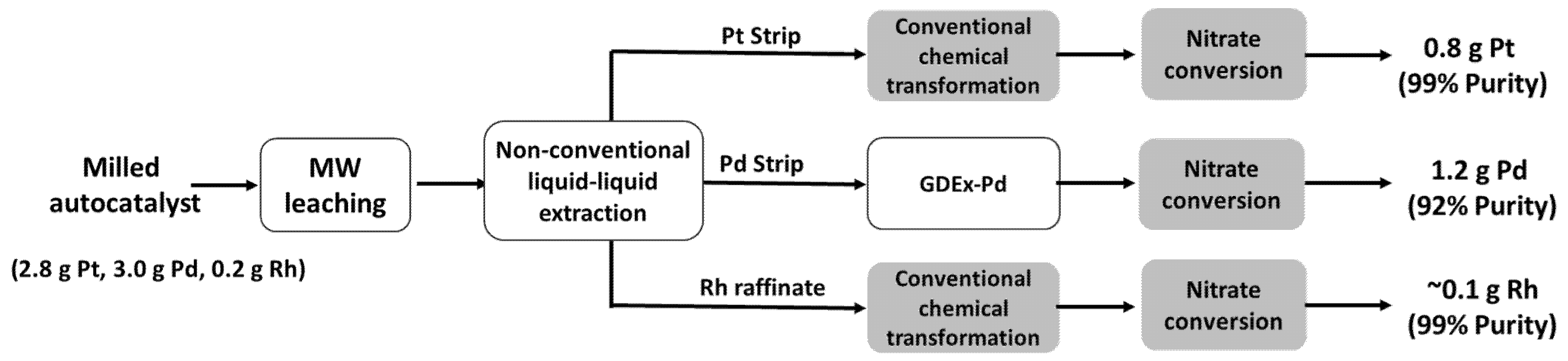
11]. In brief, the process started with a ~1.3 kg feedstock that consisted of a mixture of diesel oxidation catalysts and Three-Way Catalysts (milled, blended, and characterized). The resulting powder contained 2066 ± 24 ppm palladium, 2574 ± 15 ppm platinum, and 179 ± 5 ppm rhodium. Three technologies (namely microwave-assisted leaching [
36], non-conventional liquid-liquid extraction, and gas-diffusion electrocrystallisation) were selected and used in the context of the PLATIRUS project for the process of the feedstock, finally producing 1.2 g palladium, 0.8 g platinum, and 0.1 g rhodium in nitrate form with a purity of 92–99% (
Figure 14).
The metal nitrate solutions obtained with the PLATIRUS process are summarized in
Table 4.
The impurities, as determined by ICP analysis, were determined for the Pt and Pd samples (performed by Johnson Matthey, UK) and are presented in
Table 5 and
Table 6.
4.2. Catalyst Synthesis
The coating employed for the preparation of the catalysts was 5% Pd/Rh on CeO2/ZrO2 (75%, 25%) for the Three-Way Catalysts (TWC) and 5% Pt/Pd on CeO2/ZrO2 (75%, 25%) for the Diesel Oxidation Catalysts (DOC). The metal ratios were, respectively, Pd/Rh 55:5 and Pt/Pd 3:1 and are representative of commercial automotive catalysts.
The metal precursors used for the catalyst synthesis have been described in
Section 4.1. All the other chemicals used for the preparation of the catalysts in the present study, except the secondary PGMs precursors, were commercial. The chemical reagents used were: Ce
0.68Zr
0.32O
2 mixed inorganic oxide (CZ, Wanfeng Technology, Shaoxing, China), ammonium hydroxide solution (Merck, 25 wt.%) for pH adjustment, and γ-Al
2O
3 boehmite (Disperal P2, Sasol), as a binder for the impregnation of cordierites.
The catalytic powder was produced following the patented PROMETHEUS protocol [
37,
38]. According to the synthetic protocol, for the production of the benchmark catalysts, the commercially available metal precursors (Pt and Pd nitrates or Pd and Rh nitrates for DOC and TWC catalysts, respectively) were dissolved in water. In the case of catalysts synthesized by the recovered materials, the received metal nitrate solutions (
Table 4) were mixed. Then, the mixed inorganic oxide (CeO
2-ZrO
2) support was added under stirring at room temperature. The pH was continuously adjusted to acidic (≤4.0). The solvent (H
2O) was then removed by heating the mixture to 80–85 °C under continuous stirring. The resulting catalytic powders were dried at 105 °C and then calcined at 500 °C for 1 h.
Using the produced catalytic powders, the monolithic catalysts were then prepared by the wet impregnation method, as described in the following section.
4.3. Preparation of Monolithic Carrots
A state-of-the-art 900 cells per square inch (cpsi) thin-walled ceramic (cordierite) substrate with hexagonal cell shape was used for the preparation of the TWC catalysts. The cordierite had 2.5 inches length and 4.17 inches diameter. Three carrots were extracted from each cordierite using a cylindrical drill with the desired dimensions. The dimensions of the extracted carrots were 1.5 inches diameter and 2.5 inches length.
For DOC catalysts, a state-of-the-art 400 cpsi thin-walled ceramic (cordierite) substrate was used. The cordierite had 3.0 inches length and 4.6 inches diameter. Two carrots were extracted from this cordierite using the same procedure described previously. The dimensions of the carrots were 1.5 inches diameter and 2 inches length.
For the deposition of the previously synthesized catalyst on the walls of the aforementioned ceramic monolithic carrots, the slurry method was followed, as described in detail elsewhere [
11,
38]. The final loading of each synthesized catalyst was calculated by considering the final weight increase of the monolithic carrots. For each carrot, the dimensions (diameter and length) and weight were measured to achieve higher precision at the step of impregnation and the calculation of the final loading in g PGMs/ft
3. The carrots were used for determining the catalytic efficiency either as fresh samples or aged samples, following the procedures described in the next sections.
4.4. Ageing of Catalysts
One of the most common procedures to simulate the thermal ageing of a catalyst at a laboratory scale is using accelerated thermal treatments in humidified air flow. This approach is called hydrothermal ageing. In the present study, a tubular oven equipped with a heated gas line to flux the gases through the sample during the operation was employed. A peristaltic pump was used to add the water to the air stream. The system can be operated with a flexible air/H2O ratio and a maximum ageing temperature of 1200 °C.
4.5. Physicochemical Characterization of the Synthesized Catalysts
The prepared catalysts were characterized in terms of their metal loading and structural properties. The structural characteristics of the produced carrots were determined with the use of optical microscopy. In this respect, pieces of 2.5–3″ length were cut from the carrot bodies, to be examined. The samples for the structure analysis were cross-sectionally cut so that the monolith cells could be observed. A metallurgical microscope by AmScope (ME520 series) equipped with a microscope digital camera 14 MP ultrafine color was used for the analysis. The sample was placed under the objective lenses to be moved in the vertical direction to focus and obtain a resolution suitable for measuring the thickness of the washcoat.
The metal loading of the prepared catalysts was determined with X-ray Fluorescence Analysis of the catalytic powder using a Vanta Olympus (2017, Waltham, MA, USA) XRF Analyzer. It should be noted that, for more precise loading measurements, the XRF instrument was calibrated for each metal separately (additionally to the internal calibration of the instrument), as described elsewhere [
39]. The powder was obtained after milling and sieving (at particle sizes < 250 μm) of the pieces of the carrot body used for optical microscopy. Finally, the obtained powder was dried at 120 °C for 2 h before sample preparation and XRF analysis. For the analysis, appropriate amounts of catalyst powder (~5 g) were pressed inside polyethylene cups. Each sample was then analyzed with the measurement of 10 repeated scans (1 scan/90 s).
To evaluate the degradation of the catalytic performance induced by all the ageing sources, measurements of the specific surface area (SSA) and pore diameters of the fresh and aged samples was performed, employing nitrogen physisorption at liquid nitrogen temperature. A Micromeritics ASAP 2020 Accelerated Surface Area and Porosimetry System was used to measure the specific surface area (SSA) according to the BET equation. The ASAP 2020 analyzer is equipped with two independent vacuum systems, one for sample preparation and one for sample analysis. The measurements are obtained from the nitrogen adsorption–desorption isotherms at −196 °C. Prior to the determination of the specific surface area of the sample, a pre-treatment by heating under vacuum at a high temperature (90–350 °C), designated as outgassing, is done, to remove gases and vapors that may have become physically adsorbed. The SSA calculation was performed, taking into account 10 measurement points between 0.05 and 0.3 of relative pressure (P/P0). Pore size distribution was determined using the BJH calculation. This method, described by Barrett, Joyner, and Halenda, allows one to determine the modal and average diameter from desorption (BJH des) and adsorption (BJH ads) branch of the obtained isotherms, considering the capillary condensation in cylindrical pores.
4.6. Catalytic Efficiency Testing
The lab-scale testing bench for catalytic systems used for material characterization of all the after-treatment components (catalyst, substrates), is presented in
Figure 15. The synthetic mixture of gases was selected with a composition that mimics the real working conditions; N
2 was used as carrier gas, being an inert gas that does not participate in the catalytic reaction. All gaseous species (CO, HC, NO
x, SO
2) pass through a mixer with a controlled flow accurately measured with a single mass flow controller to control the correct concentration of all the species in the final gas mixture.
The synthetic gas mixture was heated up by passing through an in-line heater, while water was dosed through a peristaltic pump and injected in a hot gas stream through a steam generator. The catalyst sample (carrot) was installed in a heated sample holder. To monitor the temperature and the oxygen concentration in the gas stream, thermocouples were placed in the sample, and a wide-band oxygen sensor supplied by UEGO was placed before the sample holder.
The composition of the gases and the stream temperature can be modified according to the requirements of the tests to be carried out. The gas mixture was fluxed through the lab scale sample and analyzed using an FT-IR Multi-gas Analyser MKS 2030 HS for the simultaneous analysis of all chemical species involved, with an uncertainty of about ±5 ppm. The concentrations of the chemical species were recorded at the inlet and at the outlet of the sample, to evaluate the variation induced by the catalytic reactions.
Because, in the real exhaust gases, a large number of different hydrocarbon species can be found, these hydrocarbons may present different chemical activity inside the catalyst. HC used in the present study is a gas mixture of short-chain hydrocarbons, ethylene-propylene-methane (volumetric ratio C2H4/C3H6/CH4 = 3.5/1/1). This specification was defined after a characterization of the HC content in the real exhaust gases downstream of a representative diesel engine.
Typically, the sample’s dimensions were 1 to 1.5 inches diameter by 2 to 6 inches long; parts could be coated as cores or cut from full-scale monoliths. Cores from larger parts, so-called carrots, represent fully formulated catalysts ideally suited for laboratory reactors. During the tests, the temperature was controlled at the inlet, the outlet, and multiple positions throughout the sample.
The tests were performed according to well-established protocols to measure the light-off temperatures and conversion efficiency (ramp-up curves) of the pollutant gases as a function of temperature.
The volumetric flow rate of the reactants entering the sample is expressed as Space Velocity (SV) (units are h−1).
The testing conditions are summarized hereafter:
Space Velocity (SV) = 50.000 h−1
Maximum temperature: 500 °C
TWC tests: two levels of gases composition, varying the CO, HC, and NO concentrations (expressed in ppm) in the feed gas:
Slightly lean gas mixture, λ ≅ 1.02;
Slightly rich gas mixture, λ ≅ 0.99.
TWC tests: Oxygen Storage procedure: only CO and O2 in the feeding gas
DOC tests: three levels of gases composition, with different CO concentrations (expressed in ppm) in the feeding gas:
Low CO;
Medium CO;
High CO.
5. Conclusions
The present paper has reported the synthesis of new, small-scale catalytic monoliths (both TWCs and DOCs) with the direct use of PGMs containing nitrate solutions (containing identified impurities) coming from the recovery of spent autocatalysts. The catalytic performance of the samples was evaluated in comparison with catalysts produced using commercially available metal nitrate precursors. The catalysts were synthesized through the wet impregnation method, while, for the deposition of the washcoat on the ceramic cordierite cells, the slurry method was used. The synthesized materials were characterized in terms of metal loading, structural properties, and surface porosity. The study of the effect of thermal ageing revealed a detrimental decrease of the specific surface area caused by the sintering phenomena, upon exposure of the catalysts to high temperatures. The T50 values of the TWC samples obtained from recycled PGMs (T50~220 °C for CO oxidation, T~240 °C for HC oxidation, and T~250 °C for NO reduction) were better than those of TWC synthesized using commercial metal nitrates, and similar to the results previously obtained from commercial catalysts. The structural effects observed upon ageing were found to influence the catalytic efficiency of the TWCs, reducing their ability for the abatement of pollutant gases, while decreasing significantly the OSC of the catalysts. Similar results were obtained for the Diesel Oxidation Catalysts, as the samples prepared from recycled PGMs solutions, both fresh and aged, showed better performance than the reference samples prepared by commercial metal nitrates, concerning T50 values for CO, HC, and NO to NO2 oxidation reactions, reaching values similar to those reported for commercial DOC autocatalysts.