Simultaneous Galvanic Generation of Fe2+ Catalyst and Spontaneous Energy Release in the Galvano-Fenton Technique: A Numerical Investigation of Phenol’s Oxidation and Energy Production and Saving
Abstract
:1. Introduction
2. Results and Discussion
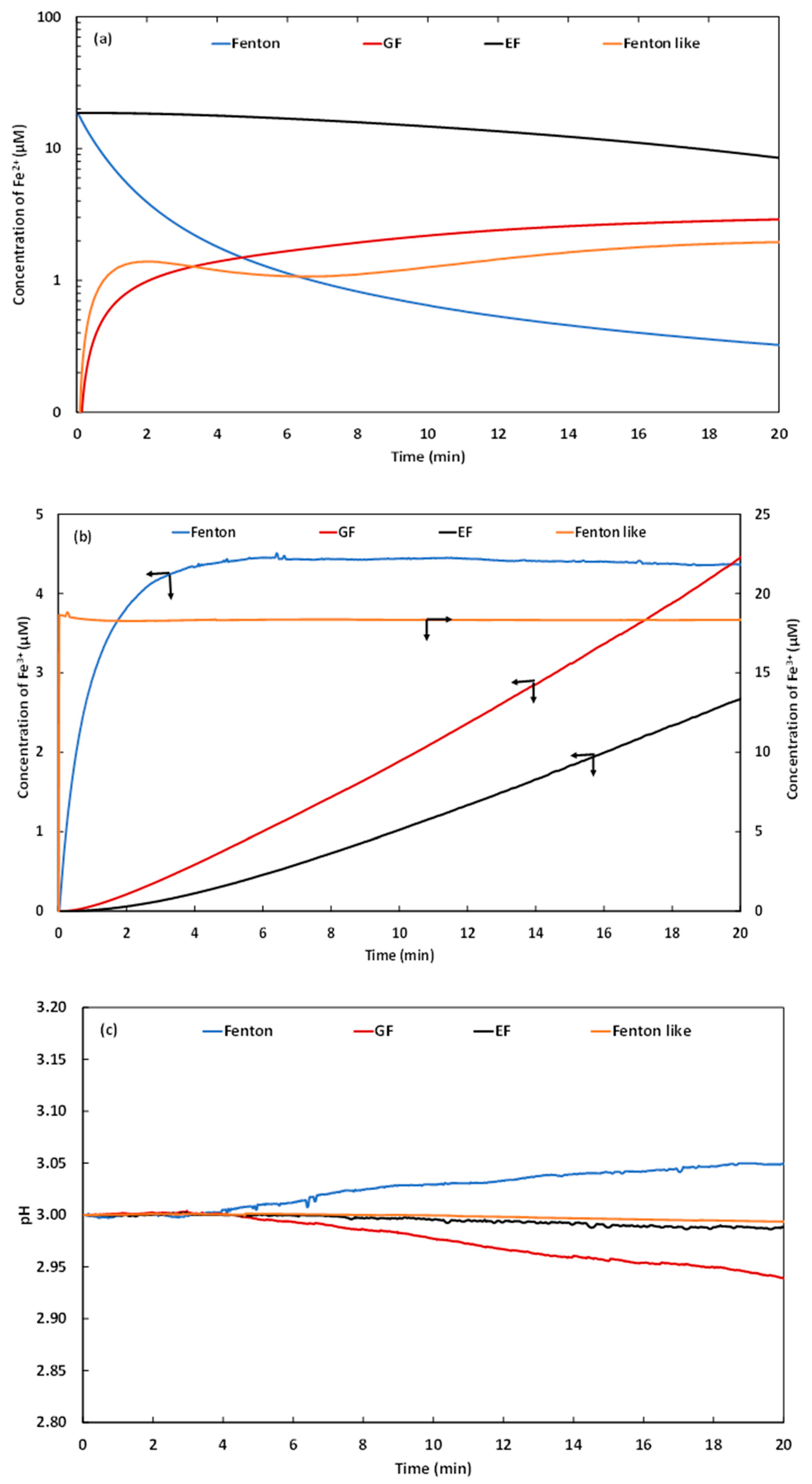
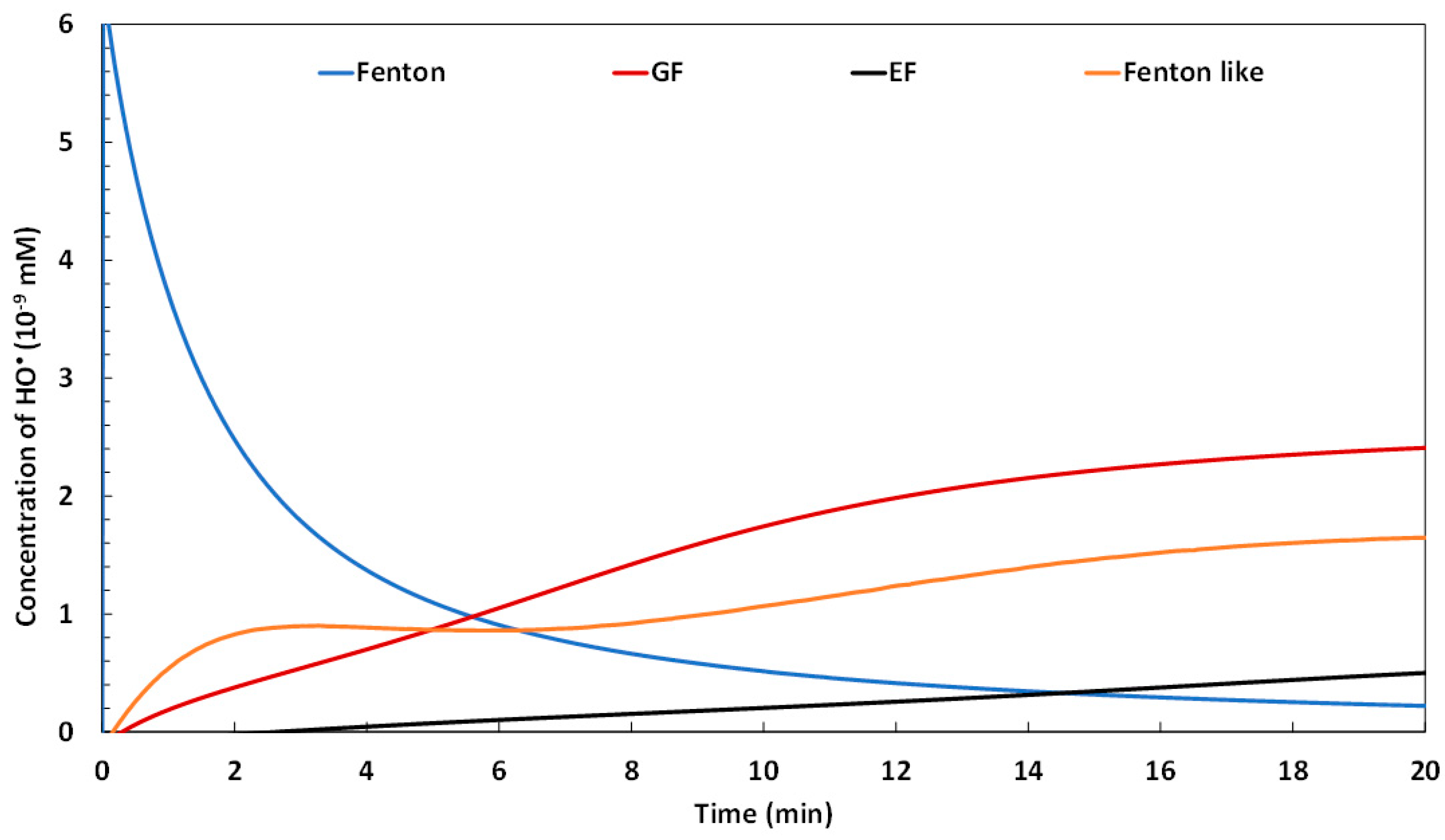
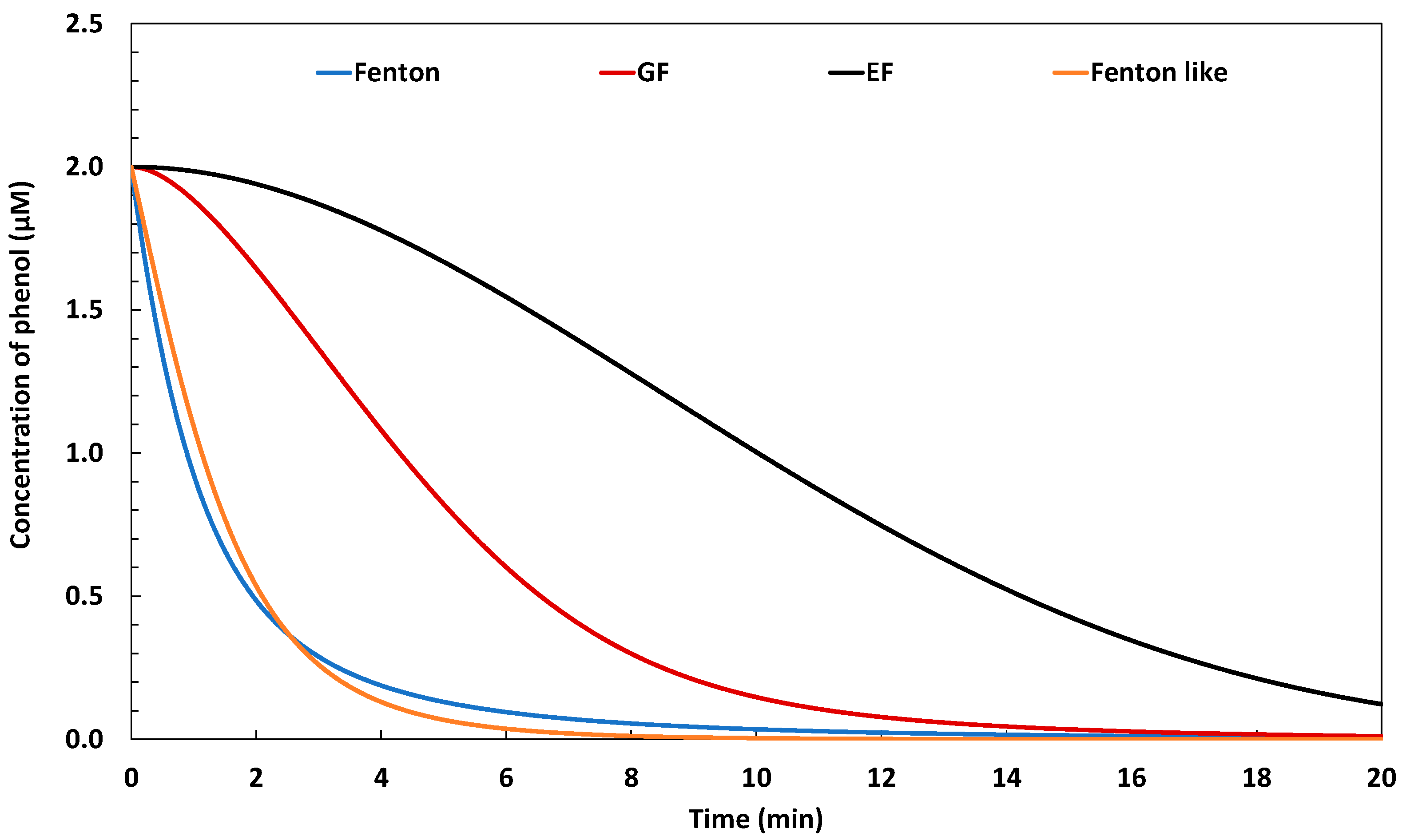
2.1. Comparison of the Performances of the Galvano-Fenton Technique to the Different Fenton-Based Processes
2.1.1. Evolution of Ionic Species
2.1.2. Evolution of Hydroxyl Radical Yield
2.1.3. Efficiency in Terms of Phenol Degradation
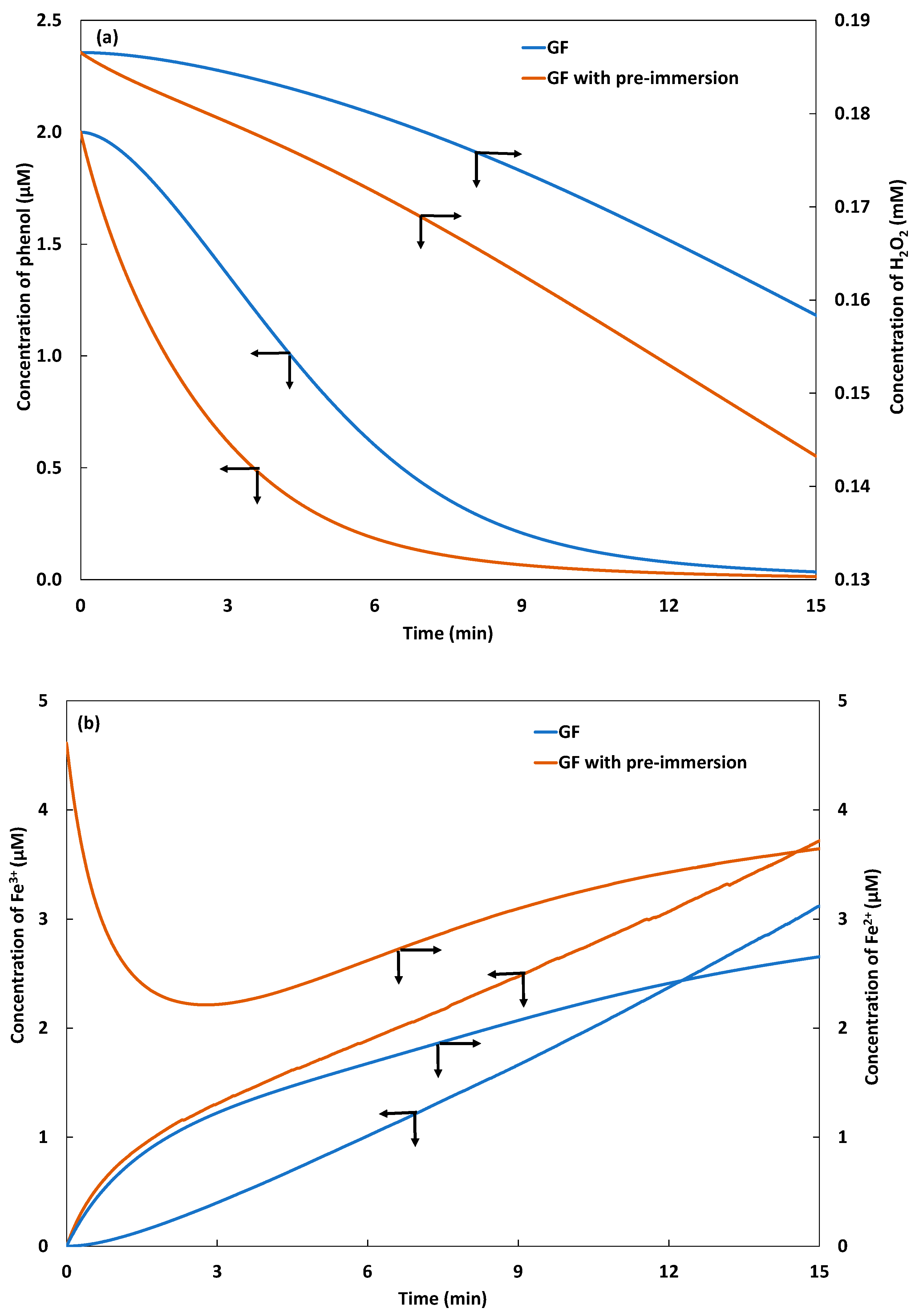
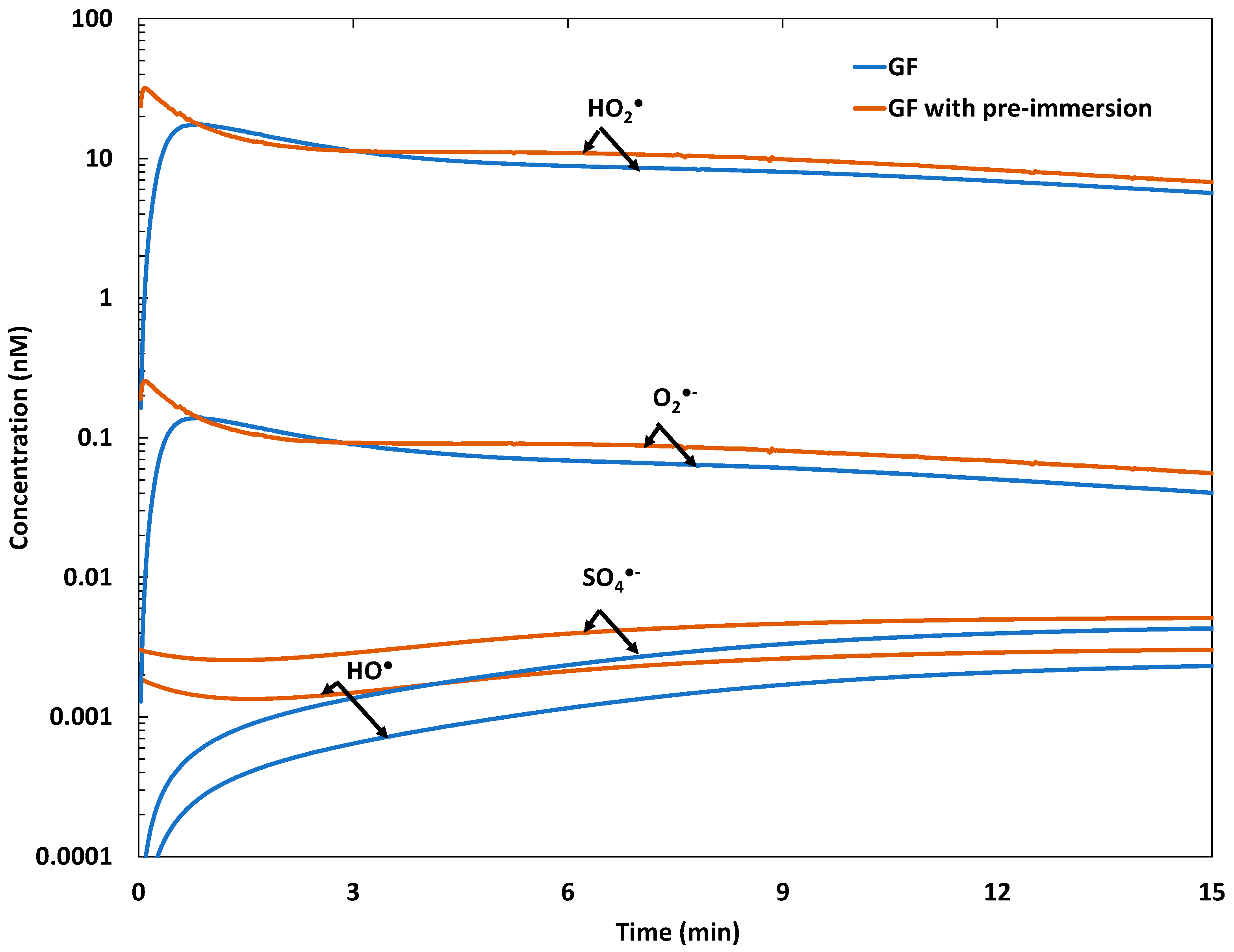
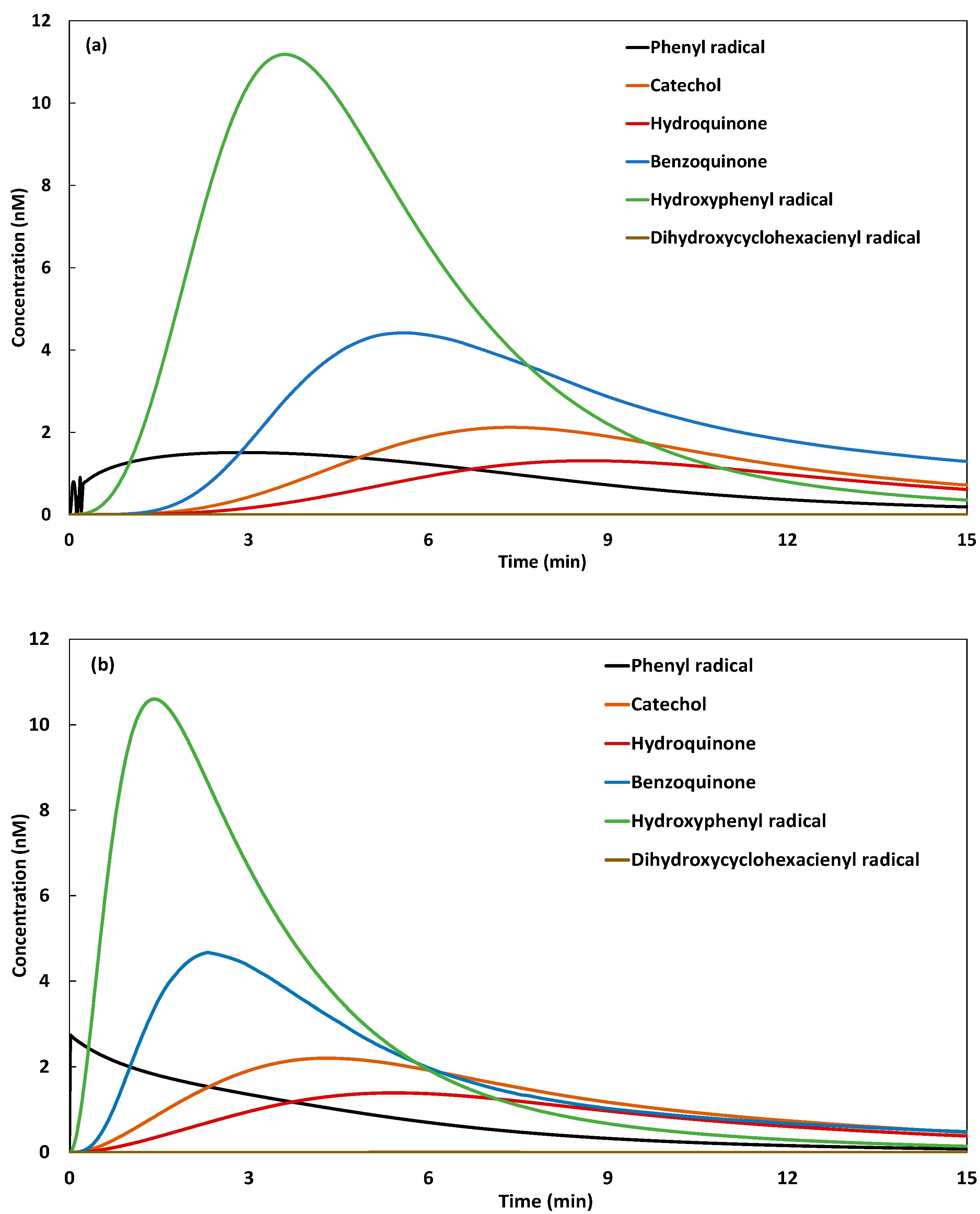
2.2. Galvano-Fenton and Pre-Immersion Effects
2.2.1. Fenton Reagent, Catalyst, and Phenol Degradation
2.2.2. Evolution of Free Radicals’ Yields
2.2.3. Mechanistic Insights
2.2.4. Energetic Insights
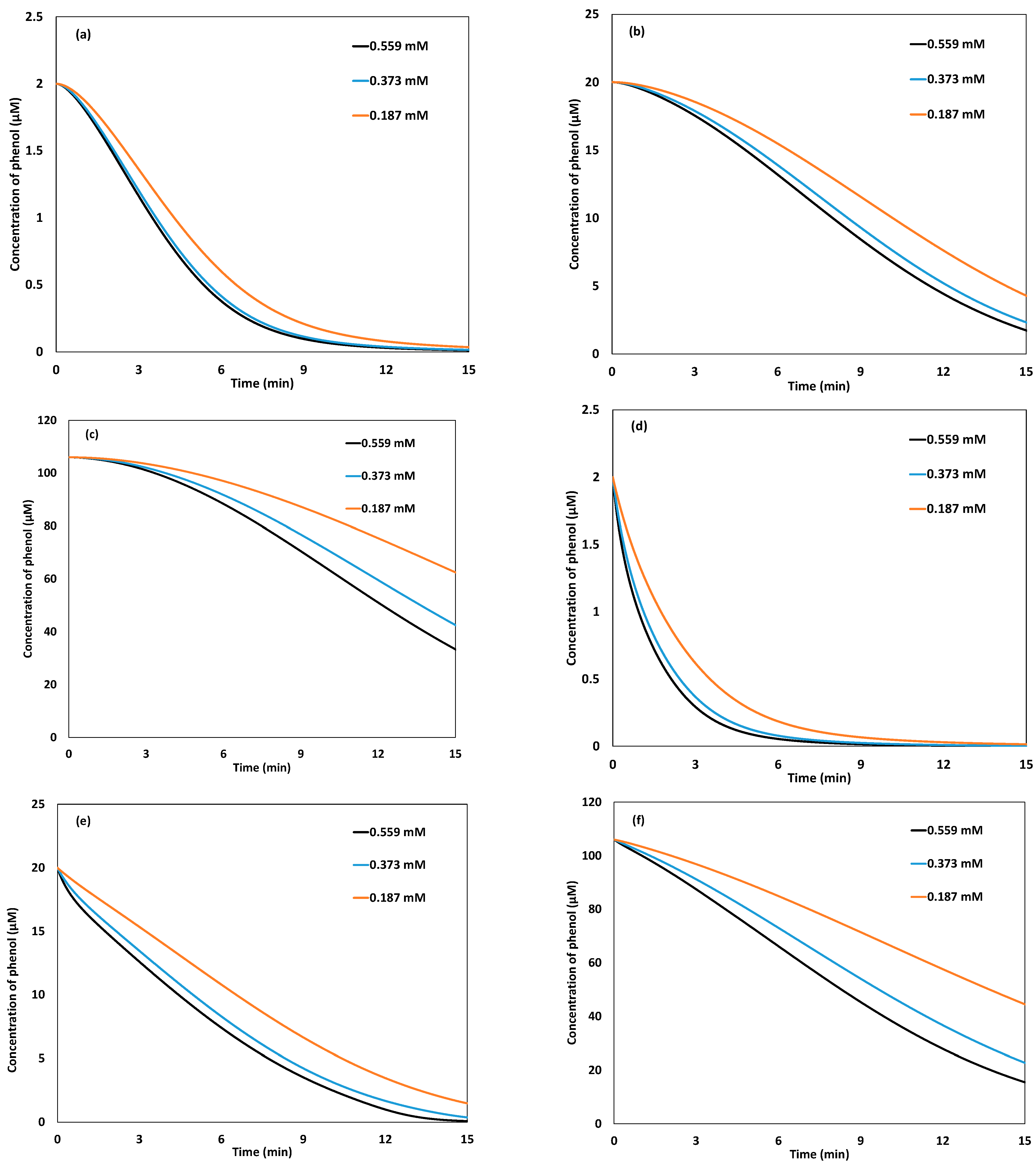
2.3. Parametric Analysis of Galvano-Fenton and Galvano-Fenton with Pre-Immersion Techniques
2.3.1. Effect of the Ratio of H2O2 to Total Released Iron
2.3.2. Effect of Phenol Concentration
3. Materials and Methods
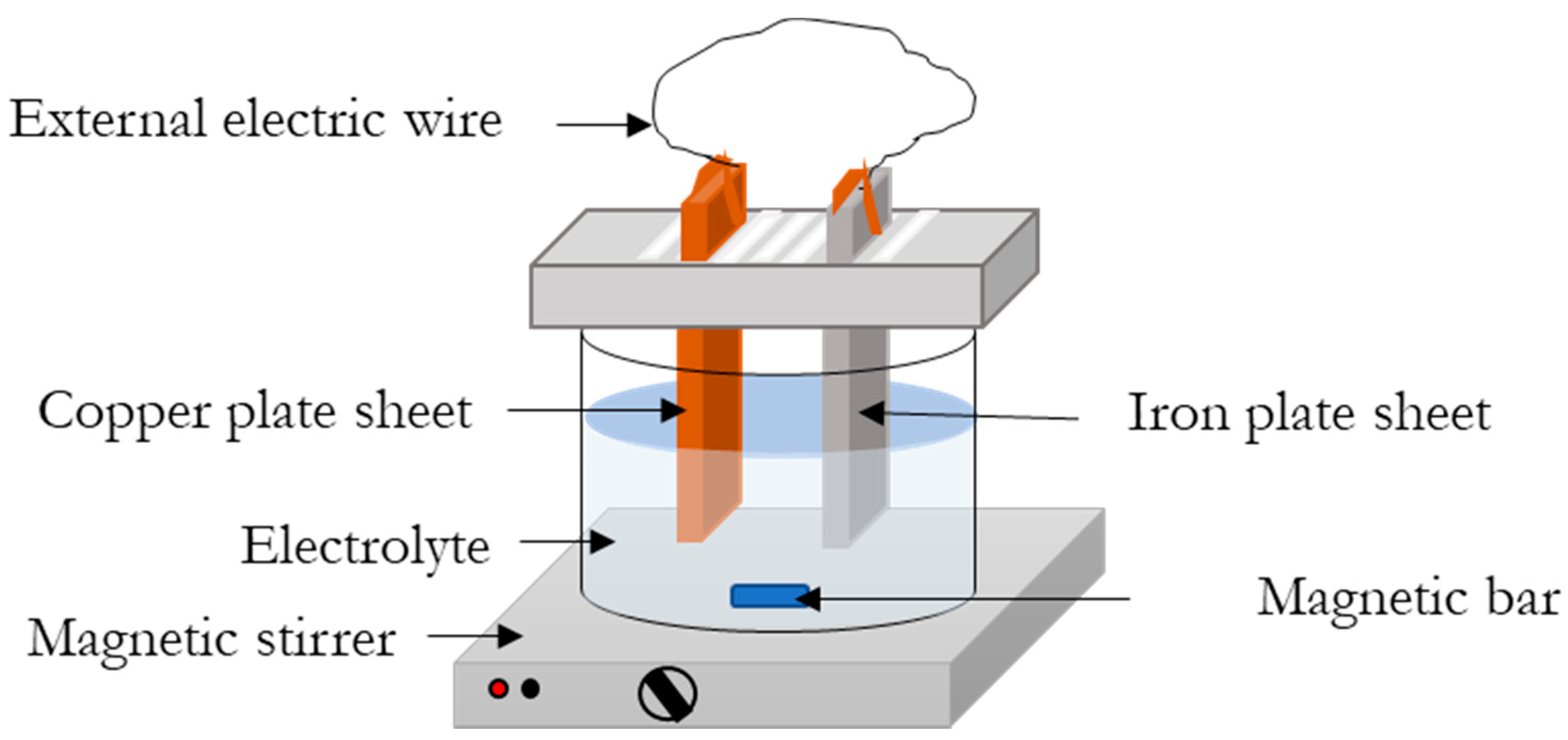
3.1. Processes’ Configuration
3.2. Numerical Approach
3.2.1. Galvano-Fenton Process
3.2.2. Galvano-Fenton Process with Pre-Immersion Time
3.2.3. Electro-Fenton Process
3.2.4. Classic Fenton and Fenton-Like Processes
3.2.5. Chemical Mechanism in the Electrolyte
| i | ith Reaction | ||
|---|---|---|---|
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| 8 | |||
| 9 | |||
| 10 | |||
| 11 | |||
| 12 | |||
| 13 | |||
| 14 | |||
| 15 | |||
| 16 | |||
| 17 | |||
| 18 | |||
| 19 | |||
| 20 | |||
| 21 | |||
| 22 | |||
| 23 | |||
| 24 | |||
| 25 | |||
| 26 | |||
| 27 | |||
| 28 | |||
| 29 | |||
| 30 | |||
| 31 | |||
| 32 | |||
| 33 | |||
| 34 | |||
| 35 | |||
| 36 | |||
| 37 | |||
| 38 | |||
| 39 | |||
| 40 | |||
| 41 | |||
| 42 | |||
| 43 | |||
| 44 | |||
| 45 | |||
| 46 | |||
| 47 | |||
| 48 | |||
| 49 |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Sarmento, A.P.; Borges, A.C.; de Matos, A.T.; Romualdo, L.L. Phenol degradation by Fenton-like process. Environ. Sci. Pollut. Res. 2016, 23, 18429–18438. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- De Laat, J.; Truong Le, G.; Legube, B. A comparative study of the effects of chloride, sulfate and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe(III)/H2O2. Chemosphere 2004, 55, 715–723. [Google Scholar] [CrossRef] [PubMed]
- De Laat, J.; Le, T.G. Effects of chloride ions on the iron(III)-catalyzed decomposition of hydrogen peroxide and on the efficiency of the Fenton-like oxidation process. Appl. Catal. B Environ. 2006, 66, 137–146. [Google Scholar] [CrossRef]
- De Laat, J.; Gallard, H. Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: Mechanism and kinetic modeling. Environ. Sci. Technol. 1999, 33, 2726–2732. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J. Hazard. Mater. 2010, 178, 202–208. [Google Scholar] [CrossRef]
- Martins, A.F.; Wilde, M.L.; Vasconcelos, T.G.; Henriques, D.M. Nonylphenol polyethoxylate degradation by means of electrocoagulation and electrochemical Fenton. Sep. Purif. Technol. 2006, 50, 249–255. [Google Scholar] [CrossRef]
- Tarkwa, J.B.; Oturan, N.; Acayanka, E.; Laminsi, S.; Oturan, M.A. Photo-Fenton oxidation of Orange G azo dye: Process optimization and mineralization mechanism. Environ. Chem. Lett. 2019, 17, 473–479. [Google Scholar] [CrossRef]
- Lu, M.; Wang, J.; Wang, Y.; He, Z. Heterogeneous photo-fenton catalytic degradation of practical pharmaceutical wastewater by modified attapulgite supported multi-metal oxides. Water 2021, 13, 156. [Google Scholar] [CrossRef]
- Monteil, H.; Péchaud, Y.; Oturan, N.; Oturan, M.A. A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Application to the treatment of pharmaceutical pollutants in water. Chem. Eng. J. 2019, 376, 119577. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Cihanoǧlu, A.; Gündüz, G.; Dükkanci, M. Influence of ultrasound on the heterogeneous Fenton-like oxidation of acetic acid. Water Sci. Technol. 2017, 76, 2793–2801. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current advances and trends in electro-Fenton process using heterogeneous catalysts—A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmann, S.; Keller, H. Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye Orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl. Catal. B Environ. 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Bremner, D.H.; Burgess, A.E.; Houllemare, D.; Namkung, K.C. Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl. Catal. B Environ. 2006, 63, 15–19. [Google Scholar] [CrossRef]
- GilPavas, E.; Correa-Sánchez, S.; Acosta, D.A. Using scrap zero valent iron to replace dissolved iron in the Fenton process for textile wastewater treatment: Optimization and assessment of toxicity and biodegradability. Environ. Pollut. 2019, 252, 1709–1718. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of pH on zero valent iron performance in heterogeneous Fenton and Fenton-like processes: A review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Li, Y.; Ji, J.; Wong, F.S.; Lu, X. Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: Kinetic, pathway and effect factors. Sep. Purif. Technol. 2008, 62, 551–558. [Google Scholar] [CrossRef]
- Haddour, N.; Kherrat, R. Procédé de Traitement d’un Effluent Liquide Comprenant un Polluant Organique, Assurant la Production D’énergie Électrique et Dispositif Associé. Patent WO2016097601, 18 December 2014. [Google Scholar]
- Gasmi, I.; Kerboua, K.; Haddour, N.; Hamdaoui, O.; Alghyamah, A.; Buret, F. Kinetic pathways of iron electrode transformations in Galvano-Fenton process: A mechanistic investigation of in-situ catalyst formation and regeneration. J. Taiwan Inst. Chem. Eng. 2020, 116, 81–91. [Google Scholar] [CrossRef]
- Gasmi, I.; Kerboua, K.; Haddour, N.; Hamdaoui, O.; Alghyamah, A.; Buret, F. The Galvano-Fenton process: Experimental insights and numerical mechanistic investigation applied to the degradation of acid orange 7. Electrochim. Acta 2021, 373. [Google Scholar] [CrossRef]
- Cook, W.G.; Olive, R.P. Pourbaix diagrams for the iron-water system extended to high-subcritical and low-supercritical conditions. Corros. Sci. 2012, 55, 326–331. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Li, X.; Li, F.; Zhou, S. Photodegradation of 2-mercaptobenzothiazole in the γ-Fe2O3/oxalate suspension under UVA light irradiation. J. Hazard. Mater. 2008, 153, 426–433. [Google Scholar] [CrossRef]
- Chen, H.Y. Why the Reactive Oxygen Species of the Fenton Reaction Switches from Oxoiron(IV) Species to Hydroxyl Radical in Phosphate Buffer Solutions? A Computational Rationale. ACS Omega 2019, 4, 14105–14113. [Google Scholar] [CrossRef] [Green Version]
- Gasmi, I.; Haddour, N.; Hamdaoui, O.; Kerboua, K.; Alghyamah, A.; Buret, F. A Novel Energy-from-Waste Approach for Electrical Energy Production by Galvano—Fenton Process. Molecules 2021, 26, 4013. [Google Scholar] [CrossRef]
- Ahmad, Z. Corrosion kinetics. In Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann: Oxford, UK, 2006; pp. 57–119. [Google Scholar] [CrossRef]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. Oxygen Solubility, Diffusion Coefficient, and Solution Viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Qiu, S.; He, D.; Ma, J.; Liu, T.; Waite, T.D. Kinetic Modeling of the Electro-Fenton Process: Quantification of Reactive Oxygen Species Generation. Electrochim. Acta 2015, 176, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Thirugnanasambandham, K.; Kandasamy, S.; Sivakumar, V.; Kumar, R.K.; Mohanavelu, R. Modeling of by-product recovery and performance evaluation of Electro-Fenton treatment technique to treat poultry wastewater. J. Taiwan Inst. Chem. Eng. 2015, 46, 89–97. [Google Scholar] [CrossRef]
- Ting, W.; Lu, M.; Huang, Y. The reactor design and comparison of Fenton, electro-Fenton and photoelectro-Fenton processes for mineralization of benzene sulfonic acid (BSA). J. Hazard. Mater. 2008, 156, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. The Basics of Reaction Kinetics for Chemical Reaction Engineering. In Fundamentals of Chemical Reaction Engineering; McGraw Hill: New York, NY, USA, 2003; pp. 1–52. Available online: http://resolver.caltech.edu/CaltechBOOK:2003.001 (accessed on 1 August 2021).
- Koprivanac, N.; Loň, A. Photo-assisted Fenton type processes for the degradation of phenol: A kinetic study. J. Hazard. Mater. 2006, 136, 632–644. [Google Scholar] [CrossRef]
- Bray, W.C. The mechanism of reactions in aqueous solution Examples Involving Equilibria and Steady States. Chem. Rev. 1931, 10, 161–177. [Google Scholar] [CrossRef]
- Machulek, A., Jr.; Moraes, J.E.F.; Okano, L.T.; Silvério, C.A.; Quina, F.H. Photolysis of ferric ions in the presence of sulfate or chloride ions: Implications for the photo-Fenton process. Photochem. Photobiol. Sci. 2009, 8, 985–991. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerboua, K.; Hamdaoui, O.; Haddour, N.; Alghyamah, A. Simultaneous Galvanic Generation of Fe2+ Catalyst and Spontaneous Energy Release in the Galvano-Fenton Technique: A Numerical Investigation of Phenol’s Oxidation and Energy Production and Saving. Catalysts 2021, 11, 943. https://doi.org/10.3390/catal11080943
Kerboua K, Hamdaoui O, Haddour N, Alghyamah A. Simultaneous Galvanic Generation of Fe2+ Catalyst and Spontaneous Energy Release in the Galvano-Fenton Technique: A Numerical Investigation of Phenol’s Oxidation and Energy Production and Saving. Catalysts. 2021; 11(8):943. https://doi.org/10.3390/catal11080943
Chicago/Turabian StyleKerboua, Kaouther, Oualid Hamdaoui, Naoufel Haddour, and Abdulaziz Alghyamah. 2021. "Simultaneous Galvanic Generation of Fe2+ Catalyst and Spontaneous Energy Release in the Galvano-Fenton Technique: A Numerical Investigation of Phenol’s Oxidation and Energy Production and Saving" Catalysts 11, no. 8: 943. https://doi.org/10.3390/catal11080943
APA StyleKerboua, K., Hamdaoui, O., Haddour, N., & Alghyamah, A. (2021). Simultaneous Galvanic Generation of Fe2+ Catalyst and Spontaneous Energy Release in the Galvano-Fenton Technique: A Numerical Investigation of Phenol’s Oxidation and Energy Production and Saving. Catalysts, 11(8), 943. https://doi.org/10.3390/catal11080943








